Abstract
Pediatric vascular disease is rare, and remains a big challenge to vascular surgeons. In contrast to adults, surgery for pediatric vascular disease is complicated by issues related to small size, future growth, and availability of suitable vascular conduit. During the last 30 years, 131 major vascular operations were performed in a tertiary referral center, Seoul National University Hospital, including aortoiliac aneurysm, acute or chronic arterial occlusion, renovascular hypertension, portal venous hypertension, trauma, tumor invasion to major abdominal vessels, and others. Herein we review on the important pediatric vascular diseases and share our clinical experiences on these rare diseases.
Keywords: Children, Aortic aneurysm, Renovascular hypertension, Portal hypertension, Thrombectomy, Autotransplantation
INTRODUCTION
Pediatric vascular disease is very rare. Throughout the literature, only small case series or single case reports can be found. The diseases are quite heterogeneous in the causes and clinical manifestations. In contrast to adults, surgery for pediatric vascular disease is complicated by issues related to small size, future growth, and availability of suitable vascular conduit.
We would like to share our experiences with the pediatric vascular diseases in a tertiary hospital and to provide a brief literature review on these rare diseases. Our 30-year experience with major pediatric vascular surgery in Seoul National University Hospital (SNUH) and Seoul National University Children’s Hospital are summarized in the Table 1. The list includes major vascular surgeries performed by vascular surgeons only or in collaboration with pediatric surgeons. We hope this review be a good opportunity for the readers to have some insights on the unique pediatric vascular diseases and special considerations in treating pediatric patients with vascular diseases. We also hope more vigorous communications of experiences on treating these rare diseases in the real world would be activated in the future issues of the journal.
Table 1.
Major pediatric vascular surgeries performed in Seoul National University Hospital during the last 30 years (n=131)
| Diagnosis (n) | Operation (n) |
|---|---|
| Aorto-iliac Aneurysm (4) | Endoaneurysmal graft replacement (3) Excision & primary repair (1) |
| Renovascular hypertension (21) | Bypass (11) Autotransplantation (6) Nephrectomy (3) Reimplanatation of renal artery (1) |
| Acute arterial occlusion (3) | Image-guided thrombectomy (2) Mechanical thrombectomy (1) |
| Chronic arterial occlusion (2) | Vein bypass (1) Myotomy & vein bypass (1) |
| Portal hypertension (20) | Distal splenorenal shunt (18) Mesocaval shunt (1) Stent (1) |
| Glycogen storage disease (13) | Portocaval shunt (13) |
| Varicose vein (7) | High ligation & stripping (7) |
| IVC thrombosis due to tumor (4) | Thrombectomy (3) Inferior vena cava excision & interposition graft (1) |
| Trauma (5) | Primary repair/bypass (5) |
| End stage renal disease (52) | Autologous arterio-venous fistula (52) |
AAA
Pediatric abdominal aortic aneurysm (AAA) is quite rare, especially in infancy and early childhood. The etiology varies widely with heterogenic presentations. The cause of pediatric AAA can be categorized as infected, inflammatory, congenital (prenatal), genetic (tuberous sclerosis, inherited vasculopathy), trauma, or idiopathic [1]. Infected AAA is the most common type of AAA in children, frequently associated with umbilical artery catheterization or cardiac catheterization [2,3]. Because of the rarity, the natural history of pediatric AAA is not well-known. The timing and indications for interventions as well as the optimal surgical treatment of pediatric AAA pose a therapeutic dilemma. Small size of the involved vessels and the effect of later growth on the reconstructed aorta should be considered. Many ruptured cases were reported in the untreated AAA or in a stillborn [4,5]. Therefore, a broad understanding of the surgical options is particularly important in treating these AAAs.
After aneurysmectomy, prosthetic grafts such as Dacron or expanded polytetrafluoroethylene (ePTFE) can be used for revascularization. Eliason et al. [1] reported that they favored ePTFE over Dacron grafts because of frequent post-implantation dilation of Dacron graft. In some cases, allografts were used, but the long-term results are unknown with possible risks of graft deterioration and pseudoaneurysm formation [6,7]. Kaye et al. [8] reported that children younger than 6 years old rarely have adequate autologous options for graft construction and benefit from the use of cryopreserved arterial allografts of aorto-biiliac artery or superficial femoral artery.
