Abstract
Setting: All health centres in Macenta District, rural Guinea.
Objective: To compare stock-outs of vaccines, vaccine stock cards and the administration of various childhood vaccines across the pre-Ebola, Ebola and post-Ebola virus disease periods.
Design: This was an ecological study.
Results: Similar levels of stock-outs were observed for all vaccines (bacille Calmette–Guérin [BCG], pentavalent, polio, measles, yellow fever) in the pre-Ebola and Ebola periods (respectively 2760 and 2706 facility days of stock-outs), with some variation by vaccine. Post-Ebola, there was a 65-fold reduction in stock-outs compared to pre-Ebola. Overall, 24 facility-months of vaccine stock card stock-outs were observed during the pre-Ebola period, which increased to 65 facility-months of stock-outs during the Ebola outbreak period; no such stock-out occurred in the post-Ebola period. Apart from yellow fever and measles, vaccine administration declined universally during the peak outbreak period (August–November 2014). Complete cessation of vaccine administration for BCG and a prominent low for polio (86% decrease) were observed in April 2014, corresponding to vaccine stock-outs. Post-Ebola, overall vaccine administration did not recover to pre-Ebola levels, with the highest gaps seen in polio and pentavalent vaccines, which had shortages of respectively 40% and 38%.
Conclusion: These findings highlight the need to sustain vaccination activities in Guinea so that they remain resilient and responsive, irrespective of disease outbreaks.
Keywords: health systems strengthening, SORT IT, prevention, operational research, health service utilisation
Abstract
Contexte: Tous les centres de santé de la Préfecture de Macenta, en Guinée rural.
Objectif: Comparer la rupture en vaccins, en cartes de stock de vaccins et l'administration des différents vaccins d'enfance pendant les périodes pré-Ebola, Ebola et post-Ebola.
Schéma: Une étude écologique.
Résultats: Des niveaux similaires de rupture étaient observés pour tous les vaccins (bacille Calmette-Guérin [BCG], pentavalent, polio, rougeole, fièvre jaune) dans les périodes pré-Ebola et Ebola (respectivement 2760 et 2706 jours-structure de rupture), avec quelques variations par vaccin. Post-Ebola, il y avait 65 fois plus de réduction en rupture, comparé à la période pré-Ebola. Un total de 24 mois-structure de rupture en cartes de stock de vaccins était observé pendant la période pré-Ebola, qui a augmenté à 65 mois-structure de rupture pendant la période Ebola ; une telle rupture ne s'est pas produite dans la période post-Ebola. Excepté la fièvre jaune et la rougeole, l'administration de vaccin a diminué universellement pendant la période de pointe de l'épidémie (août–novembre 2014). L'arrêt complet de l'administration de vaccin pour le BCG et une baisse marquée pour la polio (diminution de 86%) étaient observés en avril 2014, correspondant à une rupture de vaccins. Post-Ebola, l'administration globale de vaccins n'a pas atteint les niveaux pré-Ebola, avec les plus grands écarts observés aux niveaux de la polio et du pentavalent (respectivement des baisses de 40% et 38%).
Conclusion: Ces résultats soulignent le besoin de maintenir les activités de vaccination en Guinée afin qu'elles restent résilientes et réactives, indépendamment de l'épidémie d'une maladie.
Abstract
Marco de referencia: Todos los centros de atención de salud del distrito de Macenta en una zona rural de Guinea.
Objetivo: Comparar el desabastecimiento de vacunas, las tarjetas de existencias de vacunas y la administración de las diversas vacunas de la infancia durante diferentes períodos, en función de la epidemia de fiebre hemorrágica del Ébola, a saber: antes, durante el brote y después del mismo.
Método: Un estudio ecológico.
