Abstract
We examined sera from 42 patients 1 to 30 months of age for rotavirus immunoglobulin M (IgM), IgA, IgG, and IgG subclasses and sought to determine if serum antibody could serve as a reliable marker for prediction of disease severity. Infants in the first few months of life usually had high maternal IgG titers and, when they were infected with rotavirus, had low IgM titers or no IgM in acute-phase sera and poor seroconversions 3 weeks later, suggesting that maternal antibodies had inhibited viral replication and antibody responses. All patients ≥6 months of age had IgM in acute-phase sera, indicating that IgM is a good marker for acute rotavirus infection. IgG was the best overall predictor of an infection, as the convalescent-phase sera of 81% of the patients had a fourfold rise in the IgG titer. IgA titers in convalescent-phase sera and conversion rates were higher among patients ≥12 months of age than among children younger than 12 months. IgG1 was the predominant subclass detected in the acute-phase sera of some children and in all 28 convalescent-phase serum samples examined. Patients with preexisting acute-phase IgG titers of ≥100 or ≥200 had diarrhea that was less severe or of a shorter duration. These results indicate that serum IgG is the most reliable marker for seroconversion and is a consistent proxy for protection against severe disease.
Previous studies have demonstrated that children infected with rotavirus develop systemic and local immune responses and are protected from severe disease upon reinfection (5, 6, 22, 38). However, our understanding of the true correlates of protection, essential for vaccine development, and the mechanisms of protection is still incomplete. At present, antibodies are generally considered a good marker for infection and a proxy for protection, but which antibodies (intestinal or serum, or both) are needed for protection remains unclear (25). While local immunity in the gut is believed to play a key role in protection, measurement of a local immune response in children is a challenge. Coproantibodies are subject to proteolytic degradation and are not considered a reliable marker for infection, and intestinal fluids are difficult to obtain from children, so we are left with measuring serum immunoglobulin A (IgA) titers as a proxy for local immunity (7, 18, 25, 36). Because of differences among the reagents and assays used in different laboratories and the lack of detailed clinical information concerning individual patients in most studies (4, 12, 13, 24, 30, 32, 37), diverse opinions exist about the responses and roles of serum antibodies in children with acute rotavirus diarrhea.
IgG is the most abundant Ig isotype and constitutes approximately 80% of the total Igs in human sera. The four IgG subclasses, IgG1 to IgG4, have different physiochemical, biological, and functional properties, such as the ability to activate complements (IgG1 and IgG3) and to cross the placenta and mediate opsonization of antigens by macrophages and neutrophils (IgG1, IgG3, and IgG4) (16). Measurement of the levels of these Ig subclasses in serum could help us understand their origin (i.e., maternal source or active infection) and the types of T-helper responses and may help predict disease outcomes in children with rotavirus infection or vaccine efficacy in clinical trials.
In this study, we examined sera from a cohort of children with severe acute diarrhea due to rotavirus for Ig isotype (IgM, IgA, and IgG) and IgG subclass responses. We further examined if the levels of these antibodies could serve as markers for prediction of the severity of symptoms, such as diarrhea and vomiting. We demonstrated an age-dependent antibody response and identified IgG as the most reliable and consistent marker for seroconversion. We also documented that preexisting IgG in acute-phase serum was associated with protection against severe disease. Our findings may provide useful guidance for the development and testing of live or parenteral rotavirus vaccines.
MATERIALS AND METHODS
Study population and specimen collection.
From March 1999 to March 2002, we collected blood and fecal specimens from 42 children less than 3 years of age admitted for treatment for acute gastroenteritis due to rotavirus at Children's Healthcare of Atlanta, Atlanta, Ga., and Hasbro Children's Hospital, Providence, R.I. All children were otherwise in generally good health. The patients in the two hospitals were enrolled according to the same study protocol. They had not received rotavirus vaccines and had no prior history of diarrhea due to rotavirus. For children enrolled at Hasbro Children's Hospital, we collected detailed symptom data, including fever (the temperature was measured rectally), vomiting, diarrhea, and dehydration, and calculated composite severity scores on the basis of those symptoms (15) during the entire period of illness and on the day of the first blood sample collection. At the time of enrollment, all but one patient had a documented onset of illness that ranged from 1 to 10 days earlier. Two blood samples were obtained from each patient: the first one was obtained within 72 h of enrollment, and the second one was obtained 21 to 44 days later. The sera were aliquoted and stored at −70°C. Fecal specimens collected from all patients tested positive for rotavirus by enzyme immunoassay (EIA) and PCR and were stored at −20°C.
