Abstract
Introduction
Epidermal growth factor receptor (EGFR) mutations in non–small-cell lung cancers (NSCLC) may be more common in patients with brain metastases. Previous studies, however, did not adjust for effects of confounding variables.
Methods
This retrospective study included 1,522 consecutive patients with NSCLC, whose tumors were diagnosed and tested for EGFR mutations at the University of Nebraska Medical Center (Omaha, NE) and Tata Memorial Hospital (Mumbai, India). Multivariate logistic regression was used to identify any association between EGFR status and clinical factors.
Results
EGFR mutations were more common in females than males (38.7% v 24.8%), Asians than whites (31.3% v 13.4%), nonsmokers than smokers (40.2% v 14.6%), alcohol nonconsumers than users (32.4% v 15.8%), adenocarcinoma than other histology types (32.7% v 10.3%), and patients with brain metastases than extracranial or no metastases (39.4% v 29.8% v 15.1%; P < .001 for all comparisons). There was a higher likelihood of an EGFR mutation among patients with brain metastases (odds ratio, 1.8; P < .001). The median overall survival (OS) was 19.8 months. Patients with brain metastases had a shorter median OS (15 v 20.6 months; P = .02). However, in the cohort of EGFR mutation–positive patients, there was no difference in median OS between patients with and without brain metastases (20.8 v 25.1 months; P = .11).
Conclusion
There is a nearly two-fold higher incidence of EGFR mutations in NSCLC among patients with brain metastases at diagnosis. EGFR mutations did not predict for outcomes from brain metastases.
INTRODUCTION
Approximately 40% of patients with lung cancer develop brain metastases; lung cancer accounts for up to half of all secondary brain tumors.1 Conventional chemotherapy regimens are associated with significant toxicities and modest response rates in metastatic non–small-cell lung cancer (NSCLC), particularly among patients with brain metastases.1,2 Consequently, some patients forego therapy because of anticipated intolerance.3 In particular, older patients or those unfit for treatment may discontinue therapy as a result of toxicities. In recent years, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have emerged as powerful therapeutic agents with significant improvements in response rates and progression-free survival in EGFR-mutant NSCLC.2 Identifying patients with a higher probability of having tumors that harbor EGFR mutations has therefore become important. Although EGFR testing has been advocated in all advanced-stage adenocarcinoma regardless of clinical features,4 this approach may not be cost effective, especially in low-resource settings. The ability to identify additional subgroups of patients, whose tumors are more likely to harbor an EGFR mutation and benefit from EGFR TKI therapy compared with conventional chemotherapy, may improve the cost effectiveness of EGFR testing.
The most common EGFR mutations in lung cancer include in-frame deletion within exon 19 (45%) and point mutation within exon 21 (p.L858R; 40%); exon 18 point mutations and exon 20 insertions or point mutations account for most of the remainder of mutations. The presence of activating EGFR mutations (exon 19 deletion, p.L858R mutation in exon 21, and codon 719 mutations in exon 18) predicts a response to EGFR TKIs (erlotinib, afatinib, and gefitinib).5,6
The incidence of EGFR-mutated lung cancer is approximately 16% among whites,7 but there seems to be differences on the basis of histology type, sex, and smoking status. EGFR mutations were more frequent in adenocarcinoma compared with other NSCLC (21% v 2%), women compared with men (20% v 9%), and Asians compared with North Americans (26% v 2%).8 A few studies assessing the role of targeted therapy as a single agent or in combination with radiation among patients with NSCLC with brain metastases have shown that the prevalence of EGFR mutation is higher among patients with NSCLC with brain metastases.9 Given the poor survival of patients with brain metastases, such findings have therapeutic implications and may also help elucidate a possible role of EGFR mutation in the development of brain metastases. However, these studies were largely descriptive. A study with a sufficient sample size to adjust for sex, smoking status, ethnicity, and histology10 could provide a closer estimate of the incidence of EGFR mutations in patients with NSCLC presenting with different stages, including those with and without brain metastases.
METHODS
A retrospective study of consecutive patients with NSCLC, who were examined for the presence of EGFR mutations, diagnosed between 2007 and 2014 at the University of Nebraska Medical Center (UNMC; Omaha, NE) and Tata Memorial Hospital (TMH; Mumbai, India) was conducted. A total of 1,609 patients were identified; however, 87 patients were excluded as a result of missing data.
Patient Selection
The diagnoses were based on microscopic examination of the biopsy of lung mass or metastases, pleural fluid cytology, or bronchoscopic brushing. Information obtained at diagnosis included age, sex, ethnicity, smoking status, history of alcohol consumption, and histology type. Ethnicity was divided into Asian, white, and others because the other categories included only a small number of patients. On the basis of patients’ self-reports, smoking status was categorized as smoker versus nonsmoker, and alcohol consumption was divided into consumer versus nonconsumer. The categorization of patients into smoker and alcohol consumer required current or prior use of tobacco and alcohol consumption, regardless of the quantity.
Staging evaluation included computed tomography, bone scan and/or positron emission tomography, as well as imaging of brain (magnetic resonance imaging or contrasted computed tomography). The American Joint Committee on Cancer, 7th edition of tumor-node-metastasis classification system was used for tumor staging. Patients were divided into those without metastasis and with extracranial and/or brain metastases. Overall survival was defined as the time between diagnosis and date of death or last contact. Patients who were alive at last contact were censored for survival analysis. The study was conducted in accordance with institutional guidelines at both participating institutions.