The diameter of the graft should be selected considering the later growth of the patient. Using the larger diameter graft with spatulated anastomosis is usually recommended [1]. Barral et al. [9] reported a series of aortic surgery for children and recommended correction should be deferred, if possible, until the child reaches 8 to 10 years so that a prosthesis of sufficient diameter (8-mm or greater) can be used. In their series, a 6-mm aortic graft had to be replaced at the end of the growth period because of recurrent hypertension. However, if the rupture risk of AAA seems to be great, earlier repair with smaller graft or cryopreserved allograft is warranted. They also recommended surgical tips to compensate for the traction force applied to the graft due to the normal growth of the vertebrae [9]. The first consists of reinforcing the anastomoses externally with a band of Dacron. The second measure is to oversize the length of the grafts, taking care not to produce a kink and cause stenosis. The angulation of the graft decreased as the patient grew. Kaye et al. [8] recommended artificial grafts be placed end-to-side both proximally and distally and made with a redundant C-shape to allow growth. To allow for circumferential growth, all suture lines should be at least partly comprised interrupted sutures.
The role of currently popular endovascular aneurysm repair (EVAR) in pediatric AAA is very limited. Due to the fixed geometry of the stent-graft, EVAR is not appropriate for a growing child. It can be tried only in older adolescent, in whom further growth is unexpected, or a bridge procedure before definite open surgery in case of a critically ill child [8,10,11].
The authors performed surgeries of 3 AAAs and 1 iliac aneurysm in children. The causes of AAA were congenital, tuberous sclerosis, and idiopathic and the operations were performed at the ages of 15 months, 7 years, and 8 months, respectively. Endoaneurysmal graft replacement with a Dacron graft was performed in all cases, and two I-type and one Y-type grafts were used. The case of AAA in tuberous sclerosis had been reported elsewhere, a 3-cm infrarenal aneurysm, much larger than the normal average size of 6-mm in her age of 7 years [12]. The pathology showed a loss of elastic fibers in the media of the aorta. She underwent an open repair with a 10-mm-sized Dacron graft (Fig. 1), which was patent for more than 5 years. A case of idiopathic iliac artery aneurysm in a 4-year-old girl was reported elsewhere [13]. The girl presented with a 5-cm-sized pulsatile abdominal mass without pain or leg discomfort. The aneurysm located at the ostium of right external iliac artery and the distal external iliac artery was not found (Fig. 2). The ipsilateral leg was perfused by the enlarged deep circumflex iliac artery and inferior hypogastric artery with normal palpable pulsation on the foot. The orifice of the aneurysm was suture-ligated inside the aneurysm and the aneurysm was excised. Because of the well-developed collaterals, reconstruction of the external iliac artery was not necessary.
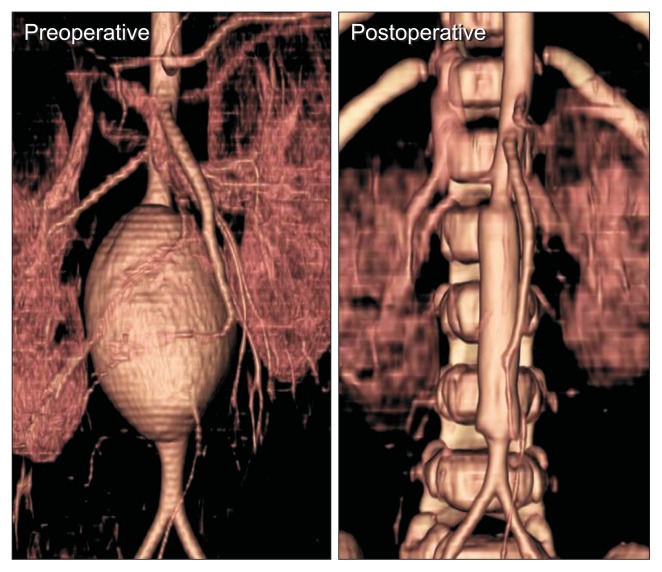
Fig. 1.
An infrarenal aortic aneurysm in a 8-month-old girl with tuberous sclerosis. Open repair was done with a 10-mm Dacron graft. Postoperative computed tomography after 4 months showed the graft patent.
