Abstract
Aims
To synthesise evidence from UK-based randomised trials of psycho-educational interventions in children and young people (CYP) with Type 1 Diabetes (T1D) to inform the evidence-base for adoption of such interventions into the NHS.
Methods
We searched Medline, Embase, Cochrane, PsycINFO, CINAHL, and Web of Science up to March 2016. Two reviewers independently selected UK-based randomised trials comparing psycho-educational interventions for improving management of T1D for CYP with a control group of usual care or attention control. The main outcome was glycaemic control measured by percentage of glycated haemoglobin (HbA1c); secondary outcomes included psychosocial functioning, diabetes knowledge, adverse and other clinical outcomes. A narrative synthesis and meta-analysis were conducted. Pooled effect sizes of standardised mean difference (SMD) were calculated.
Results
Ten eligible trials of three educational and seven psycho-educational interventions were identified. Most interventions were delivered by non-psychologists and targeted adolescents with more than one year duration of diabetes. Meta-analysis of nine of these trials (N = 1,838 participants) showed a non-significant reduction in HbA1c attributable to the intervention (pooled SMD = -0.06, 95% CI: -0.21 to 0.09). Psycho-educational interventions aiming to increase children’s self-efficacy had a moderate, beneficial effect (SMD = 0.50, 95% CI: 0.13 to 0.87). No benefits on diabetes knowledge and other indicators of psychosocial functioning were identified.
Conclusions
There is insufficient evidence to recommend the use of particular psycho-educational programme for CYP with T1D in the UK. Further trials with sufficient power and reporting standards are needed. Future trials could consider active involvement of psychological specialists in the delivery of psychologically informed interventions and implementation of psycho-educational interventions earlier in the course of the disease.
Systematic review registration
PROSPERO CRD42015010701
Introduction
Type 1 Diabetes (T1D) is one of the most common chronic diseases in childhood and adolescence, with an incidence of 28.2 new cases per 100,000 children under the age of 14 in the United Kingdom (UK) every year [1]. The UK has the fourth largest paediatric diabetes population in Europe and the fifth largest paediatric diabetes population in the world [2, 3] with the most recent estimates indicating at least 29,000 children under 19 years have T1D in the country [4, 5].
In the UK, children and young people (CYP) with T1D are usually managed by multi-disciplinary teams in hospital-based diabetes clinics. T1D management primarily aims to optimise glucose control, whilst also maintaining quality of life. The gold standard for assessing average glucose control over the preceding 2–3 months is glycated Haemoglobin A1c (HbA1c) and regular testing is recommended to guide management advice. The National Institute for Health and Care Excellence (NICE) has recently recommended a target for HbA1c of 6.5% (48 mmol/mol) or lower [6]. Although it is widely accepted that intensive management aiming for lower glycaemic targets confers a significant reduction in risk of diabetes-related complications [7], only 6.4% of children cared for in clinical services in England and Wales meet this target [5].
Although the mainstay of T1D management is through insulin and dietary modifications, the need for structured educational programs at diagnosis has been highlighted as a priority by government bodies and diabetes organisations in the UK [6, 8, 9]. Such programs constitute an integral part of diabetes management since they are necessary to integrate the complex demands of diabetes self-management into daily life. However, it is well accepted that education is a necessary, but not sufficient component of diabetes care. A distinction has been made between traditional education programs that aim to teach diabetes-related knowledge and skills, and those that incorporate psychological elements and provide support in areas such as problem-solving, goal-setting, stress management, coping, motivation, and counselling. Although a successful educational programs have been introduced across the UK for adults with T1D [10, 11], there is a lack of evidence-base for equivalent programs for children and adolescents with no agreed standardised package available in the UK [12].
Over the last few years several systematic reviews have examined the effect of these programs on metabolic and psychological outcomes in CYP with T1D. In a review commissioned by the NHS Health Technology Assessment programme in 2001, Hampson et al. made the first comprehensive attempt to systematically review literature on the effectiveness of psycho-educational interventions among adolescents [13]. They summarised intervention effects using the standardised mean difference (SMD) (i.e. difference in mean change-from-baseline scores between groups divided by the pooled standard deviation) which allows for a direct comparison across trials that used different scales to assess outcomes. They concluded that psycho-educational interventions had a small, non-significant effect on glycaemic control corresponding to a decrease of 0.6% in HbA1c (SMD = 0.3, 95% CI -0.04 to 0.7) but appeared to confer more substantial improvements in psychological outcomes (SMD = 0.4, 95% CI 0.2 to 0.6) [13]. The review also highlighted that evidence was predominantly derived from the USA with a notable shortage of UK-based randomised controlled trials (RCTs). An updated review by Murphy et al. in 2006 showed little progress in the development of new interventions in the UK [14]. Two subsequent meta-analyses provided evidence for a glycaemic benefit of such interventions. The first showed that children and adolescents who received a psychological intervention had reduced HbA1c levels (SMD = -0.35, 95% CI -0.66 to -0.04) and psychological distress (SMD = -0.5, 95% CI: -0.8 to -0.1) compared to controls [15]. The second meta-analysis focused on family-based psycho-educational interventions and found a beneficial effect on both glycaemic control (mean difference in % HbA1c = -0.6, 95% CI -1.2 to -0.1) and diabetes knowledge (SMD = 0.94, 95% CI 0.67 to 1.82) [16].
Evidence for the effectiveness of psycho-educational interventions in children with T1D is predominantly derived from non-UK trials. Only two, small scale RCTs conducted in the UK were included in previous reviews [17, 18], the most recent of which was published in 2002 [17]. Yet, the evidence for the effectiveness of such interventions might be context-dependent since, for example, the quality of standard care against which interventions are compared shows considerable variation between countries [19]. This suggests that the extent to which conclusions from previous reviews can be generalised to the UK health care system is unclear. Moreover, the last decade has also seen a number of large UK-based RCTs of psycho-educational interventions which have not been systematically reviewed. A need, therefore, exists for a comprehensive assessment of these interventions to determine whether there is sufficient evidence to support adoption of psycho-educational interventions into the NHS.
This systematic review aims to critically appraise and synthesise evidence from UK-based RCTs on the effectiveness of psychoeducational interventions in improving glycaemic control, psychosocial functioning, diabetes knowledge and other outcomes in CYP with T1D. It is expected that findings of this review will be used to inform the evidence-base for adoption of such interventions into the NHS.
Methods
The protocol for this review has been published in the International Prospective Register for Systematic Reviews (PROSPERO) (Registration number: CRD42015010701 –see S1 File). The conduct and report of the current systematic review is in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) guidelines (see S1 Checklist) [20].
