Abstract
Renal ischemia-reperfusion injury (IRI) is a common cause of renal dysfunction and renal failure. Histone/protein deacetylases (HDACs) regulate gene accessibility and higher order protein structures and may alter cellular responses to a variety of stresses. We investigated whether use of pan- and class-specific HDAC inhibitors (HDACi) could improve IRI tolerance in the kidney. Using a model of unilateral renal IRI, we investigated early renal function after IRI, and calculated fibrosis after IRI using an automated scoring system. We found that pan-HDAC inhibition using trichostatin (TSA) yielded significant renal functional benefit at 24–96 hours (p < 0.001). Treated mice developed significantly less fibrosis at 30 days (p < 0.0004). Class I HDAC inhibition with MS-275 yielded similar effects. Protection from fibrosis formation was also noted in a cold ischemia transplant model (p < 0.008) with a trend toward improved cold ischemic survival in TSA-treated mice. These effects were not accompanied by induction of typical ischemic tolerance pathways or by priming of heat shock protein expression. In fact, heat shock protein 70 deletion or overexpression did not alter renal ischemia tolerance. Micro-RNA 21, known to be enhanced in vitro in renal tubular cells that survive stress, was enhanced by treatment with HDACi, pointing to possible mechanism.
Introduction
Ischemia-reperfusion injury (IRI) is a significant source of morbidity in renal transplantation, as well as other medical scenarios including cardiac arrest, cardiopulmonary bypass and trauma. Despite maneuvers to mitigate this process, IRI and manifestations of early allograft dysfunction occur in 30% of renal transplant recipients and are associated with poorer long-term outcomes (1–3). Organ shortage has led to acceptance of grafts with greater degrees of baseline ischemic insult, secondary to either the mode of donor death or to overall donor co-morbidities (4,5). This may contribute to the fact that while short-term graft survival has steadily improved, long-term graft survival has been essentially unchanged over nearly two decades (6–9). Loss of renal transplant function is a predictor of death, including death from cardiovascular causes (10). As such, it is critical to gain a more thorough understanding of the molecular mechanisms leading to IRI, in order to develop new strategies for injury prevention and treatment. IRI, as the name implies, is a two-phase event. Initially, an ischemic insult induces ATP depletion, mitochondrial dysfunction and release of calcium, protease complexes and free radicals. Reperfusion of this damaged tissue then activates innate immune pathways causing cellular apopto-sis and adaptive immune responses, which can be locally destructive (5).
We hypothesize that histone/protein deacetylases (HDACs) contribute significantly to IRI. HDACs are a highly conserved family of proteins that remove acetyl groups from DNA-associated histone proteins around which chromosomes are supercoiled. Acetylation disrupts the association between positively charged histone tails and negatively charged DNA, and de-acetylation reverses this. In this manner HDACs, and their counterparts, the histone/protein acetyltransferases (HATs), regulate access of large transcriptional complexes to promoter sites on coiled segments of chromosome, leading to gene activation or silencing (11). HDACs are now known to also regulate the acetylation of > 1750 non-histone proteins (12).
HDACs are subdivided by homologous structure and function into several classes. Class I HDACs (HDAC-1, -2, -3, -8) are notable for ubiquitous expression and nuclear localization. Class IIa HDACs (HDAC-4, -5, -7, -9) generally have negligible or only weak deacetylase activity, shuttle between the nucleus and cytoplasm, and are regulated by intracellular kinase cascades. Class IIb (HDAC-6, -10) are distinguished by containing two separate catalytic domains (13). It is now clear that HDACs also deacetylate non-histone proteins in order to participate in more generalized signaling mechanisms and transcriptional regulatory pathways, and these effects may be more important than those involving histone acetylation (14). These targets include heat shock protein 90 (Hsp90) (15) and heat shock factor protein-1 (HSF-1) (16), both known components of renal IRI, which when acetylated during stress lead to HSF-1 nuclear translocation and induction of a number of genes including Hsp70, a protein known to be highly expressed during IRI and other modalities of cell stress (17–24).
We hypothesized that HDAC inhibition would lead to improved IRI tolerance in murine models of renal IRI, and that by assessing the class specificity of this process, we might identify whether the effects were mediated by gene regulation or by induction of heat shock responses.
Materials and Methods
IRI model
Briefly, wild-type (WT) C57BL/6 female adult mice (Jackson Laboratories, Bar Harbor, ME) weighing 18–25 g were treated with DMSO control or TSA (1 mg/kg in DMSO) (Selleck Chemicals, London, ON, Canada) (25), a pan-HDAC inhibitor, by intraperitoneal injection 16 hours before IRI and again just prior to the start of the warm ischemia procedure. Alternate experiments utilized the same administration pattern with class I-specific HDACi, MS-275, at 3 mg/kg in DMSO (Selleck Chemicals) (26–28). To achieve adequate experimental warm-ischemia, mice were anesthetized with pentobarbital sodium (65 mg/kg IP). After anesthetic, they were placed in a temperature-controlled operative apparatus. Core body temperature was continuously measured and maintained at 36.0 ± 0.5 °C. Under an operating microscope, the left renal pedicle exposed and clamped for 28 minutes with a microvascular clip (Roboz Surgical Instrument, Gaithersburg, MD). After the clamp was released, the right kidney was exposed and removed. After closure, animals were subcutaneously injected with 100 mL/kg of warm saline after the operation to ensure hydration. Animals were kept in an incubator (37 °C) until awake. Postoperatively, plasma BUN was assessed daily for 4 days, and then weekly, using an i-STAT Analyzer with 6+ cartridges (Abbott Labs, Lake Forest, IL). These cartridges have a maximum BUN reading of 140 mg/dl. Mice were given access to water ad lib post-procedure. All animal protocols adhered to the NIH Guide for the Care and Use of Laboratory Animals and were performed in an AAALAC accredited facility.
