Abstract
Btn2p, a novel cytosolic coiled-coil protein in Saccharomyces cerevisiae, was previously shown to interact with and to be necessary for the correct localization of Rhb1p, a regulator of arginine uptake, and Yif1p, a Golgi protein. We now report the biochemical and physical interactions of Btn2p with Ist2p, a plasma membrane protein that is thought to have a function in salt tolerance. A deletion in Btn2p (btn2Δ strains) results in a failure to correctly localize Ist2p, and strains lacking Btn2p and Ist2p (btn2Δ ist2Δ strains) are unable to grow in the presence of 0.5 or 1.0 M NaCl. Btn2p was originally identified as being up-regulated in a btn1Δ strain, which lacks the vacuolar-lysosomal membrane protein, Btn1p, and serves as a model for Batten disease. This up-regulation of Btn2p was shown to contribute to the maintenance of a stable vacuolar pH in the btn1Δ strain. Btn1p was subsequently shown to be required for the optimal transport of arginine into the vacuole. Interestingly, btn1Δ ist2Δ strains are also unable to grow in the presence of 0.5 or 1.0 M NaCl, and ist2Δ suppresses the vacuolar arginine transport defect in btn1Δ strains. Although further investigation is required, we speculate that altered vacuolar arginine transport in btn1Δ strains represents a mechanism for maintaining or balancing cellular ion homeostasis. Btn2p interacts with at least three proteins that are seemingly involved in different biological functions in different subcellular locations. Due to these multiple interactions, we conclude that Btn2p may play a regulatory role across the cell in response to alterations in the intracellular environment that may be caused by changes in amino acid levels or pH, a disruption in protein trafficking, or imbalances in ion homeostasis resulting from either genetic or environmental manipulation.
BTN2 in the yeast Saccharomyces cerevisiae encodes a nonessential novel coiled-coil protein, Btn2p, which was originally identified by gene expression profiling as one of only two proteins up-regulated in yeast strains lacking Btn1p (btn1Δ strains), the yeast model for Batten disease (24). This up-regulation of Btn2p in btn1Δ strains was originally presumed to occur in response to altered vacuolar pH because deletion of either BTN1 or BTN2 results in an alteration of the ability of yeast cells to maintain balanced pH homeostasis in the vacuole (1, 24). It was subsequently determined that Btn2p interacts with Rhb1p (previously designated Rsg1p), which regulates the activity of plasma membrane Can1p arginine and lysine permease (2, 27). Furthermore, deletion of BTN2 results in altered arginine uptake, a phenotype exhibited by rhb1Δ strains. This altered arginine uptake in btn2Δ strains was shown to result from a failure to localize Rhb1p to a distinct peripheral structure, thereby causing a loss of the regulation of arginine uptake by the Can1p permease (2). Therefore, Btn2p was implicated in regulating intracellular levels of arginine, an assertion supported by the fact that Btn1p has been shown to have a role in the transport of arginine across the vacuolar membrane (12). Thus, the original observation that Btn2p was up-regulated in btn1Δ strains was interpreted to be a compensatory mechanism for balancing intracellular arginine levels, although the functions of both Btn1p and Btn2p still require further study.
Btn2p has also been shown to interact with Yif1p (3), an essential protein that is a component of a complex at the Golgi apparatus that interacts with transport GTPases, such as Ypt1p, Ypt31p, and Sec4p, and that functions in transport from the endoplasmic reticulum to the Golgi apparatus (16). In the absence of Btn2p (btn2Δ strains), Yif1p becomes mislocalized to or accumulates in the vacuole. Therefore, disruption of Btn2p function alters the trafficking of both Rhb1p and Yif1p, which have different functions in different parts of the cell. These results implicate Btn2p as having a function in intracellular protein trafficking and in maintaining intracellular metabolite homeostasis and suggest that disturbances in intracellular homeostasis and protein trafficking may be linked.
We report a third protein interaction for Btn2p. We demonstrate that Ist2p, a plasma membrane protein that has been reported to show homology to sodium and calcium channel proteins (15) and to have a function in salt tolerance (5), interacts physically and biochemically with Btn2p. Similar to the situation for other Btn2p-interacting proteins, the disruption of Btn2p function results in a failure to correctly localize Ist2p. Moreover, the disruption of both Btn2p and Ist2p (btn2Δ ist2Δ strains) reveals a potential functional interaction between these two proteins through sensitivity of growth in the presence of NaCl. Therefore, btn2Δ results in the altered subcellular localization of the interacting proteins Rhb1p, Yif1p, and Ist2p, which have been implicated in a variety of cellular processes, such that btn2Δ results in pleiotrophic phenotypes. Therefore, the up-regulation of Btn2p in btn1Δ strains may represent a compensatory mechanism for balancing disturbances in each of these biological processes, namely, the regulation of amino acid levels, protein trafficking, and ion homeostasis. In addition, we demonstrate that btn1Δ ist2Δ strains also exhibit NaCl sensitivity and that ist2Δ can suppress the vacuolar arginine transport defect in btn1Δ strains. As human Cln3 has been shown to complement Btn1p function in btn1Δ strains (9, 12, 23), the implications of these results are also discussed in the context of Batten disease.
MATERIALS AND METHODS
Yeast strains and plasmids.
The S. cerevisiae strains used in this work are listed in Table 1. Strain B-11718 was acquired from the American Type Culture Collection, and strain YPH499 was obtained from Stratagene. Strains B-13048 (btn1Δ), B-14248 (rhb1Δ), B-14252 (btn1Δ rhb1Δ), and B-14399 (btn2Δ rhb1Δ) were previously described (2).