During the same study period, 17 healthy children were enrolled at Children's Healthcare of Atlanta in the same manner described above, except that only one blood specimen was collected from each child. Fecal specimens from all control subjects were tested for rotavirus by EIA and were found to be negative. Written consent was obtained from all parents or guardians, and the protocol was approved by the institutional review boards of the Centers for Disease Control and Prevention and of each institution.
Detection of rotavirus antibodies in serum.
Rotavirus-specific IgM, IgA, IgG, and IgG subclasses (IgG1, IgG2, IgG3, and IgG4) were detected by indirect EIA by protocols adapted from previous studies (8-10). Briefly, 96-well Immuno II plates (Nagle Nunc, Rochester, N.Y.) were coated with rhesus rotavirus (105 focus-forming units/well) in carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. The plates were blocked with 2% bovine serum albumin in phosphate-buffered saline (PBS), and serum samples were serially diluted (twofold) in duplicate in diluent buffer (PBS with 0.25% bovine serum albumin) starting at a dilution of 1:25. After incubation for 2 h at room temperature, the plates were washed thoroughly with PBS-0.05% Tween 20. Goat anti-human IgM, IgA, IgG, and IgG subclass horseradish peroxidase conjugates (Southern Biotech, Birmingham, Ala.) in diluent buffer were then added to the plates. The plates with all Igs except IgA were incubated for 2 h at room temperature; those with IgA were incubated overnight at 4°C. The optimal dilution of each conjugate was determined by checkerboard titration. The reaction was developed with 3,3′,5,5′-tetramethylbenzidine (Sigma, St. Louis, Mo.), and the optical density (OD) at 450 nm was read with an MRX microplate reader (Dynex, Chantilly, Va.). The antibody titers were defined as the reciprocal of the highest dilution of serum with a net OD value (OD with serum minus OD with diluent buffer) ≥0.1. A sample was considered positive if the titer was ≥25. Seroconversion was defined as a fourfold or greater rise in titer in convalescent-phase serum from that in the acute-phase serum. For IgM and IgG, a rise in titers from <25 to ≥50 was considered a seroconversion, whereas for IgA, seroconversion was defined as a rise in titers from <25 to ≥25. Positive and negative control sera were included in all assays and were tested in the same manner.
Data analysis.
Each serum specimen was tested in duplicate, and the average values were recorded and analyzed. We used nonparametric statistics for analysis because the data were not normally distributed (28). Symptom scores for the different age groups were compared for continuous variables by the Kruskal-Wallis test. The categorical variables, such as sex, vomiting (as a percentage of the study participants), and dehydration (as a percentage of the study participants), were analyzed by the chi-square test. The symptom scores and antibody titers between age groups were compared by use of the Wilcoxon rank-sum test, which was also used to compare symptom scores for patients with higher antibody titers in acute-phase sera to those for patients with low antibody titers or no antibody.
RESULTS
Characteristics of subjects.
Of the 42 patients enrolled in the study, 17 were girls and 25 were boys, and they ranged in age from 1 to 30 months. Patients were analyzed in three age groups: <6, 6 to 11, and ≥12 months. All of the patients had typical clinical symptoms of acute rotavirus gastroenteritis, including fever, vomiting, and/or diarrhea of various severities. We analyzed demographic data and symptom scores during the course of the illness for 31 of the 33 patients enrolled at Hasbro Children's Hospital by age group and demonstrated an age-dependent expression for some symptoms (Table 1).
TABLE 1.
Comparison of symptoms by age in patients with rotavirus diarrhea
| Variable | Age group (mo)
|
||
|---|---|---|---|
| <6 (n = 10) | 6-11 (n = 9) | ≥12 (n = 12) | |
| Age (mo)a | 2.5 (1-5) | 9 (6-11) | 16.5 (14-21) |
| Sex (no. of males/no. of females) | 8/2 | 5/4 | 8/4 |
| Maximum temp (°C)a | 38.8 (37.0-39.7) | 38.9 (36.7-39.4) | 39.0 (36.8-39.6) |
| No. of stools per daya | 8.5 (4-20) | 5 (2-20) | 7.5 (2-30) |
| No. of days of diarrheaa | 2.5 (2-5) | 4 (2-10) | 2.5b (1-6) |
| No. of patients with vomiting/total no. (%) | 6/10 (60)c | 9/9 (100)c | 11/12 (92) |
| No. of days of vomitinga | 1d,e (0-3) | 3d (1-5) | 2e (0-5) |
| No. of patients with dehydration/total no. (%) | 2/10 (20)f,g | 6/9 (67)f | 11/12 (92)g |
| Severity scorea,h | 7.5i (4-11) | 10 (4-11) | 10.5i (7-12) |
| LOSj (days)a | 3 (2-6) | 3 (2-4) | 3 (2-4) |
Data are presented as medians (ranges).
