Abstract
Purpose
To report experiences of the sandwich technique (ST) for preservation of pelvic flow during endovascular repair of complex aortic or aortoiliac aneurysms.
Materials and Methods
Eight patients underwent elective endovascular aneurysm repair (EVAR) using the ST between March 2013 and February 2017. The anatomic indications for the ST were complex aortoiliac aneurysms (5 cases), abdominal aortic aneurysms (AAA) with non-diseased short common iliac arteries (2 cases) and AAA with unilateral occluded iliac artery (1 case). The ST was performed through both femoral and brachial approach. Patient clinical and radiologic data were collected and analyzed.
Results
Eight patients (7 male; mean age, 73.4 years) were followed over a mean period of 277 days (range, 9–1,106 days). The technical success rate was 100%. The primary patency rate of the iliac stent-grafts was 88% (14/16 cases). One internal iliac and 1 external iliac stent-graft occlusion was observed during the early postoperative period. There was 1 gutter endoleak which disappeared spontaneously within 4 days, and there were 2 type II endoleaks: one treated by coil embolization after 13 months, and the other observed without treatment. There were no cases of sac growth or aneurysm-related deaths, and no cases of buttock claudication or impotence.
Conclusion
The ST is a safe and feasible technique to preserve pelvic circulation during endovascular treatment of complex aortoiliac aneurysms. The need to expand the indications for complex EVARs with adjunctive procedures, such as the ST is highlighted in situations where branched/fenestrated device availability is limited.
Keywords: Aortic aneurysm, Endovascular, Double barrel, Sandwich, Endovascular aneurysm repair
INTRODUCTION
Endovascular aneurysm repair (EVAR) is accepted as the first-line treatment for abdominal aortic aneurysms (AAA) due to decreased operative mortality and morbidity rates and shorter patient recovery time [1]. However, there are many cases that are difficult to treat with standard EVAR, such as aneurysms with hostile short or angled proximal neck, tortuous iliac artery, or co-existence of iliac aneurysms. Especially, AAA combined with common iliac artery (CIA) aneurysms is found in 55% to 65% of AAA patients in Asia [2,3], and requires a more demanding procedure due to potential difficulties in obtaining adequate distal landing zones for stent-graft limb fixation. Additionally, mean CIA lengths have been reported to be shorter in Asians than in Caucasians [3]. AAAs with bilateral short, healthy common iliac artery (AAA wSCIA) are difficult to manage [3] because secure landing of the distal stent-grafts in the CIA is often difficult, while extending the iliac limbs into the external iliac artery to achieve secure landing zone requires coverage of the internal iliac artery (IIA) flow. IIA occlusion is known to cause complications, especially when it is done bilaterally (12% to 45%) [4]. Buttock claudication, ischemic colitis, bowel or bladder dysfunction, and erectile dysfunction are common complications that might be elicited by IIA occlusion. Hence, preservation of flow to at least one IIA is recommended [5,6]. To overcome some of these limitations, adjunctive endovascular techniques during EVAR have been developed. The use of new techniques and/or devices has led to increased success rates of complex EVARs for treating AAAs with iliac aneurysms (AIA), isolated CIA aneurysms (CIAA), or AAA wSCIA over the past decade [7,8]. These include the bell-bottom technique [7], iliac branched devices (IBD), and the sandwich technique (ST) to avoid IIA occlusion [8].
With the introduction of commercial IBDs, the use of the ST has been challenged especially for the purpose of iliac flow preservation. However, the ST is still an attractive option in situations where IBDs are not available. The ST also has the advantage of being technically easy to perform under experienced hands and does not need require elaborate equipment except for an additional covered stent. The ST was first reported in 2008 as a new technique to safely address AAA encumbered by adverse iliac anatomies, including AIA, CIAA, AAA wSCIA [9]. Despite the various reports regarding the feasibility of the ST, the number of reports in Korea is still lacking. We therefore report our single center early experiences for treatment of complex AIA or AAA w/SCIA using the ST.
MATERIALS AND METHODS
1) Study design
This retrospective study enrolled 8 consecutive patients undergoing elective EVAR with the ST to treat AIA, AAA wSCIA, or AAA with uni-CIA at a single center between March 2013 and February 2017. Indications for the ST were (a) AIA with no bilateral distal landing zones in the CIA; (b) AIA with no unilateral distal landing zone plus contralateral IIA aneurysm (IIAA) or contralateral IIA with occlusion or severe stenosis; (c) AAA with bilateral healthy but short CIA; (d) bilateral isolated CIAA with no bilateral distal landing zone [10]. Follow-up assessment included routine outpatient visits with imaging by computed tomographic angiography (CT angiography) or duplex ultrasonography. The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1706/402-107) in Seongnam, Korea.