EGFR Mutation Analysis at UNMC
Genomic DNA was extracted from formalin-fixed paraffin-embedded tissue containing at least 20% tumor tissue. When necessary, macrodissection was performed to obtain the threshold level of tumor. Sections were deparaffinized and then digested from a minimum of 4 hours to overnight. DNA was extracted and simultaneous real-time polymerase chain reaction amplification and mutation detection was performed using Amplification Refractory Mutation System technology and Scorpions dual primer-probes available in the EGFR RGQ PCR Kit (QIAGEN, Valencia, CA). Seven separate mutation amplification reactions and one wild-type control reaction were performed, allowing for the detection of 29 somatic mutations in exons 18 to 21. The limit of detection of this assay is 2.5% to 10%, depending on the specific mutation (point mutation or deletion/insertion).
EGFR Mutation Analysis at TMH
Formalin-fixed paraffin-embedded tissue sections containing at least 75% tumor were deparaffinized in xylene and the DNA was extracted (QIAamp FFPE Tissue Kit, QIAGEN). Mutation analysis was performed using a clinically validated real-time TaqMan assay described previously.11 Exon 20 point mutations were performed additionally on specimens from August 2012 onward. The analytical sensitivity of this assay has a limit of 1% detection, and each run consisted of a positive and a negative control to check the integrity of the assay.
Statistical Analysis
χ2 tests or Fisher’s exact test were used to determine univariate associations with patient location (TMH or UNMC) and patient characteristics, and EGFR mutation status and patient characteristics. Age distributions were compared between groups with t tests. Multivariate logistic regression was used to determine associations between EGFR status and demographic/clinical factors including sex, ethnicity, smoking status, alcohol use, stage, adenocarcinoma histology, metastatic location, and overall survival (OS). P values < .05 were considered to be statistically significant. SAS Software version 9.3 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
RESULTS
This analysis included 1,522 patients. Patients’ characteristics and differences on the basis of country of diagnosis and EGFR mutational status are depicted in Tables 1 and 2. Most patients (87%) had adenocarcinoma histology, which included pure adenocarcinoma as well as predominant adenocarcinoma with areas of neuroendocrine differentiation and adenosquamous histology. Patients diagnosed at UNMC rather than TMH were more likely to be older (median age 64 v 57 years), female (48.2% v 33.7%), white (93.4% v 0%), smokers (77.6% v 37.6%), consumers of alcohol (29.9% v 14.9%), and nonmetastatic (36% v 8%). Conversely, patients diagnosed at UNMC were less likely than those at TMH to have an EGFR mutation (13.1% v 31.3%). For the entire cohort, tumor EGFR mutations were more commonly seen in females than males (38.7% v 24.8%), Asians than whites (31.3% v 13.4%), nonsmokers than smokers (40.2% v 14.6%), alcohol nonconsumers than consumers (32.4% v 15.8%), adenocarcinoma than other histology types (32.7% v 10.3%), and patients with brain metastases than extracranial metastases or no metastasis (39.4% v 29.8% v 15.1%). Eight patients with EGFR-mutant tumors from the UNMC cohort received erlotinib as part of their treatment regimen. In the TMH cohort, 42 patients did not receive a TKI during treatment. The type of mutation (exon 19 v 21) did not correlate with the presence of metastases (Appendix Table A1); the frequency of other mutations was too small to conduct an analysis.
Table 1.
Patient Characteristics on Basis of Country of Origin
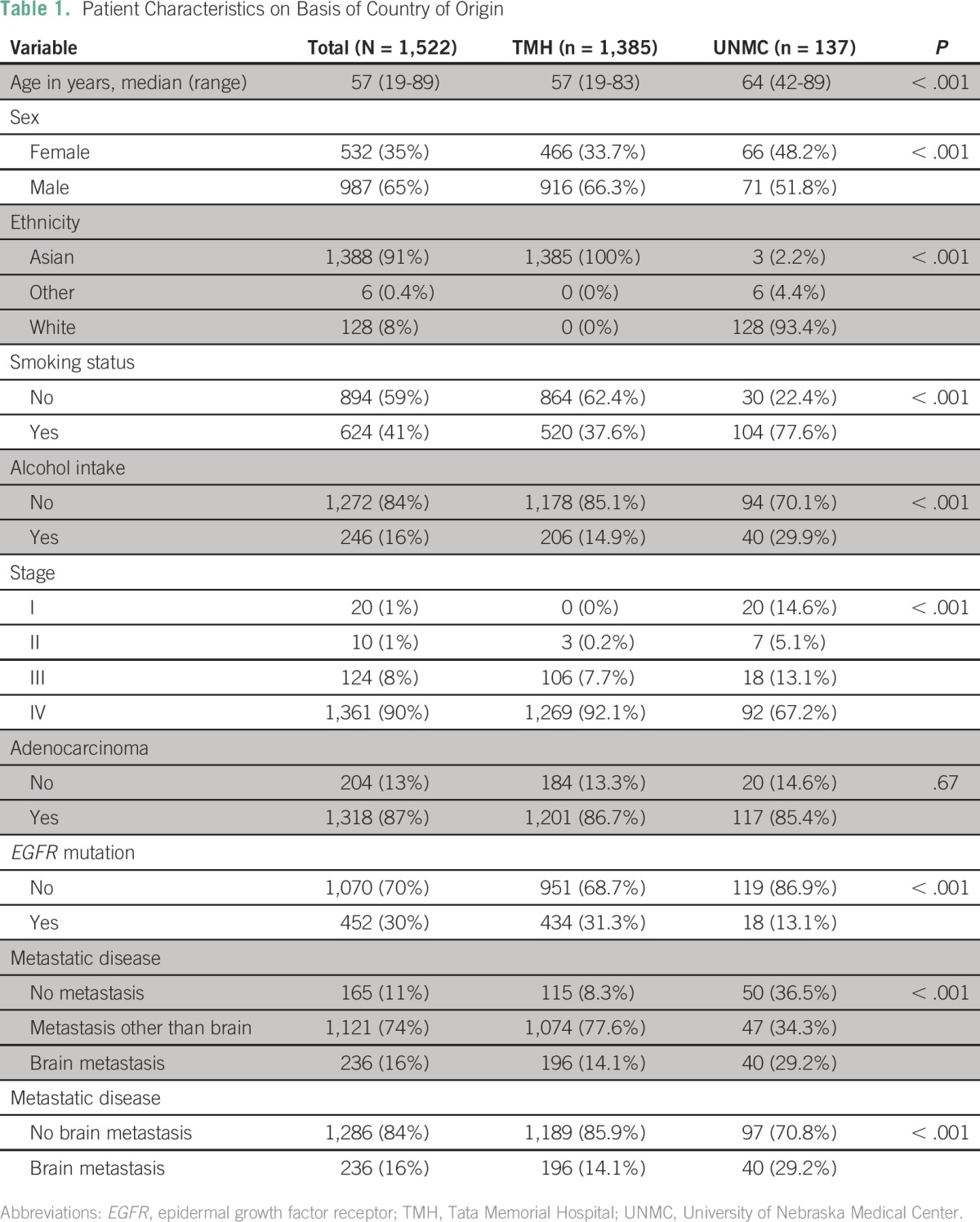
Table 2.