TABLE 1.
Yeast strains used in this study
| Genotype | Strain |
|---|---|
| MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-11718 |
| MATα btn1Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-13048 |
| MATα btn2Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-14847 |
| MATα rhb1Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-14248 |
| MATα ist2Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-14884 |
| MATα btn2Δ::loxP rhb1Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-14399 |
| MATα btn1Δ::loxP rhb1Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-14252 |
| MATα btn2Δ::loxP ist2Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-15013 |
| MATα btn1Δ::loxP ist2Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-14830 |
| MATα btn1Δ::loxP btn2Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-13049 |
| MATα leu2-3,112 ura3-52 his3-200 ade2-101 lys2-801 trp1-901 cdc25-2 | CDC25H |
| MATaleu2Δ1 ura3-52 his3-200 ade2-101 lys2-801 trp1Δ63 | YPH499 |
| MATα ist2Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 + pDAP124 | B-14915 |
| MATα btn2Δ::loxP ist2Δ::KanMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 + pDAP0124 | B-14983 |
Strains constructed for this study were generated by using the loxP-KanMX-loxP disruption cassette (7). The btn2::loxP-KanMX-loxP disruption cassette was amplified by using forward primer 5′-CAA CCA AAA GAA AAT AAC TAA TAG ACC CCA TTA CAA TAT AGA AGC ATA GGC CAC TAG TGG ATC TG-3′ and reverse primer 5′-GCC GTA AAA ATG AAA GAT GGG GAG TAT GTA TTA TCA CCC ACA GCT GAA GCT TCG AC GC-3′. The ist2::loxP-KanMX-loxP disruption cassette was amplified by using forward primer 5′-ATG TCG CAG ACA ATT ACA TCT CTA GAT CCG AAT TGT GTT AGC ATA GGC CAC TAG TGG ATC TG-3′ and reverse primer 5′-AGA GGA TTC GGT TGT CTT AGA ATT GTT CGT GAC ATT CTT CAC TCC AGC TGA AGC TTC GTA CGC-3′. The btn1::loxP-KanMX-loxP disruption cassette was amplified by using forward primer 5′-GTA CTT AAA CAC ATA TGA AGA TAT AGC GCA AGT AAA TAT AAG CAT AGG CCA CTA GTG GAT CTG-3′ and reverse primer 5′-CAC TTT ATT TCA ATC TCC TAT TTA ATA TCA CAA CAA AAC TCA GCT GAA GCT TCG TAC GC-3′. Plasmid pCP132 served as the template to generate all loxP-KanMX-loxP disruption cassettes.
Strains B-14847 (btn2Δ) and B-14884 (ist2Δ) were constructed by transforming B-11718 competent cells with PCR-generated btn2::loxP-KanMX-loxP and ist2::loxP-KanMX-loxP gene disruption cassettes, respectively. Strain B-14830 (btn1Δ ist2Δ) was generated by transforming B-14884 (ist2Δ) competent cells with the PCR-generated btn1::loxP-KanMX-loxP gene disruption cassette. Strain B-15013 (btn2Δ ist2Δ) was constructed by transforming B-14847 with ist2::loxP-KanMX-loxP followed by excision of the KanMX selection marker by using the Cre-loxP recombination event. The transformants were selected on YPD medium (2% glucose, 1% Bacto yeast extract, 2% Bacto peptone) supplemented with 200 μg of Geneticin (G-418 sulfate; Invitrogen)/ml.
All integration events were verified by PCR analyses. The primers used in the PCR to validate replacement of the genes were kanFW (5′-CCT CGA CAT CAT CTG CCC-3′) and kanRE (5′-GGA TGT ATG GGC TAA ATG-3′). These primers anneal to regions inside the two loxP sequences. Also, primers annealing to regions 350 to 500 bp upstream of the ATG translational start site and 350 to 500 bp downstream of the stop codon of BTN1, BTN2, or IST2 were used in combination with primers kanFW and kanRE to confirm homologous integration at the desired locus.
Double-deletion strains were constructed by using the Cre-loxP recombination technique. Plasmid pCP133 contains the Cre recombinase gene under the control of the GAL1 promoter. Expression of the Cre recombinase results in precise excision of the KanMX marker, allowing for the selection marker to be reused for the construction of double-deletion strains. The primers used to validate homologous integration (see above) were used to confirm excision of the KanMX marker at the BTN2 or IST2 locus. Plasmid pCP133 was removed from each of the strains lacking the KanMX marker by streaking 106 cells onto a solid medium (2% glucose and 0.67% yeast nitrogen base with amino acids) containing 5-fluoroorotic acid and selecting for uracil auxotrophs.
For complementation studies, IST2-c-myc and enhanced green fluorescent protein (EGFP)-IST2 were independently subcloned into centromeric single-copy expression vector pAB625 (1).
NaCl sensitivity.
To assay NaCl sensitivity, growth on agar-solidified media was assessed by spotting 5 μl of cell suspension containing approximately 105, 104, 103, 102, or 101 cells onto YPD medium, YPD medium-0.5 M NaCl, or YPD medium-1.0 M NaCl. The plates were incubated for 2 to 3 days at 30°C.
Two-hybrid studies and coimmunoprecipitation of Btn2p and Ist2p.
The Stratagene Cytotrap system was used for two-hybrid screening of interaction partners for Btn2p. The manufacturer's instructions were essentially followed with BTN2-pSOS, a bait plasmid screened as previously described against a yeast cDNA library constructed in the pMyr or trap plasmid (2).