Cold ischemia renal transplant model
TSA (1 mg/kg in DMSO) was administered I.P. to donors and recipients of renal transplants 16 hours and just prior to the ischemia event (donor) and 16 hours and just prior to the renal implantation (recipient). DMSO alone was administered to control donors and recipients. The organ procurement and transplant procedure was used per published technique with the exception that we implanted the ureter directly into the bladder with a purse string bladder suture (29). After donor procurement, the donor kidney was flushed with 0.5 mL of UW solution and stored in an ice bath in a cold room for 18 hours. Implantation was timed to be reperfused at 18 hours after procurement. Graft sew-in time was standardized at 30 minutes. Due to extensive cold ischemia injury, native nephrectomy was performed at 1 week after transplant in one group and 30 days after transplant in another group to allow for determination of transplant graft function or long-term fibrosis respectively.
Tissue collection
Under terminal general anesthesia, the left kidney was harvested at the indicated time and divided into four parts and frozen in liquid nitrogen for the extraction of protein or RNA, paraffin embedded for histopathology, and snap-frozen for immunoperoxidase staining (30).
RNA isolation and quantitative PCR
RNA extraction and purification was performed using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was reverse transcribed to cDNA using TaqMan reverse transcription reagents (ABI, Grand Island, NY). Quantitative PCR was performed using gene-specific primers and TAMRA-labeled FAM probes. Samples were run on an ABI PRISM 7000 Sequence Detection System with comparative method normalized to ribosomal RNA and analyzed using Sequence Detector 7.1 (ABI).
Western blotting
Cell lysates prepared from mouse kidneys were separated by SDS-PAGE, transferred to PVDF membranes and immunoblotted using the primary antibody anti-Hsp70 (1:1000, Millipore) and second antibody (Santa Cruz).
Histopathology analysis
Nephrectomy specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Histologic sections (4 μm) were stained with hematoxylin and eosin (H&E), and trichrome, and reviewed by a pathologist (TRB) blinded to conditions. Additional sections were stained as a single group with Sirius Red for quantification of fibrosis by digital image analysis. Sirius Red-stained sections were scanned using the Aperio ScanScope®CS slide scanner (Aperio Technologies, Vista, CA). Whole slide digitized images were analyzed using the Aperio ImageScope software (version 10.0.1346.1807; Aperio Technologies, Vista, CA; http://www.aperio.com/download.asp) using an algorithm optimized for detection of fibrosis (red staining of collagen fibers) as a percentage of the total volume of tissue on the slide. Subcapsular areas were excluded to limit the analysis to parenchymal changes.
Statistical analysis
BUN was compared between groups at various time points using the student t-test and curves were compared using ANOVA – a log scale was used to correct for flattening of the curve due to a cap on peak detectable BUN. Cold ischemia survival was determined by Kaplan–Meier analysis. Sirius red percentage by group was compared pairwise using Mann–Whitney U analysis, and among all groups using ANOVA. Statistical analysis performed with GraphPad Prism software (La Jolla, CA).
Results
Pan-HDAC inhibition and early renal function after warm IRI
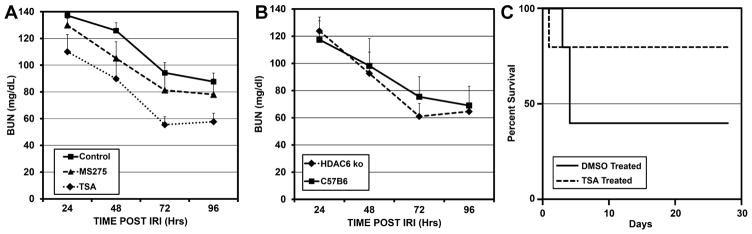
Unilateral renal IRI with contralateral nephrectomy was carried out under tightly controlled temperature conditions. Female C57BL/6 adult mice were used throughout. Twenty-eight minutes of warm ischemia yielded a consistently survivable and significant injury, with a mean 24-hour BUN peak of 137 ± 8.9 (n = 23). To assess the effect of pan-HDAC inhibition, 1 mg/kg of trichostatin (TSA) in dimethylsulfoxide (DMSO) was administered intraperitoneally 16 hours before, and again just prior to, renal IRI. Controls received DMSO alone at the same time points. Blood urea nitrogen (BUN) was assessed every 24 hours for the first 4 days after IRI (Figure 1A). TSA-treated mice (n = 11) had a lower mean BUN peak at 24 hours, and area-under-curve analysis demonstrated significantly lower BUN levels over the entire curve up to 96 hours, compared to DMSO treated controls (n = 23) (p < 0.001). The maximum detectable level of BUN using the cartridge-based assay was 140, so that any level above 140 would yield a reading of 140–this caps the maximum detectable value.