Patient Characteristics on Basis of Epidermal Growth Factor Receptor Mutational Status
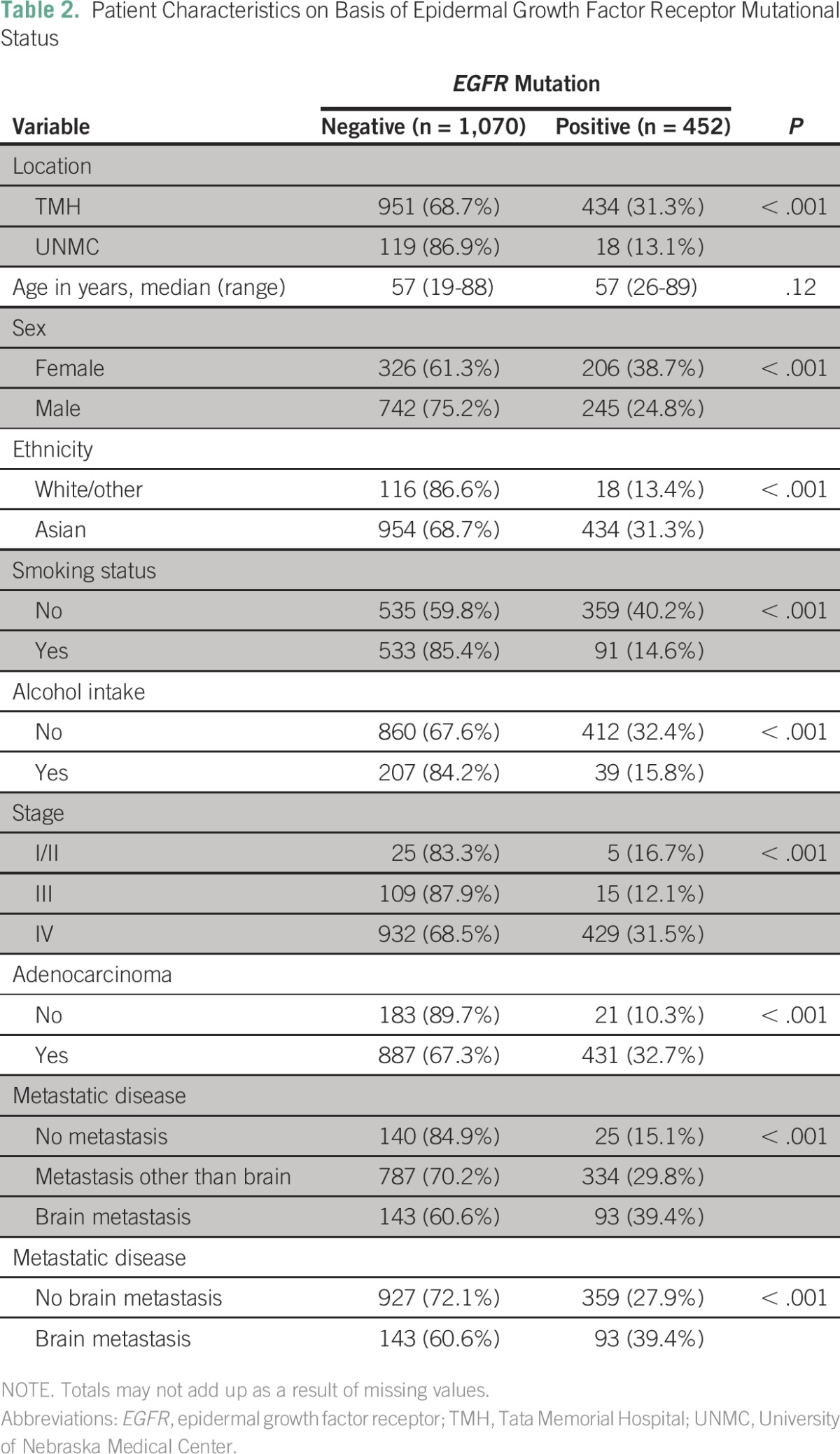
In a multivariate analysis of all patients (presented in Table 3), ethnicity, smoking status, alcohol intake, adenocarcinoma, and metastatic disease were all significant predictors of having a tumor with an EGFR mutation. Pairwise comparisons revealed that patients with brain metastases were 1.9 times more likely to have EGFR mutations than patients with extracranial metastases (P < .001) or those with none and extracranial metastases (odds ratio [OR], 1.8; P < .001). The outcomes were largely similar when the data from the institutions were analyzed separately (Appendix Tables A2 and A3). For patients at UNMC, the incidence of EGFR mutation was significantly higher for nonsmokers than smokers.
