Abstract
PURPOSE
To determine whether patients who had a positive repeated culture was predictive of worse clinical outcome than those who achieved microbiological cure at 6 days in the Mycotic Ulcer Treatment Trial I (MUTT-I).
DESIGN
Secondary analysis from a multicenter, double-masked, randomized clinical trial.
METHODS
Setting
Multiple hospital sites of the Aravind Eye Care System, India.
Study Population
Patients with culture-positive filamentous fungal ulcers and visual acuity of 20/40 to 20/400 re-examined 6 days after initiation of treatment
Intervention
Corneal scraping and cultures were obtained from study participants at day-6 after enrollment.
Main outcome Measures
We assess 3-month best spectacle corrected visual acuity (BSCVA), 3-month infiltrate/scar size, corneal perforation and re-epithelialization rates stratified by culture positivity at day 6.
RESULTS
Of the 323 patients with smear positive ulcers enrolled in MUTT-I, 299 (92.6%) were scraped and cultured six days after enrollment. Repeat culture positivity was 31% (92/299). Among patients who tested positive at enrollment, those with positive 6-day cultures had significantly worse 3-month BSCVA (0.39 LogMAR; 95% CI: 0.24 to 0.44; P<0.001), larger 3-month scar-size (0.39 mm; 95% CI: 0.06 to 0. 73; P=0.02), were more likely to perforate or require therapeutic penetrating keratoplasty (OR: 6.27; 95% CI: 2.73 to 14.40; P<0.001), and were slower to re-epithelialize (HR: 0.33; 95% CI: 0.21 to 0.50; P<0.001) than those with a negative 6-day culture result.
CONCLUSIONS
Early microbiological cure on culture is a predictor of clinical response to treatment.
TRIAL REGISTRATION clinicaltrials.gov Identifier
INTRODUCTION
Fungal keratitis represents up to 50% of corneal infections in the tropics and remains one of the most challenging categories of ocular infection to treat.1,2 Although corneal cultures are still the gold standard for diagnosis of keratitis, their use for assessing treatment response and clinical prognosis may prove to be valuable.3 Monitoring response to therapy is often complicated by toxicity of topical drops, and/or host immune/inflammatory response, which may appear to worsen the corneal opacity, although they are controlling the infection.4–6 Typical characteristics of ulcer healing such as epithelialization do not always indicate that a fungal ulcer is responding; and in fact may even hinder the penetration of topical fungicide.7
Clinicians also look for ways to determine which patients are at highest risk of a poor outcome and need closer monitoring, particularly in resource poor settings.4 One study of both suspected fungal and bacterial corneal ulcers found that baseline smear-negative and culture-negative microbial keratitis had a decreased risk of requiring surgical intervention compared with culture-positive keratitis. In the Steroids for Corneal Ulcer Trial (SCUT), baseline culture positivity in bacterial ulcers despite prior appropriate antibiotic treatment was associated with worse visual acuity outcomes.8–12 Here, we investigate the utility of repeat culture for determining prognosis and management of fungal ulcers.
METHODS
The methods for the MUTT-I have been discussed in detail previously.13 Briefly, patients presenting to the Aravind Eye Hospital or the University of California San Francisco Eye Clinics with a smear-positive fungal corneal ulcer and baseline vision of 20/40 to 20/400 were enrolled and randomized to receive either topical natamycin 5% (Natacyn; preserved with benzalkonium chloride, 0.01%) or topical voriconazole, 1% (Vfend IV; reconstituted in sterile water for injection with benzalkonium chloride, 0.01%, by Aurolab). Baseline and 6-day scraping and cultures were obtained and detailed methods for the handling of microbiological specimens have been outlined in a prior publication.13 Ethical approval was obtained from the Aravind Eye Care System Institutional Review Board, the University of California, San Francisco Committee on Human Research, and the Dart-mouth-Hitchcock Medical Center Committee for the Protection of Human Subjects. Informed written consent was obtained from all participants, and the trial conformed to the Declaration of Helsinki. MUTT-I was registered at clinicaltrials.gov under NCT00996736.
OUTCOME MEASURES
The primary outcome for this non- pre-specified secondary analysis was best spectacle-corrected visual acuity (BSCVA) at 3 months. Secondary outcomes included infiltrate/scar size at 3 months, the occurrence of corneal perforation and/or the need for therapeutic penetrating keratoplasty (TPK) and rate of re-epithelialization.