Figure 1.
Serial BUN measurements at 24–96 hours after standardized 28 minutes of renal IRI in female mice (±SEM), comparing A those treated with pan-HDAC inhibitor TSA (n = 11, p < 0.001), class I specific HDAC inhibitor MS-275 (n = 5, p < 0.01), or DMSO control (n = 23), and B mice lacking HDAC6 (n = 5; p = 0.72) or control B6 mice (n = 5).
Pan HDAC inhibition and fibrosis formation after warm IRI
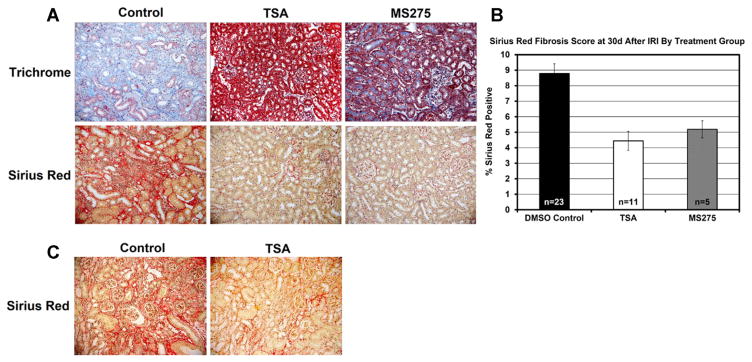
At 30 days after IRI, mice were sacrificed and kidney tissue was preserved as described. Kidneys that had been treated with TSA prior to IRI demonstrated lower tubular injury and inflammation by a four-stage scoring system, and showed less fibrosis by trichrome staining. To better quantify differences in fibrosis, a whole slide computer-scored, Sirius Red-based fibrosis assessment was performed (Figure 2). Mice treated with TSA prior to IRI developed significantly less fibrosis than DMSO treated controls (p < 0.0004).
Figure 2. Assessment of fibrosis formation in samples taken 30 days after renal IRI from mice treated with HDAC inhibitor or control mice.
(A) Trichrome and Sirius Red staining for renal fibrosis in mice treated with TSA (n = 11), MS-275 (n = 5), or DMSO controls (n = 23) (200×). (B) Computer-automated assessment of Sirius Red positivity from whole renal sections mice treated with TSA (p < 0.0004), MS-275 (p < 0.01), or DMSO control. (C) Sirius Red staining for renal fibrosis after isograft transplantation with 18 hours cold ischemia in mice treated with TSA (n = 6) or DMSO controls (n = 7) (200×).
Lymphocyte migration after warm IRI
We have shown that pan-HDACi use can accentuate the function of T-regulatory (Treg) cells (30–34). To assess whether IRI was accompanied by an influx of T cells generally, or Tregs specifically, we performed immunohistochemistry on kidney sections taken at various times from 1 to 28 days after IRI, comparing DMSO-treated controls to TSA-treated mice. There was a modestly increased infiltration of T cells into the kidneys at 4 days after IRI in TSA-treated mice, compared to controls (Figure S1). However, at 14 and 28 days, T cell infiltrates in TSA-treated mice were diminishing, whereas the DMSO-treated controls continued to have significant T cell-rich infiltrates for up to 28 days post-IRI. Treg were rarely seen in either condition, making determinative effects unlikely.
Histone acetylation after warm IRI
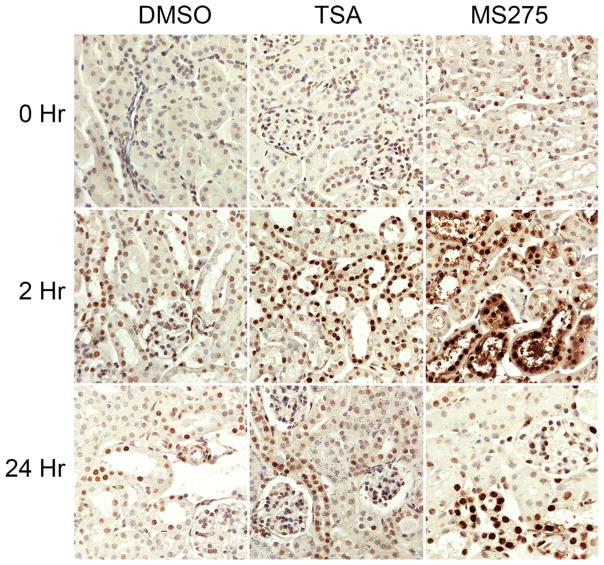
To assess the effects of TSA on histone acetylation in the kidneys of treated mice, we stained renal sections for acetylated histone 3 (acH3) at several time-points within 24 hours of IRI. As anticipated, we found that H3 lysine acetylation (acH3K9) was significantly increased in TSA-treated samples relative to control (Figure 3).
Figure 3. Immunohistochemistry of renal cortical tissue showing acetylated histone 3 at designated hours after IRI in mice treated with TSA, MS-275 or DMSO control.
Increased acetylated histone 3 is seen in HDAC inhibitor-treated kidneys after IRI.