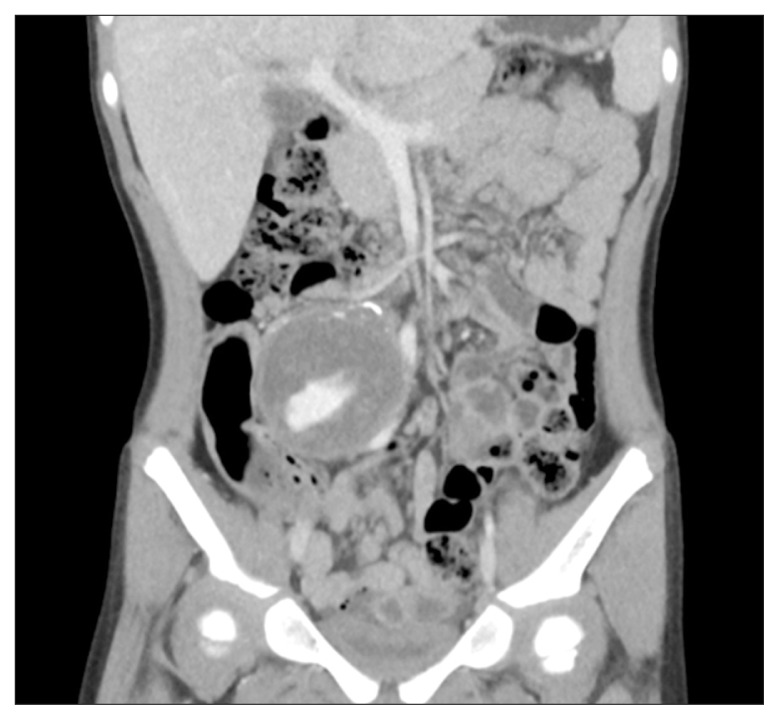
Fig. 2.
A huge aneurysm in right common iliac artery in a 4-year-old girl. Aneurysm excision and primary repair was done successfully.
RVHT
Pediatric renal arterial occlusive disease is rare, but remains an important cause of surgically correctable hypertension. Renal artery stenosis represents the most common correctable cause of severe hypertension in the pediatric population, except neonates and infants (<1 year of age) in whom aortic coarctation is the leading cause of hypertension [14]. Renovascular hypertension (RVHT) in childhood is estimated to account for 10% of those with marked hypertension [15]. The etiology of renovascular disease is quite variable. Stanley et al. [15] reported that developmental renal artery stenosis at the origin was the most common (80%) in children, which was mainly treated by renal artery reimplantation. They mentioned that a preponderance of developmental renal artery stenoses in North American white children is very different than the stenoses related to inflammatory aortoarteritis that dominate reports from the Asian subcontinent. Various diseases are associated with childhood RVHT, including fibromuscular dysplasia, syndromic causes (neurofibromatosis type I, tuberous sclerosis, Williams’ syndrome, Marfan’s syndrome), vasculitis (Takayasu arteritis, polyarteritis nodosa, Kawasaki disease, Moyamoya disease), extrinsic compression (neuroblastoma, Wilms’ tumor), and other causes (radiation, umbilical artery catheterization, trauma, congenital rubella syndrome) [16].
After the diagnosis of RVHT, all children should be treated with antihypertensive drugs. If the patient is refractory to the medical therapy, renal artery intervention or surgery can be tried. Percutaneous transluminal renal angioplasty (PTRA), with or without stenting, is a preferred treatment option for RVHT in children. However, elastic recoil and restenosis is not infrequent. Clinical improvement is reported to be 28% to 94%. Possible complications after PTRA includes arterial spasm, dissection, arterial rupture with retroperitoneal extravasation, and acute thrombosis. Renal artery stenting in children is controversial, and it should be used in selected cases. Many patients develop in-stent restenosis, which needs additional intervention or surgery. Stenting may limit future surgery, and the extent of surgery is usually longer after stenting. The primary indications for surgery are refractory RVHT for which medical treatment and angioplasty have failed. Surgical treatment includes revascularization or nephrectomy. Revascularization can be done by renal artery reimplantation, aorto-renal bypass, extra-anatomic bypass (from hepatic artery in the right, or splenic artery in the left), or autotransplantation. Autogenous saphenous vein can be used as the conduit, but there is a risk of late aneurysmal degeneration in nearly 20% of the vein graft [17]. Internal iliac artery is a useful conduit. If the conduit is not appropriate, a strategy to wait with endovascular treatment until a definitive revascularization procedure with prosthetic graft in the adolescent may lead to the desired outcome.
Authors had operated 21 renal arterial diseases in 13 pediatric patients with RVHT during the last 30 years. Mean age was 12.2 years, ranging from 8 to 18 years. The baseline characteristics of pediatric RVHT are quite different from those of adult patients. All pediatric RVHT was caused by non-atherosclerotic disease, including Takayasu arteritis, Moyamoya disease, fibromuscular dysplasia, or Alagile syndrome. Main indication for operation was uncontrolled hypertension. Associated renal functional impairment (serum creatinine level greater than 1.2 mg/dL) was found in only one patient. The center protocol for the treatment of RVHT in SNUH is to try endovascular intervention first, if applicable (Fig. 3). If the lesion is refractory to percutaneous transluminal angioplasty (PTA) or recurrent after PTA, surgical correction is considered. Various surgical treatments were performed in 21 renal arterial lesions; 11 bypasses, 6 autotransplantations, 3 nephrectomies, and 1 reimplantation of renal artery. Hypertension improved in 11/13 (84.6%), restenosis/occlusion developed later in 2, which were treated by PTA and left nephrectomy. The result of vein surgical correction after failed PTA (Fig. 4).