Resultados: Se observaron niveles equivalentes de desabastecimientos de todas las vacunas (BCG, pentavalente, antipoliomielítica, antisarampionosa y antiamarílica) antes de la epidemia del Ébola y durante la misma (2760 y 2706 días de desabastecimiento por establecimiento, respectivamente), con alguna variación en función de las vacunas. En el período posterior a la epidemia se presentó una tasa de desabastecimientos 65 veces menor, en comparación con el período anterior a la epidemia. En general, se observaron 24 meses-centro de desabastecimiento en las tarjetas de existencias vacunales durante el período pre-Ébola, que aumentaron a 65 meses-centro de desabastecimiento durante la epidemia; en el período posterior al brote no ocurrió este tipo de desabastecimiento. Con la excepción de la vacuna antiamarílica y la antisarampionosa, la administración de vacunas disminuyó globalmente durante el período de máxima actividad de la epidemia (de agosto a noviembre del 2014). Se observó una interrupción total de la administración de BCG y una tasa considerablemente baja de administración de vacuna antipoliomielítica (disminución de un 86%) en abril del 2014, que correspondió con el desabastecimiento de vacunas. Después de la epidemia del Ébola, la administración general de vacunas no recuperó el nivel anterior al brote y las mayores carencias se observaron con la vacuna antipoliomielítica y la pentavalente (40% y 38% de déficit, respectivamente).
Conclusión: Los resultados del presente estudio destacan la necesidad de sostener las actividades de vacunación en Guinea, de manera que conserven su capacidad de recuperación y de respuesta, con independencia de los brotes epidémicos.
The 2014–2015 Ebola virus disease outbreak in West Africa was the worst in history.1,2 Guinea was one of the most affected countries, with a total of 3811 Ebola cases and 2543 deaths.2
Assuring the health of children requires the provision of curative and preventive services through functional and accessible health facilities. Several West African studies have reported that the Ebola outbreak was responsible for significant disruptions to the provision of health care3–6 and an up to four-fold increase in avoidable all-cause mortality, mostly in children aged <5 years.7 Substantial reductions in the administration of anti-malarials have also been reported.3 Less information is available on the impact of the Ebola outbreak on preventive child health services, such as vaccination; the one published study assessing vaccine administration in relation to the Ebola outbreak identified a significant decline in vaccine uptake in the Forest Region of Guinea.8
Vaccinating all children against vaccine-preventable diseases through implementation of the Expanded Programme on Immunization (EPI) is a national health priority in Guinea.9 Maintaining vaccination activity during sustained infectious disease outbreaks is vital to prevent secondary epidemics of vaccine-preventable diseases and associated mortality.10 Thus, understanding the patterns of childhood vaccination during the Ebola outbreak is important for guiding the health system response in future epidemics.
Various factors, such as fear of contracting Ebola in health facilities, health care worker (HCW) deaths and community distrust, may have affected vaccination uptake in children.10–13 It is also possible that vaccination, much of which relies on the use of needles, was hampered because HCWs were instructed to minimise or avoid using needles where possible during the Ebola outbreak.12,13 Furthermore, HCW availability for vaccination outreach activities may have been compromised due to the need to repurpose health facility staff to Ebola-related activities. Finally, shifts in priority in national logistics towards Ebola control efforts might have negatively influenced vaccine procurement, the supply system and the cold chain, resulting in stock-outs.
A rapid method of quantifying the impact of the Ebola outbreak on childhood vaccination is to assess the trend in vaccination activity and vaccine stock-outs across the pre-Ebola, Ebola and post-Ebola periods. We thus assessed these parameters in relation to vaccination of children aged <5 years at the health facility and community levels in Macenta District, which had the highest Ebola caseload in Guinea.
METHODS
Study design
This was an ecological study.
Setting
General setting
Guinea, located in West Africa, is bordered by six countries, two of which, Sierra Leone and Liberia, recorded Ebola cases during the 2014–2015 outbreak. Guinea is a low-income country with a population of nearly 11 million, of whom 17% are aged <5 years. Under-five mortality was estimated to be 100 per 10 000 population in 2013.14 Vaccination of children is guided by the EPI programme,9 and is offered at most health facilities, regardless of level, including community-based outreach activities. Vaccination coverage was estimated at 62% for measles and 63% for DTP3 (diphtheria, pertussis, tetanus), HepB3 (hepatitis type B), and Hib3 (Haemophilus influenzae type B) in 2013.14
Specific setting
The study was conducted in Macenta District, one of 35 rural districts in southern Guinea, with a population of 278 456, which is composed of 13 subdistricts and the urban town of Macenta. The district health system consists of 17 health centres and one district hospital.