STATISTICAL ANALYSIS
Patients enrolled in MUTT-I who did not have a repeat corneal scraping and culture were excluded from the analysis. Baseline characteristics were compared using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Multiple linear regression predicting patient’s 3-month BSCVA with covariates including 6-day culture-positivity (yes/no), treatment arm, and baseline BSCVA was performed. The geometric mean of the longest diameter and the longest perpendicular was used to assess infiltrate/scar size and epithelial defect size. Multiple linear regression was fit predicting patient’s 3-month infiltrate/scar size using 6-day culture positivity, treatment arm, and enrollment infiltrate/scar size as covariates. Time to re-epithelialization was analyzed using a Cox proportional hazards model with 6-day culture positivity, treatment arm, and enrollment epithelial defect size as covariates. A logistic regression model with covariates for 6-day culture positivity, treatment arm, and baseline infiltrate depth was used to assess the odds of corneal perforation and/or TPK. Separate sensitivity analyses of the models were performed by controlling for baseline culture positivity (yes/no), bacterial organism and baseline clinical characteristics including BSCVA, infiltrate/scar size, epithelial defect size, presence of hypopyon, and depth of ulcer.
RESULTS
Six days after trial enrollment, 299 of the 323 patients were scraped and cultured again, resulting in a repeated culture positivity of 31% (92/299). Table 1 compares the baseline characteristics of study participants who were repeat culture positive versus repeat culture negative. There were 130 males (43.5%) with a median age was 47 (IQR 38, 56). Median baseline visual acuity was logMAR 0.66 (IQR 0.38, 0.90), and median baseline infiltrate/scar size was 3.19 (IQR 2.50, 4.00). Those who did not have a negative culture by day 6 were slightly younger and somewhat less likely to have been on topical antifungals at presentation. They also had overall slightly worse baseline clinical features such as decreased visual acuity, increased scar size, increased epithelial defect and more likely to have a hypopyon than those who were culture negative at 6 days.
Table 1. Baseline Characteristics of Patients by Repeated Culture Results.
Baseline characteristic data for 299 patients with a repeated fungal culture performed 6 days after enrollment in the Mycotic Ulcer Treatment Trial-I. Patient characteristics are stratified by their day-6 culture results.
| Baseline Characteristic | Fungal Culture Positive on Day 6 (N=92) | Fungal Culture Negative on Day 6 (N=207) |
|---|---|---|
| Gender, N | ||
| Male | 43 (14%) | 87 (29%) |
| Female | 49 (16%) | 120 (40%) |
| Age (years), median (25th, 75th Percentile) | 45 (38, 55) | 48 (38,58) |
| Occupation, N | ||
| Agriculture | 34 (28%) | 100 (33%) |
| Non-Agriculturea | 58 (19%) | 107 (36%) |
| Medication use at enrollmentb, N | ||
| Topical ocular antifungals | 37 (12%) | 101 (34%) |
| Other topical ocular dropsc | 50 (17%) | 128 (43%) |
| Systemic antifungals | 1 (1%) | 10 (3%) |
| Other systemic | 26 (9%) | 54 (18%) |
| Trauma/Injury, N | ||
| Vegetative Matter/Wood | 25 (8%) | 47 (16% ) |
| Metal/Otherd | 33 (11%) | 74 (25%) |
| Unknown Object | 3 (1%) | 11 (4%) |
| Contact Lens | 0 (0%) | 0 (0%) |
| Affected Eye, N | ||
| Right | 115 (38%) | 40 (13%) |
| Left | 92 (31%) | 52 (17%) |
| Visual Acuity (logMAR), median (25th, 75th percentiles) | 0.72 (0.46, 1.02) | 0.64 (0.38, 0.88) |
| Infiltrate/Scar Size (mm1), median (25th, 75th percentiles) | 3.39(2.95, 4.08) | 3.14(2.40, 3.99) |
| Hypopyon | ||
| no | 50 (17%) | 147 (49%) |
| <0.5mm | 20 (7%) | 32 (11%) |
| >0.5mm | 22 (7%) | 28 (9%) |
| Depth | ||
| >0–33% | 47 (16%) | 111 (37%) |
| >33–67% | 36 (12%) | 76 (25%) |
| >67–100% | 9 (3%) | 19 (6%) |
| Epithelial Defect (mm1), median (25th, 75th percentiles) | 2.97 (2.00,3.66) | 2.30 (1.41,3.39) |
| Duration of Symptoms, days, median (25th, 75th percentiles) | 5 (4,10) | 6 (3,10) |
| Systemic disease, N | 9 (3%) | 31 (10.4%) |
Includes unemployed, retired, etc.
