Abstract
Mice enter bouts of daily torpor, drastically reducing metabolic rate, core body temperature (Tb), and heart rate (HR), in response to reduced caloric intake. Because central adenosine activation has been shown to induce a torpor-like state in the arctic ground squirrel, and blocking the adenosine-1 (A1) receptor prevents daily torpor, we hypothesized that central activation of the A1 adenosine receptors would induce a bout of natural torpor in mice. To test the hypothesis, mice were subjected to four different hypothermia bouts: natural torpor, forced hypothermia (FH), isoflurane-anesthesia, and an intracerebroventricular injection of the selective A1 receptor agonist N6cyclohexyladenosine (CHA). All conditions induced profound hypothermia. Tb fell more rapidly in the FH, isoflurane-anesthesia, and CHA conditions compared to torpor, while mice treated with CHA recovered at half the rate of torpid mice. FH, isoflurane-anesthesia, and CHA-treated mice exhibited a diminished drop in HR during entry into hypothermia as compared to torpor. Mice in all conditions except CHA shivered while recovering from hypothermia, and only FH mice shivered substantially while entering hypothermia. Circulating lactate during the hypothermic bouts was not significantly different between the CHA and torpor conditions, both of which had lower than baseline lactate levels. Arrhythmias were largely absent in the FH and isoflurane-anesthesia conditions, while skipped beats were observed in natural torpor and periodic extended (>1 sec) HR pauses in the CHA condition. Lastly, the hypothermic bouts showed distinct patterns of gene expression, with torpor characterized by elevated hepatic and cardiac Txnip expression and all other hypothermic states characterized by elevated c-Fos and Egr-1 expression. We conclude that CHA-induced hypothermia and natural torpor are largely different physiological states.
Introduction
Targeted temperature management is perhaps one of the most promising new treatments for patients suffering from acute bouts of toxicity, ischemia, or myocardial infarction (Gordon 2001, Group 2002, Yenari and Han 2012, Scirica 2013, Ahmad, Wang et al. 2014). However, humans respond to exogenous cooling by generating heat to mitigate cooling. The thermoregulatory responses elicited by exogenous cooling, such as shivering, brown fat activation and vasoconstriction via the sympathetic nervous system (SNS) might lessen the benefits of targeted temperature management. In contrast, many small mammals and birds naturally lower core body temperature (Tb) as they enter bouts of torpor. Despite decades of research into both peripheral compounds and central brain pathways involved in a torpor bout, our understanding of the mechanism of induction and arousal from torpor is still rudimentary (Bouma, Verhaag et al. 2011).
Adenosine, a ubiquitous nucleoside and purinergic signaling molecule, has garnered recent attention as a potential torpor mediator. Adenosine is involved in thermoregulation, energy signaling, and sleep (Fredholm, Johansson et al. 2011). Centrally, adenosine inhibits GABA release in the arcuate nucleus (ARC) of the hypothalamus through the adenosine A1 and A2 receptors (Chen and van den Pol 1997). The ARC is essential for entry into torpor and acts as a center of integration for hunger and satiety signals from the periphery (Gluck, Stephens et al. 2006, Pelz, Routman et al. 2008, Minor, Chang et al. 2009). Additionally, central levels of adenosine relay peripheral energy levels, as minor fluctuations in ATP levels cause large perturbations on adenosine levels (Dunwiddie and Masino 2001). Furthermore, adenosine plays a role in controlling sleep (Bjorness and Greene 2009), and long term electroencephalogram recordings of hibernating ground squirrels revealed that bouts of torpor are entered through a sleep state (Walker, Glotzbach et al. 1977, Heller and Ruby 2004).