2) Technique
The ST comprises four easy-to-perform steps. (a) The main body of a bifurcated aortic stent-graft is inserted using an ipsilateral femoral approach and deployed with the distal end of the iliac limb placed above the iliac bifurcation. (b) The ipsilateral IIA is cannulated using a long sheath via a left brachial approach with the tip of the long sheath located around the level of the iliac bifurcation. (c) A self-expandable covered stent (SECS, Viabahn; W. L. Gore & Associates, Flagstaff, AZ, USA) is placed at least 1.5 cm inside the nondiseased IIA, with at least 5 cm overlapping into the ipsilateral limb of the bifurcated graft. (d) The external iliac limb extension is inserted and the proximal end is positioned at the same level as the cranial end of the SECS, deployed first and accommodated with a semi-compliant balloon prior to deploying the SECS. When a second SECS was used as the external iliac limb extension to form the sandwich configuration, both SECSs were deployed at the same time and accommodated simultaneously with balloon catheters. In cases where a contralateral CIAA was also found, they were treated by either coil-embolization of the IIA and limb stent-graft extension to the external iliac artery (EIA) or with physician-modified IBDs in selected cases.
3) Definitions and statistical analysis
Primary endpoints included technical success rate, IIA patency rate, size change of the aneurysm sac, and procedure-related or unrelated early (less than 30 days) and late mortality rates (more than 30 days). Technical success was defined as successful implantation of the iliac stent-grafts for anatomic reconstruction of the iliac artery bifurcation with preservation of antegrade flow to the internal iliac arteries. Additional variables included complication rates (pelvic ischemia, endoleak, access complications, end-organ complications, and aneurysm rupture) and performance data (procedure and fluoroscopy times, transfusion, hospital length of stay, and conversion rate to open aneurysm repair and secondary procedures). Statistical analysis was performed using PASW Statistics ver. 18.0 software (IBM Co., Armonk, NY, USA). Data are presented as values and percentages, mean±standard deviation, or median with interquartile range.
RESULTS
1) Patients and procedure
Patient demographic features and comorbid conditions are shown in Table 1. Eight patients (7 male) with a mean age of 73.4 years underwent EVAR with the ST. Two patients underwent physician-modified IBD placement on the contralateral limb at same time. The mean follow-up duration was 277 days (range, 9–1,106 days). The mean number of follow-up images (CT angiography or duplex ultrasound) was 1.9. Table 2 shows the indications for the ST, aneurysmal sizes, adjunctive procedures for the contralateral limb, and clinical course of each patient. The AIA extended to both CIA bifurcations in 3 patients and to only one in 2 patients. In patients with AIA and both CIAA, two underwent unilateral ST and contralateral IIA embolization with iliac limb extension to the EIA, while one underwent unilateral ST and contralateral physician-modified IBD procedure. In the two patients that had AIA with unilateral CIAA, one underwent unilateral ST only, while another patient underwent unilateral ST and contralateral physician-modified IBD due to the presence of bilateral IIAA. Two patients had AAA with short nondiseased CIA (<20 mm in length). One patient had bilateral short CIA, and the other had unilateral short CIA. These patients underwent unilateral ST plus contralateral iliac limb stent placement at the iliac bifurcation. Finally, one patient had AAA with a single CIA due to total occlusion of the contralateral CIA. This patient underwent ST and crossover femoro-femoral bypass operation.
Table 1.
Patient demographics and comorbidities (n=8)
| Parameter | Value |
|---|---|
| Male | 7 (87) |
| Age (y) | 73.4 (63–90) |
| Hypertension | 6 (75) |
| Diabetes | 2 (25) |
| PAOD | 1 (12) |
| CKD (eGFR <30 mL/min/1.73 m2) | 0 (0) |
| Ischemic heart disease | 3 (37) |
| BMI (kg/m2) | 26.9 (21.1–35.3) |
Values are presented as number (%) or median (range).
PAOD, peripheral arterial occlusive disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; BMI, body mass index.
Table 2.