Search strategy
Six databases (Medline, Embase, Cochrane, PsycINFO, CINAHL, and Web of Science) were systematically searched for relevant citations published up until March 2016. The search strategy was developed with the assistance of a professional librarian. A combination of free-text words and medical subject heading (MeSH) terms were used to generate five subsets of citations relating to population, intervention, outcomes of interest, randomised controlled trials and studies conducted in the UK (see S2 File). Results were limited to CYP up to 24 years. The search was not limited by language or year of publication. A number of “snowballing” techniques were also used to minimise the potential of publication bias and to increase the sensitivity of our search. These included hand-searching reference lists of all selected articles, and contacting experts and corresponding authors of selected articles for any known published or unpublished relevant trials.
Eligibility criteria
We included trials conducted in the UK that examined the effectiveness of educational or psycho-educational intervention in CYP up to 24 years with T1D. A broad definition of psycho-educational interventions was used; we included interventions targeting CYP, their families and/or health care professionals that aimed to improve management of diabetes in children. Interventions including any type of teaching diabetes-related knowledge or skills and/or providing any form of psychosocial training or support were eligible. Studies were not excluded based on setting, delivery or duration of the intervention. Interventions had to be randomised controlled trials that involved a non-intervention arm of children with T1D receiving standard care. Trials in which the control group was matched for the extra contact time (attention control) were also included. Studies combining type 1 and type 2 diabetes or children and young people (≤24 years old) with adults (>24 years) were excluded unless results were stratified by type of diabetes or age group respectively. Finally, we excluded letters, commentaries, editorials, reviews, conference proceedings, intervention development protocols, pilot trials and qualitative studies.
Types of outcome measures
The primary outcome of interest was glycaemic control, as measured by levels of HbA1c. Secondary outcomes included indicators of psychosocial functioning, diabetes knowledge, insulin regimen, adverse events (episodes of hypoglycaemia and diabetes ketoacidosis-DKA), and service utilisation.
Study selection and data extraction
Retrieved citations were entered into a reference management library (EndNote), and duplicates were removed. Initially, titles and abstracts of unique citations were screened and full texts of potentially eligible articles were then retrieved and screened. Titles, abstracts, and full texts were independently reviewed by 2 reviewers (DC and KRH). In parallel, the same reviewers then independently extracted data from all eligible trials using a pre-piloted data extraction form (see S3 File) as per guidelines by the Centre for Review and Dissemination (CRD) for systematic reviews in healthcare [21]. At all stages, any discrepancies were resolved by joint discussion. We extracted data on study design and methodology, intervention characteristics and type of care received by controls. We also extracted data on sample size, baseline characteristics, recruitment and study completion rates, reasons for attrition, power of the study, baseline and follow-up outcome data for each trial arm, and information for assessment of the risk of bias. Corresponding authors of included studies were contacted by email for clarification on trial methods or data whenever there was insufficient data reported (three authors were contacted, all provided further information).
Interventions were categorised according to their primary methodology as educational (i.e. those targeting diabetes-related knowledge and skills), psychological (i.e. those providing any form of psychosocial support) or psycho-educational (those combining educational with psychological elements). Psycho-educational interventions were further classified into the following categories: supportive or counselling therapy (including motivational interviewing, non-directive counselling, and solution-focused therapy); cognitive behavioural therapy (including techniques such as goal setting, activity scheduling, problem solving, cognitive restructuring, and stress management); family systems therapy; psychotherapy (including psychodynamic or interpersonal approaches) and other interventions (including eclectic approaches).
Quality assessment
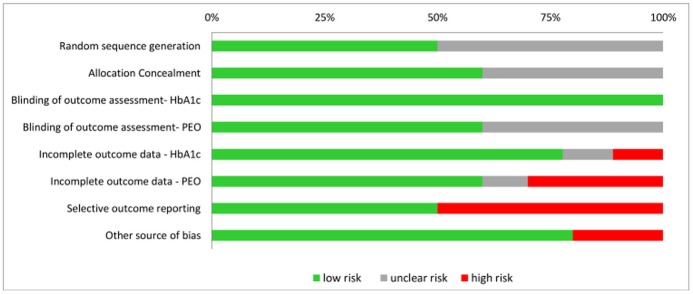
Quality assessment was conducted independently by two reviewers (DH and KRH) and disagreements were resolved by consensus. Quality of individual trials was assessed using six domains of the Cochrane Collaboration’s tool for assessing risk of bias [22], including sequence generation; allocation concealment; blinding of outcome assessors; completeness of outcome data; selective reporting of outcomes; and other sources of bias. Since blinding of participants and personnel to knowledge of the intervention was not possible, this domain was excluded from the assessment. Assessment of the two domains relating to blinding of outcome assessors and data completeness was made separately for glycaemic and psycho-educational outcomes. For each domain, studies were classified as being at low, high or unclear risk of bias.
Data synthesis and calculation of effect sizes
Data were analysed through narrative synthesis and meta-analysis. We used the SMD to summarise intervention effects on continuous outcomes, calculated by dividing the between group difference in mean change-from-baseline scores (or follow-up scores adjusted for baseline values) by the pooled standard deviation of the change scores [23]. We calculated the intervention effect using the follow-up interval set a priori for the definition of the primary outcome. Four trials provided multiple follow-up measurements without stating any primary time point, in which case we used the longest follow-up measurement available. To examine whether results were sensitive to selection of time point, we repeated the meta-analyses, where possible, by using the shortest follow-up measurement that was available immediately after the end of the intervention; no differences in the summary estimates were observed (see S4 File). If standard deviations of change scores were not available from the published report, we obtained them by correspondence with the authors, or by hand calculating on the basis of available published data. For seven trials none of the above was feasible and standard deviations of change scores were imputed assuming a conservative correlation coefficient of 0.5 [24]. We varied the assumed correlation of r = 0.5 between baseline and follow-up measurements from r = 0.3 to r = 0.7 to see if this has any effect on the summary estimates; results were robust to these variations.
For trials with multiple intervention arms, the intervention arm which was directly comparable to the control arm (i.e. without any co-intervention or change in routine care) was chosen. In cross-over trial designs we only used data from the first period. For cluster-randomised trials we used effect sizes adjusted for clustering effect and baseline values, or if not available, we adjusted sample sizes for the “design effect” [23].
To avoid unit of analysis errors, each trial contributed only one estimate per psychosocial construct. For example, where studies reported both patient and parent/carer reports of the same measure the former were used in the meta-analysis. Moreover, if studies reported multiple comparisons for different participants (such as for younger and older children), these measures were combined within each study before being entered in the meta-analysis. Finally, for comparisons that were not independent of one another (such as when studies reported several dimensions of quality of life for the same participants), we calculated a synthetic effect size for each study. This was defined as the weighted mean of the multiple effects with a variance that takes account of the correlation between the outcomes [25], again assuming it to be r = 0.5 if not stated.
Calculating overall summary effects
We combined effect sizes from individual studies using a random effects model to account for differences in the interventions and settings across studies. Results were provided as pooled SMD with 95% confidence intervals. A standardised mean difference of ~0.2, ~0.5, and ~0.8 was considered as small, medium and large respectively [26]. To facilitate clinical interpretation of intervention effects on glycated haemoglobin, we re-expressed the pooled SMD into absolute units by multiplying the estimate by the pooled standard deviation of all included studies. We generated forest plots, sorted by level of precision, to visually assess intervention effects across studies. All analyses were performed using STATA 12 (StataCorp, College Station, Texas).
