Abstract
The role of immunoglobulin A (IgA) anti-tissue transglutaminase antibodies (IgA-tTG) as predictors of untreated celiac disease (CoD) is well documented, and the presence and levels of these antibodies are most accurately monitored with native or recombinant human antigens. However, IgA-deficient CoD patients are not identified by IgA serology, and conflicting results concerning the diagnostic validity of IgG antibodies against gliadin (IgG-AGA), endomysium (IgG-EmA), and tTG (IgG-tTG) have been reported. The aim of the present study was to evaluate the utility of IgG-tTG for the detection of CoD in IgA-deficient patients. Samples from 115 IgA-deficient and 200 IgA-sufficient subjects were collected and tested for the presence of IgA and IgG antibodies against tTG, EmA, and AGA. Antibodies against tTG were measured by an enzyme-linked immunosorbent assay based on recombinant human tTG, and antibodies against EmA were determined by immunofluorescence. The values for IgG-tTG showed a higher correlation (correlation coefficient [r] = 0.91) with those for IgG-EmA for the IgA-deficient subjects than for the IgA-sufficient subjects (r = 0.88). The overall concordance of the positive and negative results between IgG-tTG and IgG-EmA was 97%, and the IgG-tTG assay discriminated between IgG-EmA-positive and -negative subjects with IgA deficiency at a rate of 100%. Elevated levels of IgG-tTG and IgG-EmA were measured in 70% of the IgA-sufficient subjects. IgG-tTG detection with recombinant human tTG is a good alternative to IgG-EmA detection, and the addition of IgG-tTG assessment to present screening methods may improve the ability to identify IgA-deficient subjects with CoD.
Celiac disease (CoD) is a gluten-induced inflammation of the small intestine strongly associated with the HLA DQ2 or DQ8 haplotype (30). The manifestations may vary from overt enteropathy to extraintestinal forms, and the symptoms may even be silent (8). Mandatory for the diagnosis of CoD is a small-bowel biopsy, in which the biopsy specimen displays the characteristic changes of the mucosal structure, villous atrophy and crypt elongation, which are restored when gluten is excluded from the diet (13). The active phase of CoD is accompanied by elevated levels in serum of immunoglobulin A (IgA) autoantibodies against endomysium (IgA-EmA) and tissue transglutaminase (IgA-tTG) (7, 12, 31), and the presence of these antibodies is frequently used as a selection criterion for jejunal biopsy.
Selective IgA deficiency occurs in Caucasians with a frequency of 1:400 to 1:500 (10, 17), and 2.6% of patients with CoD are also IgA deficient (6). Consequently, individuals with IgA deficiency have a 10- to 15-fold increased risk of the development of CoD, and these subjects are not detected by conventional IgA serology. The general clinical presentation of CoD does not differ between IgA-deficient patients and other patients, but an overrepresentation of silent and atypical symptoms was observed among IgA-deficient CoD patients (6, 9).
Determination of the IgG class of antibodies against AGA (IgG-AGA), EmA (IgG-EmA), and tTG (IgG-tTG) has been suggested as an alternative for the identification of IgA-deficient subjects with CoD, but the accuracies of these assays vary. IgG-AGA has been shown to have a low specificity for CoD and, hence, has not enabled a reduction of the number of biopsies performed (6, 7, 23, 27). Additionally, the sensitivity was low, leaving a high number of cases of CoD undetected by this assay.
IgG-EmA detection in IgA-deficient patients was equivalent to the IgA-EmA detection in subjects with normal serum IgA levels, despite the technical difficulties and subjective means of titer assessment associated with the immunofluorescence method (19, 20). IgG-tTG measurement by an enzyme-linked immunosorbent assay (ELISA) based on guinea pig transglutaminase, on the other hand, has limited relevance for CoD (14, 31). However, it has recently been shown that the detection of IgG-tTG with recombinant human tTG of high purity was a useful marker for CoD in IgA-deficient subjects (18). The aim of the present study was to evaluate whether the detection of IgG-tTG is compatible with the detection of IgG-EmA for the diagnosis of CoD in patients with IgA deficiency.
MATERIALS AND METHODS
Patient sera.
Serum samples collected from 1999 to 2001 from 315 Swedish subjects suspected of having CoD were included in this retrospective study. All sera were examined under ordinary diagnostic conditions at the Department of Clinical Microbiology and Immunology, Lund University Hospital, Lund, Sweden. The serum samples were stored at −20°C until they were analyzed for additional CoD-specific antibodies.