Table 3.
Multivariate Analysis of Positive Epidermal Growth Factor Receptor Mutational Status for the Entire Cohort of Patients
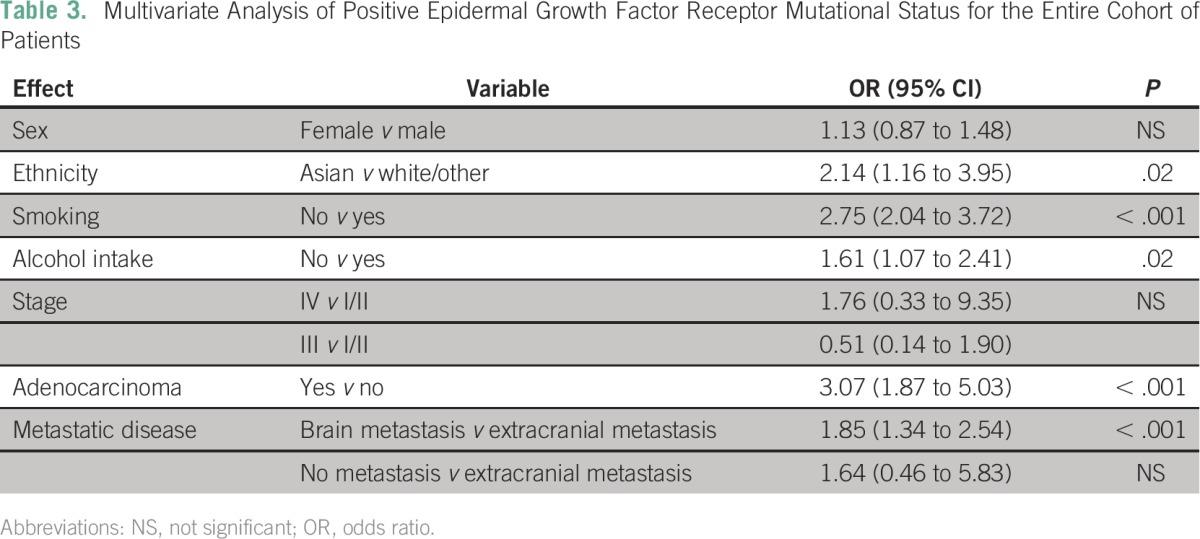
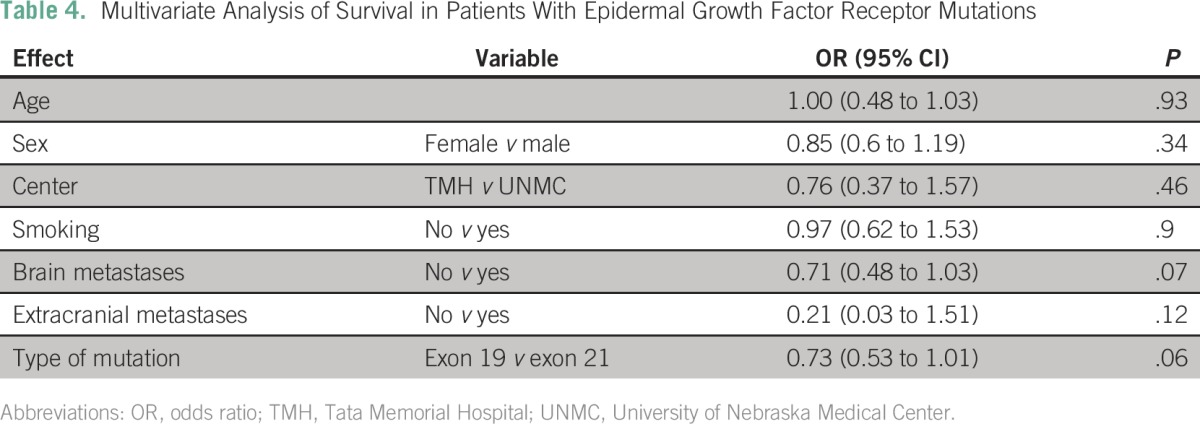
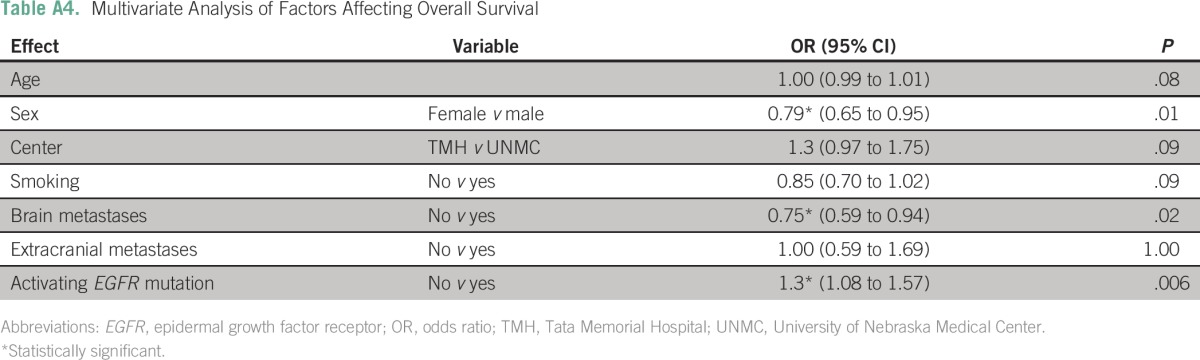
The median OS of the entire cohort was 19.8 months (95% CI, 17.9 to 21.7 months). The OS was similar at the two sites (TMH, 19.7 months; UNMC, 21.6 months; P = .2). Patients with an EGFR mutation had a longer median OS compared with those without (25.1 v 16.8 months; P < .001; Appendix Table A4). Patients with tumors harboring an exon 19 deletion had a numerically better median OS compared with those with tumors containing an L858R mutation (27.1 v 21.1 months), but this difference was not statistically significant (odds ratio, 0.73; 95% CI, 0.53 to 1.01; P = .056; Table 4). Patients with brain metastases had a shorter median OS compared with those without brain metastases (15 months; 95% CI, 11.7 to 18.4 v 20.6 months; 95% CI, 18.3 to 22.8 months; P = .02). However, in the cohort of EGFR mutation–positive patients, there was no difference in median OS between patients with and without brain metastases (20.8 v 25.1 months; P = .11).
