Abstract
Cryptosporidium is an important cause of diarrhea in developed and developing countries, and its epidemiology is of interest. The methodologies used in the detection of Cryptosporidium-specific antibodies vary widely, which complicates comparison of results. This study assesses the performance of a Cryptosporidium recombinant protein (rCP41) in a serological assay compared to that of a crude antigen preparation. The 41-kDa protein from the oocyst wall was previously cloned and expressed in Escherichia coli. Sera from 192 healthy adults from the Texas Medical Center (Houston) were tested for anti-Cryptosporidium antibody reactivity using both crude and recombinant antigen preparations in an enzyme-linked immunosorbent assay. Immunoglobulin G reactivity was highly concordant (88%; P < 0.0001) between the two antigen preparations, with 110 positive (57%) and 59 negative (31%) by both tests. Regression analysis revealed a high correlation between the absorbance values generated with both antigen preparations and suggests that the rCP41 may be used in place of crude antigen. These results indicate that the use of the recombinant CP41 antigen in a standardized serodiagnostic assay could provide a reliable and cost-effective method for assessing human exposure to Cryptosporidium.
Cryptosporidiosis is a common cause of diarrhea around the world, but the serological identification of those who have been exposed in the past is sadly wanting. This paper reports the use of a recombinant protein in an enzyme-linked immunosorbent assay (ELISA) format, an approach that is amenable to the adoption of a more standardized assay system among laboratories involved in epidemiological studies.
Cryptosporidium infection is self limited in immunocompetent individuals, but it can cause profuse, watery diarrhea and abdominal symptoms which typically resolve in 7 to 14 days without medical care (10). In immunocompromised individuals, especially those with AIDS, the disease can cause persistent, voluminous diarrhea, which can lead to death. The causative agent, Cryptosporidium, is a protozoan parasite that is infectious to humans. Two major species are associated with human infections, Cryptosporidium parvum and C. hominis (11). C. hominis is primarily transmitted among humans, while C. parvum is zoonotic and can infect multiple mammalian species (21). Transmission of both species is through the fecal-oral route and has been associated with the ingestion of contaminated recreational or drinking water (19). Reports of recreational water-associated outbreaks of cryptosporidiosis in the United States have steadily increased since 1990 (17).
Seroprevalence surveys provide the best measure of community exposure to Cryptosporidium. The present standard for Cryptosporidium antigen used in seroprevalence studies is a crude preparation, consisting of disrupted oocysts (1). Major advances have been made recently in the technology to maintain C. parvum in vitro (2, 12). However, until this methodology becomes widely accepted, researchers will continue to rely on in vivo models to produce adequate numbers of the organism. Various animal models and purification of oocysts from feces have been reported (1, 7, 9). The inherent bacterial and other impurities in such preparations can result in an assay yielding reduced sensitivity (6), significant nonspecific reactivity, and difficulties in comparing results from different laboratories.
To overcome these difficulties, immunodominant antigens rather than whole-parasite extracts could be used for serological detection to standardize the assay and improve its sensitivity and specificity (20). Indeed, recombinant antigens have been successfully employed in serodiagnostic tests for other parasitic diseases, including American trypanosomiasis, amebiasis, toxoplasmosis, and leishmaniasis (16, 28, 29, 32). Several candidate antigens for Cryptosporidium have been identified and successfully cloned (4, 8, 13-15, 18, 23-28, 30, 31, 33).
The present study evaluated one of these recombinant antigens, rCP41, a 41-kDa protein previously isolated from the oocyst wall (15). The native CP41 antigen appears to be associated with the oocyst wall. Further, the CP41 gene sequence has been identified in the genomes of multiple Cryptosporidium species (15), and antiserum raised against C. parvum oocyst proteins identified a 41-kDa band which was specific to Cryptosporidium and was shared by several Cryptosporidium species (C. parvum, C. baileyi, C. meleagridis, and C. serpentis) (15). It should be noted that these studies were done before C. hominis was recognized.