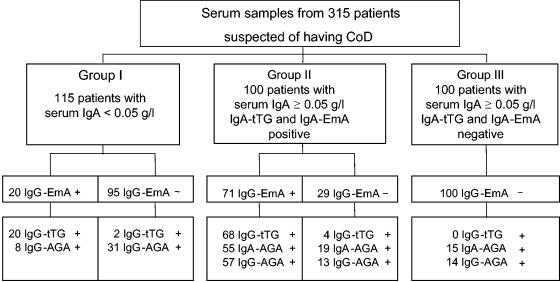
The patients were divided into three groups according to their serum IgA concentrations and EmA results (Fig. 1). Group I included 115 IgA-deficient patients (77 females and 38 males; median age, 23 years; age range, 0.5 to 92 years) with serum IgA levels <0.05 g/liter. Group II included 100 patients (60 females and 40 males; median age, 20 years; age range, 0.8 to 87 years) with serum IgA levels ≥0.05 g/liter and positivity for IgA-EmA. Group III included 100 patients (65 females and 35 males; median age, 13.5 years; age range, 0.9 to 72 years) with serum IgA levels ≥0.05 g/l and negativity for IgA-EmA. All studies of the serum samples were performed in accordance with the ethical rules of the hospital.
FIG. 1.
Serology results for 315 patients suspected of having CoD tested for serum IgA and CoD-related antibodies against recombinant human tTG (IgA-tTG and IgG-tTG), EmA (IgA-EmA and IgG-EmA), and AGA (IgA-AGA and IgG-AGA). The numbers of patients with positive (+) and negative (−) serologies by the respective IgA and IgG antibody tests are shown.
Serum IgA concentrations.
Sera were screened for low IgA concentrations by turbidometry, and sera containing <0.09 g of IgA per liter were reanalyzed by rocket immune electrophoresis. A patient was considered IgA deficient if the serum IgA level was <0.05 g/liter.
Specific IgA and IgG antibodies.
IgA-tTG and IgG-tTG antibodies were determined by an ELISA based on recombinant human tTG (Celikey and ReCombi tTG IgG antibodies, respectively; Pharmacia Diagnostics, Freiburg, Germany). The antibody levels in patient serum samples diluted 1:101 were estimated by comparison with the levels on a standard curve (antibody concentration range, 0 to 100 U/ml), and samples yielding a result greater than 100 U/ml were reinvestigated by the use of higher dilutions. The cutoff level for IgA-tTG was 5 U/ml, and the cutoff for IgG-tTG was determined by receiver operating characteristics (ROC) analysis (11).
The serum samples were evaluated for the presence of IgA-EmA and IgG-EmA by an indirect immunofluorescence assay with monkey esophagus tissue and fluorescein isothiocyanate-conjugated secondary antibodies against IgA (Euroimmun, Lübeck, Germany) or IgG absorbed with monkey IgG (The Binding Site Ltd., Birmingham, United Kingdom). Samples that showed fluorescence at a dilution of 1:10 were considered positive and were subsequently tested at higher dilutions.
IgA-AGA and IgG-AGA were detected by an ELISA based on commercial AGA as the antigen (Sigma, St, Louis, Mo.). Bound serum antibodies were detected with Ig class-specific alkaline phosphatase-conjugated antibodies. The results were expressed in arbitrary units per liter and were classified as positive or negative on the basis of the results for healthy blood donors.
Statistics.
Antibody levels are expressed as median values (5th and 95th percentiles). The Kruskal-Wallis test was used to estimate differences in antibody levels between different groups, and correlation coefficients (r values) were calculated by the Spearman rank method.
The ROC analyses for IgG-tTG were based on the assumption that IgG-EmA is a diagnostically significant reference marker of CoD. The results for patients with positive IgG-EmA results were designated true positive, and those for patients with negative IgG-EmA results were designated true negative. By using different threshold values, the fraction of positive IgG-tTG results for the true-positive group was plotted against the fraction of positive IgG-tTG results for the true-negative group (1 − number of sample with true-negative results). Thereafter, the area under the curve was calculated, and a suitable cutoff value was selected. The calculations were made for the groups of IgA-deficient and IgA-sufficient subjects separately, and one value representing the average optimal threshold of the two calculations, 4 U/ml, was selected as the cutoff for estimates of positivity for IgG-tTG.