Class-specific HDAC inhibition and warm IRI
To assess HDAC class-specificity of renal protection, we utilized a class I specific HDAC inhibitor, MS-275, with the same dosing regimen and the same renal IRI protocol as in our studies with TSA. We found that MS-275-treated mice had lower BUN in the 4 days after renal IRI (p < 0.01), and developed less fibrosis at 28 days compared to DMSO controls (p < 0.01), although the effects were somewhat diminished compared to pan-HDAC inhibition with TSA (Figures 1A and 2). Pan-HDAC inhibited mice (TSA-treated) had significantly lower BUN curves compared to class-I HDAC- (MS-275) treated mice (p < 0.01).
No class II-specific HDAC inhibitors exist at this time. HDAC6 is a prominent HDAC class II member, so to assess the role that class II HDAC has on the process of renal IRI, we performed renal IRI in mice with germ-line deletion of HDAC6 (HDAC6−/−) compared to wild-type controls (Figure 1B). HDAC6−/− mice had equivalent BUN at four daily time-points after renal IRI compared to wild-type controls, (p = 0.72). HDAC6 plays a significant role in HSF-1 localization and trafficking, diminishing the likely import of HSF-1 effects here (35). Thus the majority of the benefit of using pan HDAC inhibitors in renal IRI seems to reside in the class I-specific effects.
HDAC inhibition and cold IRI
In order to further test the impact of HDAC inhibition on ischemia tolerance, we utilized a renal transplant model of cold ischemia, with 18 hours of cold storage in UW solution in an ice bath and then standardized timing of renal transplantation (30 minutes) from B6 female donors to B6 female recipients. Experimental donors and recipients were given TSA I.P. and controls received treatment with DMSO alone. This model induces greater injury that the warm IRI model so early graft dysfunction can compromise survival. Thus, we compared two groups: in the first, native nephrectomies were performed 7 days after transplant and subsequent renal function of the transplanted kidney and recipient survival were tabulated. TSA-treated pairs had 4/5 recipients survive to 30 days after native nephrectomy.
Controls had 2/5 survive to 30 days. This trend to survival benefit with TSA treatment did not meet significance due to small numbers, limited by the complexity of the procedure (p = 0.32) (Figure 1C). Because there was significant mortality in the control group, we wished to assess a complete second group of survivors for fibrosis development at 30 days after transplant. We thus delayed native nephrectomy until 30 days after transplant to allow for fibrosis to develop in the transplanted kidney. At 30 days, we sacrificed the animals, then compared Sirius Red percentage in the grafts. Tissue damage and fibrosis was more significant in this model than in the warm IRI model and strong positive Sirius Red percentage was judged to be most representative of fibrosis extent by a blinded pathologist. Sirius Red percentage in the TSA-treated group (n = 6) was significantly less than in the control group (n = 7) (p = 0.008), indicating substantial protection from fibrosis with TSA (Figure 2C).
Gene expression in renal tissue
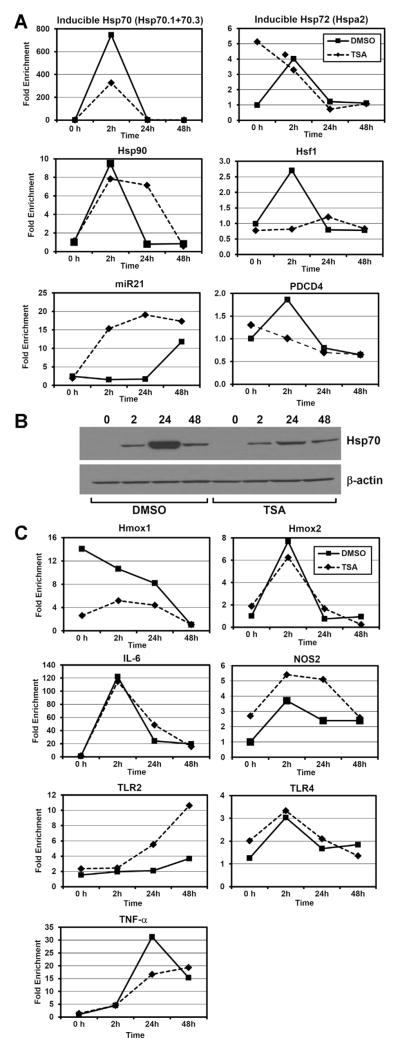
Since HDAC inhibition can, in theory, alter the expression of any gene, we wished to pursue a targeted analysis of genes known to be associated with IRI tolerance or heat shock, as opposed to broad genome-wide analysis that would be prone to identify a large number of differences in expression of unknown significance. Utilizing quantitative PCR, we determined that a number of identified heat shock proteins and modulators of heat shock response (Hsp70, Hsp72, Hsp90, Hsf1) had either no change or significantly lower expression in TSA-treated animals at a number of time points after IRI relative to controls (Figure 4A). Constitutively expressed Hsp72 did have higher expression before IRI in TSA-treated mice compared to controls, but post-ischemia expression was nearly identical. To confirm the lack of induction of Hsp70 in HDAC inhibitor-treated mice, we performed Western blotting and confirmed significant diminution of Hsp70 protein in post-IRI kidney specimens treated with HDAC inhibitor compared to controls (Figure 4B). We also did not find enhanced Hsp70 protein expression by immunohistochemistry on sections of kidneys from TSA-treated mice compared to DMSO-treated mice (Figure S2).
Figure 4. Quantitative PCR and Western blot analysis at designated hours after IRI in mice treated with TSA or control.