The purpose of this study was to compare the recombinant CP41 antigen to a crude antigen preparation, which is the present standard, in an enzyme-linked immunosorbent assay designed to detect anti-Cryptosporidium antibodies in human sera. One-hundred ninety-two serum samples from adults were tested for specific immunoglobulin G (IgG) and IgM antibodies using each antigen preparation.
MATERIALS AND METHODS
Human serum specimens.
Serum samples were collected from 192 volunteers as part of a separate, ongoing study (5). These volunteers were healthy adults, aged 18 to 50, seen at The University of Texas Health Science Center at Houston. Blood was drawn at the General Clinical Research Center (Memorial Hermann Hospital) only after informed consent was obtained. Serum was immediately separated, aliquoted, and stored at −86°C. The study was approved by the Committee for the Protection of Human Subjects, University of Texas Houston Health Science Center.
Antigen preparations.
Crude antigen was purified from disrupted oocysts (Cryptosporidium parvum, Iowa isolate) in the laboratory of Charles R. Sterling (University of Arizona, Tuscon, Ariz.) as previously described (3). Recombinant CP41 antigen was expressed from the pTrcHis expression vector (Invitrogen, Inc.) as a polyhistidine fusion protein and purified by nickel-nitrilotriacetic acid affinity chromatography as described previously (15). Both antigen preparations were frozen and shipped on dry ice to The University of Texas School of Public Health (Houston). Upon arrival, the preparations were aliquoted and stored at −86°C prior to use.
ELISA testing protocol.
The ELISA testing procedure to detect anti-Cryptosporidium antibodies was previously described (5). Crude antigen was diluted in 0.05 M sodium carbonate buffer, pH 9.6, for a final concentration of 2 μg/ml. Recombinant antigen was diluted in 0.1 M sodium carbonate buffer, pH 9.5, for a final concentration of 144 μg/ml. In each case, 100 μl of antigen (0.2 μg of crude antigen/well and 14.4 μg of recombinant antigen/well, respectively) was added to each well of a 96-well microtiter plate (Immunosorp; Nunc, Roskilde, Denmark) and incubated overnight at 4°C. Microtiter wells were thoroughly washed with 0.1% Tween 20 in a 0.15 M phosphate-buffered saline solution (pH 7.2) between each step.
Wells were blocked at 37°C for 1 h with a 1% nonfat dry milk solution. Sera were diluted (1:2) in PBS and applied at 50 μl/well for 1 h at 37°C. This was followed by addition of biotinylated mouse anti-human IgG or IgM (1:1,000 dilution) (Zymed Laboratories Inc., San Francisco, Calif.) and incubation for 1 h at 37°C. Horseradish peroxidase-conjugated streptavidin (1:1,000 dilution) was then applied to each well and incubated as before. The reaction was visualized by the addition of 0.03% peroxidase-activated 2.2′-azino-di-[3-ethylbenzthiazolinsulfonate-(6)] (Boehringer-Mannheim Biochemicals, Indianapolis, Ind.) to each well, and the plate was read spectrophotometrically (414 nm) at 10 to 15 min postincubation.
Triplicate wells of positive and negative control sera were included on each plate for each antigen. Unknown sera were tested in duplicate against each antigen. Data were expressed as net absorbance, which was calculated by subtracting the mean reagent control wells from the serum-containing wells. Cutoff values for anti-Cryptosporidium antibody positivity were defined as a mean absorbance ≥1.5 times that of the mean negative control serum for each plate.
Sample size calculations.
A pilot study using 32 human serum samples was performed with both rCP41 and the crude antigen preparations. Results provided variability estimates for a sample size calculation (Instat software version 2.0; Graphpad Software, San Diego, Calif.). The desired power level was set at 0.80 (α = 0.05, two sided), and the minimum difference detectable as statistically significant was set at 15%. The estimated sample size was 178 and 185 for the IgG and IgM tests, respectively. Thus, the final sample size of 192 sera was greater than the minimum number indicated to validate the results.
Data analysis.