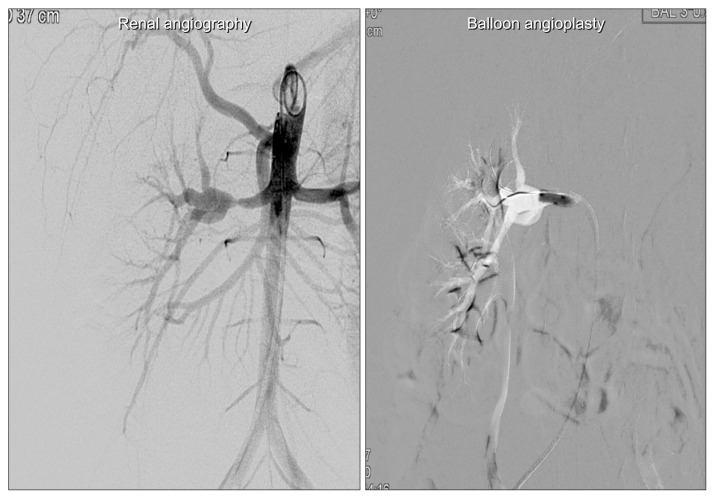
Fig. 3.
Right renal artery stenosis was treated successfully by balloon angioplasty.
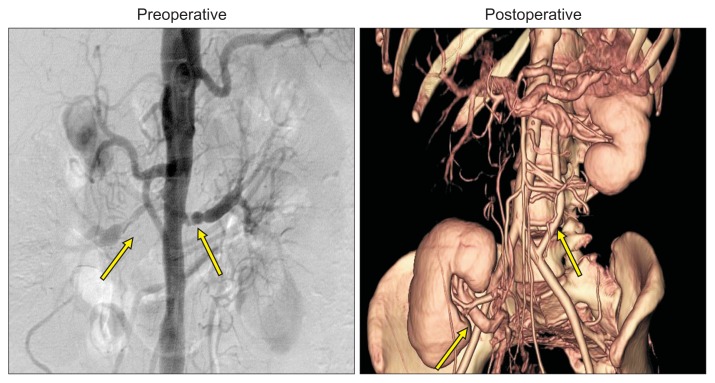
Fig. 4.
Both renal artery stenoses (arrows) due to Takayasu arteritis. Right kidney autotransplantation and left common ilio-renal bypass with a polytetrafluoroethyle (ePTFE) graft was done successfully.
AAO
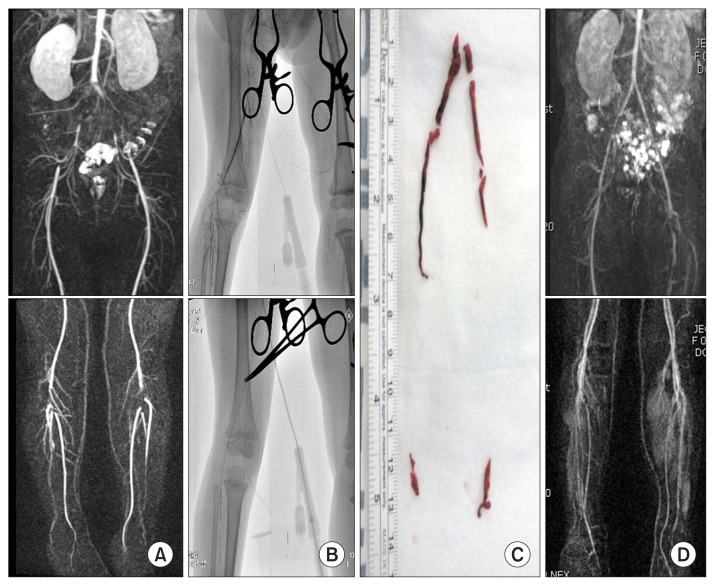
Acute arterial occlusion (AAO) in childhood is rare, but this medical emergency requires immediate treatment. Delayed treatment often results in mortality or significant morbidity. Especially in pediatric population, the diagnosis and treatment of AAO is difficult due to frequent delay in diagnosis, technical difficulties in accessing small-caliber vessels, and poor conduit for possible bypass. If acute leg pain developed, careful assessment of the arterial pulsation should be done by physical examinations and hand-held Doppler. Duplex ultrasonography or computed tomography/magnetic resonance angiography can confirm the diagnosis. Kayssi et al. [18] reported a total of 151 cases of AAO in children. The most common cause was vessel catheterization (91%), followed by idiopathic (5%), traumatic (4%), and congenital (1%). In contrast to adults, AAO in pediatric population could generally be managed nonoperatively with anticoagulation, likely because of their greater ability to develop arterial collaterals. However 11 cases deteriorated and required surgery, which resulted in 2 mortalities and 3 major amputations. Recent advances in endovascular surgery, many alternative therapies can be tried; open surgery, endovascular intervention, or hybrid operation. Recently authors published the result of image-guided thrombectomy in two cases of AAO in children [19]. Using over-the-wire Fogarty catheter under C-arm imaging, selective and less traumatic thromboembolectomy can be performed successfully (Fig. 5). Lim et al. [20] reported a case of aortobrachial bypass with autogenous saphenous vein in a 12-year-old girl with AAO from the subclavian artery due to tumor embolization. The graft was patent at 8-year follow-up.