In addition to the correlative evidence listed above, experimental evidence of manipulating adenosine signaling suggests the involvement of adenosine in mediating a pathway for torpor induction. For example, peripheral administration of adenosine (or adenosine receptor agonists) causes a drop in Tb, MR, and HR in animals that utilize torpor and those that do not (Miller and Hsu 1992, Anderson, Sheehan et al. 1994, Swoap, Rathvon et al. 2007, Yang, Tiselius et al. 2007, Olson, Jinka et al. 2013, Jinka, Combs et al. 2015). Similarly, central administration of cyclohexyladenosine (CHA), an A1 adenosine receptor specific agonist or adenosine monophosphate (AMP), causes a fall in Tb (Jinka, Tøien et al. 2011, Muzzi, Blasi et al. 2013, Tupone, Madden et al. 2013). Importantly, central administration of the non-selective adenosine receptor antagonist 8-sulfophenyltheophylline into torpid mice, or the A1 receptor specific antagonist 8-cyclopenthyltheophylline into torpid hamsters, causes a return to normothermia from a bout of torpor (Bruns, Daly et al. 1983, Tamura, Shintani et al. 2005, Iliff and Swoap 2012). What is not known, however, is whether CHA initiates a bout of natural torpor and not just unregulated hypothermia.
Due to the diverse inhibitory and sleep-promoting effects of central A1 adenosine receptors, the necessity of adenosine signaling during entry into torpor, and the known hypothermic response to A1 adenosine receptor activation, we hypothesized that central administration of CHA would induce entry into torpor in mice. Our objective was to characterize the physiological fingerprints of four hypothermic states at multiple levels to distinguish between a natural torpid state and unregulated hypothermia. We measured physiological changes in HR, HR variability, rate of Tb changes, shivering, circulating metabolites, and gene expression during natural bouts of torpor and during acute central injections of CHA. Further, we used isoflurane-anesthesia and forced hypothermia (FH) as additional points of comparison because they impose hypothermia on the organism when used in targeted temperature management in humans (Group 2002, Scirica 2013, Ahmad, Wang et al. 2014).
Materials and Methods
Mice
All experiments were approved by the Williams College Institutional Animal Care and Use Committee and were performed in accordance with the guidelines described by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. C57Bl/6J female mice were ordered pre-cannulated from Taconic Biosciences (Hudson, NY) with a guide cannula implanted into the lateral ventricle of the mouse. The mice were singly housed with Teklad sani-chip bedding (Envigo #7090) at 22°C, except during forced hypothermia, with a 12 hour dark/light cycle. Food and water were provided ad libitum in all conditions except torpor.
Electrocardiogram Radiotelemeter Implantation
All telemeter implantations were performed at Williams College. Mice were initially anesthetized under 5% isoflurane in oxygen and then transferred to 2% isoflurane in oxygen through a nose cone. A small midline incision of the peritoneum revealed the body cavity into which a sterilized radiotelemeter was placed (TA10ETAF-20; Data Sciences International, St Paul, MN). This telemeter provides an electrocardiogram (ECG), Tb, and activity. The ribs of the telemeter were sutured with a non-absorbable suture (5-0 Ethilon; Johnson & Johnson, New Brunswick, NJ) to the peritoneum in order to hold the telemeter in place. The skin was further separated from the peritoneum both ventral-laterally and ventral-medially up to the shoulder in order to place the ECG leads in approximately a Lead II configuration. The peritoneum was closed with additional sutures. The skin was closed with 7 mm wound clips (Reflex Clips; Fine Science Tools, Foster City, CA) and dressed with a triple antibiotic ointment. The mice were allowed to recover for at least 10 days before experimental testing.
Data Collection
Tb, ECG, and locomotor activity were sampled for a period of 10 seconds once per minute. All outputs from the ECG radiotelemeters were collected through receiver pads and D.S.I. acquisition software. The data were subsequently analyzed by D.S.I. analysis software or Ponemah analysis software. HR was calculated from ECG traces. Shivering was quantified using the Ponemah software by enabling the “noise” feature as we have shown previously (Maher, Barbash et al. 2015).