Assessment of heterogeneity and publication bias
Heterogeneity between studies was assessed by the I2 statistic, which quantifies the percentage of total variation that can be attributed to heterogeneity [27, 28]. Values of I2 ≤50%, 50–75%, and ≥75% were considered as indicative of low, moderate and high heterogeneity respectively [27]. Individual studies were removed one at a time from the meta-analysis to explore whether heterogeneity could be reduced. We also investigated potential sources of heterogeneity by conducting subgroup analyses, where possible, against potentially modifying factors (type of intervention, study quality and age group). Funnel plot was constructed to explore the possibility of publication bias for the primary outcome.
Results
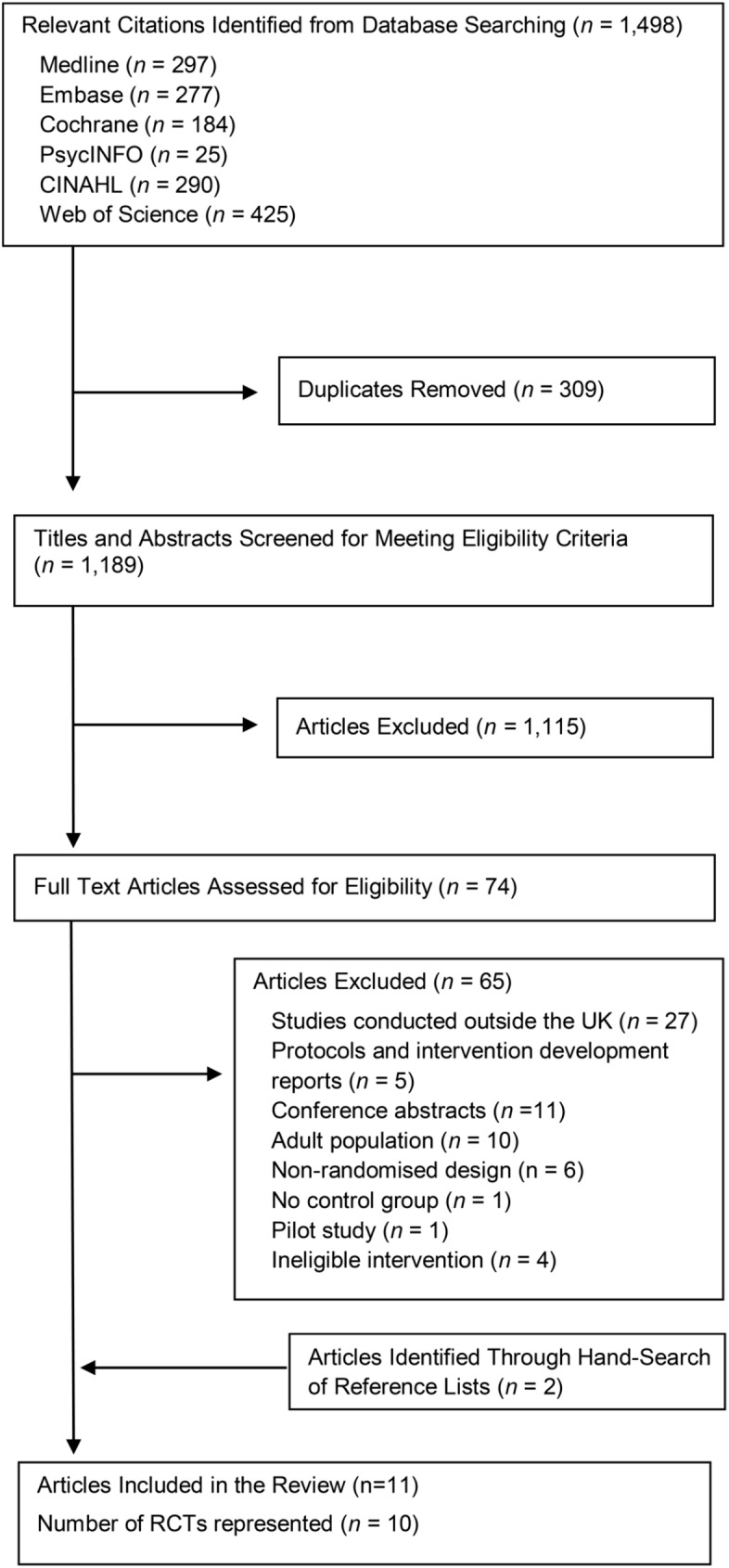
The search strategy yielded 1,189 potentially relevant papers, of which 74 were read in full. Two additional articles were identified from reference lists. As per the eligibility criteria, we excluded a small pilot trial which examined the feasibility of a UK psychoeducational intervention [29]. Results of the same intervention were reported in a subsequent main trial which was included in the current review [30]. In total, eleven studies [17, 18, 30–37] representing ten randomised controlled trials were found to meet the eligibility criteria and were included in the current review (see Fig 1).
Fig 1. Flow diagram of study selection.
Characteristics of included RCTs are shown in Table 1 and S5 File. In all RCTs participants were analysed by intention to treat. Six trials had a parallel group design [17, 30, 31, 33–35], one trial had a cross-over design [18] and three were cluster randomised [32, 36, 37]. Sample sizes ranged from 48 to 693 with a median of 113. Participation rates were generally low, ranging from 31% to 70.2% (median 50%). Six studies recruited only adolescents [17, 30, 31, 33, 34, 36] one of which also included young people up to 24 years [17]. All but three trials [17, 18, 34] targeted children who had been diagnosed with T1D for more than one year. Median duration of diabetes was 5.6 years and ranged from 2.8 to 9.2 years.
Table 1. Characteristics of included trials.