Fig. 5.
(A) Magnetic resonance angiography (MRA) showed the embolic occlusion of both iliac and popliteal arteries in an 8- year-old child. (B) Image-guided thromboembolectomy was done successfully. (C) Removed thromboemboli was demonstrated. (D) Postoperative MRA showed patent arteries from the iliac to the calf.
CHRONIC PAOD
Chronic arterial occlusion in children is rare. Because atherosclerotic disease does not prevail, iatrogenic or community trauma, vasculitis, congenital anomaly, or chronic embolism can be the cause of peripheral arterial occlusive disease (PAOD) in children. It may result in claudication or critical limb ischemia. Frequently collateral circulations are well developed in children, arterial occlusion is usually tolerable without significant symptoms and lower extremity ischemia in children is seldom immediately limb threatening. A unique indication for operation in children with PAOD is growth retardation or leg length discrepancy (LLD).
Cardneau et al. [21] reported a long-term follow-up results of 16 vein bypasses in children. The indications for operation included LLD in 6 patients, claudication in 4, both LLD and claudication in 3, markedly diminished ankle-brachial indexes with a potential for LLD in 2, and a traumatic dissection with hemorrhage in 1. The most common cause of arterial occlusion was cardiac catheterizations in 11 patients, followed by each case of arteritis, dialysis cannulation and penetrating trauma. Except one graft occlusion and one follow-up loss, the vein graft reconstruction in preadolescent children were durable, and in 70% of children their preexisting LLDs were reduced.
Authors experience 2 cases of vein bypass grafts in children. Both cases presented with popliteal arterial occlusion, and the causes of arterial occlusion were popliteal entrapment syndrome and chronic trauma. The former was treated by musculotendinous section and vein interposition graft and the later by popliteal-tibial vein bypass. During the follow-up, thrombectomy and patch angioplasty was done for the second case.
PHT
In the current era of transplantation and therapeutic endoscopy, the role of shunt operation for portal hypertension (PHT) has decreased. Endoscopic therapy including endoscopic variceal ligation or endoscopic variceal clipping, are now widely practiced to control hemorrhage secondary to portal hypertension. Liver transplantation (LT) not only relieves PHT but also treats the underlying liver disease. In case of intractable PHT, LT can be the treatment of choice, although the problems of donor shortage and long waiting list remains. Therefore shunt operation is becoming the exception rather than the rule nowadays. However, there are still problems than can be resolved only by shunt surgery, such as severe hypersplenism and massive splenomegaly in patients who do not need transplantation.
The purpose of shunt operation in adults is mainly to control the variceal bleeding. But in pediatric patients, another important goal is to preserve the hepatic portal flow and encourage the normal growth of the liver. In this aspect, meso-Rex shunt is the optimal approach to extrahepatic portal vein obstruction. The meso-Rex shunt is a restorative procedure in which, unlikely a portosystemic shunt that creates a nonphysiologic connection between portal and systemic circulation, the mesenteric flow is redirected back into the liver through the Rex venous recessus (portion of left portal venous system joining the umbilical vein). Unfortunately we had no experience of Rex shunt during the period. There are some reports showing the advantages of Rex shunt including a Korean case series [22–26].