The first Ebola case in Macenta District was reported on 14 March 2014 and the last case occurred on 25 February 2015.15 After Guinea was declared Ebola free by the World Health Organization (WHO) on 29 December 2015, a few sporadic cases were reported in 2016 in a different district, leading to a new official declaration of the outbreak's end on 1 June 2016.16 Macenta District reported a total of 717 confirmed cases and a case fatality rate of 66%. Thirteen HCWs were infected in the district, with 11 deaths.15
Vaccination activities in Macenta District
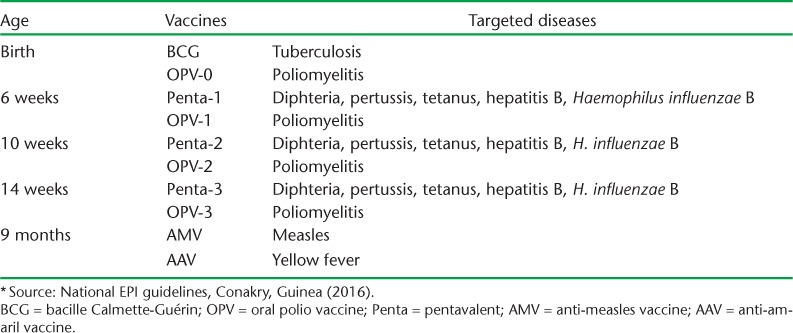
EPI activities are conducted according to national guidelines9 and offered in all 17 health centres in the district (the district hospital did not conduct vaccination). The national childhood vaccination schedule is shown in Table 1. In Guinea, the vaccination strategy involves both fixed (i.e., facility-based) and outreach activities in order to target universal coverage. Mass vaccination campaigns are occasionally conducted, but none were held during the Ebola outbreak.17 The vaccine supply chain originates at the central pharmacy located in Conakry, and is sequentially linked to all district pharmacies and health facilities. The supply chain follows the same vaccine supply link, and at the health facility level there are solar or kerosene-powered refrigerators. Routine vaccination activities are performed by health professionals and trained vaccinators.
TABLE 1.
Expanded Programme of Immunization (EPI * ) schedule in Guinea
Study population and period
We captured data from all 17 public health centres conducting vaccination in Macenta District over three distinct periods: the pre-Ebola (1 March 2013–28 February 2014), Ebola (1 March 2014–28 February 2015) and post-Ebola (1 March 2016–31 July 2016) periods. The pre-Ebola and Ebola periods were defined according to how the outbreak was experienced in Macenta District. The post-Ebola period refers to the country situation, based on the WHO's first official declaration for the end of the outbreak in Guinea.
Data variables, validation and analysis
The data on routine vaccination activities are compiled in standardised vaccination tally sheets, which are collated at health facility level and reported to the district health office on a monthly basis. The health district committee supervises EPI activities and prepares monthly district-level reports. The data abstracted for the current analysis included the number of vaccine doses administered per month, the number of days of vaccine stock-outs per month and whether or not there were stock-outs of the vaccine stock cards (used to document vaccine stocks at facility level) each month. The data were collected for five vaccines: oral polio (for poliomyelitis), pentavalent (for diphteria, pertussis, tetanus, hepatitis B and Haemophilus influenzae type B), bacille Calmette-Guérin (BCG, for tuberculosis), measles, and yellow fever. Data on the Ebola cases were collected from national Ebola reports.
The data were entered into an EpiData database, v. 2.0.7.22 for entry (EpiData Association, Odense, Denmark) and cross-checked with health facility vaccination registers and tally sheets. Statistics on Ebola were sourced from the national Ebola situation report.15 The data were collected from May to July 2016.
Vaccine stock-outs were measured in terms of stock-outs per facility per day (facility-days of stock-outs), and stock-outs of the vaccine stock card per facility (facility-month of stock-outs). Comparisons between pre-Ebola and Ebola periods included all months of data, whereas comparisons between the pre- and post-Ebola periods were limited to 5 months of the same period of each respective year, which limited the possible influence of seasonal differences. Trends were explored separately by vaccine strategy (facility-based vs. community) to determine whether the two strategies were differentially impacted by the outbreak. Mean numbers of vaccine doses administered per month were compared between the pre-Ebola and the post-Ebola periods to identify any recovery gap (failure to regain the pre-Ebola level). Differences in the means were estimated using paired sample t-tests, with a level of significance set at P ≤ 0.05 and a 95% confidence level (CI).