P < 0.05 (significant differences between variables are shown by the same letter).
P < 0.01.
P < 0.01.
P < 0.01.
P < 0.05.
P < 0.01.
Symptom scores over the course of illness were collected from 31 patients with acute diarrhea and were analyzed by age group.
P < 0.01.
LOS, length of stay in hospital.
Seventeen healthy children ranged in age from 2 to 23 months and were age matched with the rotavirus-infected patients (means, 12 and 11 months, respectively; medians, 16 and 10 months, respectively; P > 0.1). The healthy children did not have diarrhea at the time of enrollment.
Antibody isotype responses.
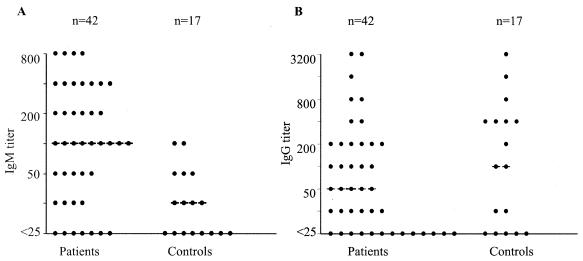
We examined the acute-phase sera from the 42 patients for IgM titers; IgM titers could be measured in 35 (83%) patients; and the 7 children with no IgM, including 5 from whom blood was obtained within 2 days of illness onset, were all young (age, 1 to 5 months). These 42 subjects had a median IgM titer of 100 (mean, 201), which was significantly higher (P < 0.01) than that for the 17 healthy controls (median titer, 25; mean titer, 27) (Fig. 1A). In contrast, the acute-phase sera of the patients and the sera of the controls had similar median and mean IgG titers, indicating that IgG was already present in most patients before rotavirus infection (Fig. 1B). The acute-phase sera of eight patients and two controls had detectable IgA titers.
FIG. 1.
Serum antibodies in children with acute rotavirus diarrhea and healthy controls. Acute-phase sera from 42 patients and sera from 17 controls were tested for rotavirus IgM (A) and IgG (B), as described in the text. The median antibody titers in patients and controls are indicated by dashed lines. Patients with acute rotavirus diarrhea had a significantly higher median IgM titer than the controls.
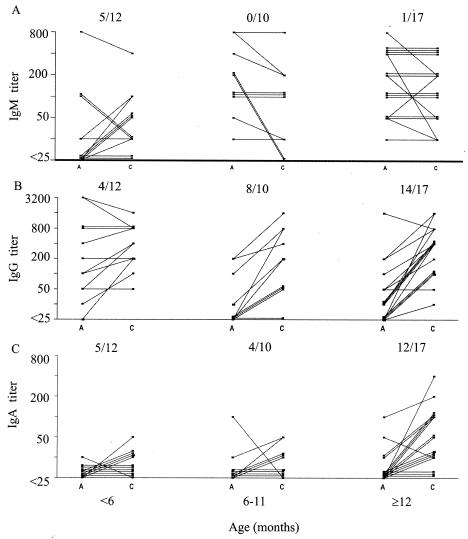
Of the original 42 patients, paired serum samples were available from 39 to test for seroconversion (Fig. 2). The IgM titers in 33 patients remained unchanged or decreased; 5 of 12 patients <6 months of age and 1 of 27 children ≥6 months of age seroconverted (Fig. 2A). Blood was obtained during the first 2 days of illness from the five younger children and the one older child with IgM conversions.
FIG. 2.
Antibody profiles in sera of patients with rotavirus diarrhea. Paired serum samples were collected from 39 children <3 years of age and were examined for Ig isotypes, as described in the text. The data shown are antibody titers and seroconversion rates, as defined by a fourfold or greater rise in antibody titers in convalescent-phase serum samples (C) over those in acute-phase serum samples (A).
Rotavirus-specific IgG was the best overall predictor of an acute infection in children ≥3 months of age. Among infants <6 months old, the acute-phase sera of 11 (92%) of the 12 infants had IgG titers ≥25 (median titer, 150), including 6 infants ≤2 months of age whose acute-phase sera had high IgG titers (median titer, 600) (Fig. 2B). None of these six young subjects with high IgG titers had seroconverted 3 weeks later. In contrast, the acute-phase sera of six subjects 3 to 5 months of age had much lower IgG titers (median titer, 50), and four of the six subjects went on to develop a fourfold rise in titer in their convalescent-phase sera (Fig. 2B). The acute-phase sera of subjects ≥6 months of age generally had lower IgG titers, as evidenced by median values of <25 and 25 for those of 6 to 11 and ≥12 months of age, respectively. Most of these subjects (8 of 10 subjects aged 6 to 11 months and 14 of 17 subjects aged ≥12 months) had seroconverted (Fig. 2B).