Experimental design
A total of 26 mice were used in the current study. Physiological data were collected on at least five mice for each condition. However, not every mouse experienced each condition. The four experimental conditions were natural torpor, FH, isoflurane-anesthesia, and CHA. After any one intervention, mice were allowed to recover for three to seven days before further testing to allow for sufficient time to re-establish circadian rhythms (1 day) and normal food intake (2 days post caloric restriction). To account for potential residual effects of previous treatments in our analysis, a random crossover design was used. To achieve a bout of natural torpor, mice were calorically restricted to 70% of standard chow per day, administered at the onset of the dark phase. After a bout of torpor (approximately 2-3 days post-initiation of caloric restriction) the mice were fed ad libitum. For forced hypothermia, mice were anesthetized for 20 seconds under 5% isoflurane in oxygen and placed in a restrainer (Product RSTR551; Kent Scientific). The restrainer was subsequently placed resting in between two diagonally oriented ice packs with the animal's tail touching the packs (0-4°C) at an ambient temperature of 13°C. After Tb reached approximately 21°C, mice were placed in their home cages unrestrained and recovery was monitored. For anesthesia, mice were anesthetized at a Ta of 22°C under 2% isoflurane in oxygen in an anesthesia chamber. After Tb reached approximately 28°C, mice were placed in their home cages and recovery was monitored. For CHA treatment, CHA (Sigma Chemical, St. Louis, MO) was dissolved in 25% cyclodextrin (Sigma Chemical) solution in sterile saline. A 24 cm long PE50 polyethylene tubing (Plastics One, Roanoke, VA) with a 2.3 mm injector attached (Plastics One, Roanoke, VA) was prefilled with a CHA solution or vehicle (25% cyclodextrin in sterile saline). 50 pmol in 2 μL of CHA solution or 2 μL of vehicle were administered over a 1 minute period. The injector was left in the guide cannula for 3 minutes. All CHA injections were performed during the first two hours of the light phase, in accordance with the natural circadian rhythm of torpor. Mice were injected and monitored in their home cages until they fully aroused. The mice spontaneously aroused from the CHA injection without provocation from the experimenter or a warming of the ambient temperature.
Lactate Measurements
Blood lactate levels were measured from the tail vein using a commercially available lactate meter (www.lactate.com). Lactate was measured before the bout of hypothermia and when Tb was approximately 30°C.
Gene expression
Mice were euthanized under brief exposure to isoflurane anesthesia when Tb was approximately 30°C across all conditions (natural torpor n=6, FH n=6, isoflurane-anesthesia n=4, CHA n=5). Control naïve, euthermic mice were also euthanized (n=5). Liver and heart were removed (hearts were not removed in the isoflurane-anesthesia condition), quick frozen in liquid nitrogen, and stored at -80°C. Total RNA was extracted from liver and heart samples with an RNeasy mini kit (Qiagen). After extraction, RNA was stored at -80°C. mRNA in the total RNA pool was converted to cDNA using a RT2 first strand kit (Qiagen) and stored at -20°C. A RT2 Profiler PCR array for the mouse hypoxia signaling pathway (Qiagen PAMM-032Z) was used for qRT-PCR (Bio Rad CFX96). Cycle numbers were normalized to the average of five housekeeping genes (beta actin, beta 2 microglobulin, glyceraldehyde dehydrogenase, beta glucoronidase, and HSP90ab1). qRT-PCR was performed in duplicate. Each sample included a no-reverse transcriptase control and a no-template control. Relative changes in gene expression were calculated using the 2-ΔΔCt method.
Statistical Analyses
Data are reported as mean ± SE. Statistical tests were performed using IBM SPSS v21. Univariate ANOVAs followed by Tukey post-hoc tests were performed. A p-value of less than 0.05 was accepted as statistically significant.
Results
To test the hypothesis that central administration of CHA engages the physiological processes involved in caloric restriction (CR)-induced torpor in mice and to assess the differences between CHA-induced hypothermia and other hypothermic states, mice were subjected to four conditions: CR-induced torpor, FH, isoflurane-anesthesia, and central CHA injection. Behaviorally, changes in the posture of the mice were observed in CR-induced torpor and central CHA injected mice. CR-induced torpid animals reduced exposed body surface area by curling over and remaining motionless for the entirety of the bout. Central CHA injected mice and isoflurane-anesthetized mice tended to be splayed across the cage floor. All four conditions showed a drop in Tb, and associated drop in HR (Figure 1A). Vehicle injection caused no change in Tb (data not shown).