BTN2 and IST2 were cloned into yeast epitope tagging vector pESC-TRP (Stratagene). Plasmid DAP114 contains the BTN2 open reading frame (ORF) tagged with the FLAG epitope at the 3′ terminus and the IST2 ORF tagged with the c-myc epitope at the 3′ terminus. BTN2 was cloned as previously described (2). For IST2, an ApaI restriction site was introduced at the 5′ end and a SalI site was introduced at the 3′ end of a PCR-amplified fragment of IST2 by using primers with the sequences 5′-GGG CCC ATG TCG CAG ACA ATT AC-3′ and 5′-GTC GAC AAG CTT CTT TTT CAG CTT ATG-3′. Amplified IST2 was cloned into pCR-Blunt (Invitrogen), resulting in DAP106.
The plasmid containing BTN2-FLAG and IST2-c-myc was transformed into yeast strain YPH499 (Table 1). Protein-protein interactions were confirmed by immunoprecipitation followed by Western analysis as previously described (2). The blot was probed with anti-c-myc mouse monoclonal antibody (1:1,000; Neomarkers) or anti-FLAG mouse monoclonal antibody (Sigma) and with horseradish peroxidase-tagged anti-mouse secondary antibody (1:3,000; Amersham). The blot was stained by using an ECLplus kit (Amersham) and developed on ECL film.
Localization of EGFP-Btn2p and EGFP-Ist2p.
EGFP-Btn2p was previously described (2). Plasmid DAP124 contains yeast EGFP at the N terminus of the IST2 ORF, which is downstream of the MET promoter. To construct plasmid DAP124, a 2,885-bp BamHI/SalI fragment containing the IST2 ORF was isolated from plasmid DAP106 and ligated into the corresponding sites of pUG34. Cells were grown to stationary phase in synthetic dextrose minimal medium (0.67% yeast nitrogen base without amino acids, 2% dextrose, 1.3 g of amino acid dropout powder/600 ml) lacking histidine or both methionine and histidine on a rotary shaker at 30°C. Cells were diluted 1:100 in synthetic dextrose minimal medium lacking histidine or both methionine and histidine and grown to an optical density at 600 nm of 0.2. Vacuoles were stained with FM4-64 (Molecular Probes) and prepared for confocal microscopy as previously described (2). Confocal microscopy was performed with a Leica TCS SP microscope equipped with argon, krypton-argon, and UV lasers and 100 × 1.3NA lenses. Images were processed by using Photoshop 7.0 (Adobe).
Isolation of yeast vacuoles.
Yeast cells grown to an optical density at 600 nm of 0.6 to 0.7 in 1 liter of medium were washed once with sterile distilled water and then once with 1.0 M sorbitol. Cells were converted to spheroplasts by suspension of the cell pellet in 100 ml of 1.0 M sorbitol containing 400 U of Zymolyase 100T (ICN Pharmaceuticals). The culture was gently shaken for 90 min at 30°C. Spheroplasts were collected by centrifugation at 800 × g for 5 min and then washed twice with 1.0 M sorbitol. All subsequent manipulations were carried out at 4°C. The pellet was suspended in 25 ml of buffer A (10 mM morpholineethanesulfonic acid [MES]-Tris [pH 6.9], 0.1 mM MgCl2, 12% Ficoll 400) and homogenized by six or seven strokes in a Dounce homogenizer. The lysate was cleared by centrifugation at 26,600 × g for 35 min. The top wafer layer was collected and placed into a Dounce homogenizer containing 6 ml of buffer A, and clumps were broken up by six or seven strokes. The homogenate was transferred to an ultracentrifuge tube and layered with 6 ml of buffer B (10 mM MES-Tris [pH 6.9], 0.1 mM MgCl2, 8% Ficoll 400). The mixture was centrifuged at 26,600 × g for 30 min. The top wafer layer was collected and placed into a tube containing 6 ml of buffer C (10 mM MES-Tris [pH 6.9], 5 mM MgCl2, 25 mM KCl). Vacuoles were converted to vesicles by the addition of 2 volumes of buffer C, and a pellet was obtained by centrifugation at 26,600 × g for 20 min. The purity of the vacuoles obtained by this isolation procedure was verified by confocal microscopy, and vacuolar enrichment was confirmed by Western analysis with vacuolar markers (1, 12).
Assays of arginine transport.
Arginine uptake assays were performed as previously described (13, 17, 18, 12). The accumulation of 14C-arginine by vacuolar vesicles at each time point, at time zero, and at 30-s increments was assayed with a 100-μl reaction mixture composed of 25 mM Tris-MES (pH 7.4), 4 mM MgCl2, 25 mM KCl, 0.3 mM ATP, 3.33 μCi of 14C-arginine (348 mCi/mmol), and 30 μg of protein. The reaction was carried out at room temperature and was stopped by the addition of 5 ml of cold buffer C. Vacuolar vesicles were collected on a 0.22-μm-pore-size nylon membrane (Millipore, Burlington, Mass.). The radioactivity retained on the membrane was quantified with a scintillation counter (Beckman). The protein content of vacuolar preparations was assayed by using the Bradford assay (Bio-Rad).
RESULTS
Btn2p interacts with Ist2p.