Previously Moon et al. [25] reported the result of distal spleno-renal shunt (DSRS, Warren shunt) in 15 patients, showing DSRS is an effective and reliable procedure for children with portal hypertension and is still useful for selected pediatric patients. DSRS can be used as a bridge to LT if PHT is secondary to intrahepatic problems. DSRS is still a useful procedure when meso-Rex shunt is not feasible. It should be considered for selected patients with PHT; 1) not a candidate for LT 2) severe thrombocytopenia and leukopenia refractory to medial management.
During the last 30 years, surgical treatment for portal hypertension was performed for 20 patients in SNUH, including 18 DSRS, 1 mesocaval shunt, and 1 stenting (Table 2). The most common cause was extrahepatic portal vein stenosis or obstruction in 12 cases (60.0%), followed by congenital hepatic fibrosis in 6 and liver cirrhosis in 2. There was no perioperative mortality or severe morbidity. During the follow-up, recurrent variceal bleeding developed in 4 patients, who were treated conservatively. Among these patients, additional mesocaval shunt was performed 12 years after the first shunt operation. LT was done in three patients due to hepatocellular carcinoma and pulmonary arteriovenous fistula.
Table 2.
Surgery for portal hypertension in Seoul National University Hospital for 30 years (n=20)
| Cause of portal hypertension (n) | Surgical treatment (n) |
|---|---|
| Portal vein thrombosis (10) | Distal spleno-renal shunt (18) |
| Congenital hepatic fibrosis (6) | |
| Liver cirrhosis (2) | |
| Portal vein thrombosis (1) | Mesocaval shunt (1) |
| PV stenosis/portosystemic shunt (1) | Balloon angioplasty/stenting (1) |
PV, pulmonary valve.
GSD
Recently Choi et al. [27] reported a collective review of the long-term result of the patients with glycogen storage disease (GSD) type I treated in SNUH. In our center, practice for the management of GSD is as follows. A pediatrician starts dietary management as soon as GSD is diagnosed. When the patient does not respond to dietary treatment resulting in persistent metabolic derangement or growth retardation, porto-caval shunt (PCS) is recommended. PCS reportedly makes glucose more readily available to peripheral tissues, relieves hypoglycemia, deglycogenates the liver, and palliates other metabolic abnormalities [28].
After activation of LT program in the 1990s, LT becomes the primary recommendation. But considering the organ shortage and long waiting list for LT, PCS continues to be an important management option for GSD. Surgery yielded greater growth improvement than dietary management. However, after PCS, metabolic abnormalities remained unresolved and the de novo hepatocellular adenoma (HCA) rate was high. De novo HCA was detected in 13% of patients with dietary modification, in 100% who underwent PCS, and in none who underwent LT. PCS can be used to improve growth in GSD type I patients when LT is not possible, but close observation for metabolic abnormalities and HCA is essential.
During the last 30 years, 13 PCS was performed for GSD in SNUH. Five patients are still alive without complications or additional surgery. Four patients underwent LT due to hepatic malignancy, 2 were lost to follow-up, and 2 expired due to duodenal ulcer bleeding and Hodgkin’s lymphoma.
VASCULAR ACCESS FOR HEMODIALYSIS
In the pediatric population, vascular access for hemodialysis is a challenge to vascular surgeons. Operations require general anesthesia, and microscopic surgical technique is frequently required due to the small size of the vessels. Kidney Disease Outcomes Quality Initiative (K-DOQI) guidelines recommend the placement of permanent access in dialysis patients aged 0 to 19 years who weigh >20 kg and are unlikely to receive a transplant within 1 year [29]. Children weighing less than 10 kg are better suited for peritoneal dialysis.
Recently authors reported the outcome of arteriovenous fistula (AVF) in pediatric patients [30]. Most vascular access in children are done by pediatric surgeons in SNUH, but the vascular access for hemodialysis is still referred to the vascular surgeons. A total of 52 AVFs were created in 47 pediatric patient between January 2000 and June 2014. All were done with autologous vein, and no prosthetic graft was used. Most common type of AVF was radial-cephalic AVF in 43 patients, followed by brachial-cephalic AVF in 7, and basilic vein transposition in 2. Primary patency rates were 61%, 51%, and 48% at 1, 3, and 5 years. Secondary patency rates were 83%, 79%, and 79%, respectively. Primary maturation failure rate was 17% (9 cases) and low body weight was the independent risk factor. Mean duration of maturation was about 10 weeks. During the mean follow-up of 50 months, 43% of the patients underwent kidney transplantation. AVF in children has acceptable long-term durability, primary failure rate and maturation time.