Figure 1. Body temperature (Tb) and heart rate (HR) relationship during bouts of hypothermia.
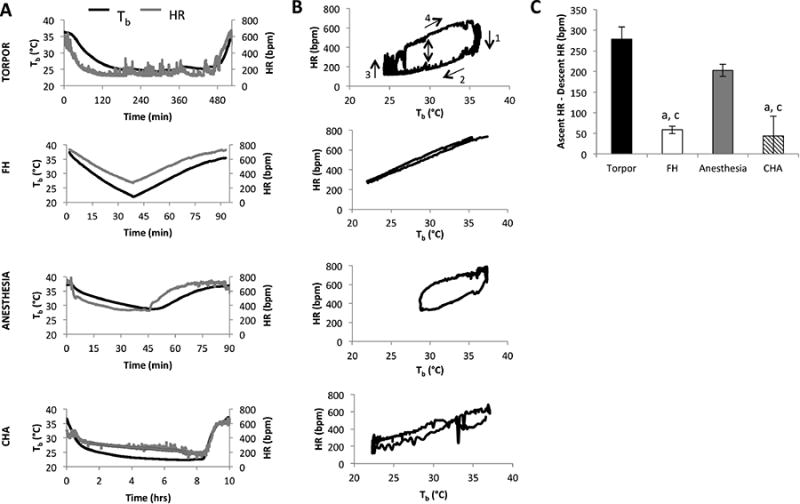
Mice were implanted with an ECG/Tb telemeter and underwent four different bouts of hypothermia. 1) Natural torpor, induced by chronic 70% caloric restriction at an ambient temperature (Ta) of 22°C. 2) Forced hypothermia (FH), where mice were restrained and exposed to a cold temperature (see Methods). 3) Isoflurane-anesthesia, where mice were kept anesthetized with 2.5% isoflurane in an O2 stream at an Ta of 22°C. 4) ICV injection of the adenosine receptor 1 agonist, cyclohexyladenosine (CHA) at an Ta of 22°C. A. Tb and HR (derived from the ECG tracing) over the bout of hypothermia. B. HR for each condition is plotted as a function of the concurrent Tb. A typical Tb/HR relationship is observed in natural torpor, with time in a clockwise direction, with the beginning of the torpor bout in the top right section of the graph and moving through each of the four steps shown. For the FH condition, the fall in HR during Tb decline closely tracked the rise in HR during Tb rise. Mice treated with CHA also lacked a discernible breadth to their Tb/HR loop with the HR following the same trajectory during body cooling or warming. The Tb/HR relationship in isoflurane-anesthetized mice showed an intermediate breadth. These qualitative assessments were quantified in part C as the difference between HR at a Tb of 30°C while the mouse was recovering from hypothermia and the HR at a Tb of 30°C as the mouse was entering hypothermia.
a = p < 0.05 vs. torpor
b = p < 0.05 vs. FH
c = p < 0.05 vs. isoflurane-anesthesia
Mice in natural torpor exhibited a complex relationship between Tb and HR (Figure 1B), as observed previously in mice and hamsters (Mertens, Stiedl et al. 2008, Swoap and Gutilla 2009). The Tb/HR relationship in CR-induced torpor shown in Figure 1B can be divided into four periods (labeled 1-4): initially, HR decreased with little change in Tb, then Tb decreased with a gradual slowing of HR, then these two phases were reversed. HR increased with little change in Tb and finally Tb returned to baseline with a gradual quickening of HR. The Tb/HR relationship in the FH, isoflurane-anesthesia, and CHA conditions was qualitatively different from the Tb/HR relationship in CR-induced torpor (Figure 1B). For FH-treated mice, the fall in HR during Tb cooling tracked the rise in HR during Tb warming. In isoflurane-anesthetized mice, HR did not decrease as much during cooling as it did in CR-induced torpor. In CHA-treated mice, the fall in HR with decreasing Tb was similar to that of FH-treated mice. The difference in HR at 30°C during recovery from hypothermia and the HR at 30°C during descent into hypothermia showed a diminished drop in HR in all three conditions relative to CR-induced torpor (Figure 1C).