The comparability of the recombinant antigen to the crude antigen preparation was evaluated using the Fisher's exact test (two sided) for a binomial comparison of positive and negative IgG and IgM absorbances from each antigen preparation. Likewise, Fisher's exact test (two sided) for analysis of proportions was used to compare the number of sera per absorbance value category. Linear regression was used to determine the strength of the correlation between the antigen preparations for IgG or IgM. These calculations were done using Minitab (Minitab, Inc., State College, Pa.) or Instat (GraphPad Software, Inc.); P ≤ 0.05 was considered statistically significant.
RESULTS
Reproducibility of the assays.
Positive and negative control sera were selected on the basis of their reactivity, or lack thereof, to crude antigen used to screen individuals for evidence of prior infection with Cryptosporidium. Mean net absorbance values (i.e., after subtracting the reagent control) of negative control sera tested against crude antigen (± standard deviation) were 0.058 ± 0.019 for IgG and 0.155 ± 0.060 for IgM. In comparison, mean net absorbance values of negative controls tested against the recombinant antigen were 0.055 ± 0.031 for IgG and 0.099 ± 0.065 for IgM. The mean coefficient of variation (CV) among plates for the negative control serum was ≤12.7%, including tests of both antigen preparations and both antibody isotypes. Because there was variability from plate to plate (albeit low), the absorbances of unknown sera were compared to the mean absorbance of the negative control on the same plate. Positive controls were also included on each plate to monitor performance of the reagents. For positive control sera, the overall CV was ≤11.0%.
Unknowns were tested in duplicate. The variability between the two test wells was low, with a mean percent difference of 4.3% (range, 2.5 to 9.1%) and 4.4% (range, 2.8 to 6.9%) for crude and recombinant antigens, respectively, when tested for IgG reactivity. In comparison, the variation between wells when testing for IgM reactivity to crude and recombinant antigens was 4.9% (range, 1.9 to 6.8%) and 5.5% (range, 1.7 to 8.0%), respectively. In only 6 (3.1%) of the 192 samples, differences between wells fell on either side of the cutoff value. These six samples include both antibody isotypes and antigen preparations. In all other samples, duplicates were both in the clearly positive or clearly negative ranges.
Percentages of IgM- and IgG-reactive sera.
Two antigen preparations (crude and recombinant) were compared by testing antibody reactivity (IgM and IgG) in 192 serum samples. Overall agreement in antibody reactivity using the two different antigens was high. Specifically, concordance (positives and negatives combined) between antigen preparations was seen in 79% of sera tested for IgM (Table 1) and 88% of sera tested for IgG reactivity (Table 2).
TABLE 1.
IgM reactivity (ELISA) to Cryptosporidium recombinant CP41 and crude oocyst antigensa
| rCP41 result | Crude antigen
|
||
|---|---|---|---|
| No. positive (%) | No. negative (%) | Total | |
| Positive | 30 (16) | 9 (5) | 39 (20) |
| Negative | 32 (17) | 121 (63) | 153 (80) |
| Total | 62 (32) | 130 (68) | 192 (100) |
Positive results for each test were defined as those sera that registered an absorbance value greater than 1.5 times the mean of their respective IgM low-absorbance control wells for that plate.
TABLE 2.
IgG reactivity (ELISA) to Cryptosporidium recombinant CP41 and crude oocyst antigensa
| rCP41 result | Crude antigen
|
||
|---|---|---|---|
| No. positive (%) | No. negative (%) | Total | |
| Positive | 110 (57) | 17 (9) | 127 (66) |
| Negative | 6 (3) | 59 (31) | 65 (34) |
| Total | 116 (60) | 76 (40) | 192 (100) |
Positive results for each test were defined as those sera that registered an absorbance value greater than 1.5 times the mean of their respective IgG low-absorbance control wells for that plate.
Significant differences, however, emerged when the percent positive values were compared between antigen preparations. That is, a higher percentage (32.3% versus 20.3%; P = 0.011) of sera were IgM positive when the crude antigen was used rather than rCP41. Specifically, 62 of 192 sera were IgM positive against the crude antigen; however, only 30 (48.4%) of these were positive when tested with rCP41. In comparison, of the 130 sera that were IgM-negative with crude antigen, most (121; 93%) were also negative with rCP41. These data show that a substantial proportion (>50%) of the IgM reactivity against crude antigen was not detected when rCP41 was used.