RESULTS
IgG-tTG levels in IgA-deficient and IgA-sufficient patients.
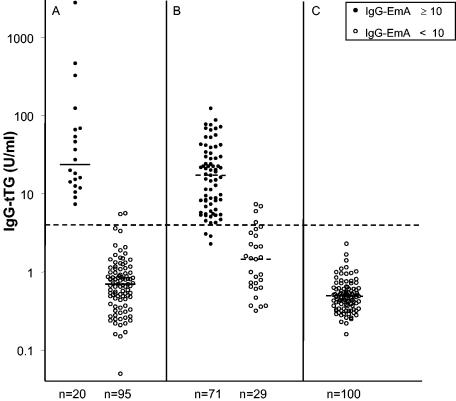
The serum IgG-tTG levels for the three groups evaluated in this study are shown in Fig. 2. There was no difference in the median IgG-tTG level between the IgG-EmA positive subjects in group I (23.7 U/ml; range, 9 to 587 U/ml) and those in group II (17.34 U/ml; range, 4 to 74 U/ml). The median IgG-EmA titers for the two groups were also equal (200 U/ml). The median IgG-tTG level for the IgG-EmA-negative patients in group II was higher (P < 0.05) than those for the IgG-EmA-negative patients in groups I and III.
FIG. 2.
Serum IgG-tTG levels in patients with IgA deficiency (A), IgA-sufficient patients with positive test results for IgA-EmA (B), and IgA-sufficient patients with negative test results for IgA-EmA (C). Positivity (titer, ≥10) and negativity (titer, <10) for serum IgG-EmA are shown. Horizontal lines represent median values, and the cutoff is 4 U/ml.
Correlation between IgG-tTG levels and IgG-EmA titers.
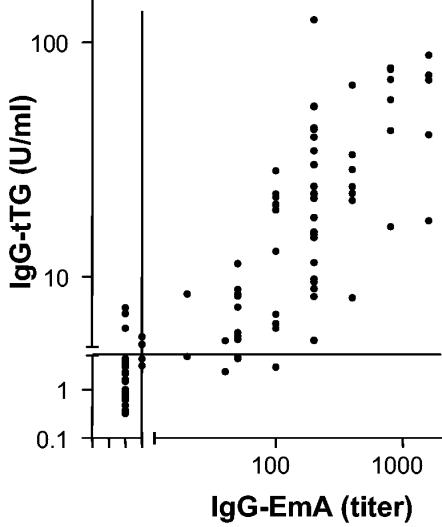
There was a positive correlation between the IgG-EmA titers and IgG-tTG levels for group I (r = 0.908; 95% confidence interval [CI] = 0.773 to 0.965) and group II (r = 0.883; 95% CI = 0.829 to 0.920). The correlation between the IgA-EmA titers and the IgA-tTG levels for group II was 0.961 (95% CI, 0.948 to 0.970). Figure 3 shows the correlation between the IgG-tTG levels in groups I and II and the IgG-EmA titers (r = 0.871; 95% CI = 0.834 to 0.901).
FIG. 3.
Relation between serum IgG-tTG levels and IgG-EmA titers. The Spearman correlation coefficient was 0.87. The lines represent cutoff levels.
Cutoff level for IgG-tTG.
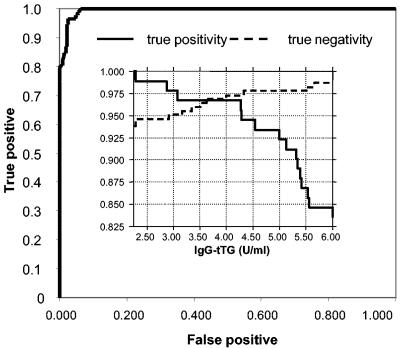
The area under the curve for the IgG-tTG ELISA was 100% for the subjects with IgA deficiency and 99.4% for those without IgA deficiency. Optimal IgG-tTG threshold levels differed between the IgA-deficient subjects (5.5 U/ml) and the IgA-sufficient subjects (2.5 U/ml). The observed overlap in IgG-tTG levels in the range from 2 to 8 U/ml (Fig. 2 and 4) comprised 5% of all patients included in the study. For simplicity, the threshold value of 4 U/ml was selected as the cutoff for calculations of positive IgG-tTG levels for all groups.