Table 4.
Multivariate Analysis of Survival in Patients With Epidermal Growth Factor Receptor Mutations
DISCUSSION
To our knowledge, the current study is the largest to date to demonstrate that EGFR mutations are significantly more frequent among tumors of patients with NSCLC with brain metastases at diagnosis. In multivariate analysis, the presence of brain metastases independently predicted a two-fold increased likelihood of an EGFR mutation compared with tumors in patients without brain metastases. Several published studies have indicated the possibility of a higher rate of EGFR mutations in NSCLC metastatic to the brain.10 These studies with small sample sizes did not adjust for effects of potential confounding variables. A more recent Korean study demonstrated similar findings, with a strong association between EGFR mutation status and brain metastases (OR, 3.8; P = .001), but not between EGFR mutation status and extracranial metastases (OR, 1.73; P = .079). The authors also reported a higher number of brain metastases in patients with EGFR mutations and a higher risk of brain metastases after resection of lung primary tumors.12 In another study, from Slovenia, patients with EGFR mutation–positive disease had a nonsignificant trend toward higher risk of brain metastases at diagnosis.13
No differences in the distribution of type of EGFR mutation (exon 19 v 21) on the basis of the presence or absence of brain metastases were identified in the current study. A Japanese study (N = 57) suggested a correlation between miliary brain metastases and exon 19 deletion, but not exon 21 point mutation.14
The development of an EGFR mutation seems to be an early event in the development of NSCLC,15 whereas EGFR amplification is a relatively late event more prevalent in metastatic lesions15-17 and may contribute to the development of brain metastases.18 Other mechanisms for increased brain metastases in EGFR-mutant NSCLC may include Met activation19 and epithelial-mesenchymal transition.20,21 Studies performed in patients resistant to EGFR TKIs have demonstrated epithelial-mesenchymal transition in a subset of EGFR-mutant NSCLC.20,21 CRIPTO1 is an oncofetal gene related to the EGF–Cripto-1/FRL-1/Cryptic family, which has been noted in EGFR-mutant NSCLC to activate SRC and ZEB1 to promote epithelial-mesenchymal transition.21 Loss of epithelial markers such as E-cadherin and cell-cell adhesion during epithelial-mesenchymal transition may result in increased motility and invasiveness of lung cancer cells.20 A subset of patients with EGFR-mutant NSCLC may undergo epithelial-mesenchymal transition; therefore, their cancer may be more likely to metastasize to the brain. These findings would encourage increased surveillance for brain metastases even in asymptomatic patients, to treat them early, possibly with a local therapy (such as stereotactic radiosurgery rather than whole-brain radiation therapy), thereby avoiding significant morbidity.
The presence of brain metastases decreases OS, with a reported median survival in patients of only 3 to 5 months.22 The results of the current study suggest that patients with tumors harboring an activating EGFR mutation have a better OS and that the presence of brain metastases does not necessarily worsen prognosis. Previous studies of prophylactic cranial irradiation (PCI) in NSCLC have shown that it decreases the risk of brain metastases without survival improvement.23,24 Identification of patients with NSCLC at high risk of brain metastases may improve the beneficial effects of PCI. Prior studies have demonstrated clinical features (including increased size of primary tumor,25 higher nodal stage,25-27 and histology [adenocarcinoma or nonsquamous v squamous])25-27 that are associated with an increased incidence of brain metastases in patients with NSCLC. One study found that PCI improved disease-free survival among patients with NSCLC with a higher risk of brain metastases; OS was similar, at least partly related to early discontinuation of therapy and relatively small sample size.28
Similar to previous reports, the presence of an EGFR mutation in this study predicted better OS.29,30 However, for patients whose tumors had an EGFR mutation, none of the common factors considered (including the presence of brain metastases) predicted for survival. These results differ from those recently published by Li et al,31 who found that the presence of EGFR mutations, especially exon 19 deletions, predicted for outcomes in patients with brain metastases. These differences may be a result of the sample size in each study. Li et al included 66 patients with EGFR mutations (33 patients each with exon 21 point mutation and exon 19 deletion), whereas the current study includes 452 patients with tumors harboring an EGFR mutation (exon 21 point mutation, n = 190; exon 19 deletion, n = 229; uncommon mutations, n = 32). In addition, the ethnicity of the patients could also contribute to the disparity. The study by Li et al included East Asian patients, whereas the current study consisted of South Asian and white patients.