In contrast to the IgM results, the percentages (60.4 versus 66.1) of sera that were IgG positive against crude versus rCP41 antigens were not significantly different (P = 0.289). Further, the majority (110; 94.8%) of the 116 sera that were IgG positive against crude antigen were also positive with rCP41. However, of the 76 sera that were IgG-negative with crude antigen, only 59 (77.6%) were negative against rCP41. rCP41 could identify IgG reactivity in 17 (22.4%) sera that were IgG negative with crude antigen. Taken together, these data indicate that both antigen preparations yielded similar results for determining anti-Cryptosporidium IgG seroprevalence. In contrast, IgM reactivity was different between the two preparations and substantial numbers of sera became IgM negative when rCP41 was used as antigen.
Paired comparison of absorbance values.
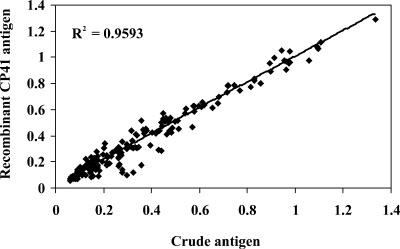
To examine these findings in more detail, a paired analysis of all 192 sera was done by plotting the mean absorbance result from the crude antigen against the mean absorbance from the recombinant. For IgG reactivity, absorbance values among the 192 sera showed a strong linear association (r2 = 0.959, P < 0.001) between the crude and recombinant antigens (Fig. 1) . Further, the distribution of absorbances was similar when IgG reactivity was determined with either of the preparations. Specifically, 44 and 42% of sera had net absorbance values of <0.20 to crude and recombinant antigens, respectively. Similarly, 43% (crude antigen) and 45% (recombinant antigen) of absorbance values fell within the 0.20 to 0.70 optical density range, and 13% of sera had absorbance values of >0.70 against both antigens.
FIG. 1.
Linear regression of absorbance values for anti-Cryptosporidium IgG reactivity to crude and recombinant antigens. The solid line represents the line of best fit, and dotted lines indicate the 95% confidence interval. The r2 value is indicated.
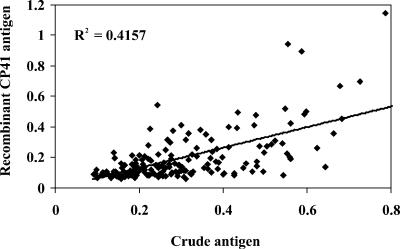
For anti-Cryptosporidium IgM reactivity, absorbance values revealed a weaker association (r2 = 0.416, P < 0.001) between the crude and recombinant antigens (Fig. 2). Likewise, the distribution of absorbances also varied between the two tests. A greater number of sera yielded low absorbance values when sera were tested against the recombinant versus crude antigen preparation. Specifically, only 1% versus 27% (P < 0.001) of sera for crude and recombinant antigens, respectively, had absorbance values that were <0.10. A large proportion, 95 and 71% (P < 0.001) for crude and recombinant antigens, respectively, had absorbance values ranging from 0.10 to 0.60. The remaining sera, 4% for crude and 3% for recombinant, had absorbance values of >0.60.
FIG. 2.
Linear regression of absorbance values for anti-Cryptosporidium IgM reactivity to crude and recombinant antigens. The solid line represents the line of best fit, and dotted lines indicate the 95% confidence interval. The r2 value is indicated.
Sensitivity and specificity.
With crude antigen as the gold standard, the sensitivity and specificity of results with the CP41 antigen were determined. IgM results yielded a sensitivity of 48.4% and a specificity of 93.1%. In comparison, IgG results yielded a sensitivity of 94.8% and a specificity of 77.6%.