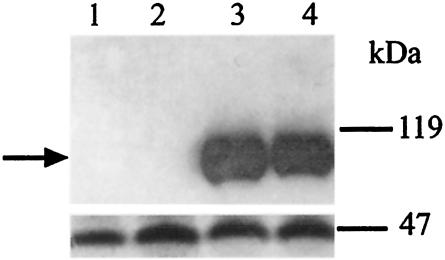
We used the Cytotrap two-hybrid system to screen for Btn2p interactions by cotransformation of yeast strain CDC25H with BTN2-pSOS and a yeast cDNA library in pMyr as previously described (2, 3). We had previously characterized Rhb1p and Yif1p as the most common candidates for interactions with Btn2p (2, 3). In addition, we isolated a single clone of IST2 as a candidate for interactions (data not shown). As with all two-hybrid systems, biochemical or in vivo confirmation of interactions is required. Figure 1 shows Western analysis with an anti-c-myc monoclonal antibody and the same blot stripped and reprobed with an anti-FLAG monoclonal antibody for various yeast strains: a whole-cell extract from strain YPH499 that expresses only Btn2p tagged with FLAG at the C terminus (lane 1); a whole-cell extract from a strain expressing Btn2p-FLAG, which was immunoprecipitated with anti-FLAG monoclonal antibody (lane 2); a whole-cell extract from strain YPH499 that expresses both Ist2p tagged with c-myc and Btn2p tagged with FLAG and that shows the presence of Ist2p-c-myc (lane 3); and a whole-cell extract from a strain expressing both C-terminally c-myc-tagged Ist2p and C-terminally FLAG-tagged Btn2p, which was immunoprecipitated with anti-FLAG monoclonal antibody (lane 4). Immunoprecipitation with anti-FLAG antibody, which binds to FLAG-tagged Btn2p, brings with it c-myc-tagged Ist2p, confirming that Btn2p and Ist2p do physically interact in vivo. It should be noted that these tags do not alter the function of Btn2p (2) or Ist2p, as determined by the ability of the tagged proteins to complement the NaCl sensitivity of the growth of btn2Δ ist2Δ strains, a phenotype that is described later (see Fig. 3).
FIG. 1.
Btn2p interacts with Ist2p in vivo. Immunoprecipitation was performed with anti-FLAG antibody and cell extracts derived from strain YPH499 expressing Btn2p-FLAG or both Btn2p-FLAG and Ist2p-c-myc. (Upper panel) Western analysis performed with anti-c-myc monoclonal antibody. (Lower panel) Western analysis performed with anti-FLAG monoclonal antibody. Lanes: 1, cell extract of YPH499 expressing Btn2p-FLAG; 2, cell extract of YPH499 expressing Btn2p-FLAG and immunoprecipitated with anti-FLAG antibody; 3, cell extract of YPH499 expressing Btn2p-FLAG and Ist2p-c-myc; 4, cell extract of YPH499 expressing Btn2p-FLAG and Ist2p-c-myc and immunoprecipitated with anti-FLAG antibody. Size markers are indicated on the right. The arrow indicates the Ist2p-c-myc band.
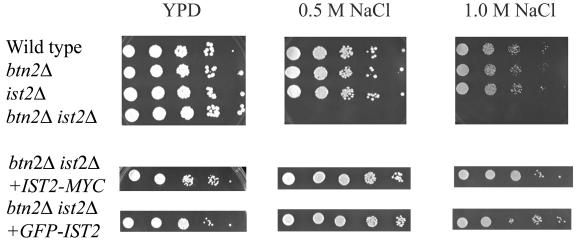
FIG. 3.
btn2Δ ist2Δ strains are sensitive to NaCl. Wild-type (B-11718), ist2Δ (B-14884), btn2Δ (B-14847), and btn2Δ ist2Δ (B-15013) strains were serially diluted. Wild-type, ist2Δ, and btn2Δ cells grew normally, whereas btn2Δ ist2Δ cells exhibited sensitivity of growth. Plates were incubated at 30°C for 2 days. For complementation studies, IST2-c-myc and EGFP-IST2 were independently subcloned into centromeric single-copy expression vector pAB625 and similarly plated (1).
btn2Δ strains fail to localize Ist2p.
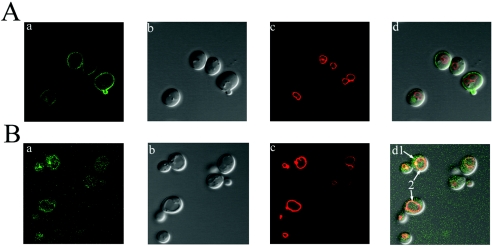
We previously reported that functional Btn2p-EGFP localized to the cytosol (2). Because the disruption of Btn2p function alters the localization of its interacting partners, Rhb1p and Yif1p, we tested whether the absence of Btn2p affected the localization of Ist2p. Ist2p has been reported to be localized at the plasma membrane (26). We confirmed that EGFP-Ist2p (N-terminal fusion of EGFP to Ist2p) in ist2Δ strains localizes to the plasma membrane (Fig. 2A). Furthermore, we demonstrated that in btn2Δ ist2Δ strains, EGFP-Ist2p is no longer localized to the plasma membrane but appears predominantly as a punctate entity in the cytosol (Fig. 2B). Interestingly, a small amount of EGFP-Ist2p appears to be localized in the vacuolar membrane; however, subcellular fractionation studies will be required to confirm this finding. These data suggest that the absence of Btn2p alters the localization of Ist2p and that Btn2p may therefore be involved in the trafficking or localization of Ist2p to the plasma membrane. Each image shown is typical of what was seen for the entire cell population for each strain. EGFP-Ist2p is functional, as determined by functional complementation of a phenotype that is described later (see Fig. 3). Note that Btn2p localization to the cytosol is not altered in ist2Δ strains (data not shown).
FIG. 2.