Indications for the sandwich technique, anatomic details, devices used and clinical course
| Patient No. | Indication for ST | ØAo (mm) | ØCIA (R/L) (mm) | ØEIA (R/L) (mm) | ØIIA (R/L) (mm) | EVAR stent-graft | Procedure for contralateral limb | Remarks |
|---|---|---|---|---|---|---|---|---|
| 1 | CIAA+IIAA | 64 | 48/25 | 10/11 | 22/18 | Gore (Excluder) | Coil embo, EIA extensiona | Persistent endoleak (IIb) treated by coil embolization of lumbar artery |
| 2 | CIAA+IIAA | 34 | 31/26 | 9/9 | 7/32 | Cook (Zenith) | IBD | |
| 3 | CIAA+IIAA | 64 | 47/19 | 11/11 | 26/43 | Cook (Zenith) | IBD | IIA stentgraft occlusion |
| 4 | CIAA | 53 | 35/23 | 10/11 | 7/8 | Gore (Excluder) | Limb stentb | Endoleak (IIa) |
| 5 | CIAA | 46 | 35/27 | 10/9 | 10/8 | Cook (Zenith) | Coil embo, EIA extensiona | EIA stent-graft occlusion treated by reinterventionc |
| 6 | Short CIA | 53 | 10/10 (length 25/18) | 9/9 | 6/7 | Cook (Zenith) | Limb stentb | |
| 7 | Short CIA | 51 | 20/18 (length 18/18) | 11/12 | 7/6 | Cook (Zenith) | Limb stentb | |
| 8 | Unilateral CIA | 54 | x/12 | x/7 | x/8 | Medtronic (Endurant) |
ST, sandwich technique; Ø, diameter; Ao, aorta; CIA, common iliac artery; EIA, external iliac artery; IIA, internal iliac artery; EVAR, endovascular aneurysm repair; R/L, right/left; CIAA, CIA aneurysms; IIAA, IIA aneurysm; Gore, W. L. Gore & Associates; Cook, Cook Inc.; embo, embolization; IBD, iliac branch device.
Coil embolization in IIA and stent-graft extension into the external iliac artery;
CIA limb stent placement above iliac bifurcation;
Aspiration thrombectomy plus urokinase thrombolysis plus ballooning and stenting.
2) Device selection
The aneurysmal diameter of the aorta, iliac arteries and type of devices used to treat each of the three types of aneurysmal diseases (AIA, AAA plus short healthy CIA, AAA plus unilateral CIA) are summarized in Table 2 and Table 3. Seven aortic bifurcated stent-grafts (5 Zenith [Cook Inc., Bloomington, IN, USA]) and 2 Excluder [W. L. Gore & Associates]), 2 aorto-uniiliac stent-grafts (Endurant; Medtronic, Minneapolis, MN, USA), 19 SECS, 13 iliac limb stent-grafts, and 2 physician-modified IBDs were used.
Table 3.
Anatomic features and size of endografts used
| Varible | Aortoiliac aneurysm (n=5) | AAA+short healthy CIA (n=2) | Unilat-CIA total occlusion (n=1) |
|---|---|---|---|
| Ao main body bifurcated stent-graft (®/Ø×L) (mm) | #Excluder 31-14×170 #Excluder 28-14×160 #Zenith 28-12×111 #Zenith 24-12×82 #Zenith 30-12×111 |
#Zenith 28-12×82 #Zenith 26-12×82 |
#Endurant 25-14×150+16×80 |
| EIA stent-graft/IIA stent-graft (product name/(®/Ø×L) (mm) | #Excluder 12×140/Viabahn 8×150 #Excluder 16-14×100/Viabahn 8×150 #Zenith 8×150/Viabahn 10×100 #Viabahn 10×100/Viabahn 9×150 #Viabahn 11×100/Viabahn 8×150 |
#Viabahn 10×100/Viabahn 9×100 #Viabahn 11×100/Viabahn 8×100 |
#Smart 10×60/Absolute pro 8×60 |
AAA, abdominal aorta aneurysm; Unilat, unilateral; CIA, common iliac artery; Ao, aorta; EIA, external iliac artery; ®, product name; Ø, diameter; L, length; IIA, internal iliac artery.