(A) Quantitative PCR assessment of mRNA expression of heat shock proteins, heat shock protein factor, miR-21 and PDCD4 mRNA at designated hours after IRI. (B) Western blot showing inducible Hsp70 protein expression at designated hours after IRI. (C) Quantitative PCR assessment of mRNA expression of ischemia-associated pathways at designated hours after IRI.
We subsequently determined gene expression of other known mediators of IRI tolerance including heme oxygenases, nitric oxide synthase (NOS), TNF-α, IL-6 and toll-like receptors (TLR-2 and -4) in kidney tissue (Figure 4C). NOS-2 expression was slightly increased in TSA-treated kidneys relative to controls, but these differences did not meet significance. Heme oxygenase expression was significantly decreased in TSA-treated kidneys relative to controls. TSA-treated mice had greater gene expression of TLR2 compared to controls at 24 and 48 hours after IRI. Other gene expression differences were not significant. None of these expression differences appeared to be significant enough to explain the IRI differences seen phenotypically.
Lastly, we wished to determine expression levels of micro-RNA21 (miR-21), the overexpression of which has been shown to be protective of renal tubular epithelium and whose expression is diminished in ischemic kidneys (36). We determined that miR-21 was not overexpressed prior to injury, but was significantly overexpressed at 2 and 24 hours after IRI relative to control kidneys (Figure 4A). The control kidneys eventually demonstrated a rise in mir-21 at 48 hours after IRI. We then determined that programmed cell death protein-4 (PDCD-4), expression, which rises in arrested tubular cells, had a spike in mRNA expression in control mice at 2 hours after IRI, but diminished in TSA-treated mice after IRI (Figure 4A).
Heat shock protein-70 and IRI
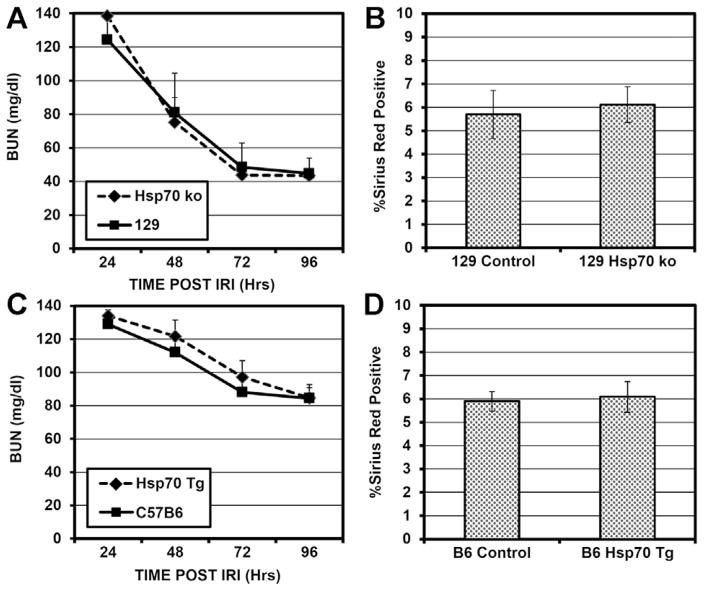
Because we demonstrated diminished inducible Hsp70 mRNA and protein expression in kidneys treated with HDAC inhibitors and these kidneys exhibited significantly improved IRI tolerance and less fibrosis formation, we wished to directly assess the effects of Hsp70 on renal IRI. Using mice either overexpressing Hsp70 (22) or deficient in both Hsp70.1 and Hsp70.3 due to germline gene deletions (37), we assessed the impact of Hsp70 modulation on renal IRI. As our mice lacking Hsp70 were on the B6.129 background, which has an intrinsically improved IRI tolerance compared to B6, we utilized 50 minutes of warm IRI in the B6.129 Hsp70−/− mice and B6.129 controls to generate equivalent injury as in the B6 mice at 28 minutes. There was no significant difference in renal function or recovery in mice lacking Hsp70, compared to controls, after standardized IRI (p = 0.95). We then assessed fibrosis formation and again found no significant difference between control and mice lacking both inducible Hsp70 genes (p = 0.75) (Figure 5A–B). In mice overexpressing Hsp70, we saw no significant improvement in renal IRI tolerance either by early renal function after IRI (p = 0.53) or in terms of fibrosis formation after IRI, compared to controls (p = 0.81) (Figure 5C–D). In mice overexpressing Hsp70 or lacking Hsp70 there was no significant compensatory modulation in Hsp90 protein level as detected by Western blotting (Figure S3). These findings call into question the importance of inducible Hsp70 in renal IRI tolerance.
Figure 5. Renal functional assessment and fibrosis quantification after 28 minutes standardized renal IRI in female mice overexpressing or lacking inducible Hsp70 (±SEM) A Serial BUN measurements at 24–96 hours after IRI in mice lacking inducible Hsp70 (n = 4) or control B6.
29 mice (n = 4) (p = 0.95). (B) Computer automated assessment of Sirius Red positivity from whole renal sections 30 days after IRI in mice lacking Hsp70 or B6.129 controls (p = 0.75). (C) Serial BUN measurements at 24–96 hours after IRI in mice overexpressing inducible Hsp70 (n = 12) or control B6 mice (n = 22) (p = 0.53). (D) Computer-automated assessment of Sirius Red positivity from whole renal sections 30 days after IRI in mice overexpressing inducible Hsp70 or control B6 mice (p = 0.81).