Some patients were on more than one medication at enrollment
Includes topical antibiotics, dilating drops, glaucoma medication, lubricating drops
Includes dust, finger, kerosene, cement, fingernail, chili powder, sand, cow’s tail, insect
Table 2 outlines the infectious organisms isolated in the 299 patients undergoing repeat culture, which included 122 (40.8%) Fusarium, 49 (16.4%) Aspergillus and 72 (24.1%) other filamentous fungi. Fifty-six (18.7%) patients tested fungal culture negative both at baseline and repeat culture. Baseline cultures did not predict 3-month visual acuity (P = 0.11), 3-month infiltrate/scar size (P = 0.30), rate of re-epithelialization (P=0.08) or rate of corneal perforation or the need for TPK (P = 0.07) after correcting for baseline values and treatment arm (Table 3a &3b).
Table 2. Microbiological Fungal Culture Results.
Fungal microorganisms identified in isolates obtained from patients for cultures performed at enrollment and 6 days after enrollment in the Mycotic Ulcer Treatment Trial-I.
| Fungal Culture Results | Fusarium (N=122) | Aspergillus (N=49) | Other (N=49) | |
|---|---|---|---|---|
| Enrollment | 6 Days After Enrollment | N (%) | N (%) | N (%) |
| + | + | 41 (34%) | 28 (23%) | 16 (13%) |
| + | − | 79 (20%) | 20 (16%) | 52 (43%) |
| − | + | 2 (1%) | 1 (1%) | 4 (3%) |
Not included are 56 patients who tested positive for fungal smear (inclusion criterion for trial enrollment) but tested negative for fungal culture at enrollment and 6 days after enrollment.
Table 3a.
Multiple Linear Regression assessing Best Spectacle-Corrected Visual Acuity.Results from three separate multiple linear regression models predicting 3-month Best Spectacle-Corrected Visual Acuity (logMAR) for patients testing positive (vs. negative) for presence of fungus at day-6, while correcting for treatment arm and baseline Best Spectacle-Corrected Visual Acuity.
| Predictor | N | 3-month BSCVAa (logMAR) | 95% CI | P |
|---|---|---|---|---|
| Enrollment Culture Positivity | 292 | 0.12 | (−0.03 to 0.27) | 0.11 |
| Day-6 Culture Positivity | 274 | 0.42 | (.28 to 0.56) | <0.001 |
| Day-6 Smear Positivity | 275 | 0.35 | (0.22 to 0.48) | <0.001 |
Best Spectacle-Corrected Visual Acuity;
Table 3b.
Regression Models assessing secondary outcomes: 3-month infiltrate/scar and corneal perforation (or need for Therapeutic Penetrating Keratoplasty).Results from models predicting 3-month infiltrate/scar size and corneal perforation (or need for Therapeutic Penetrating Keratoplasty) among patients testing positive for presence of fungus from baseline cultures, day-6 cultures, and day-6 smear, while correcting for treatment arm and baseline measurements.
| Multiple linear regression a predicting 3-month infiltrate/scar (mm) | Logistic regressionb predicting perforation or need for TPK,c | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | N | Coef. | 95% CI | P | N | HR | 95% CI | P | |
| Enrollment Culture Positivity | 290 | 0.17 | (−0.15 to 0.48) | 0.30 | 299 | 2.35 | (0.94 to 5.89) | 0.07 | |
| Day-6 Culture Positivity | 282 | 0.39 | (0.09 to 0.70) | 0.01 | 299 | 7.15 | (3.38 | to 15.13) | <0.001 |
| Day-6 Smear Positivity | 273 | 0.49 | (0.20 to 0.77) | 0.001 | 300 | 4.36 | (2.21 to 8.60) | <0.001 | |
Model included baseline infiltrate/scar size and treatment arm as covariates
Model included baseline depth and treatment arm as covariates
Therapeutic Penetrating Keratoplasty
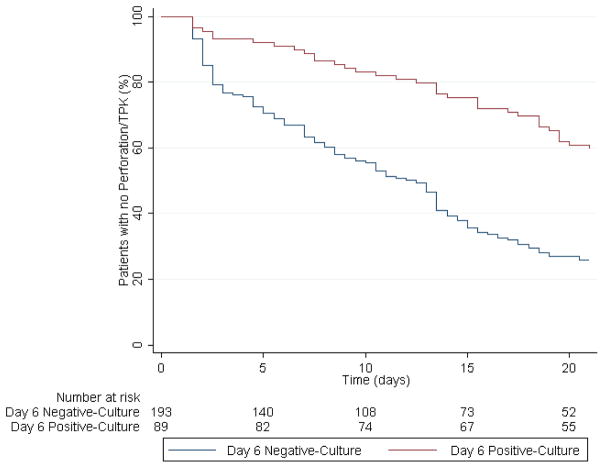
Study participants with positive 6-day cultures had on average 0.42 LogMAR lines worse BSCVA at 3-month after correcting for baseline visual acuity and treatment arm (95% CI: 0.28 to 0.56; P<0.001; Table 3a). They also had 0.39 mm larger 3-month scar-size (95% CI: 0.09 to 0. 70; P=0.01), had 7.15 times the odds of full thickness corneal perforation or the need for therapeutic penetrating keratoplasty (95% CI: 3.38 to 15.13; P<0.001; Table 3b). Finally, they were also slower to re-epithelialize after correcting for baseline values and treatment arm than those with a negative 6-day culture result (HR: 0.32; 95% CI: 0.22 to 0.47; P<0.001; Figure 1).