Localization of Ist2p to the plasma membrane and alteration of the localization of Ist2p in btn2Δ strains. (A) EGFP-Ist2p in ist2Δ strain B-14915. (B) EGFP-Ist2p in btn2Δ ist2Δ strain B-14983. (a) EGFP fluorescence. (b) Differential interference contrast images. (c) FM4-64 staining showing the vacuolar membrane. (d) Merged images. EGFP-Ist2p in the ist2Δ strain localizes to the plasma membrane. EGFP-Ist2p in the btn2Δ ist2Δ strain appears to be mislocalized to the cytoplasm and the vacuolar membrane (arrows 1 and 2, respectively). Each image is presented at a magnification of ×100 and is typical of that seen for the entire cell population.
The growth of btn2Δ ist2Δ strains is NaCl sensitive.
Ist2p has been reported to show similarity to higher eukaryotic sodium and calcium channel proteins (15). We therefore tested the sensitivity of the growth of ist2Δ strains to elevated concentrations of sodium or calcium in the media. Deletion of IST2 was previously reported to result in salt tolerance (15); however, we found that ist2Δ strains did not show an obvious growth defect in the presence of elevated levels of sodium or calcium. However, btn2Δ ist2Δ strains failed to grow in media containing 0.5 or 1.0 M NaCl (Fig. 3). There was no effect on growth for the same strains in the presence of elevated calcium levels (data not shown). We also found that deletion of Rhb1p, which also interacts with Btn2p, does not result in an NaCl sensitivity phenotype. Furthermore, deletion of Ist2p does not result in the canavanine resistance phenotype previously reported for strains lacking Rhb1p or Btn2p (2). The NaCl sensitivity phenotype exhibited by btn2Δ ist2Δ strains could be complemented by the expression of plasmid-borne BTN2 or IST2 (data not shown) and by c-myc- or EGFP-tagged IST2, as shown in the coimmunoprecipitation and localization studies described above, respectively (Fig. 3).
The growth of btn1Δ ist2Δ strains is also NaCl sensitive.
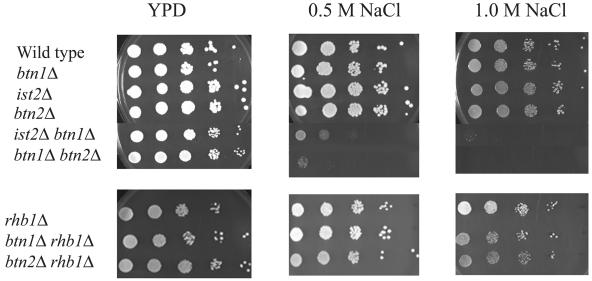
Btn2p was originally identified as being up-regulated in a btn1Δ strain. We therefore tested whether btn1Δ results in sensitivity of growth in media containing NaCl. Although the growth of btn1Δ strains did not show NaCl sensitivity, btn1Δ btn2Δ and btn1Δ ist2Δ strains were both unable to grow in media containing 0.5 or 1.0 M NaCl (Fig. 4). Because Btn2p has been shown to interact with Rhb1p, we demonstrated that the growth of btn1Δ rhb1Δ and btn2Δ rhb1Δ strains did not show NaCl sensitivity. Therefore, the NaCl sensitivity phenotype is not a result of interactions with Btn2p but is a result of the loss of distinct interactions between Btn2p and Ist2p or Btn1p and Ist2p. Strains lacking Yif1p, which also interacts with Btn2p, could not be tested, as this is an essential protein.
FIG. 4.
btn1Δ ist2Δ strains are sensitive to NaCl. Wild-type (B-11718), ist2Δ (B-14884), btn1Δ (B-13048), btn2Δ (B-14847), btn1Δ ist2Δ (B-14830), and btn1Δ btn2Δ (B-13049) strains were serially diluted. Wild type, ist2Δ, btn2Δ, and btn1Δ cells grew normally, whereas ist2Δ btn1Δ and btn1Δ btn2Δ strains exhibited sensitivity of growth. Note that strains with a deletion of RHB1 (rhb1Δ, btn1Δ rhb1Δ, and btn2Δ rhb1Δ strains) did not show a similar genetic interaction. Plates were incubated at 30°C for 2 days.
We also performed FM4-64 staining to test the vacuolar integrity and morphology of cells exposed to NaCl. We found that strains containing btn1Δ appear to have more fragmented vacuoles. However, as the dynamics of this technique have not been fully characterized in the presence of high salt concentrations, we report this finding as a qualitative assessment while we work to establish a reliable means of isolating vacuoles from salt-exposed strains to quantify the possible effects that NaCl may have on vacuoles of btn1Δ strains as opposed to other strains.
ist2Δ suppresses the defect in vacuolar arginine transport in btn1Δ strains.
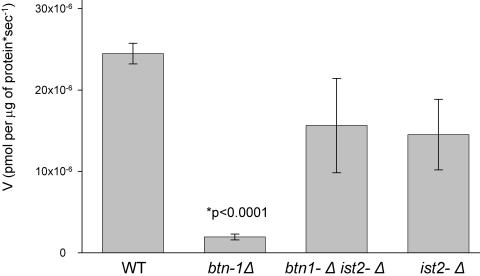
We previously reported that vacuoles isolated from btn1Δ strains have a decreased ability to transport arginine into the lumen of this organelle (12). To test whether Ist2p exerts an effect on the transport of arginine into the vacuole, we examined the effect of deleting IST2 (ist2Δ) on vacuolar arginine transport. Compared to the wild type, ist2Δ did not significantly alter arginine transport into the vacuole (Fig. 5). However, btn1Δ ist2Δ strains showed nearly normal levels of arginine transport into the vacuole, indicating that ist2Δ suppressed this defect in btn1Δ strains and revealing a distinct interaction between Btn1p and Ist2p.