During entry into hypothermia, torpor showed a significantly slower maximum rate of Tb decline compared to FH, isoflurane-anesthesia, and CHA (Figure 2). FH exhibited the fastest decrease in Tb (p<0.05). The rate of decline in Tb after CHA injection was three-fold greater than that observed in natural torpor (-0.62 ± 0.13 vs. -0.18 ± 0.02 °C/min respectively; p < 0.05). During recovery from hypothermia, there was no difference in the rate of Tb increase between torpor, FH, and isoflurane-anesthesia. However, CHA exhibited a significantly slower maximal rate of recovery as compared to torpor and FH (0.29 ± 0.06 vs. 0.43 ± 0.03 and 0.53 ± 0.04 °C/min respectively; p < 0.05).
Figure 2. The rate of Tb decline into hypothermia was slowest for natural torpor, while the rate of Tb rise from hypothermia was slowest for CHA-treated mice.
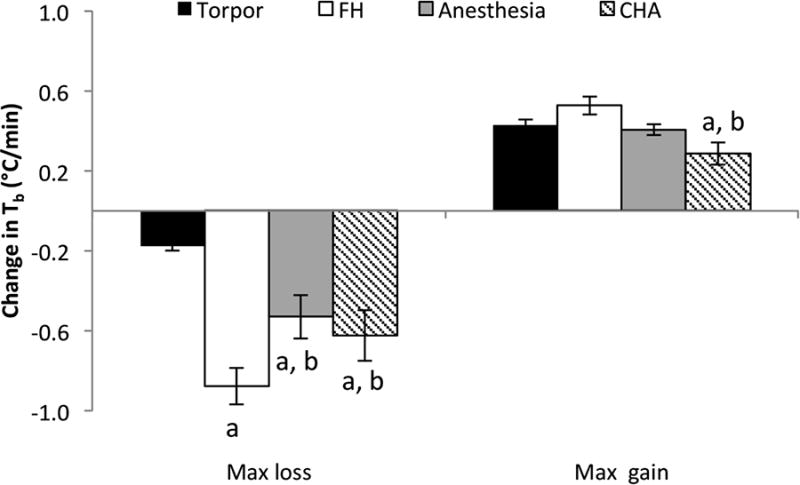
Maximal rate of Tb change was identified and calculated in 10-minute windows for each mouse and then averaged per condition. The maximum rate of Tb decline showed 3-4 fold higher rates in isoflurane-anesthesia, FH, and CHA treated mice as compared to torpor. During recovery, the maximum rate of Tb rise in CHA-treated mice was significantly slower the recovery in torpor and FH.
a = p < 0.05 vs. torpor
b = p < 0.05 vs. FH
Shivering was approximated by quantifying the noise on ECG tracings (Figure 3). Torpid mice exhibited periodic bouts of shivering during entry into torpor and while they maintained a low Tb. As they recovered, torpid mice shivered vigorously. In contrast, FH mice shivered consistently throughout the hypothermic bout, both during cooling and rewarming. Anesthetized mice did not shiver while under isoflurane-anesthesia, but did so vigorously once they were removed from the anesthesia chamber. CHA mice showed minimal shivering throughout the bout.
Figure 3. Shivering profiles of the four hypothermic conditions.