Ethics approval
Ethics approval was obtained from the National Ethics Committee in Health Research of Guinea, Conakry, Guinea. The study fulfilled the exemption criteria of the Ethics Review Board (ERB) of Médecins Sans Frontières (MSF, Geneva, Switzerland) for a posteriori analysis of routinely collected data, and thus did not require MSF ERB review. Approval was also received from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. As this was an aggregate data review study with anonymised data, informed patient consent was not required.
RESULTS
Stock-outs of vaccine stock cards and vaccines
Table 2 shows the duration of vaccine stock card and vaccine stock-outs during the pre-Ebola, Ebola and post-Ebola periods. Respectively four and 10 health centres experienced stock-outs of stock cards in the pre-Ebola and Ebola periods. This represented a total of 24 facility-months of vaccine stock card stock-outs across all vaccine types during the pre-Ebola period, which increased during the Ebola outbreak to 65 facility-months of stock-outs (a 2.7 fold increase). In the post-Ebola period, there were no vaccine stock card stock-outs.
TABLE 2.
Indication of stock-outs of vaccine stock cards and duration of vaccine stock-outs during the pre-Ebola, Ebola and post-Ebola periods, Macenta District, Guinea, March 2013–July 2016
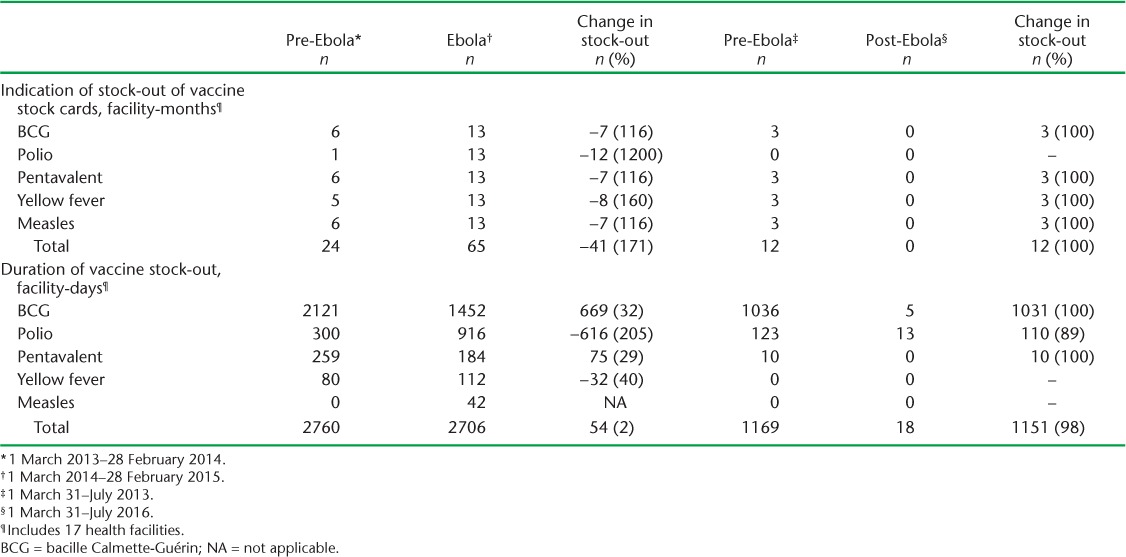
All 17 health centres experienced a vaccine stock-out in the pre-Ebola and Ebola periods, whereas post-Ebola only two reported a vaccine stock-out. Vaccine stock-outs were observed for BCG, polio, pentavalent and yellow fever in the pre-Ebola period, representing a total of 2760 facility-days of total vaccine stock-out. A similar number of facility-days of total vaccine stock-outs were observed during the Ebola period (2706 facility-days). Total vaccine stock-out during the post-Ebola period, at 18 facility-days, was dramatically lower than in the pre-Ebola period, representing a 65-fold decrease.