FIG. 5.
ist2Δ suppresses the decrease in the rate of vacuolar arginine uptake in isolated vacuoles caused by btn1Δ. The rates of uptake of 14C-arginine into isolated vacuoles from wild-type (WT) (B-11718), btn1Δ (B-13048), ist2Δ (B-14884), and btn1Δ ist2Δ (B-14830) strains are shown. Note that all isolated vacuoles were prepared from strains grown on YPD medium.
DISCUSSION
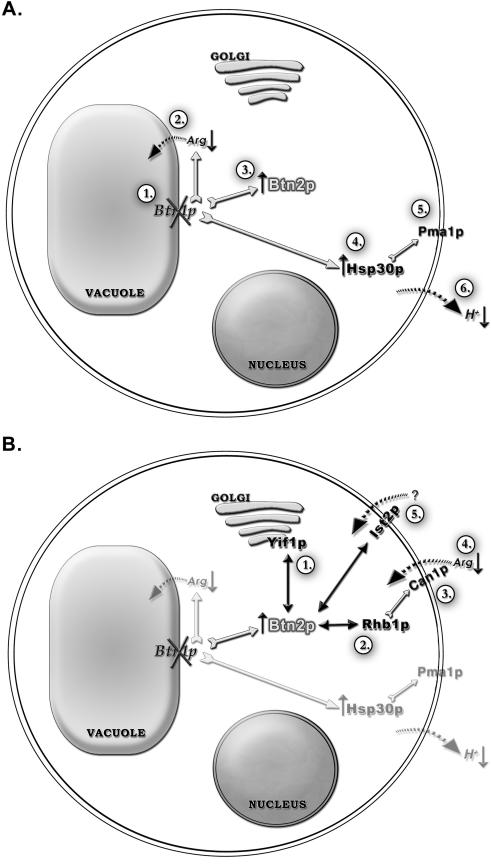
BTN2 encodes a 410-amino-acid novel coiled-coil protein that has a role in localizing proteins to different subcellular compartments. Btn2p was originally identified as being up-regulated in a btn1Δ strain. The functional implications of Btn1p and Btn2p interactions as a result of btn1Δ are summarized in Fig. 6. Btn1p is a vacuolar protein, and btn1Δ results in altered vacuolar pH and decreased sequestration of the basic amino acids arginine and lysine in the vacuole (Fig. 6A) (4, 12, 24). A previous study revealed that btn2Δ results in elevated activity of the vacuolar H+-ATPase, suggesting that the up-regulation of BTN2 expression in btn1Δ strains may contribute either directly or indirectly to normalization of the vacuolar pH in btn1Δ strains (24). However, btn2Δ does not result in altered vacuolar pH, and btn1Δ does not result in altered vacuolar H+-ATPase activity, suggesting that there is no direct correlation between vacuolar pH and vacuolar H+-ATPase activity (1). Interestingly, the only other gene up-regulated in btn1Δ strains in addition to BTN2 is HSP30 (24). The up-regulation of HSP30 was shown to down-regulate the activity of the plasma membrane H+-ATPase, which was presumed to result in elevated use of ATP as well as elevated proton pumping out of the cell (Fig. 6A). However, as we discuss below, we now believe that btn1Δ results in an alteration in ion homeostasis, perhaps due to altered amino acid levels, and this alteration in the regulation of plasma membrane H+-ATPase activity through Hsp30p could be interpreted as a means of balancing the ionic content of cells.
FIG. 6.
Schematic representation of the cellular roles of Btn1p and Btn2p. (A) Step 1 shows the deletion of btn1Δ. Step 2 shows that btn1Δ results in decreased arginine uptake into the vacuole (12). Step 3 shows that btn1Δ results in the up-regulation of Btn2p (23). Step 4 shows that btn1Δ results in the up-regulation of Hsp30p (11, 23). Step 5 shows that the up-regulation of Hsp30p down-regulates overactive Pma1p (23). Step 6 shows that the up-regulation of Hsp30p decreases excess proton pumping across the plasma membrane (23). (B) Step 1 shows that Btn2p interacts with Yif1p and is involved in correctly localizing this protein to the Golgi apparatus (3). Step 2 shows that Btn2p interacts with and is involved in correctly localizating Rhb1p (2). Step 3 shows that Rhb1p negatively regulates the Can1p permease, which transports arginine and lysine into the cell (27). Step 4 shows decreased uptake of arginine into the cell (2). Step 5 shows that Btn2p interacts with Ist2p, which shows homology to ion channel proteins and may be involved in the transport of an as-yet-unidentified cation across the plasma membrane.