Figure 1. Kaplan-Meier Survival Curve for Corneal Re-Epithelialization.
Kaplan-Meier survival curve comparing the probability of corneal re-epithelialization between patients enrolled in the Mycotic Ulcer Treatment Trial–I who had culture-positive results after 6 days of treatment (red) compared to those who had culture-negative results (blue).
Enrollment culture results showed a higher percentage of positivity (79%; 252/323) as compared to day-6 cultures 31% (92/299) and culture-positivity between baseline and 6-days was significantly correlated (R=0.22; P<0.001). Sensitivity of our models were tested by adjusting for enrollment culture-positivity, microorganism, and baseline clinical characteristics (BSCVA, infiltrate/scar size, epithelial defect size, presence of hypopyon, and depth of ulcer). Sensitivity models produced very similar results and culture-positivity remained statistically significant.
Day 6 smear-positive results were highly correlated with day-6 culture-positive results (R=0.48; P<0.001). Smear positive patients had on average 0.35 LogMAR lines worse BSCVA at 3-month (95% CI: 0.22 to 0.48; P<0.001, Table 3a), 0.49 mm larger 3-month scar-size (95% CI: (0.20 to 0.77); P=0.001), were slower to re-epithelialize (HR: 0.40; 95% CI: 0.28 to 0.57; P<0.001), and had 4.36 times the odds of full thickness corneal perforation or the need for therapeutic penetrating keratoplasty (95% CI: 2.21 to 8.60; P<0.001) after correcting for baseline values and treatment arm than those with a negative 6-day smear result. (Table 3b).
DISCUSSION
The importance of corneal cultures in the diagnosis of infectious keratitis has been well established. Here, we demonstrate that there is a potential role for corneal cultures in assessing treatment response and determining clinical prognosis as well. In our study, corneal ulcers that did not have negative cultures results by day 6 had worse 3-month visual acuity, larger scar size, increased risk of corneal perforation and slower rates of re-epithelialization. Although our ultimate goal in treatment of corneal ulcers is ulcer healing, our immediate objective of therapy is to eliminate the infection. Baseline culture positivity despite antibiotic therapy has been associated with poor outcomes in bacterial and fungal keratitis8,9,12,14 Similarly, positive fungal donor rim cultures have also been shown to predict increased risk of fungal endophthalmitis after corneal transplant.15 By contrast in our fungal smear positive population, baseline culture status did not predict clinical outcomes.
Obtaining repeat cultures allows clinicians to directly assess treatment response. If the patient has a positive repeated culture, clinicians could then consider augmenting current therapy by adding another medication topically, starting oral antifungal, or using innovative treatment techniques such as collagen cross-linking or intrastromal antifungal injection. In addition to altering therapy, positive repeat cultures identify patients who are at greatest risk of a negative outcome such as corneal perforation or the need for TPK and would benefit from closer monitoring. This is particularly important in resource poor settings where these types of infections are most prevalent. Remarkably, a simple smear was also highly correlated with clinical outcomes in our study making it a quick and inexpensive alternative to full corneal cultures.
The potential for repeat culture status to be used as surrogate markers in clinical trials is also noteworthy. The use of surrogate endpoints has become increasingly common in clinical trials, particularly in the fields of oncology and infectious disease.16 Advantages of surrogate trial endpoints include smaller sample sizes and faster trial completion as they allow detection of response to treatment at an early stage. The fact that repeat culture is easily obtained at an early stage of treatment and so highly correlated with all clinical outcomes of interest make it an excellent choice.
Limitations to this study include the fact that all patients in the study were enrolled in south India therefore organisms in this region may not be representative of infectious organisms in other regions or countries. Although all organisms in this study were filamentous fungi, a variety of species were represented, making it difficult to draw conclusions about the significance of culture positivity for each organism subtype.