FIG. 4.
ROC analysis curves for recombinant human IgG-tTG in all patients. Positive, patients positive for IgG antibodies against EmA (IgG-EmA; titer, ≥10); negative, patients with IgG-EmA titer <10. (Inset) True positivity and negativity for IgG-tTG levels in relation to cutoff values.
Frequency of IgG-tTG and concordance with the presence of IgG-EmA.
The presence of IgG-EmA and IgG-tTG antibodies was concordant in 98.3% of the IgA-deficient patients and 96.5% of the IgA-sufficient patients, giving a 97.1% agreement for all patients.
True positivity and true negativity were observed in 100 and 97% of the IgA-deficient subjects, respectively. The corresponding values calculated for IgA-sufficient subjects were 95.8 and 96.9%. For all patients the rate of true positivity for IgG-tTG was 96.7% and the rate for true negativity was 97.3% (Fig. 4).
Relation between IgG-tTG, IgG-EmA, and IgG-AGA.
IgG-AGA was detected in 39 of 115 (40%) of the subjects in group I. IgG-AGA was found in 70 and 14% of the subjects in groups II and III, respectively. The corresponding values for IgA-AGA in group I and in groups II and III were 74 and 15%. The distributions of IgG-AGA and IgA-AGA in relation to those of IgG-EmA and IgG-tTG are shown in Fig. 1, and the conjunct occurrence of IgG-AGA, IgG-EmA, and IgG-tTG is shown in Table 1. Concordant positive and negative results between IgG-AGA, IgG-EmA, and IgG-tTG were found in 71% of all subjects with suspected CoD, whereas 26% discordant results between IgG-AGA and IgG-EmA or IgG-tTG were observed.
TABLE 1.
Distributions of positive and negative results for IgG antibodies against recombinant human tTG, EmA, and AGA among patients suspected of having CoD
| IgG-tTG result | IgG-EmA result | IgG-AGA result | No. (%) of patientsa
|
|||
|---|---|---|---|---|---|---|
| Group I (n = 115) | Group II (n = 100) | Group III (n = 100) | All patients (n = 315) | |||
| − | − | − | 63 | 14 | 86 | 163 |
| − | − | + | 30 | 11 | 14 | 55 |
| − | + | − | 0 | 0 | 0 | 0 |
| − | + | + | 0 | 3 | 0 | 3 |
| + | − | − | 1 | 1 | 0 | 2 |
| + | − | + | 1 | 2 | 0 | 3 |
| + | + | − | 12 | 14 | 0 | 26 |
| + | + | + | 8 | 54 | 0 | 62 |
| IgG-tTG and IgG-EMA concordance | 113 (98.3) | 93 (93.0) | 100 (100.0) | 306 (97.1) | ||
n, total number of patients.
DISCUSSION
The role of IgA-tTG antibodies as a marker of untreated CoD is well documented (1, 2, 12, 22, 32), and their presence is most accurately monitored with native or recombinant human antigens (34). However, IgA-deficient CoD patients are not identified by conventional IgA serology, and inconsistent results concerning the diagnostic validity of IgG-EmA and IgG-tTG in different patient groups have been reported (14, 20, 31).
One of a few studies with IgA-deficient patients showed that IgG-EmA is a highly specific marker for CoD and that detection of IgG-tTG with recombinant human tTG can be used as a reliable alternative to the detection of IgG-EmA for the diagnosis of CoD in patients with IgA deficiency (18).
In our study, the performances of IgG-tTG and IgG-EmA were determined in IgA-deficient and IgA-sufficient patients suspected of having CoD. A high proportion (17%) of the IgA-deficient subjects included in the study was positive for both IgG-EmA and IgG-tTG. This is a higher prevalence of CoD than that reported earlier in IgA-deficient subjects (25), and the increased rate observed here is probably a result of the preselection of samples, which was based on previous serology. The detection of IgG-tTG was as efficient as that of IgG-EmA in both categories of patients, and the concordance between the methods was 97%. Since no intestinal biopsy results were available for the patients, we evaluated the IgG-tTG titer against the IgG-EmA titer. By taking the high diagnostic accuracy of the IgA- and IgG-EmA titers into account (2, 18, 20, 24), it would be reasonable to assume that increased levels of EmA strongly indicate CoD, even if the absence of EmA does not exclude the possibility of disease.