Btn2p is a cytosolic protein that has now been implicated in localizing Rhb1p to the cell periphery, Yif1p to the Golgi apparatus, and now Ist2p to the plasma membrane (Fig. 6B). There is little to link Rhb1p, Yif1p, and Ist2p at the functional level other than the fact that each interacts with Btn2p and that each is mislocalized in the absence of Btn2p. We have therefore discovered a novel link among Rhb1p, Yif1p, and Ist2p that implies that Btn2p has the potential to affect several cellular processes. Disruption of Btn2p function can result in a variety of different phenotypes that are associated with the mislocalization of Btn2p-interacting proteins. In this study, we have shown that Btn2p physically interacts with Ist2p and that Btn2p is necessary for the correct localization of Ist2p. In addition, we have shown that btn2Δ or btn1Δ in combination with ist2Δ results in a similar phenotype, sensitivity to NaCl. The role of Btn2p and its multiple interactions are summarized in Fig. 6B. Btn2p may have a role in regulating the trafficking around the cell of a very specific set of proteins that have a functional link essential to maintaining a biological balance. In this sense, Btn2p may be a sensor of an imbalance or an effector that acts upon an imbalance. What Btn2p senses or responds to requires further study. However, clues that further identify the role of Btn2p come from studies of btn1Δ strains that up-regulate Btn2p, which are further discussed below and which suggest that Btn2p may have a role in sensing or responding to changes in cation levels in cells.
We demonstrate that btn1Δ strains lacking either Ist2p or Btn2p show decreased growth in the presence of high salt concentrations. Several Na+/H+ antiporters present in the vacuolar membrane have been implicated as functioning in vacuolar transport, vacuolar acidification, and ion homeostasis (10). Furthermore, several studies have concluded that H+ antiporters represent the principal mechanism of transporting both inorganic and organic cations across the vacuolar membrane (19). In addition, a family of seven proteins has been identified as mediating bidirectional amino acid transport at the vacuolar membrane (25). Btn1p has been shown to be involved in the transport of arginine into the vacuole (12), and arginine transport across the vacuolar membrane requires the generation of a proton motive force and an intact vacuolar ATPase (12, 17, 25). Disruption of Btn1p function disrupts the transport of arginine into the vacuole, resulting in a 10-fold decrease in the levels of sequestered arginine and lysine in the vacuole (12). Therefore, one could predict that an environmental insult, such as high salt concentrations, would alter the activity of vacuolar Na+/H+ antiporters and precipitate further disruptions in transport processes at the vacuolar membrane. Thus, disruption of both Btn1p at the vacuole and Ist2p at the plasma membrane (btn1Δ ist2Δ strains) results in cells being unable to maintain cellular cationic balance upon exposure to high salt concentrations. Similarly, the lack of Btn2p and its multiple roles in cells precipitate a similar situation in btn1Δ btn2Δ and btn1Δ ist2Δ strains.
It is fascinating that ist2Δ suppresses the vacuolar arginine transport defect exhibited by btn1Δ strains. Although further studies are required, it is apparent that disruptions that result in altered cation levels, whether they are organic, such as arginine, or inorganic, such as salt, have revealed a link that indicates that yeast cells may work to maintain an overall ionic balance of all or some of these ions. Although there appears to be no apparent correlation between vacuolar pH and vacuolar H+-ATPase activity, future experiments will focus on determining whether cation levels influence both vacuolar pH and vacuolar H+-ATPase activity. We note that studies of other organisms have revealed that plants have been shown to mediate salt tolerance through ion uptake into the tonoplast (28). A complete understanding of this phenomenon, in particular, in btn1Δ strains, will require a complete understanding of which proteins facilitate the transport of all molecules into and out of cells and also into and out of each subcellular compartment.
It was previously demonstrated that the protein associated with Batten disease, Cln3, and Btn1p have conserved functions. Btn1p is 39% identical and 59% similar to human Cln3 protein, mutations in which result in the lysosomal storage disorder Batten disease (11, 22). Batten disease is characterized by accumulation of lipopigments in the lysosome (6, 8, 14, 20, 21). In summary, btn1Δ cells are known to have an altered regulation of vacuolar pH and a decrease in the ability to transport basic amino acids into the vacuole, features which presumably account for the decreased levels of basic amino acids in the vacuole. Our new findings suggest that these alterations in vacuolar content might result in these cells having an altered ability to maintain cellular and vacuolar ionic homeostasis. A limited ability to utilize the vacuolar compartment as a means to maintain ionic homeostasis might precipitate further vacuolar-lysosomal dysfunction. It is obviously difficult to compare ion homeostasis mechanisms between single-celled organisms, such as yeasts, and humans. However, a change in lysosomal function mediated by altered ionic content could conceivably result in the accumulation or aggregation of proteins that are usually targeted to the lysosome for degradation and might contribute to the characteristic accumulation of storage materials in the lysosome in Batten disease.
Acknowledgments
We thank Tim Curran for technical support and Denia Ramirez-Montealegre and Jared Benedict for help in preparation of the manuscript.
This work was supported by NIH grant R01 NS36610. S.J.L. was supported by grant NSF9986712 from the REU Program in Cellular and Molecular Biology at the University of Rochester.