CONCLUSION
For the treatment of vascular diseases in children, the decision to operate or the operative procedures should be carefully executed. For arterial reconstruction, the small size, future growth, abundancy of collateral circulation and availability of suitable vascular conduit need to be considered. Because of the higher development of collateral circulation and regenerative capacity, the prognosis is usually good in case performed in a dedicated high volume center.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Eliason JL, Coleman DM, Criado E, Stanley JC. Surgical treatment of abdominal aortic aneurysms in infancy and early childhood. J Vasc Surg. 2016;64:1252–1261. doi: 10.1016/j.jvs.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Mendeloff J, Stallion A, Hutton M, Goldstone J. Aortic aneurysm resulting from umbilical artery catheterization: case report, literature review, and management algorithm. J Vasc Surg. 2001;33:419–424. doi: 10.1067/mva.2001.109739. [DOI] [PubMed] [Google Scholar]
- 3.Benrashid E, McCoy CC, Rice HE, Shortell CK, Cox MW. Mycotic saccular abdominal aortic aneurysm in an infant after cardiac catheterization: a case report. Ann Vasc Surg. 2015;29:1447.e5–1447.e11. doi: 10.1016/j.avsg.2015.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Knirsch W, Hillebrand D, Horke A, Lewin MA, Rein J, Uhlemann F. Aortic aneurysm rupture in infantile Marfan’s syndrome. Pediatr Cardiol. 2001;22:156–159. doi: 10.1007/s002460010185. [DOI] [PubMed] [Google Scholar]
- 5.Ball RY. Ruptured abdominal aortic aneurysm in a stillborn fetus. Virchows Arch A Pathol Anat Histopathol. 1983;399:237–244. doi: 10.1007/BF00619584. [DOI] [PubMed] [Google Scholar]
- 6.Cho YP, Kim SC, Kim SA, Jun H, Kwon TW. An idiopathic congenital abdominal aortic aneurysm with impending rupture in a 23-month-old boy. J Vasc Surg. 2013;57:508–510. doi: 10.1016/j.jvs.2012.08.106. [DOI] [PubMed] [Google Scholar]
- 7.Kwon H, Kwon H, Hong JP, Han Y, Park H, Song GW, et al. Use of cryopreserved cadaveric arterial allograft as a vascular conduit for peripheral arterial graft infection. Ann Surg Treat Res. 2015;89:51–54. doi: 10.4174/astr.2015.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye AJ, Slemp AE, Chang B, Mattei P, Fairman R, Velazquez OC. Complex vascular reconstruction of abdominal aorta and its branches in the pediatric population. J Pediatr Surg. 2008;43:1082–1088. doi: 10.1016/j.jpedsurg.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Barral X, de Latour B, Vola M, Lavocat MP, Fichtner C, Favre JP. Surgery of the abdominal aorta and its branches in children: late follow-up. J Vasc Surg. 2006;43:1138–1144. doi: 10.1016/j.jvs.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Khan MS, Moore JW. Treatment of abdominal aortic pseudoaneurysm with covered stents in a pediatric patient. Catheter Cardiovasc Interv. 2000;50:445–448. doi: 10.1002/1522-726X(200008)50:4<445::AID-CCD17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Halaweish I, Patel H, Si MS. Giant aortic aneurysm in a child with Takayasu arteritis. Cardiol Young. 2016;26:593–595. doi: 10.1017/S1047951115001511. [DOI] [PubMed] [Google Scholar]
- 12.Moon SB, Shin WY, Park YJ, Kim SJ. An abdominal aortic aneurysm in an 8-month-old girl with tuberous sclerosis. Eur J Vasc Endovasc Surg. 2009;37:569–571. doi: 10.1016/j.ejvs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Oh C, Youn JK, Han JW, Kim HY, Jung SE. Right iliac arterial aneurysm in a 4-year-old girl who does not have a right external iliac artery. Ann Surg Treat Res. 2016;91:265–268. doi: 10.4174/astr.2016.91.5.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piercy KT, Hundley JC, Stafford JM, Craven TE, Nagaraj SK, Dean RH, et al. Renovascular disease in children and adolescents. J Vasc Surg. 2005;41:973–982. doi: 10.1016/j.jvs.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Stanley JC, Criado E, Upchurch GR, Jr, Brophy PD, Cho KJ, Rectenwald JE, et al. Pediatric renovascular hypertension: 132 primary and 30 secondary operations in 97 children. J Vasc Surg. 2006;44:1219–1228. doi: 10.1016/j.jvs.2006.08.009. discussion 1229. [DOI] [PubMed] [Google Scholar]