This study did not find a significant difference in outcomes between patients whose tumors had exon 19 deletions and those with exon 21 mutations, although the former group had a 27% decreased risk of death compared with those with an exon 21 mutation. Older studies have not found a difference in OS,32 but recent studies found a better prognosis with the presence of an exon 19 deletion. Survival analyses of the LUX-Lung 3 and the LUX-Lung 6 trials (afatinib v chemotherapy [cisplatin and pemetrexed, LUX-Lung 3; cisplatin and gemcitabine, LUX-Lung 6]) demonstrated a significant improvement in OS with afatinib compared with cisplatin-based chemotherapy in patients with tumors that harbored exon 19 deletions, but not L858R mutations.33 A recent meta-analysis of 4,835 patients enrolled in clinical trials of EGFR TKIs suggested that patients with an exon 19 deletion have a better prognosis (hazard ratio, 0.61; 95% CI, 0.43 to 0.86).34 Again, this discrepancy may reflect the sample size differences.
Integration of EGFR mutation status with other clinical predictors may identify patients with a higher risk of developing brain metastases, who may benefit from close monitoring and serve as candidates for enrollment in future trials of PCI. Further investigation into the role of EGFR mutations in the development of brain metastases in patients with NSCLC may also provide insights into the molecular biology of brain metastases for lung cancer and other malignancies that commonly metastasize to the brain.
A retrospective study design has inherent limitations. Patients more likely to have a tumor with an EGFR mutation on the basis of clinical features are more likely to be tested. A multivariate analysis was performed to adjust for known confounding variables, but unknown variables may exist. The incidences of EGFR mutations in each cohort are similar to those reported in the literature for the two ethnicities studied in this analysis.35,36 A few studies have shown a difference in the incidence of EGFR mutation in never-smokers and former or current smokers (51% v 19% v 4%, respectively) as well as with the history of smoking pack-year numbers and smoke-free years.37 The retrospective nature of this study did not allow such categorization of smoking status. Conversely, to our knowledge, this is the largest study to have assessed the incidence of EGFR mutation in NSCLC with brain metastases and effects of EGFR mutation status on OS. In conclusion, patients whose tumors harbored an EGFR mutation had a nearly two-fold increased risk of brain metastases, but EGFR status did not predict for survival in patients who had brain metastases.
Appendix
Table A1.
Impact of Epidermal Growth Factor Receptor Mutation Type on Metastasis for the Entire Cohort of Patients
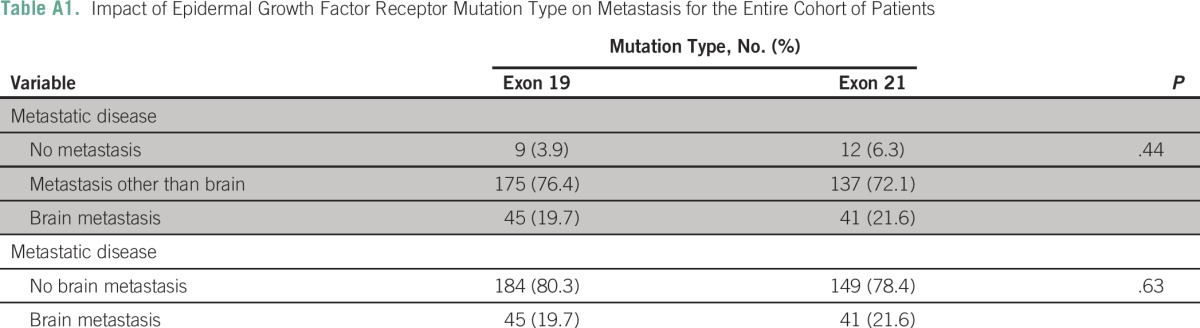
Table A2.
Multivariate Analysis of Positive Epidermal Growth Factor Receptor Mutational Status (Tata Memorial Hospital cohort)
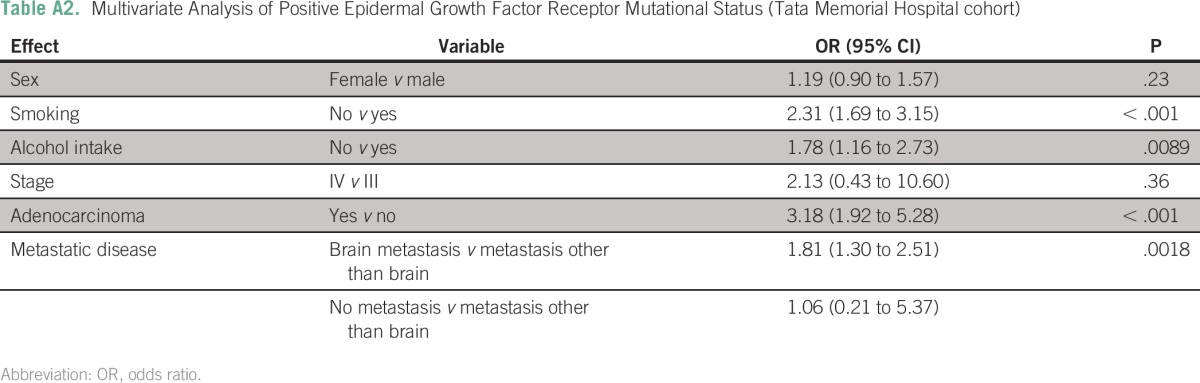
Table A3.