The duration of BCG vaccine stock-out was most prominent in the pre-Ebola period, contributing to 2121 (77%) of the total 2760 facility days of vaccine stock-outs. BCG and polio were the most important contributors to the total vaccine stock-out in the Ebola outbreak period, with BCG contributing 1452 (54%) and polio 916 (34%) of total 2706 facility-days of stock-outs.
Trends in vaccine administration in health facilities
Figure 1 shows the monthly trends in under-five vaccinations by vaccine type administered at health-facility level during the pre-Ebola, Ebola and post-Ebola periods. Except for yellow fever and measles vaccines, the trend of administration for all vaccines varied across these time periods, with an overall decline seen around the peak period of the outbreak. For BCG, two lows indicating complete cessation of vaccine administration occurred in both the pre-Ebola and Ebola periods, which corresponded to vaccine stock-outs in all 17 health centres. For polio, two prominent troughs were observed during the Ebola period, also corresponding to vaccine stock-outs in respectively 12 and 11 health centres. In the first trough (April 2014), the average monthly number of polio vaccines administered decreased to 478 from an average of 3503 in the pre-Ebola period (an 86% decrease), recovered shortly thereafter, but was followed by a similar reduction in August 2014.
FIGURE 1.
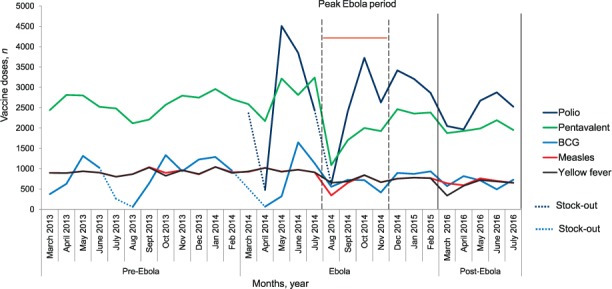
Trends in vaccination administration by vaccine type for children aged <5 years in health facilities during the pre-Ebola, Ebola and post-Ebola periods, Macenta District, Guinea, March 2013–July 2016. BCG = bacille Calmette-Guérin.
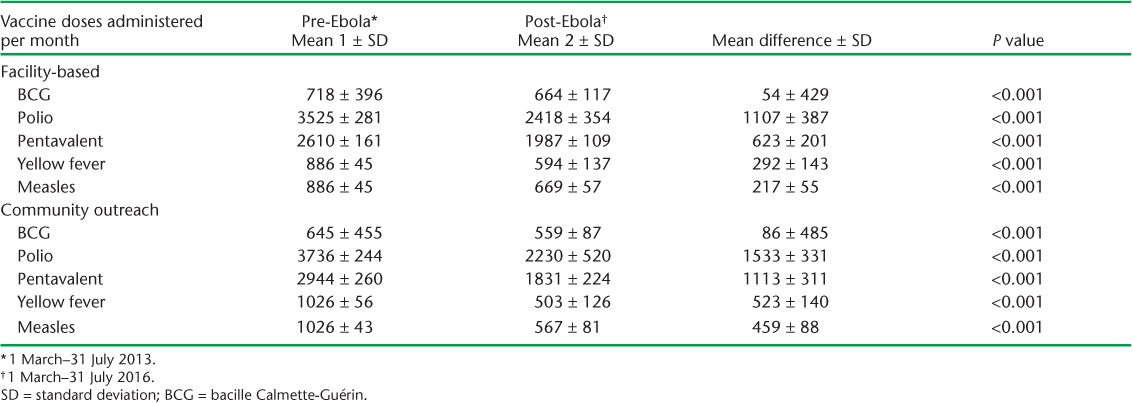
The mean number of vaccines administered per month at health facilities during the pre-Ebola period was significantly higher for all vaccine types compared to the post-Ebola period (Table 3), with the largest mean difference across the two periods observed for polio, at 1107.
TABLE 3.