3) ST procedure data
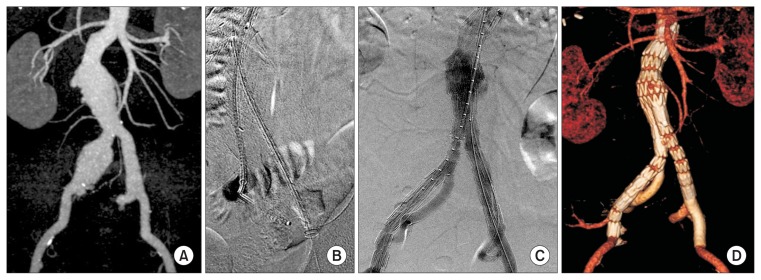
All eight patients underwent ST under general anesthesia, and 100% of them had bilateral femoral access. An additional left brachial artery open access was performed. The mean operating time was 251 minutes, mean contrast volume used was 316 mL, and mean hospital length of stay was 7.3 days. Red blood cell was transfused in two patients (Table 4). Ancillary procedures were 2 physician-modified IBDs due to contralateral CIAA and/or IIAA and 1 femoro-femoral bypass due to contralateral CIA total occlusion. Two contralateral IIA embolization and limb stent-graft extension into the EIA were performed (Table 2). Representative illustrations of the procedure performed in one of the AIA patients are shown in Fig. 1.
Table 4.
Early results of the ST (≤30 days)
| Parameter | Value | Remarks |
|---|---|---|
| Hospital day (d) | 7.3 | |
| Mortality | 0 | |
| Rupture | 0 | |
| Conversion to open | 0 | |
| Technical success | 8 (100) | |
| Stent occlusion | 2 (EIA 1, IIA 1) (12) | EIA occlusion (POD 8) >Thrombectomy (POD 9) d/t tortuous EIA stent facing vessel wall IIA occlusion (POD 7) d/t tortuous IIA stent facing vessel wall |
| Endoleak | 0 | |
| Blood transfusiona | 2 pts (400 mL) | |
| Others | 0 |
Values are presented as number (%) unless otherwise indicated.
ST, sandwich technique; EIA, external iliac artery; IIA, internal iliac artery; POD, postoperative day; d/t, due to; pts, patients.
Number/amount per patient.
Fig. 1.
Representative images of a patient with aortoiliac aneurysm treated with the ST. (A) Initial CT image showing an aortoiliac aneurysm, with aneurysmal changes in both CIA and IIA. (B) The limb of the bifurcated stent-graft is deployed in the left CIA, a SECS is introduced from the brachial access into the left IIA, another SECS is introduced from the left femoral access into the EIA. (C) Final angiography after EVAR with ST on the left iliac limb side, and physician-modified iliac-branched device on the right side. (D) Follow-up CT angiography showing intact flow in both external/internal iliac arteries. ST, sandwich technique; CT, computed tomographic; CIA, common iliac artery; IIA, internal iliac artery; SECS, self-expandable covered stent; EIA, external iliac artery; EVAR, endovascular aneurysm repair.
4) Patency of iliac stent-grafts
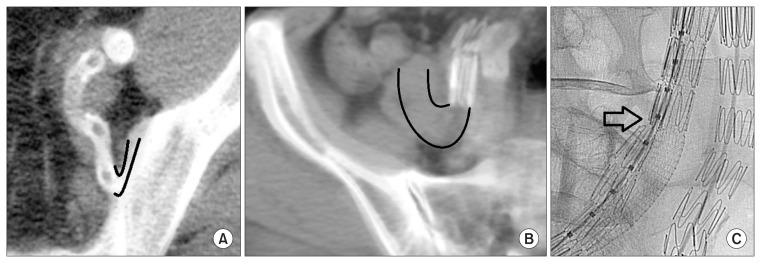
The technical success rate was 100%. Primary iliac stent-graft patency rate was 87.5% (14/16 cases), with one IIA stent occlusion and one EIA occlusion occurring during the early (≤30 days) postoperative period. In the IIA stent occlusion case, the end of the SECS was facing the wall of a tortuous internal iliac artery, which was found on postoperative day 7 on follow-up CT angiography (Fig. 2A). This patient had a simultaneous IBD procedure on the contralateral side, so the flow in the contralateral IIA was well maintained. In the EIA stent occlusion case, the proximal overlapping zone was found to be stenotic (probably due to the difference in radial force between the 2 stents inside the sandwich), while the distal end of the stent-graft was again facing a tortuous EIA wall, findings that were discovered at postoperative day 8 on follow-up CT (Fig. 2B). The EIA stent-graft occlusion was treated by thrombolysis with urokinase, aspiration thrombectomy, balloon dilatation (7×100 mm), and two additional stent (8×39 mm balloon expandable stents) implantations overlapping the stenosis lesion and tortuous stent-graft ends (Fig. 2C). A follow-up CT angiography five days after the reintervention showed no evidence of occlusion. With the exception of the above two cases there were no further occlusions in the sandwich iliac stent-grafts during the follow-up period. The iliac aneurysm sacs treated with the ST showed a decrease in diameter in 3 cases (60%) and no change in 2 cases (40%).
Fig. 2.