Microbiological positivity on repeat culture appears to be a clinically useful tool for assessing treatment response and risk of poor clinical outcome. This simple measure also may serve as a valuable surrogate endpoint for corneal ulcer clinical trials given how highly it is correlated with clinical outcomes of interest.
Supplementary Material
Acknowledgments
a) FUNDING/SUPPORT. This work was supported by grants U10 EY018573 (Lietman and Acharya) and K23 EY025025 (Rose-Nussbaumer) from the National Eye Institute and grants from That Man May See, the Harper/Inglis Trust, the South Asia Research Foundation, and Research to Prevent Blindness (McLeod, Lietman and Acharya). Natamycin and voriconazole were donated by Alcon and Pfizer, respectively. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
b) FINANCIAL DISCLOSURES. There are no financial conflicts of interest to report. Kathryn Ray, Tom Lietman and Jennifer Rose-Nussbaumer had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
c) OTHER ACKNOWLEDGMENTS. Kathryn Ray and Jennifer Rose-Nussbaumer contributed to the data analysis and writing of this manuscript. Thomas Lietman, Stephen McLeod, N. Venkatesh Prajna, Prajna Lalitha, and Nisha Acharya contributed to the design and implementation of this study and editing of the manuscript. Drs. N. Venkatesh Prajna, Prajna Lalitha, Tiruvengada Krishnan, Revathi Rajaraman, and Muthiah Srinivasan contributed to the study implementation and editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman MR, Johnson GJ, Husain R, Howlader SA, Minassian DC. Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br J Ophthalmol. 1998;82(8):919–925. doi: 10.1136/bjo.82.8.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLeod SD, Kolahdouz-Isfahani A, Rostamian K, Flowers CW, Lee PP, McDonnell P. The Role of Smears, Cultures, and Antibiotic Sensitivity Testing in the Management of Suspected Infectious Keratitis. Ophthalmology. 1996;103(1):23–28. doi: 10.1016/s0161-6420(96)30738-0. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PA. Fungal infections of the cornea. Eye. 17(8):852–862. doi: 10.1038/sj.eye.6700557. 0000. [DOI] [PubMed] [Google Scholar]
- 5.Ansari Z, Miller D, Galore A. Current Thoughts in Fungal Keratitis: Diagnosis and Treatment. Curr Fungal Infect Rep. 2013;7(3):209–218. doi: 10.1007/s12281-013-0150-110.1007/s12281-013-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 7.Shi W, Li S, Liu M, Jin H, Xie L. Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):581–586. doi: 10.1007/s00417-007-0719-x. [DOI] [PubMed] [Google Scholar]
- 8.Ray KJ, Prajna L, Srinivasan M, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol. 2013;131(3):310–313. doi: 10.1001/jamaophthalmol.2013.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenburg CE, Lalitha P, Srinivasan M, et al. Moxifloxacin susceptibility mediates the relationship between causative organism and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2013;54(2):1522–1526. doi: 10.1167/iovs.12-11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalitha Prajna, Srinivasan M, Manikandan P, et al. Relationship of in vitro susceptibility and in vivo clinical outcome in bacterial keratitis. Clin Infect Dis. 2012;54(10):1381–1387. doi: 10.1093/cid/cis189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhadange Y, Das S, Kasav MK, Sahu SK, Sharma S. Comparison of culture-negative and culture-positive microbial keratitis: cause of culture negativity, clinical features and final outcome. Br J Ophthalmol. doi: 10.1136/bjophthalmol-2014-306414. [DOI] [PubMed] [Google Scholar]
- 12.Bhadange Y, Das S, Kasav MK, Sahu SK, Sharma S. Comparison of culture-negative and culture-positive microbial keratitis: cause of culture negativity, clinical features and final outcome. Br J Ophthalmol. 2015;99(11):1498–1502. doi: 10.1136/bjophthalmol-2014-306414. [DOI] [PubMed] [Google Scholar]
- 13.Prajna NV, Krishnan KT, Mascarenhas J, et al. The mycotic ulcer treatment trial: a randomized trail comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422–429. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalitha P, Srinivasan M, Manikandan P, Bharathi MJ, et al. Relationship of in vitro susceptibility to moxifloxacin and in vivo clinical outcome in bacterial keratitis. Clin Infect Dis. doi: 10.1093/cid/cis189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilhelmus KR, Hassan SS. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology. 2007;114(3):440–445. doi: 10.1016/j.ophtha.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 16.De Gruttola VG, Clax P, DeMets DL, Downing GJ, et al. Considerations in the evaluation of surrogate endpoints in clinical trials. summary of a National Institutes of Health workshop. Control Clin Trials. doi: 10.1016/s0197-2456(01)00153-2. (0197-2456) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.