Mean vaccine doses administered per month during the pre-Ebola and post-Ebola periods, Macenta District, Guinea, March 2013–July 2016
Trends in vaccine administration in communities
The monthly trends in under-five vaccinations by type of vaccine in the community during the pre-Ebola, Ebola and post-Ebola periods are shown in Figure 2. Overall, the trends in vaccine administration at the community level were similar to those observed in health facilities, except that the decline in vaccination activity universally affected all vaccines during the peak period of the Ebola outbreak, and was followed by a slower monthly increase for the polio and pentavalent vaccines. The 4 months from August to November 2014 were considered the peak Ebola period in the district, with 89% of all confirmed cases. Troughs for BCG and polio occurred at the same time as for health facility-based administration, and corresponded to vaccine stock-outs.
FIGURE 2.
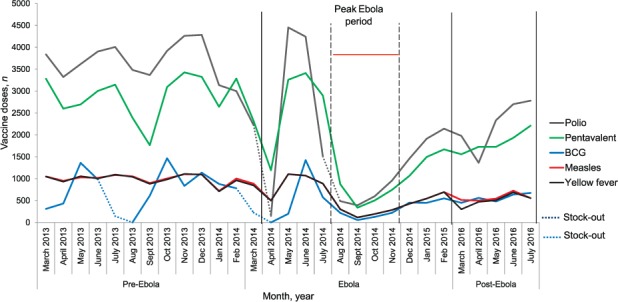
Trends in vaccination administration by vaccine type for children aged <5 years through community-based outreach during the pre-Ebola, Ebola and post-Ebola periods, Macenta District, Guinea, March 2013–July 2016. BCG = bacille Calmette-Guérin.
The mean number of vaccinations administered per month in the community during the pre-Ebola period was significantly higher for all vaccine types than during the post-Ebola period (Table 3). The largest recovery gaps between the two time points were observed for the polio and pentavalent vaccinations. For polio, the pre-Ebola mean of 3736 doses administered per month reduced to a mean of 2230 doses/month, representing a 40% recovery gap (P < 0.001). Similarly, the post-Ebola recovery gap for the pentavalent vaccine was 38%, with 1831 mean doses/month during the post-Ebola period compared to the pre-Ebola mean of 2944 doses/month (P < 0.001).
DISCUSSION
This is one of the first studies from an Ebola-affected country in West Africa to describe trends in vaccination activity before, during and after the Ebola virus disease outbreak. Our results show that stock-outs of five primary childhood vaccines and their stock cards increased considerably in Macenta District during the Ebola outbreak but, reassuringly, improved in the post-Ebola period. During the Ebola period, there was a universal decline in vaccine administration in both health facilities and communities, with recovery gaps persisting into the post-Ebola period.
Beyond the declines in vaccine administration observed during the Ebola outbreak period, the persistent recovery gaps in the administration of all vaccine types into the post-Ebola period are of particular concern and are likely to contribute to a reversal of the gains made towards achieving the Sustainable Development Goal (SDG) target of achieving universal vaccination coverage by 2030.18–20
These study findings have a number of policy and practice implications. First, the pre-Ebola rate of facility-days of vaccine stock-outs persisted into the Ebola outbreak period, and vaccine stock cards stock-outs increased 2.7-fold compared to the pre-Ebola period, suggesting that actual vaccine stock-outs may have been underestimated, as the lack of stock cards might have had a direct impact on monitoring vaccine stock-outs. In any case, these findings highlight the need to improve the regular maintenance of vaccine supplies and vaccine supply monitoring at the health facility level. In addition, introducing a safety net of vaccine stocks at provincial or facility levels could serve as a stopgap measure in future outbreaks and should be considered. Reassuringly, there was a dramatic improvement in vaccine supplies and vaccine stock cards post-Ebola, most likely related to the exemplary actions taken by the national and international health community to improve vaccination logistics in the post-Ebola period.21
Second, although overall declines were seen in vaccination activity during the Ebola outbreak, much of the reduction may be explained by stock-outs, particularly of the BCG and polio vaccines, during the pre-Ebola and Ebola periods. The pre-Ebola vaccine stock-outs may have been due to temporary supply and/or distribution dysfunctions at the central pharmacy. The multiple shortages in the number of polio vaccine doses administered were directly related to these vaccine stock-outs in the Ebola period. This seems intuitive, as the two antigens were the most significant contributors to overall stock-outs. The stock-out of polio vaccines may also be explained by preferential administration of the oral vaccine at a time when injectables were being avoided, to reduce the risk of Ebola infection. This might also have led to a relative increase in demand for oral polio vaccines which, when combined with stock-outs due to compromised distribution logistics during the outbreak, might have aggravated the situation.22 Furthermore, a striking decline in polio vaccine administration was noted 2 months prior to the stock-outs. This might have been associated with community or facility-based factors, such as redeployment of health staff to Ebola response activities. The reasons for these findings remain speculative, and need further investigation.