Representative images of the stent occlusion cases after the sandwich technique. (A) Occlusion of the IIA stent observed on cross-sectional view of follow-up CT angiography. The distal end of the IIA stent was facing the wall of a tortuous IIA. Black lines show the course of the IIA distal to the stent. The large, upper circle is the intact EIA. (B) Occlusion of the EIA stent-graft observed on cross-sectional view of follow-up CT angiography. The distal end of the EIA stent was facing the wall of a tortuous EIA. Black lines show the course of the EIA distal to the stent. (C) Angiography image during the re-intervention of the EIA stent-graft occlusion case. The EIA limb stent-graft is being squeezed by the SECS. The black arrow annotates the stenotic portion of the EIA stent-graft. IIA, internal iliac artery; CT, computed tomographic; EIA, external iliac artery; SECS, self-expandable covered stent.
5) Complications
There were no early or late aneurysm-related mortalities during the follow-up. Early and late results are displayed in Table 4 and Table 5. Overall, there were no complaints for buttock claudication and erectile dysfunction, and there were no renal, heart or cerebrovascular complications. There were no access site complications such as occlusion, hematoma or pseudoaneurysm in the 8 brachial artery open access sites and the CFA access sites (14 Perclose Proglide [Abbott Vascular, Santa Clara, CA, USA], 1 open cutdown). Only one gutter endoleak were detected on completion angiography. The gutter endoleak (type Ib) sealed spontaneously after postoperative day 4 on follow-up CT angiography (Fig. 3, Table 6). During the follow-up period, there were 2 cases of type II endoleak. One type IIa endoleak was found 2 months after the procedure on CT angiography without any change in aneurysmal sac diameter, and therefore was followed-up without intervention. The other type IIb endoleak was persistent (>6 months) with signs of aneurysmal sac growth, and therefore was intervened 13 months after the initial procedure by lumbar artery coil embolization. The reintervention was successful without evidence of recurrence. Among the 8 patients, two patients had undergone duplex ultrasonography only without CT. Due to the inaccuracy of aneurysmal sac size measurements by duplex ultrasonography, these two patients were excluded for evaluation of aneurysmal sac size. In the remaining 6 patients, there were no sac growths on follow-up CT angiography. The sac size was reduced by more than 10% in 3 patients, showing 10% reduction at 12 months, 12% reduction at 4 months, and 20% reduction at 15 months, respectively. The other 3 patients showed aneurysmal sac change of 6% reduction at 2 months, 5% reduction at 4 months, and no growth at 1 month, respectively.
Table 5.
Late results of the ST (>30 days)
| Parameter | Number | Remark |
|---|---|---|
| FU duration (d) | 277 | Mean 1.9 images per patient |
| Mortality | 0 | |
| Rupture | 0 | |
| Conversion to open aneurysm repair | 0 | |
| Buttock claudication/erectile dysfunction | 0 | |
| Stent occlusion | 0 | |
| Endoleak (type) | 2 (II) | 1 type IIb endoleak treated by coil embolization of the lumbar artery 1 type IIa endoleak |
| Others | 0 |
ST, sandwich technique; FU, follow-up.
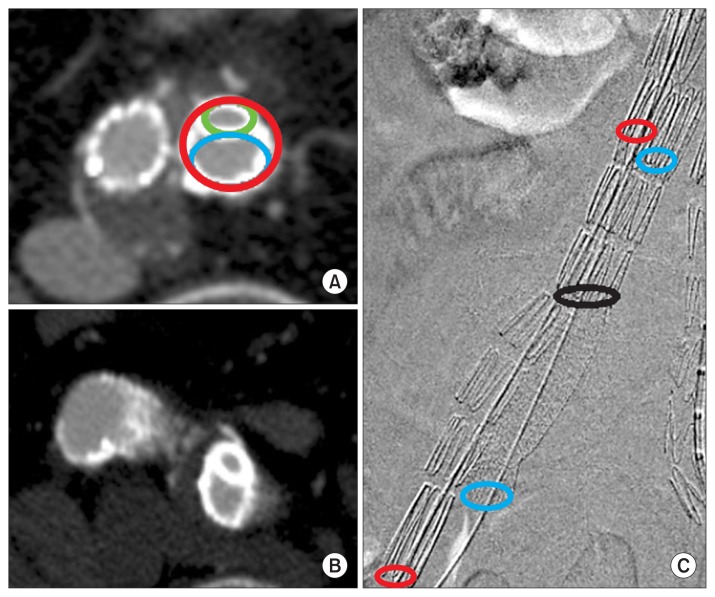
Fig. 3.