DISCUSSION
Serological assays for anti-Cryptosporidium antibodies provide an important and widely used indicator of prior exposure to the parasite. Earlier studies have established that specific serum IgG can be detected with antigens from disrupted oocysts following infection (5, 22). Further, the presence of serum IgG has been associated with at least partial resistance to infection from subsequent challenge (5). However, no standard in serological assays for Cryptosporidium antibodies presently exists among laboratories. Most investigators use disrupted oocysts, and many, but not all, use the C. parvum Iowa isolate as the antigen source. Further, oocyst purification protocols and the degree of purity of the final product may vary. These differences may lead to disparate results and complications in the comparison and interpretation of findings. Thus, a more uniform antigen source that is easier to produce while maintaining or improving the sensitivity and specificity of present methods would be of great assistance to epidemiological investigations. In this study we have compared the utility of two antigen preparations, one a complex mixture of antigens from disrupted oocysts (C. parvum Iowa isolate) and the other a recombinant protein (rCP41) from the oocyst wall.
When sera were tested for IgG reactivity, both antigen preparations yielded comparable results. This conclusion was drawn from the concordance of results when categorical data as well as paired net absorbance values were analyzed. The latter showed a similar distribution of absorbance values and a highly significant correlation. Overall, these findings strongly indicate that the recombinant CP41 antigen yields results comparable to those found with crude antigen. Further, the utility of the CP41 antigen represents an improvement in availability and uniformity of the antigen used in the serological test.
In contrast, when sera were tested for IgM reactivity with the two antigen sources, important differences were noted. The overall concordance of results was lower than that of IgG (79% versus 88%), the pattern of absorbance values was significantly different, and the correlation of absorbances between the methods was substantially lower (r2 = 0.416 versus 0.959). Further, in about half of the sera, IgM reactivity was not present when tested against the rCP41 antigen, but it was present when tested against the crude antigen. In these cases, the absorbances of the discordant values were not clustered near the cutoff point, making it unlikely that discordance is an artifact of the definition of a positive value. In sera with IgM reactivity to crude antigen, but not to rCP1, the antibody may have been directed toward carbohydrate epitopes of one or more glycoproteins in the crude antigen preparation. These specificities would not be present on the nonglycosylated rCP41 protein.
The small number (n = 23; 12%) of discordant IgG results that did occur may be related to the subjectivity in setting a cutoff value that defined a positive result. Indeed, most of the discrepant IgG results had absorbance values that fell close to the cutoff (data not shown). However, if discrepancies arose only for this reason, then one might expect an equal distribution in both directions (i.e., false positives and false negatives). That was not the case in these tests. Seventeen (74%) of those 23 discrepant sera were negative in tests with crude antigen but were positive with the recombinant. This finding may indicate that the CP41 antigen is one of several important immunogens and is represented to an unknown extent in the crude antigen preparation, where it must compete for binding sites on the microtiter plate with other lesser or nonimmunogenic antigens. Thus, the great increase in the CP41 availability in the recombinant may well result in enhanced binding and an increase in the sensitivity of the test. Alternatively, folding differences between the native and recombinant proteins may affect the number or suitability of sites that are available for antibody binding.
The reliability of crude antigen as a gold standard for Cryptosporidium antibody detection is questionable. Advances in Cryptosporidium recombinant technology now make it possible to design new, improved systems for detection of specific antibodies. Our study shows that the recombinant protein from the oocyst wall is comparable to the more commonly used crude antigen preparation for the detection of anti-Cryptosporidium IgG. Standardization of assay conditions using the CP41 recombinant could provide a useful and sensitive method that would allow for a more direct comparison of results among laboratories and studies, particularly if CP41 is present in all Cryptosporidium species that infect humans. The CP41 gene has been previously identified in C. parvum genotypes 1 (now C. hominis) and 2 as well as in C. baileyi and C. wrairi. However, antisera to rCP41 did not recognize C. baileyi (15). Thus, it is uncertain if CP41 expression is common to all Cryptosporidium species. Studies examining CP41 expression in C. hominis oocysts and the protein's cross-reactivity with the analogous protein from other Cryptosporidium species are in progress.
Acknowledgments
This work was supported in part by a cooperative agreement (CR-819814) and grants (CR-824759) from the National Center for Environmental Research (NCER) STAR Program, Environmental Protection Agency, the National Institutes of Health General Clinical Research Centers (GCRC) (M01-RR-02558), and the Food and Drug Administration (FD-U-001621-01).