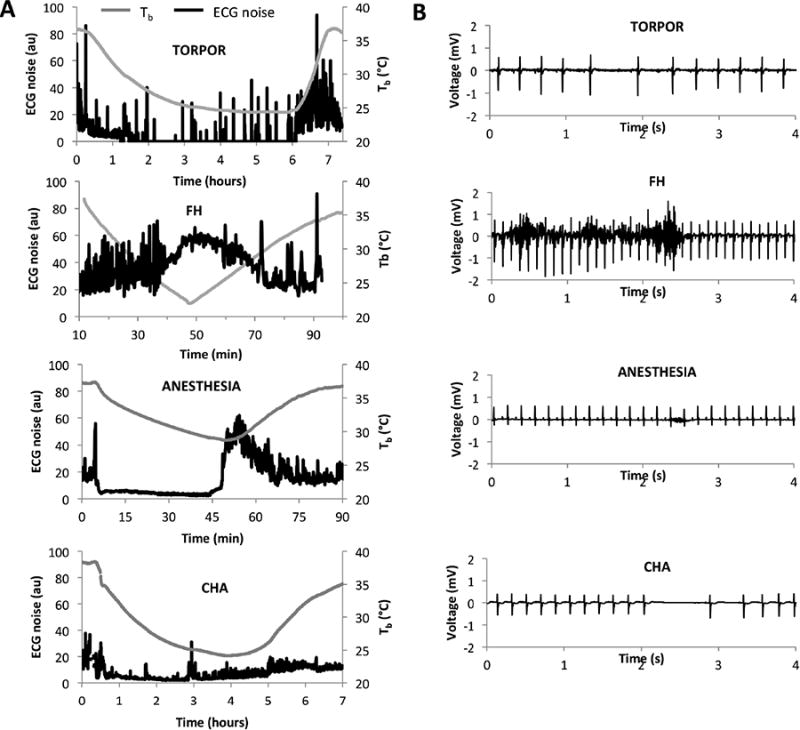
Shivering was approximated as the average noise in ECG tracings. A. Shivering profiles (ECG noise, measured in arbitrary units) for each condition. Torpid mice show periodic bouts of shivering while torpid and substantial shivering while arousing. FH mice show substantial shivering throughout the hypothermic bout. Anesthetized mice only shiver as they recover. CHA mice show minimal shivering throughout the hypothermic bout. B. Representative ECG tracings for each condition from which noise was derived
As we have shown previously (Swoap, Iliff et al. 2012), mice had significantly lower lactate levels during torpor compared to baseline lactate levels (0.53 ± 0.15 mM vs. 1.93 ± 0.13 mM respectively; p < 0.05) (Figure 4). Torpid mice also showed significantly lower lactate levels compared to FH mice (3.67 ± 0.25 mM) and anesthetized mice (5.50 ± 0.54 mM); and FH and anesthetized mice showed significantly higher lactate levels compared to baseline. There was no significant difference in circulating lactate levels between CHA-treated mice (0.84 ± 0.19 mM) and torpid mice.
Figure 4. FH and anesthetized mice show signs of anaerobic metabolism, whereas torpid and CHA-treated mice do not.
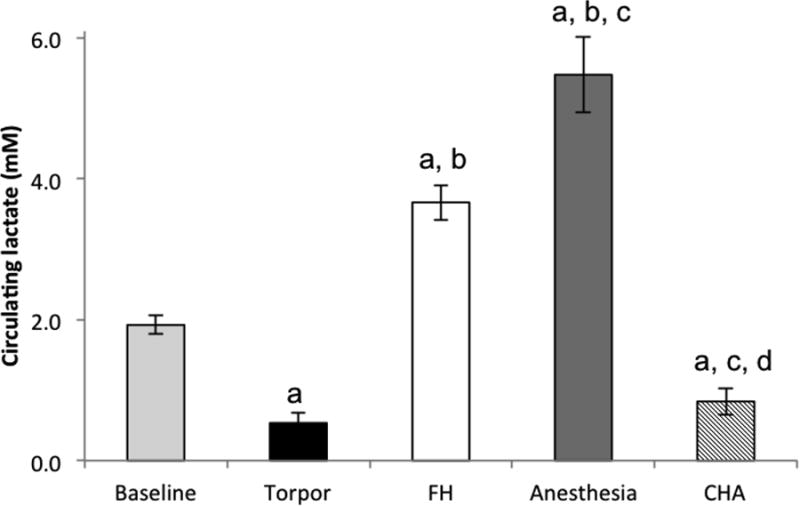
FH and anesthetized mice show higher circulating lactate levels compared to baseline (taken before treatment). CHA-treated mice and torpid mice show lower than baseline lactate levels.