The rotavirus-specific IgA response was also age dependent and was only partially reflective of infection in infants. Seven (18%) of the 39 acute-phase serum samples had IgA titers ≥25, whereas 24 (62%) convalescent-phase serum samples had IgA titers ≥25. The titers in the convalescent-phase sera of 17 children ≥12 months of age were significantly higher (P = 0.002) than those in children <12 months old, as evidenced by the median titers (50 and <25, respectively) and mean titers (78 and 7, respectively). Rotavirus-specific IgA conversion was observed in 41% (9 of 22) of the subjects <12 months of age, whereas it was observed in 71% (12 of 17) of those ≥12 months of age (Fig. 2C).
IgG subclass responses.
Of the 28 paired serum samples that had adequate volumes for determination of the titers of the IgG subclasses, IgG1 was predominant and IgG3 was present as a minor subclass (Table 2). IgG1 was detected at relatively high titers in five of the eight acute-phase serum samples and in all eight convalescent-phase serum samples from children <6 months of age. The acute-phase sera of none of the 9 children 6 to 11 months of age had detectable IgG1, and the acute-phase sera of only 3 of the 11 children ≥12 months of age had low titers of IgG1. The convalescent-phase sera of all subjects ≥6 months of age had IgG1. IgG3 was not detected in any of the acute-phase sera but was present in five convalescent-phase serum samples. Antibodies of the IgG2 and IgG4 subclasses were not detected in any serum sample.
TABLE 2.
Rotavirus-specific IgG subclasses in serum of children with acute diarrhea
| Age group (mo) | No. of children | No. of children with detectable antibody (median titer among responders)a
|
|||||
|---|---|---|---|---|---|---|---|
| IgG
|
IgG1
|
IgG3
|
|||||
| A | C | A | C | A | C | ||
| <6 | 8 | 7 (400) | 8 (800) | 5 (400) | 8 (400) | 0 | 2 (25) |
| 6-11 | 9 | 4 (38) | 9 (200) | 0 | 9 (100) | 0 | 2 (75) |
| ≥12 | 11 | 7 (25) | 11 (400) | 3 (50) | 11 (200) | 0 | 1 (100) |
Paired serum samples from 28 patients 1 to 30 months of age were examined for rotavirus-specific IgG and its subclasses, as described in the text. A, acute-phase serum; C, convalescent-phase serum.
Serum antibodies are associated with less severe disease.
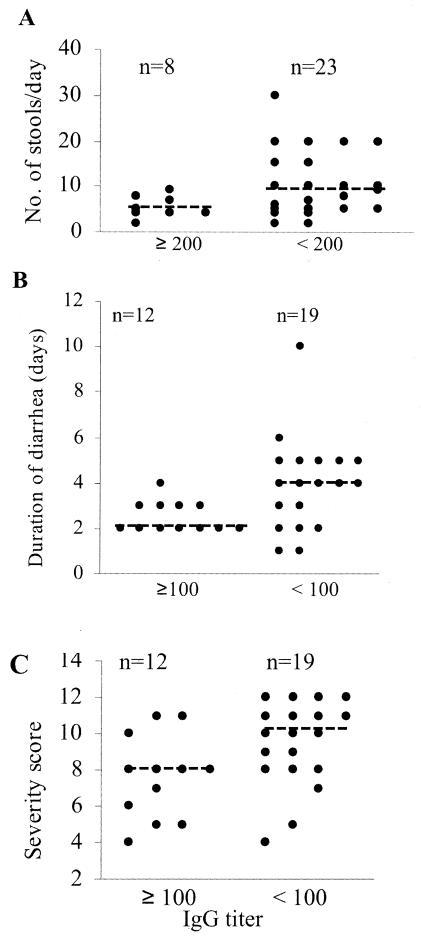
We assessed whether preexisting or newly induced antibodies in the acute-phase sera of the 31 subjects with detailed symptom scores were predictive of disease severity (Fig. 3 and 4). Compared with subjects with no IgG or lower IgG titers, subjects with IgG titers ≥200 in acute-phase sera had significantly fewer (P < 0.05) stools per day (medians, 4.5 and 9 stools per day, respectively) (Fig. 3A), and patients with IgG titers ≥100 in acute-phase sera had significantly shorter (P < 0.05) durations of diarrhea (median durations, 2 and 4 days, respectively) (Fig. 3B). Subjects with IgG titers ≥100 in acute-phase sera also had significantly lower (P < 0.05) composite severity scores than those with no IgG or lower IgG titers (median severity scores, 8 and 10, respectively) (Fig. 3C).
FIG. 3.