We especially recognize the contributions of the GCRC nursing staff, particularly Madelene Ottesen, Julie Rice, and Nai Hui Chiu, for blood collections.
This study was the thesis research work of Sonia A. Kjos as part of the requirements for the Master of Science degree.
REFERENCES
- 1.Arrowood, M. J. 1997. Diagnosis, p. 43-64. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press LLC, Boca Raton, Fla.
- 2.Arrowood, M. J. 2002. In vitro cultivation of Cryptosporidium species. Clin. Microbiol. Rev. 15:390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrowood, M. J., and C. R. Sterling. 1987. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 73:314-319. [PubMed] [Google Scholar]
- 4.Cevallos, A. M., X. Zhang, M. K. Waldor, S. Jaison, X. Zhou, S. Tzipori, M. R. Neutra, and H. D. Ward. 2000. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect Immun. 68:4108-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell, C. L., P. C. Okhuysen, C. R. Sterling, C. Wang, W. Jakubowski, and H. L. Dupont. 1999. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am. J. Trop. Med. Hyg. 60:157-164. [DOI] [PubMed] [Google Scholar]
- 6.Crowther, J. R. 1995. ELISA theory and practice. Methods in molecular biology, vol. 42. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 7.Current, W. L. 1990. Techniques and laboratory maintenance of Cryptosporidium, p. 31-50. In J. P. Dubey, C. A. Speer, and R. Fayer (ed.), Cryptosporidiosis of man and animals. CRC Press, Boca Raton, Fla.
- 8.De Graaf, D. C., H. De Coninck, F. Petry, I. B. Eeckhout, and J. E. Peeters. 2002. Specific bovine antibody response against a new recombinant Cryptosporidium parvum antigen containing 4 zinc-finger motifs. Korean J. Parasitol. 40:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entrala, E., J. Molina-Molina, M. Rosales-Lombardo, M. Sanchez-Moreno, and C. Mascaro-Lazcano. 2000. Cryptosporidium parvum: oocysts purification using potassium bromide discontinuous gradient. Vet. Parasitol. 92:223-226. [DOI] [PubMed] [Google Scholar]
- 10.Fayer, R., and B. L. Ungar. 1986. Cryptosporidium spp. and cryptosporidiosis. Microbiol. Rev. 50:458-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyot, K., A. Follet-Dumoulin, E. Lelièvre, et al. 2001. Molecular characterization of Cryptosporidium isolates obtained from humans in France. J. Clin. Microbiol. 39:3472-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hijjawi, N. S., B. P. Meloni, M. Ng'anzo, U. M. Ryan, M. E. Olson, P. T. Cox, P. T. Monis, and R. C. Thompson. 2004. Complete development of Cryptosporidium parvum in host cell-free culture. Int. J. Parasitol. 34:769-777. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins, M. C., and R. Fayer. 1995. Cloning and expression of cDNA encoding an antigenic Cryptosporidium parvum protein. Mol. Biochem. Parasitol. 71:149-152. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins, M. C., R. Fayer, M. Tilley, and S. J. Upton. 1993. Cloning and expression of a cDNA encoding epitopes shared by 15- and 60-kilodalton proteins of Cryptosporidium parvum sporozoites. Infect. Immun. 61:2377-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins, M. C., J. Trout, C. Murphy, J. A. Harp, J. Higgins, W. Wergin, and R. Fayer. 1999. Cloning and expression of a DNA sequence encoding a 41-kilodalton Cryptosporidium parvum oocyst wall protein. Clin. Diagn. Lab. Immunol. 6:912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, A. M., H. Roberts, and A. M. Tenter. 1992. Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J. Med. Microbiol. 37:404-409. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. H., D. A. Levy, G. F. Craun, M. J. Beach, and R. L. Calderon. 2002. Surveillance for waterborne-disease outbreaks-United States, 1999-2000. Morb. Mortal. Wkly. Rep. 51:1-47. [PubMed] [Google Scholar]