| First author (publication year) | Country (study name) | No of participants randomised (eligibility criteria) |
Mean (SD) % HbA1c at baseline | Mean (SD or range) duration of diabetes (years) | Mean (SD or range) age (years) | Intervention, setting, mode of delivery | Theoretical Model | Control group | Interventionist | Duration of intervention in months (except as noted) | Assessment points a (months) | Time in min spent on each session (No of sessions) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bloomfield (1990) | Scotland | 48 (children <13 years with T1D > 3 months | 9.3 (1.5) | 2.8 (2.1) | 9.0 (3.0) | Semi-structured educational program, Community, Group of families | - | Usual care | D | 12 | 12 | 210 (10) |
| Howells (2002) | Scotland | 79 (children 12–24 years) | 8.8 (1.7) | 7.0 (4.5) | 16.8 (3.4) | Negotiated telephone support, Home, Child | SLT | Usual care | D | 12 | 12 | 9 (16) |
| Franklin (2006) | Scotland (Sweet Talk) | 64 (children 8–18 years with T1D >1 year) | 10.2 (1.7) | 4.1 (1.7–8.6) | 13.5 (10.5–15.6) | Automated text message support plus goal-setting education, Home, Child | SCT | Usual care | MDT | 12 | 12 | NA |
| Channon (2007) | Wales | 80 (adolescents 14–17 years with T1D >1 year) | 9.2 (1.9) | 9.2 (1.8) | 15.3 (1.1) | Motivational interviewing, Home & community, Child | MSA | Usual care plus additional support visits | PSY + N | 12 | 6, 12, 24 | 20–60 (4) |
| Murphy (2012) | UK (FACTS) |
305 (adolescents with T1D >1 year) | 9.3 (1.9) | 5.6 (3.4) | 13.2 (2.0) | Family-cantered structured program, Clinic, Group of families | SLT | Usual care | MDT | 6 | 9, 12, 18 | 90 (6) |
| Robling (2012) | UK (DEPICTED) | 693 (children 4–15 years with T1D >1 year) | 9.3 (1.8) | 5.1 (2.7) | 10.6 (2.8) | Training healthcare practitioners in consultation skills using eclectic approach, Clinic, Child with carer | CMCS | Usual care | MDT | 12 | 12 | 100 (3.5) |
| Coates (2013) | N. Ireland (CHOICE) | 135 (adolescents 13–19 years with T1D >1 year) | 8.9 (1.5) | 6.6 (3.8) | 15.4 (1.8) | Structured educational program, Clinic, Group of families | - | Usual care | N + D | 5 | 1, 3, 6, 12, 24 | 180 (4) |
| Doherty (2013) | UK (Triple P) |
90 (Parents of adolescents aged 11–17 years) | 8.5 (1.3) | 5.1 (3.4) | 13.5 (1.0) | Self-directed, web-based behavioural intervention, Home, Parents | SLT | Usual care | NA | 2.3 | 2.3 | 60 (10) |
| Christie (2014) | England (CASCADE) | 365 (Children 8–16 years with T1D >1 year & HbA1c ≥ 8.5%) | 10.0 (1.5) | 5.9 (3.3) | 13.2 (2.1) | Motivational interviewing, solution-focused brief therapy, Clinic, Group of families | MSA | Usual care | N + O | 4 | 12,24 | 120 (4) |
| Price (2016) | UK (KICk-OFF) |
480 (adolescents 11–16 years with T1D > 1 year) | 9.2 (1.7) | 5.6 (2.0) | 13.8 (1.5) | Intensive, structured education course, Community, Group of children | - | Usual care | N + D + O | 5 days | 6, 12, 24 | 420 (5) |
Notes: NA: not applicable, D: dietitian, PSY: psychologist, N: nurse, MDT: multidisciplinary team member, O: other, FACTS: Families, Adolescents, and Children Teamwork Study, DEPICTED: Development and Evaluation of a Psychosocial Intervention in Children and Teenagers Experiencing Diabetes, CHOICE: Carbohydrate, Insulin, Collaborative Education, CASCADE: Child and Adolescent Structured Competencies Approach to Diabetes Education, KICk-OFF: Kids In Control OF Food, SLT: Social Learning Theory, SCT: Social Cognitive Theory, MSA: Menu of Strategies Approach, CMCS: Consultation Model of Communication Styles
a from start of intervention
Of the ten RCTs, seven [17, 30–37] used psycho-educational and three [18, 33, 36] purely educational interventions. Six trials [30, 32–35, 38] provided reference to the full trial protocol. However, in only four trials [30, 32, 35] was the intervention described in sufficient detail to be replicated in practice. In all RCTs, control groups received standard care which in most cases included three to five clinic visits per year; however, in one trial [31] the control group was matched for contact time by receiving additional support visits. Only one trial [32] provided a detailed description of standard care.
All psycho-educational interventions reported using an underlying theoretical model. Of the seven psycho-educational interventions, three used supportive or counselling therapy [31, 32, 35], two employed cognitive behaviour therapy strategies [17, 34], one used family therapy [30], and one [37] used an eclectic approach. Interventions targeted individual children [17, 31, 35, 37], groups of children [36], family groups [18, 30, 32, 33], and parents [34]. Four interventions [30, 32, 33, 38] were delivered in clinics and six [17, 18, 31, 34–36] in home or other community settings. Intensity of interventions varied considerably with total time spent on intervention ranging from 2.4 to 35 hours (median of 8.5 hours). Most interventions were delivered by dietitians and nurses and in only one trial [31] the interventionist had a background in psychology. Evidence for training of the interventionist was provided in half of the trials [17, 30, 32, 36, 37].
Five interventions [17, 18, 31, 35, 37] had a duration of one year with the remaining interventions [30, 32–34, 36] lasting for 6 months or less. Half of the trials had a follow-up assessment after the end of the intervention. Retention rates ranged from 43% to 100% and half of the trials [18, 31, 33–35] were deemed underpowered to detect an effect in their primary outcome. Six trials reported monitoring adherence to trial protocol [17, 31, 32, 34, 36, 37]. Eight trials [17, 18, 30, 32–34, 36, 37] provided information on intervention attendance and in three of them [30, 32, 34] attendance rates were considered as potentially insufficient to demonstrate an intervention effect (see S5 File).
Risk of bias
Risk of bias assessment is presented in Fig 2. Risk of selection bias due to inadequate sequence generation was unclear in half of the trials [18, 31–33, 37] since method of randomisation was not reported by authors. Risk of bias due to poor allocation concealment could not be assessed in four trials [18, 30, 32, 35]. Although blinding of participants and interventionists is not feasible in the context of psycho-educational interventions, risk of detection bias from outcome assessment was considered small for HbA1c (objectively measured) and for most of the psycho-educational outcomes (use of standardised scales). There was high risk of bias due to incomplete psychological data in three trials [30, 31, 34], which reflected the high attrition rate in this type of interventions. Five trials [18, 31, 33, 36, 37] did not report all psychological outcomes and were at high risk of selective outcome reporting. Other sources of bias included baseline imbalances not accounted for in the analyses [34] and inappropriate study design (cross-over) [18]. When all bias domains were considered together, one trial [18] scored low risk in only one domain, three trials [30, 31, 33] scored low risk in two or three bias categories, and the remaining studies [17, 32, 34–37] scored low risk in four or more domains (see S6 File).
Fig 2. Outcome of risk of bias assessment by type of bias (Note: PEO = psycho-educational outcomes).