Multivariate Analysis of Positive Epidermal Growth Factor Receptor Mutational Status (University of Nebraska Medical Center cohort)
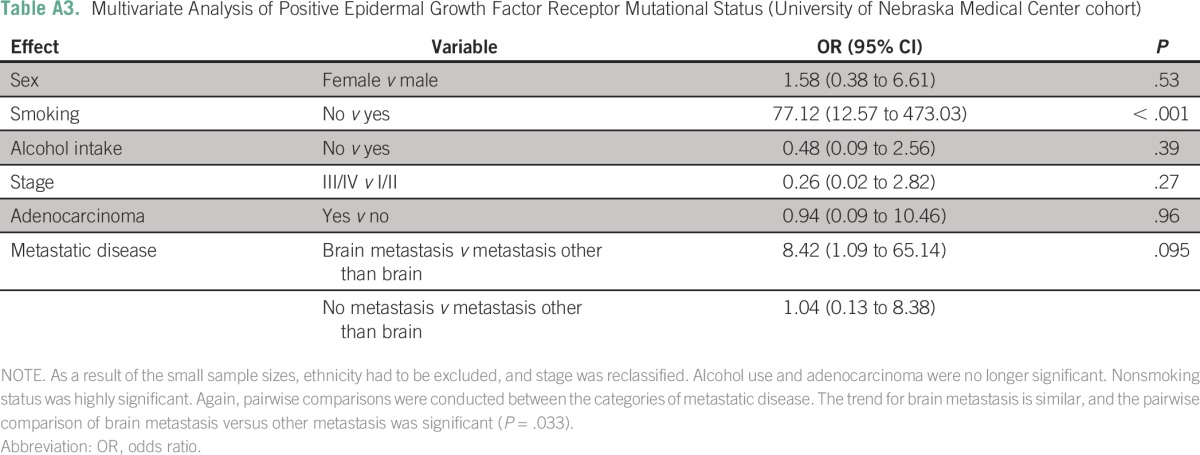
Table A4.
Multivariate Analysis of Factors Affecting Overall Survival
Footnotes
Presented at the 16th World Conference on Lung Cancer, Denver, CO, September 6-9, 2015.
AUTHOR CONTRIBUTIONS
Conception and design: Vijaya Raj Bhatt, Apar Kishor Ganti, Kumar Prabhash
Financial support: Sripad D. Banavali
Administrative support: Sripad D. Banavali, Apar Kishor Ganti, Kumar Prabhash
Provision of study materials or patients: Sanyo P. D’Souza, Allison M. Cushman-Vokoun, Vanita Noronha, Anne Kessinger
Collection and assembly of data: Vijaya Raj Bhatt, Sanyo P. D’Souza, Allison M. Cushman-Vokoun, Vanita Noronha, Vivek Verma, Amit Joshi, Anuradha Chougule, Nirmala Jambhekar, Alissa Marr, Vijay Patil, Kumar Prabhash
Data analysis and interpretation: Vijaya Raj Bhatt, Lynette M. Smith, Anne Kessinger, Alissa Marr, Vijay Patil, Sripad D. Banavali, Apar Kishor Ganti, Kumar Prabhash
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Vijaya Raj Bhatt
No relationship to disclose
Sanyo P. D’Souza
No relationship to disclose
Lynette M. Smith
No relationship to disclose
Allison M. Cushman-Vokoun
No relationship to disclose
Vanita Noronha
No relationship to disclose
Vivek Verma
No relationship to disclose
Amit Joshi
No relationship to disclose
Anuradha Chougule
No relationship to disclose
Nirmala Jambhekar
No relationship to disclose
Anne Kessinger
No relationship to disclose
Alissa Marr
Research Funding: Merck Sharp and Dohme (Inst)
Vijay Patil
No relationship to disclose
Sripad D. Banavali
No relationship to disclose
Apar Kishor Ganti
Consulting or Advisory Role: Otsuka Pharmaceutical, Boehringer Ingelheim, Biodesix, Pfizer
Research Funding: Pfizer (Inst), Amgen (Inst), NewLink Genetics (Inst), ARIAD (Inst), Astex Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Merck Serono (Inst), Merck (Inst), Janssen Biotech (Inst)
Kumar Prabhash
No relationship to disclose
REFERENCES
- 1.Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40:716–722. doi: 10.1016/j.ctrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: Is afatinib better or simply newer? J Clin Oncol. 2013;31:3303–3306. doi: 10.1200/JCO.2013.49.8782. [DOI] [PubMed] [Google Scholar]
- 3.Small AC, Tsao C-K, Moshier EL, et al. Prevalence and characteristics of patients with metastatic cancer who receive no anticancer therapy. Cancer. 2012;118:5947–5954. doi: 10.1002/cncr.27658. [DOI] [PubMed] [Google Scholar]
- 4.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seshacharyulu P, Ponnusamy MP, Haridas D, et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganti AK. Epidermal growth factor receptor signaling in nonsmall cell lung cancer. Cancer Invest. 2010;28:515–525. doi: 10.3109/07357900903476760. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 8.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 9.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt VR, Kedia S, Kessinger A, et al. Brain metastasis in patients with non-small-cell lung cancer and epidermal growth factor receptor mutations. J Clin Oncol. 2013;31:3162–3164. doi: 10.1200/JCO.2013.49.8915. [DOI] [PubMed] [Google Scholar]
- 11.Chougule A, Prabhash K, Noronha V, et al. Frequency of EGFR mutations in 907 lung adenocarcinoma patients of Indian ethnicity. PLoS One. 2013;8:e76164. doi: 10.1371/journal.pone.0076164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9:195–199. doi: 10.1097/JTO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 13.Stanic K, Zwitter M, Hitij NT, et al. Brain metastases in lung adenocarcinoma: Impact of EGFR mutation status on incidence and survival. Radiol Oncol. 2014;48:173–183. doi: 10.2478/raon-2014-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekine A, Kato T, Hagiwara E, et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: Distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77:64–69. doi: 10.1016/j.lungcan.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Gazdar AF, Minna JD. Deregulated EGFR signaling during lung cancer progression: Mutations, amplicons, and autocrine loops. Cancer Prev Res (Phila) 2008;1:156–160. doi: 10.1158/1940-6207.CAPR-08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol. 2011;29:2972–2977. doi: 10.1200/JCO.2010.33.3906. [DOI] [PubMed] [Google Scholar]