Discussion
Renal ischemic injury is intrinsic to renal transplantation but also is a common and currently untreatable clinical problem in trauma, cardiovascular surgery, and endovascular procedures. Despite decades of research into mechanism of ischemic injury, no modifiable factors have emerged into the clinical mainstream to alter the tolerance of ischemic injury.
We have determined that treatment with a pan-HDACi, TSA, just prior to renal ischemic injury is protective of renal function in the early phase after injury, and is associated with a substantial diminution of fibrosis long-term after renal IRI. Lesser protection of early renal function was engendered by the class I HDACi, MS-275, although renal fibrosis formation was mitigated similarly to TSA treatment. HDAC6-deficient mice did not have altered tolerance of renal IRI, indicating that elimination of this major class II HDAC does not improve IRI tolerance. Thus, much of the IRI tolerance improvement is derived from class I HDAC inhibition.
We also determined that pan-HDAC inhibition with TSA leads to significant protection from fibrosis and preservation of renal function in a renal transplant-based cold ischemia model and TSA-treated mice had a trend toward greater survival of 18 hours cold renal IRI that did not reach significance due in large part to the limitation of small numbers in this procedure that requires multiple survival surgeries. This lends significant support to the proof of concept that HDAC inhibition can protect versus cold ischemia injury in a transplant setting.
We subsequently investigated gene expression and determined that pan-HDAC inhibitor use does not lead to significantly altered renal expression of many of the typical genes associated with improved IRI tolerance in the laboratory setting, including heme oxygenase 1 and 2, nitric oxide synthase 2, TNF-α, and IL-6. Heme oxygenase 1 expression was actually diminished in TSA-treated kidneys after IRI, compared to the significant induction seen in control mice. Genes encoding the major inducible heat shock proteins and Hsf1 were not induced in TSA-treated kidneys after IRI, relative to control mice. In aggregate, these data indicate that the beneficial effects of HDAC inhibition are likely not mediated by many of the typical known pathways of IRI tolerance (38,39).
Some possible mechanistic data is derived from miR-21 expression, which is significantly induced in TSA-treated kidney relative to control. This induction was not present at time zero, but miR-21 expression was significantly induced at 2 and 24 hours after IRI, along with concomitant failure to induce PDCD-4 at 2 hours after IRI in renal tissue of TSA-treated mice. PDCD-4 is a marker of tubular cell cycle arrest, and preservation of cell cycling in tubular cells may be a cause or effect of renal protective effects via TSA treatment (36,40). This significant increase in miR-21 expression at early time points after IRI suggests a possible mechanistic explanation for the improved injury response seen in the HDACi-treated kidneys, as miR-21 expression is a regulatory element involved in renal injury tolerance (36,40).
MS-275-treated kidneys (class I HDACi) had substantial anti-fibrotic effects, consistent with prior reports that have demonstrated renal fibrosis protection via class I HDAC deletion or inhibition in models of unilateral ureteral obstruction (UUO)-induced renal fibrosis (41,42). While epithelial to mesenchymal transformation as studied in the ureteral obstruction models is not concordant with an ischemia response, the post-injury fibrosis expression may follow a common pathway that can be mitigated with class I HDAC inhibition (41–44).
It was clear that the major inducible form of Hsp70 was not important for renal IRI tolerance in our system, in contrast to other work (45–47). We saw neither diminution of renal tolerance of IRI in mice lacking inducible Hsp70, nor improvement of IRI tolerance in mice overexpressing Hsp70. Few studies have directly tested the effect that deletion of Hsp70 or other HSPs have on IRI tolerance. When directly tested in vivo, lack of protection has been seen even in cases where HSP induction has been protective of renal tubular cells in culture (48). It is possible that the pro-inflammatory effects of HSP release may mitigate the potential cellular benefits of HSP overexpression (49,50). Independent of the mechanism, we show that class I HDAC inhibition substantially improves renal IRI tolerance in vivo, while diminishing Hsp70 and Hsf1 expression compared to controls.
There has been one prior investigation of HDAC inhibition in renal ischemia protection (51). That study was based involved a proprietary compound (4-(phenylthio)butanoic acid [PTBA]) with HDAC inhibitory properties and which expanded renal progenitor cells in zebrafish embryos (52), provided modest renal functional protection in a mouse model of moderate IRI, and protected against renal fibrosis formation in a model of severe ischemia (51). The group did not utilize more widely studied HDACi compounds such as TSA, MS-275, or SAHA (suberoylanilide hydroxamic acid) because of concerns about nephrotoxicity in their zebrafish screening assays. The HDAC class specificity of PTBA is not publicly defined. We view this work and ours to be complementary with unique points of each.
There are several notable aspects of our work. First, we have defined the HDAC class specificity of the renal protection process, and have demonstrated that there is some greater anti-fibrotic protection relative to early renal functional preservation with class I HDAC inhibition compared to pan-HDAC inhibition. Second, we have done so with drugs that have been better studied and are more generally available than previous studies (53). Third, we have demonstrated protection from cold ischemia injury using HDAC inhibitors. Lastly, we have ruled out pre-induction of HSP responses, which would be expected if class II HDAC inhibition were responsible for these effects, as well as conventional mechanisms of renal protection such as the hemeoxygenase and nitric oxide synthase pathways as contributors to this protective effect. IRI tolerance was not affected by Hsp70 deletion or overexpression which is an important finding not previously described.