Effectiveness of interventions
Glycated haemoglobin (HbA1c)
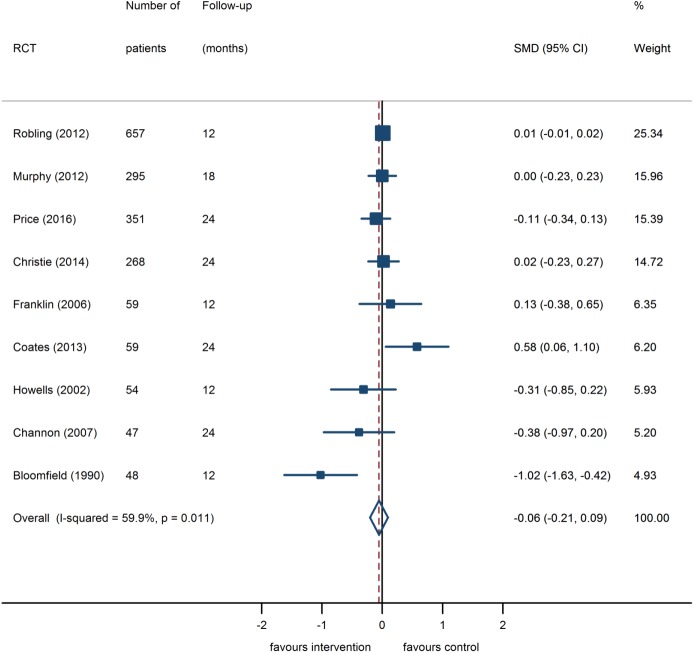
A total of nine RCTs [17, 18, 30–33, 35–37] including 1,838 participants assessed the effectiveness of educational and psycho-educational interventions in reducing HbA1c levels and were included in the meta-analysis. Effect sizes in four out of the nine trials showed a reduction in HbA1c levels attributable to the intervention (see Fig 3). The pooled analysis did not show a statistically significant glycaemic benefit (pooled SMD = -0.06, 95% CI: -0.21 to 0.09). The intervention effect was equivalent to a reduction in HbA1c of 0.1% (95% CI: -0.4% to 0.2%). There was moderate heterogeneity between the studies (I2 = 59.9%), which was fully explained by an early trial of an educational intervention [18] with a low methodological quality rating. Exclusion of this trial from the meta-analysis did not change the overall conclusion (SMD = -0.02, 95%CI: -0.13 to 0.09, I2 = 0%). The intervention effect on HbA1c remained non-significant when subgroup analyses were performed for purely educational interventions (SMD = -0.17, 95% CI -0.88 to 0.55, three studies pooled), psycho-educational interventions (SMD = 0.01, 95% CI: -0.01 to 0.02, six studies pooled), or interventions targeting adolescents (SMD = -0.05, 95% CI -0.20 to 0.10, four studies pooled).
Fig 3. Random effects meta-analysis of change scores in HbA1c (%) in psycho-educational intervention group compared with control group.
Intervention effects calculated as Standardised Mean Difference (SMD) with 95% confidence interval. A negative effect indicates improved glycaemic control attributable to intervention.
Psychosocial functioning
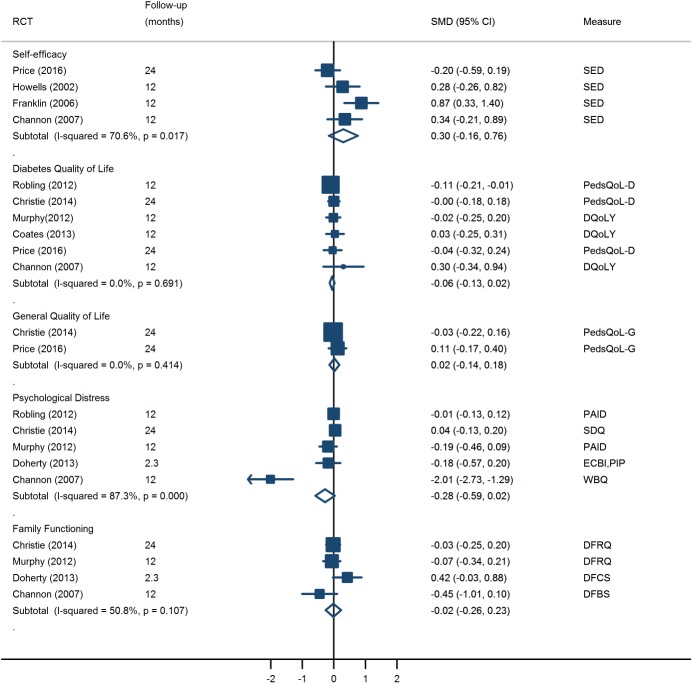
Interventions addressed various measures of psychosocial functioning (see S7 File). Four trials of one educational [36] and three psycho-educational interventions [17, 31, 35] measured the effect of interventions on increasing self-efficacy. Overall, interventions produced a small, non-significant improvement in self-efficacy (SMD = 0.30, 95% CI: -0.16 to 0.76, I2 = 70.6%). Heterogeneity was reduced when we removed the one educational intervention [36]; when it was omitted, effect of psycho-educational interventions on self-efficacy increased in magnitude and became statistically significant (SMD = 0.50, 95% CI: 0.13 to 0.87, I2 = 27.8%). There was no evidence for a beneficial effect of psycho-educational interventions on other indicators of psychosocial functioning, including diabetes-specific quality of life, general quality of life, psychological distress and family functioning (see Fig 4). Some other psychosocial outcomes were explored in isolation and showed no significant changes between the groups; these included locus of control [31], patient empowerment [33], health care climate [37], and patient enablement [37].
Fig 4. Intervention effects on psychosocial outcomes calculated as Standardised Mean Difference (SMD) of change scores with 95% confidence interval.
A positive effect in quality of life, self-efficacy, and family functioning and a negative effect is psychological distress favour intervention. The diamonds show the pooled SMD based on random effects model. Notes: SED = Self-efficacy for diabetes; PedsQoL-D = Paediatric quality of life inventory: diabetes module; DQoLY = Diabetes Quality of life measure for youths (reverse scaling); PedsQoL-G = Paediatric quality of life inventory: generic scale; PAID = Problem Areas in Diabetes scale; SDQ = Strengths and difficulties Questionnaire- impact score; ECBI = Eyberg child behavior inventory; PIP = Paediatric Inventory for parents; WBQ = Well-being questionnaire (reverse scaling); DFRQ = Diabetes Family Responsibility Questionnaire (dyadic score in Christie (2014) and parental report in Murphy (2012)); DFCS = Diabetes family conflict scale; DFBS = Diabetes Family Behavior scale.
Diabetes knowledge
Five trials (one educational [18] and four psycho-educational [17, 31, 32, 35]) measured diabetes-related knowledge using similar scales [39–41]. Four trials [17, 18, 31, 35] provided sufficient data for the meta-analysis. With a random effects model, psycho-educational interventions were associated with a non-significant reduction in diabetes knowledge, in all cases measured immediately after the end of the intervention (SMD = -0.11, 95% CI: -0.45 to 0.23, I2 = 40.5%). Heterogeneity between studies was fully explained by an early trial of an educational intervention which was the only one to show a beneficial effect [18]. One study could not be pooled in the meta-analysis but reported no difference in post-intervention knowledge scores between the two groups [32].