The concordance between IgG-EmA and IgG-tTG was slightly lower (96.5%) for the IgA-sufficient groups than for the IgA-deficient group (98%), and the observed discrepancy can probably be ascribed mainly to the technical disadvantages of the IgG-EmA assay that have been described elsewhere (18, 20). Additionally, good clinical performance of the IgG-EmA assay can be difficult to achieve with serum samples with high titers of various nonspecific IgG autoantibodies, which tend to mask the distinct EmA binding. In the present study, persistent intracellular staining of smooth muscle cells, also at high dilutions, covered the endomysial staining in two of the IgA-deficient patients and four of the IgA-sufficient patients, all of whom were found to have elevated IgG-tTG levels (data not shown).
Although the majority of IgA-sufficient patients positive for IgA-EmA concurrently had elevated IgG-tTG and IgG-EmA levels, a considerable number of the patients had neither of these antibodies. Our findings deviate from the observation that IgG1-EmA and IgG-tTG may occur in some IgA-sufficient patients who lack IgA-EmA (4, 26), and it is possible that this particular minority of CoD patients was not represented here. The specific detection of IgG-tTG was comparable to that of IgA-tTG for the IgA-sufficient patients, which is in contrast to the findings of other studies (3, 14, 33) that have shown that IgG-tTG might be found in patients with various disorders other than CoD. Most of those studies used guinea pig tTG, in which contaminating proteins might account for the nonspecific binding of antibodies. For the specific detection of IgA-tTG, it has been demonstrated that the purity and the specific origin of tTG are essential (15, 21).
The reason why not all IgA-sufficient patients with IgA-tTG had increased levels of IgG-tTG may be methodological limitations or immunological differences in the isotype response. In a previous study (16) we observed that the IgG-tTG response showed delayed kinetics compared with the IgA-tTG response in CoD children who were subjected to gluten challenge, and it is possible that a larger amount of dietary gluten is needed to elicit a detectable IgG-tTG response. The disparity in the isotypic composition of the anti-tTG response observed in our study might reflect in part individual variations in gluten intake.
Moreover, IgA-tTG seems to be directed mainly against conformational tTG-epitopes (28, 29), and it is possible that IgG-tTG is directed against the same epitopes. Hence, a competition between IgA-tTG and IgG-tTG might take place, and this competition would favor antibodies with a high avidity for tTG. The extent to which IgA-tTG and IgG-tTG might differ with respect to binding avidity and epitope specificity for tTG has not been investigated, and the clinical implications of the presence of IgG-tTG in patients with CoD remain to be resolved in future studies.
The IgG-AGA results showed a poor correlation with the IgG-tTG and IgG-EmA results. Among the IgA-deficient patients, less than half of those who were IgG-EmA positive had elevated levels of IgG-AGA. Our results are in agreement with those of other studies that demonstrated a low efficiency and a limited value of the determination of IgG-AGA levels for the diagnosis of CoD in patients with IgA deficiency (5, 7, 23, 27).
Taken together, our results indicate that the specificity of IgG-tTG is comparable to that of IgA-tTG. While the presence of IgG-tTG seems to be of limited diagnostic value in IgA-sufficient CoD patients, the presence of these antibodies without IgA-tTG might be a sign of IgA deficiency. Detection of IgG-tTG is highly compatible with the presence of IgG-EmA and may improve the possibility of identification of CoD in patients with IgA deficiency.