- 17.Yatabe Y, Takahashi T, Mitsudomi T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR-mutated lung cancer. Cancer Res. 2008;68:2106–2111. doi: 10.1158/0008-5472.CAN-07-5211. [DOI] [PubMed] [Google Scholar]
- 18.Sun M, Behrens C, Feng L, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res. 2009;15:4829–4837. doi: 10.1158/1078-0432.CCR-08-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedettini E, Sholl LM, Peyton M, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol. 2010;177:415–423. doi: 10.2353/ajpath.2010.090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res. 2014;74:309–319. doi: 10.1158/0008-5472.CAN-12-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KS, Raffeld M, Moon YW, et al. CRIPTO1 expression in EGFR-mutant NSCLC elicits intrinsic EGFR-inhibitor resistance. J Clin Invest. 2014;124:3003–3015. doi: 10.1172/JCI73048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regine WF, Scott C, Murray K, et al. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: An analysis from Radiation Therapy Oncology Group Study 91-04. Int J Radiat Oncol Biol Phys. 2001;51:711–717. doi: 10.1016/s0360-3016(01)01676-5. [DOI] [PubMed] [Google Scholar]
- 23.Xie SS, Li M, Zhou CC, et al. Prophylactic cranial irradiation may impose a detrimental effect on overall survival of patients with nonsmall cell lung cancer: A systematic review and meta-analysis. PLoS One. 2014;9:e103431. doi: 10.1371/journal.pone.0103431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lester JF, MacBeth FR, Coles B. Prophylactic cranial irradiation for preventing brain metastases in patients undergoing radical treatment for non-small-cell lung cancer: A Cochrane Review. Int J Radiat Oncol Biol Phys. 2005;63:690–694. doi: 10.1016/j.ijrobp.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: Primary tumor size, cell type, and lymph node metastases. Radiology. 2007;242:882–888. doi: 10.1148/radiol.2423051707. [DOI] [PubMed] [Google Scholar]
- 26.Na II, Lee TH, Choe DH, et al. A diagnostic model to detect silent brain metastases in patients with non-small cell lung cancer. Eur J Cancer. 2008;44:2411–2417. doi: 10.1016/j.ejca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Wang SY, Ye X, Ou W, et al. Risk of cerebral metastases for postoperative locally advanced non-small-cell lung cancer. Lung Cancer. 2009;64:238–243. doi: 10.1016/j.lungcan.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1093/annonc/mdu567. Li N, Zeng Z-F, Wang S-Y, et al: Randomized phase III trial of prophylactic cranial irradiation versus observation in patients with fully resected stage IIIA-N2 non-small-cell lung cancer and high risk of cerebral metastases after adjuvant chemotherapy. Ann Oncol 26:504-509, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Zhao J, Song SW, et al. EGFR mutations are associated with prognosis but not with the response to front-line chemotherapy in the Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2010;67:343–347. doi: 10.1016/j.lungcan.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 31. doi: 10.1007/s13277-015-3653-2. Li H, Zhang X, Cao J, et al: Exon 19 deletion of epidermal growth factor receptor is associated with prolonged survival in brain metastases from non-small-cell lung cancer. Tumour Biol 36:9251-9258, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Berry MF, Jeffrey Yang CF, Hartwig MG, et al. Impact of pulmonary function measurements on long-term survival after lobectomy for stage I non-small cell lung cancer. Ann Thorac Surg. 2015;100:271–276. doi: 10.1016/j.athoracsur.2015.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 34.Sheng M, Wang F, Zhao Y, et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: A meta-analysis. Eur J Clin Pharmacol. 2016;72:1–11. doi: 10.1007/s00228-015-1966-0. [DOI] [PubMed] [Google Scholar]
- 35.Mitsudomi T. Molecular epidemiology of lung cancer and geographic variations with special reference to EGFR mutations. Transl Lung Cancer Res. 2014;3:205–211. doi: 10.3978/j.issn.2218-6751.2014.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doval D, Prabhash K, Patil S, et al. Clinical and epidemiological study of EGFR mutations and EML4-ALK fusion genes among Indian patients with adenocarcinoma of the lung. Onco Targets Ther. 2015;8:117–123. doi: 10.2147/OTT.S74820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pham D, Kris MG, Riely GJ, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol. 2006;24:1700–1704. doi: 10.1200/JCO.2005.04.3224. [DOI] [PubMed] [Google Scholar]