Adverse and other outcomes
Seven trials [17, 30, 32, 33, 35, 36, 38] reported on the incidence of DKA and hypoglycaemic hospital admissions but none reported any increase related to the intervention. Insulin requirements were assessed in six trials [17, 18, 30, 32, 36, 38] but data were not suitable for a meta-analysis. The majority of trials reported no change in insulin regimen [17, 18, 38] or in the proportion of children who moved to pump therapy during the intervention [36]. Only two trials targeting groups of families reported a significant increase in insulin dose [32] or in frequency of insulin adjustment [30] in the intervention group. One trial assessed whether intervention increased children dietary adherence [33] but found no change. Finally, four trials assessed the impact of interventions on health service utilisation, including clinic visits [32, 35, 37], hospital admissions or contacts [18, 32, 37], and emergency hotline utilisation [35], but none found any significant change.
Publication bias
Visual assessment of the funnel plot for HbA1c showed a slightly asymmetric scatter which was mainly attributable to the presence of one small outlier study with positive effect (see S8 File).
Discussion
We identified ten UK-based RCTs comparing psycho-educational interventions for improving management of T1D for CYP with a control group of usual care or attention control. Pooled data from nine of these trials showed that psycho-educational interventions conferred no glycaemic benefits over that achieved with standard care across the populations studied. The interventions used a wide variety of approaches, from purely educational programs to interventions combining educational with psychological components. Interventions with psychological components aiming to increase children’s self-efficacy to deal with diabetes appeared to show a moderate beneficial effect. However, evidence for an improvement in other important indicators of psychosocial functioning, such as quality of life, psychological distress and family functioning was absent.
In contrast to our findings on the synthesis of UK-based interventions, two recent meta-analyses mostly based on trials from North America [15, 16] reported significant glycaemic benefits of psycho-educational interventions in children and adolescents corresponding to reductions in HbA1c by around half percentage point. They also provided evidence for significant psychological [15] and educational benefits [16]. There are a number of potential explanations for the discrepancies between our findings and that of previous reviews.
Firstly, previous reviews were mostly based on “efficacy” trials conducted in non-clinical settings by specialist interventionists with a solid background in psychology or psychiatry. In contrast, most of the interventions conducted in the UK were more pragmatic trials and delivered by non-specialist practitioners, mostly nurses and dietitians, after receiving relevant training. In fact, we found that only one UK intervention was delivered by a psychologist [31]; this was a person-centred intervention of motivational interviewing and showed the greatest beneficial effect in psychological outcomes, whilst also showing a trend for HbA1c improvement. Two other UK interventions [32, 37] attempted to incorporate components of motivational interviewing into routine clinical practice by training non-psychologists, but showed no improvement on diabetes outcomes.
Some of the most successful psychological interventions in children with T1D have been delivered by persons with a background in psychology [42–46] which seems to suggest that the discipline, training and skills of the person delivering the intervention in a paediatric population could have an impact on outcomes. Evidence from interventions on adults with Type 2 Diabetes indicates that psychological and general health professionals are equally effective in delivering psychological interventions [47], but there is little evidence available for childhood T1D. Given the shortage of psychologists in the UK diabetes services [48], “efficacy” interventions may not be easily applied into routine clinical settings, yet it might be worthwhile to ensure that future interventions are delivered by rigorously trained personnel who have a sound understanding of both diabetes and psychological matters related to child teaching and learning.
Previous reviews also used different criteria for study selection, including trials in which the control group received care other than standard, including for example intensive insulin treatment or less intensive psychological treatment. One of the UK trials included in the current review [35] also included a third arm receiving both the psycho-educational intervention and intensive insulin therapy and found a significant reduction in HbA1c by 1% as compared to standard care alone. Although a different design would be needed to disentangle the effect of the intervention from that of intensive therapy, this finding indicates that psycho-educational interventions could facilitate the uptake of intensive therapy schemes potentially enhancing their glycaemic benefits. Similar conclusions have been supported by USA trials [45, 49] which showed that psychological interventions used as an adjunct to intensive treatment conferred significant, consistent benefits in both glycaemic and psychosocial outcomes as compared to intensive treatment alone.
Although a lack of evidence for any glycaemic or psychosocial benefit of psycho-educational interventions conducted in the UK might simply reflect an absence of any “real” effect, there are other potential explanations for the negative findings. In most trials participation rates were poor which indicates that children entering trials might represent a population of children who already had a certain level of education and motivation in such a way that any additional intervention may not have a noticeable impact on their physical and psychological health (“ceiling effect”). Even the observed improvements in self-efficacy did not translate into glycaemic benefits, in most cases, measured one year after the end of the intervention. A longer duration of the intervention with provision of extended, continuous support even after the end of the program together with a longer follow-up period might be required for the behavioural changes to have an effect on the metabolic sequelae and translate into reductions in levels of HbA1c.
Findings of our review showed that most of the UK interventions are being offered to adolescents with more than one year duration of diabetes. This might be a potential reason for adolescents’ hesitance to participate as they tend to view such interventions as “non-essential”. Those individuals might have already established management strategies and behaviours that are difficult to challenge and change. Although targeting children with a shorter duration of diabetes can be challenging given the complex adaptation processes taking place, evidence from US trials suggests that implementation of psycho-educational programs earlier in the course of the disease can provide a more effective framework for such interventions [50, 51].
Low study enrolment and high withdrawal rates had also resulted in typically small sample sizes with only half of the UK trials having adequate power to detect an intervention effect. Since power calculations were mostly based on HbA1c, low sample size was particularly problematic for assessment of psycho-educational outcomes. Moreover, attendance rates were unsatisfactory and in some trials attendance was not considered sufficient to demonstrate any potential effect. Lack of intervention “reach” is a potentially important factor in the effectiveness of such interventions, and this may highlight the need to develop new and innovative strategies to decrease patient burden and encourage patient commitment in future interventions.
Educational and psychological interventions conducted in the UK also showed considerable heterogeneity in their content, intensity, selection of outcomes and delivery. This review highlights that although all of the interventions were theoretically grounded, they are poorly described, particularly with regard to the components of the intervention and the type of standard care, making it difficult to be replicated in practice. Attrition and reporting bias, especially with reference to psychosocial outcomes, was an issue in some studies and may further complicate interpretation of findings.
This is the first focused review to systematically examine the effectiveness of UK-based psycho-educational interventions on CYP with T1D. We used a rigorous protocol with high sensitivity and specificity to detect included studies. Psychosocial outcomes were grouped into conceptually homogeneous constructs, which allowed the examination of intervention effects across different aspects of psychosocial functioning. However, there are limitations. Firstly, our review was restricted to UK trials thus precluding us from making any direct comparisons between UK and non-UK interventions. Second, the variability in the scales used to measure psychological outcomes and the differences in follow-up between interventions have contributed to the observed heterogeneity across studies and warrant caution when interpreting the findings. Moreover, half of the included trials provided a single follow-up measurement which prevented us from meaningfully stratifying analyses by follow-up interval. We were also unable to assess the effect of interventions on long-term metabolic control since none of the included studies followed participants beyond two years. Third, the small number of studies did not allow us to formally examine potential modifiers, such as age, duration of diabetes and type of intervention. Fourth, the current review was limited to published studies. Although a comprehensive literature search was conducted and a number of “snowballing” techniques were used to identify eligible randomised trials, the potential of publication bias cannot be excluded. Finally, as per the eligibility criteria, we excluded one pilot trial of a UK intervention. Although some readers might consider this as a limitation, results of this small pilot study were in line with that of the subsequent main trial of the same intervention, which was incorporated into the current meta-analysis. Therefore, we believe that exclusion of this pilot study is unlikely to have affected our pooled estimates.