- 18.Mead, J. R., R. M. Lloyd, Jr., X. You, C. Tucker-Burden, M. J. Arrowood, and R. F. Schinazi. 1994. Isolation and partial characterization of Cryptosporidium sporozoite and oocyst wall recombinant proteins. J. Eukaryot. Microbiol. 41:S51. [PubMed] [Google Scholar]
- 19.Meinhardt, P. L., D. P. Casemore, and K. B. Miller. 1996. Epidemiologic aspects of human cryptosporidiosis and the role of waterborne transmission. Epidemiol. Rev. 18:118-136. [DOI] [PubMed] [Google Scholar]
- 20.Miller, H. R. 1990. Immunity to internal parasites. Rev. Sci. Technol. 9:301-344. [DOI] [PubMed] [Google Scholar]
- 21.O'Donoghue, P. J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139-195. [DOI] [PubMed] [Google Scholar]
- 22.Okhuysen, P. C., C. L. Chappell, C. R. Sterling, W. Jakubowski, and H. L. DuPont. 1998. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect. Immun. 66:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okhuysen, P. C., G. A. Rogers, A. Crisanti, F. Spano, D. B. Huang, C. L. Chappell, and S. Tzipori. 2004. Antibody response of healthy adults to recombinant thrombospondin-related adhesive protein of Cryptosporidium 1 after experimental exposure to Cryptosporidium oocysts. Clin. Diagn. Lab. Immunol. 11:235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perryman, L. E., D. P. Jasmer, M. W. Riggs, S. G. Bohnet, T. C. McGuire, and M. J. Arrowood. 1996. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol. Biochem. Parasitol. 80:137-147. [DOI] [PubMed] [Google Scholar]
- 25.Priest, J. W., J. P. Kwon, M. J. Arrowood, and P. J. Lammie. 2000. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol. 106:261-271. [DOI] [PubMed] [Google Scholar]
- 26.Priest, J. W., J. P. Kwon, D. M. Moss, J. M. Roberts, M. J. Arrowood, M. S. Dworkin, D. D. Juranek, and P. J. Lammie. 1999. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J. Clin. Microbiol. 37:1385-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagodira, S., D. Buzoni-Gatel, S. Iochmann, M. Naciri, and D. Bout. 1999. Protection of kids against Cryptosporidium parvum infection after immunization of dams with CP15-DNA. Vaccine 17:2346-2355. [DOI] [PubMed] [Google Scholar]
- 28.Shenai, B. R., B. L. Komalam, A. S. Arvind, P. R. Krishnaswamy, and P. V. Rao. 1996. Recombinant antigen-based avidin-biotin microtiter enzyme-linked immunosorbent assay for serodiagnosis of invasive amebiasis. J. Clin. Microbiol. 34:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh, S., A. Gilman-Sachs, K. P. Chang, and S. G. Reed. 1995. Diagnostic and prognostic value of K39 recombinant antigen in Indian leishmaniasis. J. Parasitol. 81:1000-1003. [PubMed] [Google Scholar]
- 30.Spano, F., L. Putignani, S. Naitza, C. Puri, S. Wright, and A. Crisanti. 1998. Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol. Biochem. Parasitol. 92:147-162. [DOI] [PubMed] [Google Scholar]
- 31.Tosini, F., S. Caccio, A. Tamburrini, G. La Rosa, and E. Pozio. 1999. Identification and characterisation of three antigenic proteins from Cryptosporidium parvum sporozoites using a DNA library expressing poly-histidine tagged peptides. Int. J. Parasitol. 29:1925-1933. [DOI] [PubMed] [Google Scholar]
- 32.Umezawa, E. S., S. F. Bastos, J. R. Coura, M. J. Levin, A. Gonzalez, R. Rangel-Aldao, B. Zingales, A. O. Luquetti, and J. F. da Silveira. 2003. An improved serodiagnostic test for Chagas' disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion 43:91-97. [DOI] [PubMed] [Google Scholar]
- 33.Winter, G., A. A. Gooley, K. L. Williams, and M. B. Slade. 2000. Characterization of a major sporozoite surface glycoprotein of Cryptosporidum parvum. Funct. Integr. Genomics 1:207-217. [DOI] [PubMed] [Google Scholar]