(A, B) Cross-sectional views of follow-up CT angiography after the sandwich technique. The smaller, upper circle (green) represents the stent in the left IIA while the bigger, lower circle (blue) represents the stent in the EIA. The red circle represents the left common iliac stent-graft. (C) Final angiography after the sandwich technique showing the location of the different stents and the length of overlap in between. The black circle represents the distal end of the right limb in the main bifurcated stent-graft, the red circles represent the external iliac stent-graft (upper proximal, lower distal), and the blue circles represent the internal iliac stent (upper proximal, lower distal). The length of overlap is shown between the upper red/blue circles and the black circle. CT, computed tomographic; IIA, internal iliac artery; EIA, external iliac artery.
Table 6.
Calculation of stent dimensions used for iliac reconstruction
| Patient with AIA | Diameter of IIA stent-graft (mm) | Diameter of EIA stent-graft (mm) | Sum of area IIA+EIA (mm2) | Diameter of CIA stent-graft (mm) | Ratio (area of CIA/area of IIA+EIA) |
|---|---|---|---|---|---|
| 1 | 8 | 12 | 163 | 14 | 0.94 |
| 2 | 8 | 16 | 251 | 14 | 0.61 |
| 3 | 8 | 10 | 128 | 12 | 0.87 |
| 4 | 9 | 10 | 142 | 12 | 0.79 |
| 5 | 8 | 11 | 145 | 12 | 0.77 |
AIA, aortoiliac aneurysm; IIA, internal iliac artery; EIA, external iliac artery; CIA, common iliac artery.
DISCUSSION
The management of AIA is challenging without standardized procedures. There are many restrictions in applying standard EVAR to manage AIA, because it is hard to secure a distal landing zone [11]. Traditionally, when at least one IIA could be saved by conventional methods, the other side was sacrificed by coil embolization with or without stent-graft extension into the EIA. However when both IIAs could not be saved by conventional EVAR techniques, a two-staged approach was used in which one IIA was occluded first and the other IIA was occluded after a certain time interval to allow for preconditioning of the pelvis from ischemia. Yet these methods can still lead to complications from pelvic ischemia, ranging from buttock claudication to necrosis or bowel infarction. Recently, with quality of life issues becoming more important after EVAR, there is a growing demand for preservation of IIA flow. In order to achieve this, several techniques have been reported which include the bell-bottom technique, the ST or the use of IBDs [8,9,11–14]. The bell-bottom technique has technical limitations in that they are difficult to apply to large aneurysms (≥24 mm) [7], and there is pathophysiologic problem when the stent adheres to the intimal layer of aneurysm. The introduction of commercially available IBDs has allowed for easier preservation of pelvic flow in complex AIAs, yet these devices are not always available or have not been approved for use in certain countries. Physician-modified IBDs can also be an alternative in cases where commercial IBDs are not available, yet this requires advanced endovascular skills and the long-term outcomes of these devices have not been demonstrated. The ST could be a good alternative method and also applicable to patients having AAA with short CIA. Also the ST using SECS has the advantage of being very flexible and can be applied to very hostile AIA anatomies, including (a) diameter <40 mm; (b) small CIA lumen (<18 mm); (c) very tortuous anatomy of the CIA; (d) isolated CIAA; (e) short CIA and (f) large IIAA [12].
In this study, EVAR with ST was performed on 8 patients including 5 AIA, 2 AAA with short CIA, and 1 AAA with unilateral CIA. The mean operating time, contrast volume, and hospital day were similar to other reports, including IBD insertions [12,14,15]. Additional procedures included 2 contralateral physician-modified IBDs, 2 coil-embolizations of the contralateral IIA and one crossover femoro-femoral bypass for the case with unilateral CIA occlusion. The patency of the iliac stent-grafts was 88% (14/16 cases) in this study, which is comparable to other studies (patency rate, 85%–95%) [12,16]. There were two cases of iliac stent-graft occlusion, one in the IIA and one in the EIA. Both of them showed very tortuous anatomies, with the distal end of the stents being landed in an acutely angulated area, in a way that the stent-graft seems to be facing the vessel wall distal to it (Fig. 2A, B). Such an abrupt change in direction of flow coming out of the stents may be related to occlusion resulting from slow-flow or swirling vortex effects, and therefore landing the distal ends of stents in acutely angulated regions should be avoided. Rotation of the C-arm for multiple projections of the distal landing zone is also important in tortuous anatomies. Another way to tackle the problem is to place a second covered stent further distally, since SECS are known to be very flexible. Another issue with occlusion may be related to the radial forces exerted by the 2 stents in the sandwich configuration. In the EIA stent occlusion case, the combination of stainless steel (Zenith Flex) stent-graft in the EIA and nitinol-based SECS (Viabahn) was used (Fig. 2C). The radial force of nitinol is relatively stronger than stainless steel [17], and therefore depending on the size of the stents used, it is possible that the limb stent-graft may have been squeezed by the SECS.