Conclusion
There is currently insufficient evidence to recommend the use of psycho-educational programmes for children and adolescents with T1D in the UK. Successful implementation of similar interventions in the USA and other countries seems to suggest that such interventions are not inherently ineffective, and evaluation of their impact on diabetes outcomes requires focusing attention on the context within which these are applied and on potential target populations. One difference between UK trials and other non-UK successful trials has been the involvement of psychologists in the delivery of psychological interventions, which may be relevant to the deferring success observed. Future randomised controlled trials in the UK could potentially benefit by considering active involvement of psychological specialists in the delivery of psychologically informed interventions and provision of rigorous training of interventionists in psychological and clinical aspects of diabetes. Greater consideration could also be given to the early implementation of psycho-educational programs in newly diagnosed children and also to the provision of innovative strategies aiming to encourage patient engagement.
Supporting information
(DOC)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank members of the Policy Research Unit for the health of children, young people and families: Catherine Law, Amanda Edwards, Ruth Gilbert, Linda Haines, Steve Morris, Helen Roberts, Cathy Street, and Miranda Wolpert. We also like to thank the librarian Miss Heather Chester for her help with the literature search strategy and all corresponding authors of included studies who provided additional data for the meta-analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This is an independent study commissioned and funded by the Children’s Policy Research Unit (CPRU), UCL (funding reference 10090001), which is funded by the Department of Health Policy Research Programme and supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. The views expressed are not necessarily those of the Department of Health.
References
- 1.International Diabetes Federation. Diabetes Atlas 2014. sixth edition (update):[Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf.
- 2.Kanavos P, van den Aardweg S, Schurer W. Diabetes expenditure, burden of disease and management in 5 EU countries. London School of Economics Health; 2012. [Google Scholar]
- 3.Group DP. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabetic medicine: a journal of the British Diabetic Association. 2006;23(8):857–66. Epub 2006/08/17. doi: 10.1111/j.1464-5491.2006.01925.x . [DOI] [PubMed] [Google Scholar]
- 4.Scottish Diabetes Survey 2013: NHS Scotland; 2014. Available from: http://www.diabetesinscotland.org.uk/Publications/SDS2013.pdf.
- 5.National Paediatric Diabetes Audit Report 2014–15: Royal College of Paediatrics and Child Health; 2016. Available from: http://www.rcpch.ac.uk/sites/default/files/page/NPDA%20Report%202014-15%20v5.2%20sent%20to%20HQIP%2025.05.2016.pdf.
- 6.NICE clinical quideline: Diabetes (type 1 and type 2) in children and young people: diagnosis and management 2015. Available from: https://www.nice.org.uk/guidance/ng18/resources/diabetes-type-1-and-type-2-in-children-and-young-people-diagnosis-and-management-1837278149317.
- 7.Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976–86. Epub 2013/11/23. doi: 10.2337/db13-1093 ; PubMed Central PMCID: PMC3837056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health Diabetes Policy Team. Making Every Young Person with Diabetes Matter: Report of the Children and Young People with Diabetes Working Group London2007. Available from: http://webarchive.nationalarchives.gov.uk/20130107105354/http:/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_073675.pdf.
- 9.Department of Health. National Service Framework for Diabetes: Delivery Strategy. London: 2003.
- 10.Group DS. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. Bmj. 2002;325(7367):746 ; PubMed Central PMCID: PMCPMC128375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen C, Woodward S. Effectiveness of dose adjustment for normal eating (DAFNE). Br J Nurs. 2012;21(4):224, 6–28, 30–2. doi: 10.12968/bjon.2012.21.4.224 . [DOI] [PubMed] [Google Scholar]
- 12.Department of Health, Diabetes UK. Structured patient education in diabetes: Report from the Patient Education Working Group. london: 2005.
- 13.Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al. Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review. Health Technol Assess. 2001;5(10):1–79. . [DOI] [PubMed] [Google Scholar]
- 14.Murphy HR, Rayman G, Skinner TC. Psycho-educational interventions for children and young people with Type 1 diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2006;23(9):935–43. doi: 10.1111/j.1464-5491.2006.01816.x . [DOI] [PubMed] [Google Scholar]
- 15.Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. Bmj. 2006;333(7558):65 doi: 10.1136/bmj.38874.652569.55 ; PubMed Central PMCID: PMC1489251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armour TA, Norris SL, Jack L Jr., Zhang X, Fisher L. The effectiveness of family interventions in people with diabetes mellitus: a systematic review. Diabetic medicine: a journal of the British Diabetic Association. 2005;22(10):1295–305. doi: 10.1111/j.1464-5491.2005.01618.x . [DOI] [PubMed] [Google Scholar]
- 17.Howells L, Wilson AC, Skinner TC, Newton R, Morris AD, Greene SA. A randomized control trial of the effect of negotiated telephone support on glycaemic control in young people with Type 1 diabetes. Diabetic medicine. 2002;19(8):643–8. CN-00409285. [DOI] [PubMed] [Google Scholar]
- 18.Bloomfield S, Calder JE, Chisholm V, Kelnar CJ, Steel JM, Farquhar JW, et al. A project in diabetes education for children. Diabetic Medicine. 1990;7(2):137–42. doi: 10.1111/j.1464-5491.1990.tb01348.x . Language: English. Entry Date: 20110610. Revision Date: 20120302. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 19.Ayling K, Brierley S, Johnson B, Heller S, Eiser C. How standard is standard care? Exploring control group outcomes in behaviour change interventions for young people with type 1 diabetes. Psychol Health. 2015;30(1):85–103. doi: 10.1080/08870446.2014.953528 ; PubMed Central PMCID: PMCPMC4270262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700 doi: 10.1136/bmj.b2700 ; PubMed Central PMCID: PMC2714672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centre for Reviews and Dissemination UoY. Systematic Reviews: CRD's guidance for undertaking systematic reviews in health care 2009. Available from: http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf.