Association of preexisting serum IgG with a less severe form of disease. The titers of rotavirus-specific IgG in acute-phase sera from 31 children for whom detailed symptom scores during the course of illness were available were determined as described in the text. IgG titers ≥100 or ≥200 were significantly associated with fewer numbers of stools per day (A), a shorter duration of diarrhea (B), or a lower severity of composite symptom scores (C). The median scores for the groups of subjects are indicated by dashed lines.
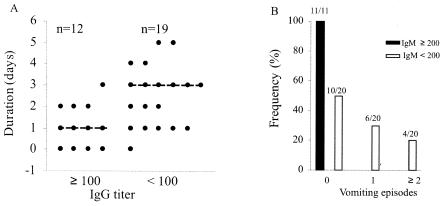
FIG. 4.
Association of preexisting serum antibodies with less severe vomiting. The titers of rotavirus-specific antibodies in acute-phase sera from 31 children for whom detailed symptom scores during the course of illness or on the day of the first blood sample collection were available were determined as described in the text. IgG titers ≥100 and IgM titers ≥200 were significantly associated with a shorter duration of vomiting (A) and a lack of vomiting (B), respectively. The median numbers of days of vomiting in the groups of subjects are indicated by dashed lines.
Serum antibody was also identified as a proxy for protection against vomiting. Children with IgG titers ≥100 during the acute phase had vomiting of a shorter duration (P < 0.01) than those with no IgG or lower IgG titers (medians, 1 and 3 days, respectively) (Fig. 4A). Children with IgM titers <200 during the acute phase were more likely (P < 0.01) to vomit than those with IgM titers ≥200 during the acute phase (Fig. 4B). None of the 11 children with IgM titers ≥200 during the acute phase experienced vomiting, while 10 of the 20 children with IgM titers <200 during the acute phase vomited at least once on the day of the first blood sample collection.
DISCUSSION
We examined the comprehensive profiles of the Ig isotypes and IgG subclasses in young children with acute diarrhea and demonstrated that the children mounted antibody responses to rotavirus in an age- or a maternal antibody-dependent manner. We detected a significantly higher median or mean IgM titer in the acute-phase sera of patients than in the sera of healthy controls and observed that the acute-phase sera of all patients ≥6 months of age had IgM, indicating that these subjects had already mounted a strong IgM response to rotavirus infection. Because the IgM titers in convalescent-phase sera usually changed little or decreased, IgM could not be used as a marker for seroconversion. Our data confirm those of earlier studies, in which 28% of the infants <6 months old developed IgM antibodies 7 days after illness (9) and a higher IgM prevalence (50%) was seen in children ≥1 year old (8, 10).
We detected high levels of rotavirus IgG in the acute-phase sera of infants with diarrhea in the first few months of life. These high preexisting IgG titers suppressed the IgM response during acute rotavirus infection and resulted in no IgG seroconversions. Children ≥3 months of age generally had lower IgG titers and IgG was detected at lower rates in acute-phase sera, but the children had high seroconversion rates. Similar results were reported in previous studies (13, 17, 18, 29). Of the 42 patients enrolled, the first blood samples were obtained from at least 36 of the patients ≤5 days after the onset of disease, suggesting that rotavirus-specific IgG might not have been adequately induced and that the majority of the patients already had IgG in their acute-phase sera prior to the present rotavirus infection. However, we could not rule out the possibility that the IgG in some older patients was induced by virus infection. The IgA response was poor in infants, and IgA titers in convalescent-phase sera and seroconversion rates increased only in children ≥12 months of age. Low IgA titers were previously reported in infants <8 months of age or in all age groups examined: the prevalence did not exceed 20% in a study conducted in Germany (10) and was 33% among children in Ecuador (8).
IgG1 was detected at high titers in the acute-phase sera of some infants <6 months of age. These high titers were most likely of maternal origin because IgG1 is the Ig subclass that is the most efficiently transported from the placenta (16). On the other hand, the IgG1 detected in the convalescent-phase sera of the 28 patients examined was mostly, if not all, induced by acute rotavirus infection. Low titers of IgG3 were detectable in only five convalescent-phase serum samples. This lower rate of detection may be due to the collection of serum specimens after the peak IgG3 response because of its short half-life in serum or to its greater susceptibility to degradation or denaturation (20). IgG3 plays a major role in acute-phase immunity, whereas IgG1 contributes to the maintenance of immunity during primary viral infections (19, 27, 31). Only a few studies have documented the presence of IgG1 and IgG3 and an earlier IgG3 response in sera of children with rotaviruses infection (2, 3, 17). It is not clear whether our lack of detection of IgG2 and IgG4 reflects their absence, their predominant association with the cell surface (16), or the young ages of these children. More studies are needed to examine the response and function of IgG subclasses in children with rotavirus diarrhea.