The main issues with the ST are related to the interaction between stents in terms of radial force and the possibility of gutter endoleaks. To reduce gutter endoleaks, an overlapping zone of more than 50 mm with the proximal mother stent-graft is recommended [12,16]. In this study, the average overlap length was 56 mm. After overlapping more than 50 mm, if the healthy distal landing zone of the iliac artery seemed to be insufficient or if type Ib endoleak was observed in final angiography, additional stents were used. There were three additional SECS implantations in the IIA for 3 patients, while no additional SECS were required in the EIA. It is also important to choose the proper diameter of the stent-grafts. Massmann et al. [16] reported that post-procedural gutter endoleaks (type Ib) were observed in 6.5% of patients at three weeks after the ST, yet in our study there was only one case of a small gutter endoleak on final angiography which spontaneously disappeared on follow-up CT angiography at postoperative day 4. On Massmann’s study [16], the inner diameter area of the CIA stent-graft (ACIAS) was about 83% to 93% of the sum of the area of the EIA stent-graft (AEIAS) and the area of the IIA stent-graft (AIIAS). In our study, the ratio of the ACIAS to the sum of AEIAS and AIIAS was 80%, which implies that we used larger EIA and IIA stent-grafts compared to the CIA stent-grafts, reflecting a tighter sandwich configuration. This sizing method seems to be the cause for the lower rate of gutter endoleaks, and even with this lower ratio (ACIAS/AEIAS+AIIA), there were no flow limitations in the IIA or EIA (Fig. 3, Table 6).
From a technical perspective, we routinely used an open brachial access, which allowed for easier approach and cannulation of the IIA. SECSs were deployed in the IIA with the support of long Flexor Ansel guiding sheaths (Cook Inc.). The tip of the Flexor sheath was usually located around the iliac bifurcation but for cases with tortuous anatomies, the sheath was inserted further in and retracted slowly as the SECS was deployed to avoid stent jumping. Another good method to prevent stent migration is to place the sheath over the SECS, deploy the SECS inside the sheath, and slowly retract the sheath for deployment of the SECS at the desired location. The use of a brachial approach is advantageous in these situations with tortuous anatomies since it allows for more direct access into the tortuous iliac artery compared to a contralateral femoral approach [15].
We had 2 cases of type II endoleak. One type IIa endoleak was observed without further treatment, while another persistent type IIb endoleak via a lumbar artery was successfully treated by coil embolization. In 6 patients with at least one follow-up CT angiography, the size of the aneurysmal sacs had decreased or did not enlarge. Due to good preservation of pelvic flow, none of the patients complained of buttock claudication or impotence during the follow-up period. These results demonstrate that the ST is effective in preventing pelvic ischemia while the primary goal of preventing rupture and sac enlargement can be safely achieved. Outcomes of this study are encouraging since it may overcome the limitations of hostile anatomy and device availability for complex EVAR in Korea. Despite the small of number of patients and the lack of a comparison group being limitations of this study, in the absence of domestic reports on ST, this study is meaningful in that it reports the result of ST in Korea and shows results comparable to other studies.