There are limitations of this work. The HDAC inhibitor treatments in this study were applied at 16 hours and 30 minutes prior to ischemia. This could provide a possible intervention in situations where renal ischemia can be predicted with some degree of reliability, such as in transplantation, cardiac bypass, and vascular surgery, but is less useful for unpredictable acute events such as trauma and sepsis. This limitation is being further studied with treatment limited to the immediate peri-ischemia event or post-ischemia treatment, although preliminary data indicates lesser effects. Additionally, since HDAC inhibition can impact the expression of any gene by altering histone-regulated gene accessibility, determining the exact mechanism involved in renal protection is challenging, although we have ruled out a number of pathways through direct testing or assessment of gene expression with and without HDAC inhibitor treatment. Our identification of the miR-21/PDCD4 pathway yields avenues for further investigation.
Renal ischemia is a prevalent and costly medical problem with no currently approved intervention. Our finding that we can substantially mitigate renal dysfunction and the formation of renal fibrosis with transient HDACi use provides a promising target for further study.
Supplementary Material
Figure S1: Immunohistochemistry showing CD3+ T cells (A) and Foxp3+ regulatory T cells (B) infiltrating kidneys at designated days after renal IRI in mice treated with TSA or DMSO controls.
Figure S2: Immunohistochemistry showing inducible Hsp70 expression at time zero and at 24 hours after renal IRI in mice treated with TSA, MS-275, or DMSO controls.
Figure S3: Western blots showing inducible Hsp70 and Hsp90 protein levels in Hsp70 deficient or control mice (top panel). Western blots showing inducible Hsp70 and Hsp90 protein levels in Hsp70 transgenic or control mice (bottom panel).
Acknowledgments
We would like to acknowledge Robin Noel for assistance with figure preparation, and Tatiana Akimova for assistance with statistical analysis. This work was in part supported by NIH/NIDDK Grant # 1K08DK092282-01 (MHL).
Abbreviations
- acH3
acetylated histone 3
- BUN
blood urea nitrogen
- DMSO
dimethylsulfoxide
- HATs
his-tone/protein acetyl transferases
- HDACi
histone/protein deacetylase inhibitors
- HDACs
histone/protein deacetylases
- HSF1
heat shock factor protein 1
- HSP
heat shock protein
- IRI
ischemia reperfusion injury
- miR
micro-RNA
- NOS
nitric oxide synthase
- PDCD-4
programmed cell death protein-4
- SAHA
suberoylani-lide hydroxamic acid
- TLR
toll-like receptor
- Treg
regulatory T cell
- TSA
Trichostatin
- UUO
unilateral ureteral obstruction
Footnotes
Author Contributions
MHL, ZW, YW, WWH conceived of and designed the experiments; MHL, ZW, YW, TRB, DDA, SM, YL, SC, RH, LW, WWH performed experiments and analyzed data; MHL, TRB, WWH wrote and edited the manuscript; MHL, ZW, YW, TRB, SM, WWH discussed and reviewed the manuscript.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Perco P, Pleban C, Kainz A, Lukas A, Mayer B, Oberbauer R. Gene expression and biomarkers in renal transplant ischemia reperfusion injury. Transpl Int. 2007;20:2–11. doi: 10.1111/j.1432-2277.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 3.Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64:945–947. doi: 10.1097/00007890-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Audard V, Matignon M, Dahan K, Lang P, Grimbert P. Renal transplantation from extended criteria cadaveric donors: Problems and perspectives overview. Transpl Int. 2008;21:11–17. doi: 10.1111/j.1432-2277.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 5.Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: Mechanisms and prevention. Transplant Proc. 2008;40:3279–3288. doi: 10.1016/j.transproceed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 8.Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346:580–590. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:961–972. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 10.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75:1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 11.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 13.Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci USA. 1999;96:4868–4873. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spange S, Wagner T, Heinzel T, Kramer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41:185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Scroggins BT, Robzyk K, Wang D, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang PL, Lun M, Schworer CM, et al. Heat shock protein expression is highly sensitive to ischemia-reperfusion injury in rat kidneys. Ann Clin Lab Sci. 2008;38:57–64. [PubMed] [Google Scholar]
- 18.Vanhooren V, Liu XE, Desmyter L, et al. Over-expression of heat shock protein 70 in mice is associated with growth retardation, tumor formation, and early death. Rejuvenation Res. 2008;11:1013–1020. doi: 10.1089/rej.2008.0783. [DOI] [PubMed] [Google Scholar]
- 19.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 20.Klettner A. The induction of heat shock proteins as a potential strategy to treat neurodegenerative disorders. Drug News Perspect. 2004;17:299–306. doi: 10.1358/dnp.2004.17.5.829033. [DOI] [PubMed] [Google Scholar]