We demonstrated associations of preexisting IgG titers in serum ≥100 or ≥200 during the acute phase with less severe diarrhea or a shorter duration of diarrhea or with lower calculated composite severity scores. Our observations agree with those of previous studies (11, 33, 37) that higher IgG titers are associated with protection against diarrheal illness. Of interest, IgG titers ≥100 and IgM titers ≥200 in acute-phase sera were associated with a shorter duration of vomiting or a lack of vomiting. A previous study (21) documented IgA as a marker for protection against vomiting. These associations, however, do not exclude the effects of other host parameters, such as age or cytokines, on protection against disease (26).
Our findings in this study are subject to several limitations. First, because we had only one acute-phase blood sample and one convalescent-phase blood sample from each child with rotavirus diarrhea, we were not able to examine the kinetics of the antibody responses. Second, we had only a small number of serum samples for which the volume was adequate for testing for neutralizing antibodies to different serotypes, and therefore, were not able to document serotype-specific immunity and protection. In addition, the types of fecal specimens collected did not allow us to reliably determine the titers of coproantibodies or address the role of intestinal antibodies in protection among this cohort of subjects. Third, since infants in the first few months of life are known to have high levels of IgG in serum (1), we assumed that the high IgG titers in the acute-phase sera of the young infants examined were predominantly of maternal origin, yet our EIA could not differentiate IgG that originated from the mothers from that induced by virus infection. Lastly, all children in this study were hospitalized, and their illnesses were already severe. Future studies will need to examine the profiles and the roles of serum antibodies in patients with the full range of illness (mild to severe).
Our study has several important implications for the testing of present and new vaccines. First, the different antibody responses or profiles and the apparently different disease outcomes observed in the children in the three age groups indicate a developmental regulation in response to rotavirus infection. Of particular interest was the lack of an IgM response in the acute-phase sera of some infants <6 months of age and the relatively poor IgA response of low titer in the acute- and convalescent-phase sera of infants <12 months old. These poor antibody responses were most likely due to the presence of relatively high titers of maternal IgG in the acute-phase sera of these infants that inhibited rotavirus infection in the gut. These observations further suggest that multiple infections are needed to induce high levels of antibody response in natural infection (14, 34, 37) and support the present immunization regimen of multiple doses to maximize immunity and protection. Second, while both IgA and IgG could be used to measure seroconversions and IgA has been the choice for determination of the antibody response to candidate vaccines in clinical trials, our findings indicate that IgG is the best indicator of seroconversion and, if it is present at critical levels, is a more reliable proxy for protection against severe disease, regardless of its origin (the mother or acute virus infection). Lastly, IgG1 is an effective complement activator and binds with a high affinity to Fc receptors on phagocytic cells (16). Whether this IgG subclass can help clear rotavirus infection through activation of complements or opsonization, as reported for other viruses (23, 35), remains to be determined. Further studies will need to examine a larger numbers of specimens to substantiate these antibody profiles and associations between the titers of antibodies in serum and disease severity.
Acknowledgments
We thank Joseph Bresee for help with the institutional review board protocol, Sara Nelson and Caminade Moscatiello for collecting and processing blood and fecal specimens, and Kathleen Murray for editorial assistance.
This study was supported by CRADA with Aventis Pasteur, Lyon, France.