- 22.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928 doi: 10.1136/bmj.d5928 ; PubMed Central PMCID: PMC3196245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- 24.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of clinical epidemiology. 1992;45(7):769–73. . [DOI] [PubMed] [Google Scholar]
- 25.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Multiple Outcomes or Time-Points within a Study. Introduction to Meta-Analysis: John Wiley & Sons, Ltd; 2009. p. 225–38. [Google Scholar]
- 26.Cohen J. Statistical power for the behavioural sciences: Hillsdale: Erlbaum; 1988. [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 ; PubMed Central PMCID: PMC192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 29.Murphy HR, Wadham C, Rayman G, Skinner TC. Approaches to integrating paediatric diabetes care and structured education: experiences from the families, adolescents, and children's teamwork study (FACTS). Diabetic Medicine. 2007;24(11):1261–8. Language: English. Entry Date: 20080418. Revision Date: 20111230. Publication Type: journal article. doi: 10.1111/j.1464-5491.2007.02229.x . [DOI] [PubMed] [Google Scholar]
- 30.Murphy HR, Wadham C, Hassler-Hurst J, Rayman G, Skinner TC. Randomized trial of a diabetes self-management education and family teamwork intervention in adolescents with Type 1 diabetes. Diabetic Medicine. 2012;29(8):e249–54. doi: 10.1111/j.1464-5491.2012.03683.x . Language: English. Entry Date: 20120817. Revision Date: 20140613. Publication Type: journal article. [DOI] [PubMed] [Google Scholar]
- 31.Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care. 2007;30(6):1390–5. doi: 10.2337/dc06-2260 . [DOI] [PubMed] [Google Scholar]
- 32.Christie D, Thompson R, Sawtell M, Allen E, Cairns J, Smith F, et al. Structured, intensive education maximising engagement, motivation and long-term change for children and young people with diabetes: A cluster randomised controlled trial with integral process and economic evaluation—The CASCADE study. Health Technology Assessment. 2014;18(20):1–202. doi: 10.3310/hta18200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coates V, Chaney D, Bunting B, Shorter G, Shevlin M, McDougall A, et al. Evaluation of the Effectiveness of a Structured Diabetes Education Programme (CHOICE) on Clinical Outcomes for Adolescents with Type 1 Diabetes: A Randomised Controlled Trial. Journal of Diabetes & Metabolism. 2013;4(6):2155–6156. doi: 10.4172/2155-6156.1000280 [Google Scholar]
- 34.Doherty FM, Calam R, Sanders MR. Positive Parenting Program (Triple P) for Families of Adolescents With Type 1 Diabetes: A Randomized Controlled Trial of Self-Directed Teen Triple P. Journal of Pediatric Psychology. 2013;38(8):846–58. doi: 10.1093/jpepsy/jst046 [DOI] [PubMed] [Google Scholar]
- 35.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabetic Medicine. 2006;23(12):1332–8. Language: English. Entry Date: 20070629. Revision Date: 20111230. Publication Type: journal article. doi: 10.1111/j.1464-5491.2006.01989.x . [DOI] [PubMed] [Google Scholar]
- 36.Price KJ, Knowles JA, Fox M, Wales JKH, Heller S, Eiser C, et al. Effectiveness of the Kids in Control of Food (KICk-OFF) structured education course for 11–16 year olds with Type 1 diabetes. Diabetic Medicine. 2016;33(2):192–203. doi: 10.1111/dme.12881 . [DOI] [PubMed] [Google Scholar]
- 37.Robling M, McNamara R, Bennert K, Butler CC, Channon S, Cohen D, et al. The effect of the Talking Diabetes consulting skills intervention on glycaemic control and quality of life in children with type 1 diabetes: Cluster randomised controlled trial (DEPICTED study). BMJ (Online). 2012;344 (7860) (no pagination)(e2359). https://doi.org/10.1136/bmj.e2359. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robling M, McNamara R, Bennert K, Butler CC, Channon S, Cohen D, et al. The effect of the Talking Diabetes consulting skills intervention on glycaemic control and quality of life in children with type 1 diabetes: cluster randomised controlled trial (DEPICTED study). BMJ (Clinical research ed) [Internet]. 2012; 344:[e2359 p.]. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/886/CN-00859886/frame.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn SM, Bryson JM, Hoskins PL, Alford JB, Handelsman DJ, Turtle JR. Development of the diabetes knowledge (DKN) scales: forms DKNA, DKNB, and DKNC. Diabetes Care. 1984;7(1):36–41. . [DOI] [PubMed] [Google Scholar]
- 40.Grossman HY, Brink S, Hauser ST. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabetes care. 1987;10(3):324–9. . [DOI] [PubMed] [Google Scholar]
- 41.Kaufman FR, Austin J, Lloyd J, Halvorson M, Carpenter S, Pitukcheewanont P. Characteristics of glycemic control in young children with type 1 diabetes. Pediatric diabetes. 2002;3(4):179–83. Epub 2004/03/16. doi: 10.1034/j.1399-5448.2002.30402.x . [DOI] [PubMed] [Google Scholar]
- 42.Anderson BJ, Wolf FM, Burkhart MT, Cornell RG, Bacon GE. Effects of peer-group intervention on metabolic control of adolescents with IDDM. Randomized outpatient study. Diabetes Care. 1989;12(3):179–83. . [DOI] [PubMed] [Google Scholar]
- 43.Mendez FJ, Belendez M. Effects of a behavioral intervention on treatment adherence and stress management in adolescents with IDDM. Diabetes Care. 1997;20(9):1370–5. . [DOI] [PubMed] [Google Scholar]
- 44.Satin W, La Greca AM, Zigo MA, Skyler JS. Diabetes in adolescence: effects of multifamily group intervention and parent simulation of diabetes. J Pediatr Psychol. 1989;14(2):259–75. . [DOI] [PubMed] [Google Scholar]
- 45.Grey M, Boland EA, Davidson M, Yu C, Sullivan-Bolyai S, Tamborlane WV. Short-term effects of coping skills training as adjunct to intensive therapy in adolescents. Diabetes Care. 1998;21(6):902–8. . [DOI] [PubMed] [Google Scholar]
- 46.Wysocki T, Harris MA, Wilkinson K, Sadler M, Mauras N, White NH. Self-management competence as a predictor of outcomes of intensive therapy or usual care in youth with type 1 diabetes. Diabetes Care. 2003;26(7):2043–7. . [DOI] [PubMed] [Google Scholar]
- 47.Alam R, Sturt J, Lall R, Winkley K. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialists and generalist clinicians on glycaemic control and on psychological status. Patient Educ Couns. 2009;75(1):25–36. doi: 10.1016/j.pec.2008.08.026 . [DOI] [PubMed] [Google Scholar]
- 48.Diabetes UK. Minding the gap: The provision of psychological support and care for people with diabetes in the UK: Diabetes UK; 2008. Available from: https://www.diabetes.org.uk/Documents/Reports/Minding_the_Gap_psychological_report.pdf. [Google Scholar]
- 49.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr. 2000;137(1):107–13. doi: 10.1067/mpd.2000.106568 . [DOI] [PubMed] [Google Scholar]
- 50.Delamater AM, Bubb J, Davis SG, Smith JA, Schmidt L, White NH, et al. Randomized prospective study of self-management training with newly diagnosed diabetic children. Diabetes Care. 1990;13(5):492–8. . [DOI] [PubMed] [Google Scholar]
- 51.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr. 2003;142(4):409–16. doi: 10.1067/mpd.2003.138 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.