REFERENCES
- 1.Baudon, J. J., C. Johanet, Y. B. Absalon, G. Morgant, S. Cabrol, and J. F. Mougenot. 2004. Diagnosing celiac disease: a comparison of human tissue transglutaminase antibodies with antigliadin and antiendomysium antibodies. Arch. Pediatr. Adolesc. Med. 158:584-588. [DOI] [PubMed] [Google Scholar]
- 2.Burgin-Wolff, A., I. Dahlbom, F. Hadziselimovic, and C. J. Petersson. 2002. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand. J. Gastroenterol. 37:685-691. [DOI] [PubMed] [Google Scholar]
- 3.Carroccio, A., G. Vitale, L. Di Prima, N. Chifari, S. Napoli, C. La Russa, G. Gulotta, M. R. Averna, G. Montalto, S. Mansueto, and A. Notarbartolo. 2002. Comparison of anti-transglutaminase ELISAs and an anti-endomysial antibody assay in the diagnosis of celiac disease: a prospective study. Clin. Chem. 48:1546-1550. [PubMed] [Google Scholar]
- 4.Cataldo, F., D. Lio, V. Marino, A. Picarelli, A. Ventura, G. R. Corazza, et al. 2000. IgG1 antiendomysium and IgG antitissue transglutaminase (anti-tTG) antibodies in coeliac patients with selective IgA deficiency. Gut 47:366-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataldo, F., V. Marino, G. Bottaro, P. Greco, and A. Ventura. 1997. Celiac disease and selective immunoglobulin A deficiency. J. Pediatr. 131:306-308. [DOI] [PubMed] [Google Scholar]
- 6.Cataldo, F., V. Marino, A. Ventura, G. Bottaro, G. R. Corazza, et al. 1998. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Gut 42:362-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catassi, C., G. Fanciulli, A. R. D'Appello, R. El Asmar, C. Rondina, E. Fabiani, I. Bearzi, and G. V. Coppa. 2000. Antiendomysium versus antigliadin antibodies in screening the general population for coeliac disease. Scand. J. Gastroenterol. 35:732-736. [DOI] [PubMed] [Google Scholar]
- 8.Collin, P. 1999. New diagnostic findings in coeliac disease. Ann. Med. 31:399-405. [DOI] [PubMed] [Google Scholar]
- 9.Collin, P., M. Maki, O. Keyrilainen, O. Hallstrom, T. Reunala, and A. Pasternack. 1992. Selective IgA deficiency and coeliac disease. Scand. J. Gastroenterol. 27:367-371. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham-Rundles, C. 2001. Physiology of IgA and IgA deficiency. J. Clin. Immunol. 21:303-309. [DOI] [PubMed] [Google Scholar]
- 11.DeLong, E. R., D. M. DeLong, and D. L. Clarke-Pearson. 1988. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837-845. [PubMed] [Google Scholar]
- 12.Dieterich, W., T. Ehnis, M. Bauer, P. Donner, U. Volta, E. O. Riecken, and D. Schuppan. 1997. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 3:797-801. [DOI] [PubMed] [Google Scholar]
- 13.Farrell, R. J., and C. P. Kelly. 2002. Celiac sprue. N. Engl. J. Med. 346:180-188. [DOI] [PubMed] [Google Scholar]
- 14.Feighery, L., C. Collins, C. Feighery, N. Mahmud, G. Coughlan, R. Willoughby, and J. Jackson. 2003. Anti-transglutaminase antibodies and the serological diagnosis of coeliac disease. Br. J. Biomed. Sci. 60:14-18. [DOI] [PubMed] [Google Scholar]
- 15.Hansson, T., I. Dahlbom, J. Hall, A. Holtz, L. Elfman, A. Dannaeus, and L. Klareskog. 2000. Antibody reactivity against human and guinea pig tissue transglutaminase in children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 30:379-384. [DOI] [PubMed] [Google Scholar]
- 16.Hansson, T., I. Dahlbom, S. Rogberg, A. Dannaeus, P. Hopfl, H. Gut, W. Kraaz, and L. Klareskog. 2002. Recombinant human tissue transglutaminase for diagnosis and follow-up of childhood coeliac disease. Pediatr. Res. 51:700-705. [DOI] [PubMed] [Google Scholar]
- 17.Koistinen, J. 1975. Selective IgA deficiency in blood donors. Vox Sang. 29:192-202. [DOI] [PubMed] [Google Scholar]