REFERENCES
- 1.Allansmith, M., B. H. McClellan, M. Butterworth, and J. R. Maloney. 1968. The development of immunoglobulin levels in man. J. Pediatr. 72:276-290. [DOI] [PubMed] [Google Scholar]
- 2.Amyes, E., J. Curnow, Z. Stark, L. Corlett, I. Sutton, and A. Vincent. 2001. Restricted IgG1 subclass of anti-Yo antibodies in a paraneoplastic cerebellar degeneration. J. Neuroimmunol. 114:259-264. [DOI] [PubMed] [Google Scholar]
- 3.Azim, T., H. Zaki, G. Podder, N. Sultana, M.A. Salam, R. Moshfiqur, S. Khuda, and D. A. Sack. 2003. Rotavirus-specific subclass antibody and cytokine responses in Bangladeshi children with rotavirus diarrhoea. J. Med. Virol. 69:286-295. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, D. I., M. M. McNeal, G. M. Schiff, and R. L. Ward. 1989. Induction and persistence of local rotavirus antibodies in relation to serum antibodies. J. Med. Virol. 28:90-95. [DOI] [PubMed] [Google Scholar]
- 5.Bhan, M. K., J. F. Lew, S. Sazawal, B. K. Das, J. R. Gentsch, and R. I. Glass. 1993. Protection conferred by neonatal rotavirus infection against subsequent diarrhea. J. Infect. Dis. 168:282-287. [DOI] [PubMed] [Google Scholar]
- 6.Bishop, R. F., G. L. Barnes, E. Cipriani, and J. S. Lund. 1983. Clinical immunity after neonatal rotavirus infection: a prospective longitudinal study in young children. N. Engl. J. Med. 309:72-76. [DOI] [PubMed] [Google Scholar]
- 7.Bouvet, J. P., and V. A. Fischetti. 1999. Diversity of antibody-mediated immunity at the mucosal barrier. Infect. Immun. 67:2687-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brussow, H., J. Sidoti, D. Barclay, J. Sotek, H. Dirren, and W. B. Freire. 1990. Prevalence and serotype specificity of rotavirus antibodies in different age groups of Ecuadorian infants. J. Infect. Dis. 162:615-620. [DOI] [PubMed] [Google Scholar]
- 9.Brussow, H., H. Werchau, L. Lerner, C. Mietens, W. Liedtke, J. Sidoti, and J. Sotek. 1988. Seroconversion patterns to four human rotavirus serotypes in hospitalized infants with acute rotavirus gastroenteritis. J. Infect. Dis. 158:588-595. [DOI] [PubMed] [Google Scholar]
- 10.Brussow, H., H. Werchau, W. Liedtke, L. Lerner, C. Mietens, J. Sidoti, and J. Sotek. 1988. Prevalence of antibodies to rotavirus in different age-groups of infants in Bochum, West Germany. J. Infect. Dis. 157:1014-1022. [DOI] [PubMed] [Google Scholar]
- 11.Clemens, J. D., R. L. Ward, M. R. Rao, D. A. Sack, D. R. Knowlton, F. P. L. van Loon, S. Huda, M. McNeal, F. Ahmed, and G. Schiff. 1992. Seroepidemiologic evaluation of antibodies to rotavirus as correlates of the risk of clinically significant rotavirus diarrhea in rural Bangladesh. J. Infect. Dis. 165:161-165. [DOI] [PubMed] [Google Scholar]
- 12.Coulson, B. S., K. Grimwood, P. J. Masendycz, J. S. Lund, N. Mermelstein, R. F. Bishop, and G. L. Barnes. 1990. Comparison of rotavirus immunoglobulin A coproconversion with other indices of rotavirus infection in a longitudinal study in childhood. J. Clin. Microbiol. 28:1367-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson, G. P., R. J. Hogg, and C. P. Kirubakaran. 1983. Serum and intestinal immune response to rotavirus enteritis in children. Infect. Immun. 40:447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinoza, F., M. Panlagua, H. Hallander, L. Svensson, and O. Strannegärd. 1997. Rotavirus infections in young Nicaraguan children. Pediatr. Infect. Dis. J. 16:564-571. [DOI] [PubMed] [Google Scholar]
- 15.Flores, J., I. Perez-Schael, M. Gonzalez, D. Garcia, M. Perez, N. Daoud, W. Cunto, R. M. Chanock, and A. Z. Kapikian. 1987. Protection against severe rotavirus diarrhea by rhesus vaccine in Venezuelan infants. Lancet i:882-884. [DOI] [PubMed] [Google Scholar]
- 16.Goldsby, R. A., T. J. Kindt, and B. A. Osborne. 1999. Immunoglobulins: structure and function, p. 83-113. In R. A. Goldsby, T. J. Kindt, and B. A. Osborne (ed.), Immunology. W. H. Freeman & Company New York, N.Y.10233681
- 17.Grauballe, P. C., A. Hornsleth, K. Hjelt, and P. A. Krasilnikoff. 1986. Detection by ELISA of immunoglobulin G subclass-specific antibody responses in rotavirus infections in children. J. Med. Virol. 18:277-281. [DOI] [PubMed] [Google Scholar]
- 18.Grimwood, K., J. C. S. Lund, B. S. Coulson, I. L. Hudson, R. F. Bishop, and G. L. Barnes. 1988. Comparison of serum and mucosal antibody response following severe acute rotavirus gastroenteritis in young children. J. Clin. Microbiol. 26:732-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gut, J. P., C. Lablache, and B. S. Kirn. 1995. Symptomatic mumps virus reinfections. J. Med. Virol. 45:17-23. [DOI] [PubMed] [Google Scholar]