Third, the recovery of vaccination administration to pre-Ebola levels is lagging behind and is of particular concern. Delamou et al. reported a continued decrease in the administration of polio, measles and yellow fever vaccines to infants in the post-Ebola period in Forested Guinea.8 Our similar findings, despite the lack of evidence of vaccine stock-outs, suggest that provider behavioural or patient demand factors may be responsible. It is also possible, although unlikely, that the observed decrease in vaccine administration was due to lower need associated with child deaths during the outbreak and/or a reduced birth rate in the post-Ebola period. These possibilities suggest a need for enhanced vaccination promotion and education at the provider and population levels as well as efforts to understand the range of possible barriers to uptake by parents. A combination of health provider, patient and community perspectives on this aspect would be useful to guide future strategies.
Finally, emergency response plans for future outbreaks need to reflect upon preparations for sustaining vaccination activities, with particular emphases on logistics, means of implementation and safety, even where existing resources are repurposed to emergencies. Likewise, the development and implementation of post-emergency plans to bring vaccination coverage up to date is necessary.
The strengths of our study include the capture of data from all facilities in one of the largest districts in the country and the assessment of trends both in health facilities and at the community level. Focusing on trends across three distinct time periods of the Ebola outbreak allowed not only an understanding of the impact of the Ebola outbreak on vaccinations, but also an assessment of the progress made since the outbreak ended, which is useful for guiding health priorities. Our reporting was also in accordance with STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.23
The study has some limitations. First, we were unable to assess vaccination coverage due to a lack of reliable population-level denominators. Second, the post-Ebola period included only 5 months of data; however, we addressed concerns around a potential seasonal impact on the pre-Ebola and post-Ebola comparison by using the same sequence of months during the pre-Ebola period as for the post-Ebola data.
In conclusion, we have highlighted important shortcomings and possible opportunities in improving and sustaining vaccination activities so that they remain resilient and responsive, irrespective of disease outbreaks.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was implemented by MSF (Brussels Operational Centre, Luxembourg) and the Centre for Operational Research, The Union. Mentorship and the coordination/facilitation of these SORT IT workshops were provided through the Centre for Operational Research, The Union; the Operational Research Unit (LuxOR) MSF, Academic Model Providing Access to Healthcare (AMPATH, Eldoret, Kenya); Institute of Tropical Medicine (Antwerp, Belgium); University of Gondar (Gondar, Ethiopia); School of Public Health, Johns Hopkins University (Baltimore, MD, USA); Luke International (Mzuzu, Malawi); The Centre for International Health, University of Bergen (Bergen, Norway); and Northern State Medical University (Arkhangelsk, Russia). The authors thank the Department of Medicine, Gamal Abdel Nasser University of Conakry (Conakry, Guinea) and the Macenta District Health Office (Macenta, Guinea) for collaboration during data collection.