- 16.Tullus K, Brennan E, Hamilton G, Lord R, McLaren CA, Marks SD, et al. Renovascular hypertension in children. Lancet. 2008;371:1453–1463. doi: 10.1016/S0140-6736(08)60626-1. [DOI] [PubMed] [Google Scholar]
- 17.Stanley JC, Zelenock GB, Messina LM, Wakefield TW. Pediatric renovascular hypertension: a thirty-year experience of operative treatment. J Vasc Surg. 1995;21:212–226. doi: 10.1016/S0741-5214(95)70263-6. discussion 227. [DOI] [PubMed] [Google Scholar]
- 18.Kayssi A, Shaikh F, Roche-Nagle G, Brandao LR, Williams SA, Rubin BB. Management of acute limb ischemia in the pediatric population. J Vasc Surg. 2014;60:106–110. doi: 10.1016/j.jvs.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Han A, Choi C, Min SI, Kim HC, Ha J, et al. Image-guided thromboembolectomy of acute arterial occlusion in children. Ann Vasc Surg. 2016;34:270.e1–5. doi: 10.1016/j.avsg.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Lim HG, Lee JR, Min SK, Park GW. Aortobrachial bypass for acute vascular insufficiency due to tumor embolization. Eur J Cardiothorac Surg. 2006;29:609. doi: 10.1016/j.ejcts.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Cardneau JD, Henke PK, Upchurch GR, Jr, Wakefield TW, Graham LM, Jacobs LA, et al. Efficacy and durability of autogenous saphenous vein conduits for lower extremity arterial reconstructions in preadolescent children. J Vasc Surg. 2001;34:34–40. doi: 10.1067/mva.2001.115600. [DOI] [PubMed] [Google Scholar]
- 22.Superina R, Shneider B, Emre S, Sarin S, de Ville de Goyet J. Surgical guidelines for the management of extra-hepatic portal vein obstruction. Pediatr Transplant. 2006;10:908–913. doi: 10.1111/j.1399-3046.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 23.Sharif K, McKiernan P, de Ville de Goyet J. Mesoportal bypass for extrahepatic portal vein obstruction in children: close to a cure for most! J Pediatr Surg. 2010;45:272–276. doi: 10.1016/j.jpedsurg.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Ha TY, Kim KM, Ko GY, Oh SH, Kwon TW, Cho YP, et al. Variant meso-Rex bypass with transposition of abdominal autogenous vein for the management of idiopathic extrahepatic portal vein obstruction: a retrospective observational study. BMC Surg. 2015;15:116. doi: 10.1186/s12893-015-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon SB, Jung SE, Ha JW, Park KW, Seo JK, Kim WK. The usefulness of distal splenorenal shunt in children with portal hypertension for the treatment of severe thrombocytopenia and leukopenia. World J Surg. 2008;32:483–487. doi: 10.1007/s00268-007-9356-0. [DOI] [PubMed] [Google Scholar]
- 26.de Ville de Goyet J, Alberti D, Clapuyt P, Falchetti D, Rigamonti V, Bax NM, et al. Direct bypassing of extrahepatic portal venous obstruction in children: a new technique for combined hepatic portal revascularization and treatment of extrahepatic portal hypertension. J Pediatr Surg. 1998;33:597–601. doi: 10.1016/S0022-3468(98)90324-4. [DOI] [PubMed] [Google Scholar]
- 27.Choi Y, Yi NJ, Ko JS, Moon JS, Suh SW, Lee JM, et al. Reappraisal of the role of portacaval shunting in the growth of patients with glycogen storage disease type I in the era of liver transplantation. Transplantation. 2016;100:585–592. doi: 10.1097/TP.0000000000000884. [DOI] [PubMed] [Google Scholar]
- 28.Starzl TE, Putnam CW, Porter KA, Halgrimson CG, Corman J, Brown BI, et al. Portal diversion for the treatment of glycogen storage disease in humans. Ann Surg. 1973;178:525–539. doi: 10.1097/00000658-197310000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Kidney Foundation. Kidney Disease Outcomes Quality Initiative guidelines for chronic kidney disease care [Internet] New York: National Kidney Foundation, Inc; 2006. [cited 2017 Feb 16]. Available from: http://www2.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/hd_guide8.htm. [Google Scholar]
- 30.Kim SM, Min SK, Ahn S, Min SI, Ha J. Outcomes of arteriovenous fistula for hemodialysis in pediatric and adolescent patients. Vasc Specialist Int. 2016;32:113–118. doi: 10.5758/vsi.2016.32.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]