- 20.Heiner, D. C. 1984. Significance of immunoglobulin G subclasses. Am. J. Med. 76:1-6. [DOI] [PubMed] [Google Scholar]
- 21.Hjelt, K., P. C. Grauballe, A. Paerregaard, O. N. Nielsen, and P. A. Krasilnikoff. 1987. Protective effect of preexisting rotavirus-specific immunoglobulin A against naturally acquired rotavirus infection in children. J. Med. Virol. 21:39-47. [DOI] [PubMed] [Google Scholar]
- 22.Hjelt, K., P. C. Grauballe, P. O. Schiotz, L. Andersen, and P. A. Krasilnikoff. 1985. Intestinal and serum immune response to a naturally acquired rotavirus gastroenteritis in children. J. Pediatr. Gastroenterol. Nutr. 4:60-66. [DOI] [PubMed] [Google Scholar]
- 23.Jahrling, P. B., R. A. Hesse, A. O. Anderson, and J. D. Gangemi. 1983. Opsonization of alphaviruses in hamsters. J. Med. Virol. 12:1-16. [DOI] [PubMed] [Google Scholar]
- 24.Jayashree, S., M. K. Bhan, R. Kumar, R. I. Glass, and N. Bhandari. 1988. Serum and salivary antibodies as indicators of rotavirus infection in neonates. J. Infect. Dis. 158:1117-1119. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, B., J. R. Gentsch, and R. I. Glass. 2002. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin. Infect. Dis. 34:1351-1361. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, B., L. Snipes-Magaldi, P. Dennehy, H. Keyserling, R. C. Holman, J. Bresee, J. Gentsch, and R. I. Glass. 2003. Cytokines as mediators for or effectors against rotavirus disease in children. Clin. Diagn. Lab. Immunol. 10:995-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junker, A. K., and P. Tilley. 1994. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J. Med. Virol. 43:119-124. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann, E. L. 1975. Nonparametrics: statistical methods based on ranks. Holden-Day, Inc., San Francisco, Calif.
- 29.Midthun, K., L. Pang, J. Flores, and A. Z. Kapikian. 1989. Comparison of immunoglobulin A (IgA), IgG, and IgM enzyme-linked immunosorbent assays, plaque reduction neutralization assay, and complement fixation in detecting seroresponses to rotavirus vaccine candidates. J. Clin. Microbiol. 27:2799-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulton, L. H., M. A. Staat, M. Santosham, and R. L. Ward. 1998. The protective effectiveness of natural rotavirus infection in American Indian population. J. Infect. Dis. 178:1562-1566. [DOI] [PubMed] [Google Scholar]
- 31.Narita, M., S. Yamada, Y. Matsuzono, O. Itakura, T. Togashi, and H. Kikuta. 1997. Measles virus-specific immunoglobulin G subclass response in serum and cerebrospinal fluid. Clin. Diagn. Virol. 8:233-239. [DOI] [PubMed] [Google Scholar]
- 32.Offit, P. A., E. J. Hoffernberg, N. Santos, and V. Gouvea. 1993. Rotavirus-specific humoral and cellular immune response after primary, symptomatic infection. J. Infect. Dis. 167:1436-1440. [DOI] [PubMed] [Google Scholar]
- 33.O'Ryan, M. L., D. O. Matson, M. K. Estes, and L. K. Pickering. 1994. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J. Infect. Dis. 169:504-511. [DOI] [PubMed] [Google Scholar]
- 34.Ray, P. G., and S. D. Kelkar. 2004. Measurement of antirotavirus IgM/IgA/IgG responses in the serum samples of Indian children following rotavirus diarrhea and their mothers. J. Med. Virol. 72:416-423. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, B. L., E. J. Knopoff, M. Saifuddin, D. M. Takefman, M.-N. Saarloos, B. E. Sha, and G. T. Spear. 1996. Susceptibility of HIV-1 plasma virus to complement-mediated lysis. J. Immunol. 157:1791-1798. [PubMed] [Google Scholar]
- 36.Velazquez, F. R., D. O. Matson, J. J. Calva, M. L. Guerrero, A. L. Morrow, S. Carter-Campbell, R. I. Glass, M. K. Estes, L. K. Pickering, and G. M. Ruiz-Palacios. 1996. Rotavirus infection in infants as protection against subsequent infections. N. Engl. J. Med. 335:1022-1028. [DOI] [PubMed] [Google Scholar]
- 37.Velazquez, F. R., D. O. Matson, M. L. Guerrero, J. Shults, J. J. Calva, A. L. Marrow, R. I. Glass, L. K. Pickering, and G. M. Ruiz-Palacios. 2000. Serum antibody as a marker of protection against natural rotavirus infection and disease. J. Infect. Dis. 182:1602-1609. [DOI] [PubMed] [Google Scholar]
- 38.Ward, R. L., and D. I. Bernstein for the US Rotavirus Vaccine Efficacy Group. 1994. Protection against rotavirus disease after natural rotavirus infection. J. Infect. Dis. 169:900-904. [DOI] [PubMed] [Google Scholar]