The programme was funded by the Department for International Development (London, UK), The Union, MSF and La Fondation Veuve Emile Metz-Tesch (Luxembourg). La Fondation Veuve Emile Metz-Tesch supported the open access publication costs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. Beeching N J, Fenech M, Houlihan C F.. Ebola virus disease. BMJ 2014; 349: g7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. . Ebola response roadmap situation report: February 2016. Geneva, Switzerland: WHO, 2017. http://apps.who.int/ebola/current-situation/ebola-situation-report-3-february-2016 Accessed May 2017 [Google Scholar]
- 3. Plucinski M M, Guilavogui T, Sidikiba S, . et al. Effect of the Ebola-virus disease epidemic on malaria case management in Guinea, 2014: a cross-sectional survey of health facilities. Lancet Infect Dis 2015; 15: 1017– 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delamou A, Delvaux T, Van Belle S, . et al. Public health impact of the recent Ebola outbreak in West Africa. BMJ Global Health 2017; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolkan H A, Bash-Taqi D A, Samai M, Gerdin M, von Schreeb J.. Ebola and indirect effects on health service function in Sierra Leone. PLOS Curr 2014; 19: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leuenberger D, Hebelamou J, Strahm S, . et al. Impact of the Ebola epidemic on general and HIV care in Macenta, Forest Guinea, 2014. AIDS 2015; 29: 1883– 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elston J W, Moosa A J, Moses F, . et al. Impact of the Ebola outbreak on health systems and population health in Sierra Leone. J Public Health (Oxf) 2015; 38: 673– 678. [DOI] [PubMed] [Google Scholar]
- 8. Delamou A, El Ayadi A M, Sidibe S, . et al. Effect of Ebola virus disease on maternal and child health services in Guinea: a retrospective observational cohort study. Lancet Glob Health 2017; 5: e448– e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. . Immunization, vaccines and biologicals. National programmes and systems, 2014. Geneva, Switzerland: WHO, 2017. http://www.who.int/immunization/programmes_systems/en/ Accessed May 2017. [Google Scholar]
- 10. Takahashi S, Metcalf C J, Ferrari M J, . et al. Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science 2015; 347: 1240– 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zachariah R, Ortuno N, Hermans V, . et al. Ebola, fragile health systems and tuberculosis care: a call for pre-emptive action and operational research. Int J Tuberc Lung Dis 2015; 19: 1271– 1275. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. . Recommendations for managing and preventing cases of malaria in areas with Ebola. Atlanta, GA, USA: CDC, 2016. www.cdc.gov/vhf/ebola/outbreaks/malaria-cases.html Accessed May 2017. [Google Scholar]
- 13. Delamou A, Beavogui A H, Konde M K, van Griensven J, De Brouwere V.. Ebola: better protection needed for Guinean health-care workers. Lancet 2015; 385: 503– 504. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. . World Health Statistics, 2015. Geneva, Switzerland: WHO, 2015. http://apps.who.int/iris/bitstream/10665/170250/1/9789240694439_eng.pdf?ua=1 Accessed May 2017. [Google Scholar]
- 15. Coordination Nationale Ebola en Guinée. . Rapport hebdomadaire de la situation de la maladie à virus Ebola dans le District Sanitaire de Macenta. Rapport du 2 Mars 2015. Macenta, Guinée: Coordination Préfectorale Ebola, 2015. [French] [Google Scholar]
- 16. World Health Organization. . Latest updates on the Ebola outbreak. May–June 2016 Geneva, Switzerland: WHO, 2017. http://www.who.int/csr/disease/ebola/top-stories-2016/en/ Accessed May 2017. [Google Scholar]
- 17. Ministry of Health. . Expanded program of immunization (EPI). Guinea. Report on vaccination. Conakry, Guinea: MoH, 2016. [Google Scholar]
- 18. United Nations. . MDG Africa Steering Group. Achieving the Millennium Development Goals in Africa. New York, NY, USA: UN, 2008. http://www.who.int/pmnch/events/2008/mdgsteeringgrouprecommendations.pdf Accessed May 2017. [Google Scholar]
- 19. United Nations. . Sustainable Development Goals. New York, NY, USA: UN, 2015. http://www.un.org/sustainabledevelopment/sustainable-development-goals/ Accessed May 2017. [Google Scholar]
- 20. National Institute of Statistics. . Enquête démographique et de santé et à indicateurs multiples, Guinée 2012. Conakry, Guinea, 2013. [French]. [Google Scholar]
- 21. World Health Organization. . Vaccination in acute humanitarian emergencies: a framework for decision making. WHO/IVB/13.07 Geneva, Switzerland: WHO, 2013. http://apps.who.int/iris/bitstream/10665/92462/1/WHO_IVB_13.07_eng.pdf Accessed May 2017. [Google Scholar]
- 22. Suk J E, Paez Jimenez A, Kourouma M, . et al. Post-Ebola measles outbreak in Lola, Guinea, January–June 2015. Emerg Infect Dis 2016; 22: 1106– 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P C, Vandenbroucke J P.. The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]