REFERENCES
- 1.Chattopadhyay, S., N. E. Muzaffar, F. Sherman, and D. A. Pearce. 2000. The yeast model for Batten disease: mutations in BTN1, BTN2, and HSP30 alter pH homeostasis. J. Bacteriol. 182:6418-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chattopadhyay, S., and D. A. Pearce. 2002. Interaction with Btn2p is required for localization of Rsg1p: Btn2p-mediated changes in arginine uptake in Saccharomyces cerevisiae. Eukaryot. Cell 1:606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chattopadhyay, S., P. M. Roberts, and D. A. Pearce. 2003. The yeast model for Batten disease: a role for Btn2p in the trafficking of the Golgi-associated vesicular targeting protein, Yif1p. Biochem. Biophys. Res. Commun. 302:534-538. [DOI] [PubMed] [Google Scholar]
- 4.Croopnick, J. B., H. C. Choi, and D. M. Mueller. 1998. The subcellular location of the yeast Saccharomyces cerevisiae homologue of the protein defective in the juvenile form of Batten disease. Biochem. Biophys. Res. Commun. 250:335-341. [DOI] [PubMed] [Google Scholar]
- 5.Entian, K. D., et al. 1999. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol. Gen. Genet. 262:683-702. [DOI] [PubMed] [Google Scholar]
- 6.Goebel, H. H. 1995. The neuronal ceroid lipofuscinoses. J. Child Neurol. 10:424-437. [DOI] [PubMed] [Google Scholar]
- 7.Guldener, U., S. Heck, T. Fielder, J. Beinhauer, and J. H. Hegemann.1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, N. A., B. D. Lake, N. N. Dewji, and N. D. Patrick. 1991. Lysosomal storage of subunit c of mitochondrial ATP synthase in Batten's disease. Biochem. J. 275:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskell, R. E., C. J. Carr, D. A. Pearce, M. J. Bennett, and B. L. Davidson. 2000. Batten disease: evaluation of CLN3 mutations on protein localization and function. Hum. Mol. Genet. 9:735-744. [DOI] [PubMed] [Google Scholar]
- 10.Hirata, T., Y. Wada, and M. Futai. 2002. Sodium and sulfate ion transport in yeast vacuoles. J. Biochem. 131:261-265. [DOI] [PubMed] [Google Scholar]
- 11.International Batten Disease Consortium. 1995. Isolation of a novel gene underlying Batten disease. Cell 82:949-957. [DOI] [PubMed] [Google Scholar]
- 12.Kim, Y., D. Ramirez-Montealegre, and D. A. Pearce. 2003. A role in vacuolar arginine transport for yeast Btn1p and human CLN3, the protein defective in Batten disease. Proc. Natl. Acad. Sci. USA 100:15458-15462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamoto, K., K. Yoshizawa, Y. Ohsumi, and Y. Anraku. 1988. Mutants of Saccharomyces cerevisiae with defective vacuolar function. J. Bacteriol. 170:2687-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kominami, E., J. Ezaki, D. Muno, K. Ishido, T. Ueno, and L. S. Wolfe. 1992. Specific storage of subunit c of mitochondrial ATP synthase in lysosomes of neuronal ceroid lipofuscinosis (Batten's disease) J. Biochem. 111:278-282. [DOI] [PubMed] [Google Scholar]
- 15.Mannhaupt, G., R. Stucka, S. Ehnle, I. Vetter, and H. Feldmann. 1994. Analysis of a 70Kb region on the right arm of yeast chromosome II. Yeast 10:1363-1381. [DOI] [PubMed] [Google Scholar]
- 16.Matern, H., X. Yang, E. Andrulis, R. Sternglanz, H.-H. Trepte, and D. Gallwitz. 2000. A novel Golgi membrane protein is part of a GTPase-binding protein complex involved in vesicle targeting. EMBO J. 19:4485-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohsumi, Y., and Y. Anraku. 1981. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 256:2079-2082. [PubMed] [Google Scholar]
- 18.Ohsumi, Y., K. Kitamoto, and Y. Anraku. 1988. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J. Bacteriol. 170:2676-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okorokov, L. A., T. V. Kulakovskaya, L. P. Lichto, and L. V. Polorotova. 1985. H+/ion antiport as the principal mechanism of transport systems in the vacuolar membrane of the yeast Saccharomyces carlsbergensis. FEBS Lett. 192:303-306. [DOI] [PubMed] [Google Scholar]
- 20.Palmer, D. N., I. M. Fearnley, J. E. Walker, N. A. Hall, B. D. Lake, L. S. Wolfe, M. Haltia, R. D. Martinur, and R. D. Jolly. 1992. Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease). Am. J. Med. Genet. 42:561-567. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, D. N., S. L. Bayliss, and V. J. Westlake. 1995. Batten disease and the ATP synthase subunit c turnover pathway: raising antibodies to subunit c. Am. J. Med. Genet. 57:260-265. [DOI] [PubMed] [Google Scholar]
- 22.Pearce, D. A., and F. Sherman. 1997. BTN1, a yeast gene corresponding to the human gene responsible for Batten's disease, is not essential for viability, mitochondrial function, or degradation of mitochondrial ATP synthase. Yeast 13:691-697. [DOI] [PubMed] [Google Scholar]
- 23.Pearce, D. A., and F. Sherman. 1998. A yeast model for the study of Batten disease. Proc. Natl. Acad. Sci. USA 95:6915-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce, D. A., T. Ferea, S. A. Nosel, B. Das, and F. Sherman. 1999. Action of Btn1p, the yeast orthologue of the gene mutated in Batten disease. Nat. Genet. 22:55-58. [DOI] [PubMed] [Google Scholar]
- 25.Russnak, R., D. Konczal, and S. L. McIntire. 2001. A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J. Biol. Chem. 276:23849-23857. [DOI] [PubMed] [Google Scholar]
- 26.Takizawa, P. A., J. L. DeRisi, J. E. Wilhelm, and R. D. Vale. 2000. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290:341-344. [DOI] [PubMed] [Google Scholar]
- 27.Urano, J., A. P. Tabancay, W. Yang, and F. Tamanoi. 2000. The Saccharomyces cerevisiae Rheb G-protein is involved in regulating canavanine resistance and arginine uptake. J. Biol Chem. 275:11198-11206. [DOI] [PubMed] [Google Scholar]
- 28.Wang, B., U. Luttge, and R. Ratajczak. 2001. Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J. Exp. Bot. 52:2355-2365. [DOI] [PubMed] [Google Scholar]