In conclusion, the ST is a safe and feasible procedure to preserve pelvic circulation during EVAR in complex aortoiliac anatomies, such as AIA, CIAA, or even AAA with short CIA, where standard EVAR is not amenable. Meticulous planning and selection of devices to match the size requirements for the ST are necessary to achieve optimal outcomes and prevent complications such as gutter endoleaks or graft occlusion. The landing of stent distal ends in acutely angulated regions was associated with graft occlusion and should therefore be avoided or extended by insertion of additional stent-grafts. This procedure can be most beneficial in situations where commercial IBDs are not available.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535–1542. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 2.Yun WS, Park K. Iliac anatomy and the incidence of adjunctive maneuvers during endovascular abdominal aortic aneurysm repair. Ann Surg Treat Res. 2015;88:334–340. doi: 10.4174/astr.2015.88.6.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng SW, Ting AC, Ho P, Poon JT. Aortic aneurysm morphology in Asians: features affecting stent-graft application and design. J Endovasc Ther. 2004;11:605–612. doi: 10.1583/04-1268R.1. [DOI] [PubMed] [Google Scholar]
- 4.Yano OJ, Morrissey N, Eisen L, Faries PL, Soundararajan K, Wan S, et al. Intentional internal iliac artery occlusion to facilitate endovascular repair of aortoiliac aneurysms. J Vasc Surg. 2001;34:204–211. doi: 10.1067/mva.2001.115380. [DOI] [PubMed] [Google Scholar]
- 5.Karch LA, Hodgson KJ, Mattos MA, Bohannon WT, Ramsey DE, McLafferty RB. Management of ectatic, nonaneurysmal iliac arteries during endoluminal aortic aneurysm repair. J Vasc Surg. 2001;33(2 Suppl):S33–S38. doi: 10.1067/mva.2001.111659. [DOI] [PubMed] [Google Scholar]
- 6.Rayt HS, Bown MJ, Lambert KV, Fishwick NG, McCarthy MJ, London NJ, et al. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Intervent Radiol. 2008;31:728–734. doi: 10.1007/s00270-008-9319-3. [DOI] [PubMed] [Google Scholar]
- 7.Torsello G, Schönefeld E, Osada N, Austermann M, Pennekamp C, Donas KP. Endovascular treatment of common iliac artery aneurysms using the bell-bottom technique: long-term results. J Endovasc Ther. 2010;17:504–509. doi: 10.1583/10-3112.1. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg RK, West K, Pfaff K, Foster J, Skender D, Haulon S, et al. Beyond the aortic bifurcation: branched endovascular grafts for thoracoabdominal and aortoiliac aneurysms. J Vasc Surg. 2006;43:879–886. doi: 10.1016/j.jvs.2005.11.063. discussion 887. [DOI] [PubMed] [Google Scholar]
- 9.Lobato AC. Sandwich technique for aortoiliac aneurysms extending to the internal iliac artery or isolated common/internal iliac artery aneurysms: a new endovascular approach to preserve pelvic circulation. J Endovasc Ther. 2011;18:106–111. doi: 10.1583/10-3320.1. [DOI] [PubMed] [Google Scholar]
- 10.EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 11.Verzini F, Parlani G, Romano L, De Rango P, Panuccio G, Cao P. Endovascular treatment of iliac aneurysm: concurrent comparison of side branch endograft versus hypogastric exclusion. J Vasc Surg. 2009;49:1154–1161. doi: 10.1016/j.jvs.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 12.Lobato AC, Camacho-Lobato L. The sandwich technique to treat complex aortoiliac or isolated iliac aneurysms: results of midterm follow-up. J Vasc Surg. 2013;57(2 Suppl):26S–34S. doi: 10.1016/j.jvs.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 13.Falkensammer J, Hakaim AG, Andrew Oldenburg W, Neuhauser B, Paz-Fumagalli R, McKinney JM, et al. Natural history of the iliac arteries after endovascular abdominal aortic aneurysm repair and suitability of ectatic iliac arteries as a distal sealing zone. J Endovasc Ther. 2007;14:619–624. doi: 10.1177/152660280701400503. [DOI] [PubMed] [Google Scholar]
- 14.Pua U, Tan K, Rubin BB, Sniderman KW, Rajan DK, Oreopoulos GD, et al. Iliac branch graft in the treatment of complex aortoiliac aneurysms: early results from a North American institution. J Vasc Interv Radiol. 2011;22:542–549. doi: 10.1016/j.jvir.2011.01.429. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler P, Avgerinos ED, Umscheid T, Perdikides T, Erz K, Stelter WJ. Branched iliac bifurcation: 6 years experience with endovascular preservation of internal iliac artery flow. J Vasc Surg. 2007;46:204–210. doi: 10.1016/j.jvs.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Massmann A, Mosquera Arochena NJ, Shayesteh-Kheslat R, Buecker A. Endovascular anatomic reconstruction of the iliac bifurcation with covered stentgrafts in sandwich-technique for the treatment of complex aorto-iliac aneurysms. Int J Cardiol. 2016;222:332–339. doi: 10.1016/j.ijcard.2016.07.226. [DOI] [PubMed] [Google Scholar]
- 17.Sabeti S, Schillinger M, Amighi J, Sherif C, Mlekusch W, Ahmadi R, et al. Primary patency of femoropopliteal arteries treated with nitinol versus stainless steel self-expanding stents: propensity score-adjusted analysis. Radiology. 2004;232:516–521. doi: 10.1148/radiol.2322031345. [DOI] [PubMed] [Google Scholar]