- 18.Korponay-Szabo, I. R., I. Dahlbom, K. Laurila, S. Koskinen, N. Woolley, J. Partanen, J. B. Kovacs, M. Maki, and T. Hansson. 2003. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut 52:1567-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korponay-Szabo, I. R., J. B. Kovacs, M. Lorincz, G. Goracz, K. Szabados, and M. Balogh. 1997. Prospective significance of antiendomysium antibody positivity in subsequently verified celiac disease. J. Pediatr. Gastroenterol. Nutr. 25:56-63. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, V., M. Jarzabek-Chorzelska, J. Sulej, K. Karnewska, T. Farrell, and S. Jablonska. 2002. Celiac disease and immunoglobulin A deficiency: how effective are the serological methods of diagnosis? Clin. Diagn. Lab. Immunol. 9:1295-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon, F., C. Camarero, R. Pena, P. Eiras, L. Sanchez, M. Baragano, M. Lombardia, A. Bootello, and G. Roy. 2001. Anti-transglutaminase IgA ELISA: clinical potential and drawbacks in celiac disease diagnosis. Scand. J. Gastroenterol. 36:849-853. [DOI] [PubMed] [Google Scholar]
- 22.Llorente, M. J., M. Sebastian, M. J. Fernandez-Acenero, S. Prieto, S. Villanueva, and G. Prieto. 2004. IgA antibodies against tissue transglutaminase in the diagnosis of celiac disease: concordance with intestinal biopsy in children and adults. Clin. Chem. 50:451-453. [DOI] [PubMed] [Google Scholar]
- 23.Lock, R. J., and D. J. Unsworth. 1999. Identifying immunoglobulin-A-deficient children and adults does not necessarily help the serologic diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 28:81-83. [DOI] [PubMed] [Google Scholar]
- 24.Maki, M., K. Mustalahti, J. Kokkonen, P. Kulmala, M. Haapalahti, T. Karttunen, J. Ilonen, K. Laurila, I. Dahlbom, T. Hansson, P. Hopfl, and M. Knip. 2003. Prevalence of celiac disease among children in Finland. N. Engl. J. Med. 348:2517-2524. [DOI] [PubMed] [Google Scholar]
- 25.Meini, A., N. M. Pillan, V. Villanacci, V. Monafo, A. G. Ugazio, and A. Plebani. 1996. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann. Allergy Asthma Immunol. 77:333-336. [DOI] [PubMed] [Google Scholar]
- 26.Picarelli, A., M. di Tola, L. Sabbatella, A. Mastracchio, A. Trecca, F. Gabrielli, T. di Cello, M. C. Anania, and A. Torsoli. 2001. Identification of a new coeliac disease subgroup: antiendomysial and anti-transglutaminase antibodies of IgG class in the absence of selective IgA deficiency. J. Intern. Med. 249:181-188. [DOI] [PubMed] [Google Scholar]
- 27.Prince, H. E., G. L. Norman, and W. L. Binder. 2000. Immunoglobulin A (IgA) deficiency and alternative celiac disease-associated antibodies in sera submitted to a reference laboratory for endomysial IgA testing. Clin. Diagn. Lab. Immunol. 7:192-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sblattero, D., F. Florian, E. Azzoni, T. Zyla, M. Park, V. Baldas, T. Not, A. Ventura, A. Bradbury, and R. Marzari. 2002. The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur. J. Biochem. 269:5175-5181. [DOI] [PubMed] [Google Scholar]
- 29.Seissler, J., U. Wohlrab, C. Wuensche, W. A. Scherbaum, and B. O. Boehm. 2001. Autoantibodies from patients with coeliac disease recognize distinct functional domains of the autoantigen tissue transglutaminase. Clin. Exp. Immunol. 125:216-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sollid, L. M. 2002. Coeliac disease: dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2:647-655. [DOI] [PubMed] [Google Scholar]
- 31.Sulkanen, S., T. Halttunen, K. Laurila, K. L. Kolho, I. R. Korponay-Szabo, A. Sarnesto, E. Savilahti, P. Collin, and M. Maki. 1998. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 115:1322-1328. [DOI] [PubMed] [Google Scholar]
- 32.Tesei, N., E. Sugai, H. Vazquez, E. Smecuol, S. Niveloni, R. Mazure, M. L. Moreno, J. C. Gomez, E. Maurino, and J. C. Bai. 2003. Antibodies to human recombinant tissue transglutaminase may detect coeliac disease patients undiagnosed by endomysial antibodies. Aliment. Pharmacol. Ther. 17:1415-1423. [DOI] [PubMed] [Google Scholar]
- 33.Volta, U., L. Rodrigo, A. Granito, N. Petrolini, P. Muratori, L. Muratori, A. Linares, L. Veronesi, D. Fuentes, D. Zauli, and F. B. Bianchi. 2002. Celiac disease in autoimmune cholestatic liver disorders. Am. J. Gastroenterol. 97:2609-2613. [DOI] [PubMed] [Google Scholar]
- 34.Wong, R. C., R. J. Wilson, R. H. Steele, G. Radford-Smith, and S. Adelstein. 2002. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J. Clin. Pathol. 55:488-494. [DOI] [PMC free article] [PubMed] [Google Scholar]