- 21.Hunt CR, Dix DJ, Sharma GG, et al. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison EM, Sharpe E, Bellamy CO, et al. Heat shock protein 90-binding agents protect renal cells from oxidative stress and reduce kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2008;295:F397–405. doi: 10.1152/ajprenal.00361.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bidmon B, Endemann M, Muller T, Arbeiter K, Herkner K, Aufricht C. Heat shock protein-70 repairs proximal tubule structure after renal ischemia. Kidney Int. 2000;58:2400–2407. doi: 10.1046/j.1523-1755.2000.00423.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol. 2009;87:195–202. doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Jung Y, Kim TY, et al. Class I histone deacetylase-selective novel synthetic inhibitors potently inhibit human tumor proliferation. Clin Cancer Res. 2004;10:5271–5281. doi: 10.1158/1078-0432.CCR-03-0709. [DOI] [PubMed] [Google Scholar]
- 27.Zhang ZY, Zhang Z, Schluesener HJ. MS-275, an histone deacetylase inhibitor, reduces the inflammatory reaction in rat experimental autoimmune neuritis. Neuroscience. 2010;169:370–377. doi: 10.1016/j.neuroscience.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 28.Chiechio S, Zammataro M, Morales ME, et al. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009;75:1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- 29.Wang JJ, Hockenheimer S, Bickerstaff AA, Hadley GA. Murine renal transplantation procedure. J Vis Exp. 2009;29:1150. doi: 10.3791/1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 31.Akimova T, Xiao H, Liu Y, et al. Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3 T-regulatory cells. Mucosal Immunol. 2014;7:1209–1220. doi: 10.1038/mi.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beier UH, Akimova T, Liu Y, Wang L, Hancock WW. Histone/protein deacetylases control Foxp3 expression and the heat shock response of T-regulatory cells. Curr Opin Immunol. 2011;23:670–678. doi: 10.1016/j.coi.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beier UH, Wang L, Hancock WW. Combination of isoform-selective histone/protein deacetylase inhibitors improves Foxp3+ T-regulatory cell function. Cell Cycle. 2012;11(18):3351–3352. doi: 10.4161/cc.21876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: Therapeutic targeting of FOXP3+regulatory T cells. Nat Rev Drug Discov. 2009;8:969–981. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA. 2010;107:14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampton CR, Shimamoto A, Rothnie CL, et al. HSP70.1 and -70.3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am J Physiol Heart Circ Physiol. 2003;285:H866–874. doi: 10.1152/ajpheart.00596.2002. [DOI] [PubMed] [Google Scholar]
- 38.Ferenbach DA, Kluth DC, Hughes J. Hemeoxygenase-1 and renal ischaemia-reperfusion injury. Nephron Exp Nephrol. 2010;115:e33–37. doi: 10.1159/000313828. [DOI] [PubMed] [Google Scholar]
- 39.Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem. 2003;278:27256–27266. doi: 10.1074/jbc.M301778200. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro MD, Bagley J, Latz J, et al. MicroRNA expression data reveals a signature of kidney damage following ischemia reperfusion injury. PLoS One. 2011;6:e23011. doi: 10.1371/journal.pone.0023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marumo T, Hishikawa K, Yoshikawa M, Hirahashi J, Kawachi S, Fujita T. Histone deacetylase modulates the pro-inflammatory and fibrotic changes in tubulointerstitial injury. Am J Physiol Renal Physiol. 2010;298:F133–F141. doi: 10.1152/ajprenal.00400.2009. [DOI] [PubMed] [Google Scholar]
- 42.Pang M, Kothapally J, Mao H, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2009;297:F996–F1005. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He W, Wang Y, Zhang MZ, et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120:1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugiura H, Yoshida T, Mitobe M, et al. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant. 2010;25:60–68. doi: 10.1093/ndt/gfp451. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Gall JM, Bonegio RG, et al. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011 doi: 10.1038/ki.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci. 2010;86:115–123. doi: 10.1016/j.lfs.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Chen SW, Kim M, Kim M, et al. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int. 2009;75:499–510. doi: 10.1038/ki.2008.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim BS, Lim SW, Li C, et al. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79:1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 50.O’Neill S, Ross JA, Wigmore SJ, Harrison EM. The role of heat shock protein 90 in modulating ischemia-reperfusion injury in the kidney. Expert Opin Investig Drugs. 2012;21:1535–1548. doi: 10.1517/13543784.2012.713939. [DOI] [PubMed] [Google Scholar]
- 51.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, et al. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24:943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Groh ED, Swanhart LM, Cosentino CC, et al. Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol. 2010;21:794–802. doi: 10.1681/ASN.2009080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glaser KB, Li J, Pease LJ, et al. Differential protein acetylation induced by novel histone deacetylase inhibitors. Biochem Biophys Res Commun. 2004;325:683–690. doi: 10.1016/j.bbrc.2004.10.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Immunohistochemistry showing CD3+ T cells (A) and Foxp3+ regulatory T cells (B) infiltrating kidneys at designated days after renal IRI in mice treated with TSA or DMSO controls.
Figure S2: Immunohistochemistry showing inducible Hsp70 expression at time zero and at 24 hours after renal IRI in mice treated with TSA, MS-275, or DMSO controls.
Figure S3: Western blots showing inducible Hsp70 and Hsp90 protein levels in Hsp70 deficient or control mice (top panel). Western blots showing inducible Hsp70 and Hsp90 protein levels in Hsp70 transgenic or control mice (bottom panel).