a = p<0.05 vs. baseline
b = p<0.05 vs. torpor
c = p<0.05 vs. FH
d = p<0.05 vs. isoflurane-anesthesia
Disparate cardiovascular responses to the hypothermic bouts were further uncovered by analysis of ECG tracings. Both torpid and CHA mice often exhibited asystoles, which were not found in FH or anesthetized mice (Figure 3B). Torpid mice regularly skipped single beats, whereas the asystoles seen in the CHA condition were often much longer. These differences in asystoles between hypothermic bouts can be visualized by Poincare plots, which graph adjacent inter-beat intervals (IBIs) against each other (Figure 5). Skipped beats characteristic of torpor appeared symmetric around the line of identity (Figure 5A), while the longer pauses between beats in CHA-treated mice were asymmetric (black arrow in Figure 5D). P-waves were not found during the longer pauses in the CHA condition, suggesting sinus arrest. The number of events where any single IBI was three times longer than the preceding IBI was determined throughout the entire bout of hypothermia in all four conditions. These events were not observed in FH or anesthetized mice. In CR-induced bouts of torpor, these events occurred 5 ± 3 times per bout, whereas these events occurred a significantly greater number of times (129 ± 18) during a bout of CHA-induced hypothermia. No other types of arrhythmias were detected in the analyses.
Figure 5. Long HR pauses are only present in torpor and CHA-induced hypothermia.
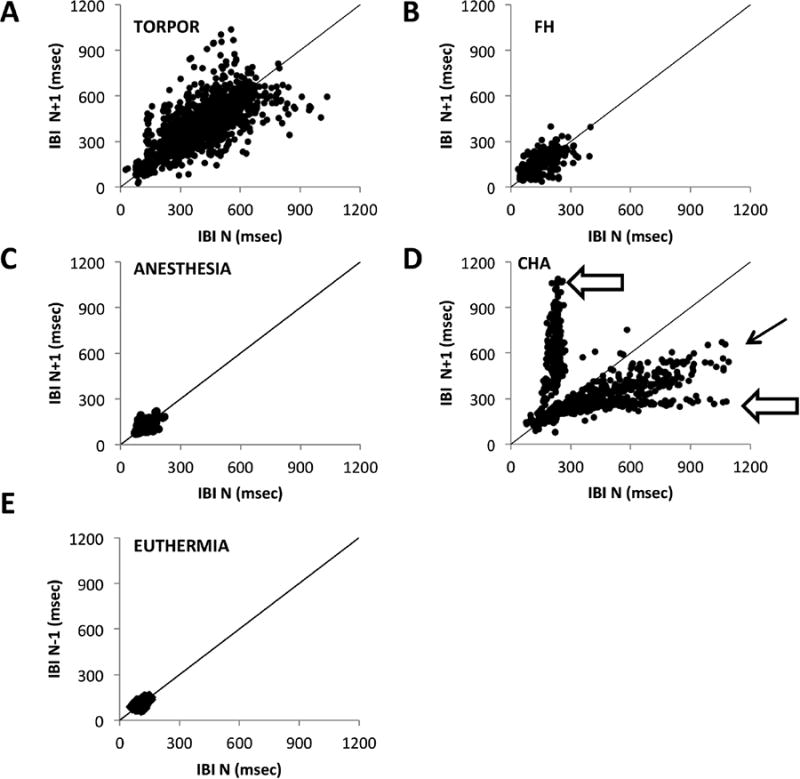
Poincare plots represent the variability in the interbeat intervals (IBI) by plotting adjacent IBIs against each other. A symmetry line is added as a reference. A. Torpor shows a symmetric pattern indicative of skipped beats. B. FH-treated mice and C. Isoflurane-anesthesia treated mice cluster near the symmetry line, indicating a relatively regular heartbeat. D. CHA shows asymmetries (black arrow) that are indicative of pauses that are not skipped beats. The open arrows point to the long pauses seen in the CHA condition. E. The IBIs of a euthermic mouse cluster tightly around the symmetry line (6 hours of data are shown).
Steady state mRNA levels in the heart and liver from mice in each condition, as assessed by qRT-PCR arrays, showed unique patterns of gene expression (Figure 6). Four genes in the liver and heart – Txnip, c-Fos, Egr1, and Hif1a – showed distinct expression patterns in the hypothermic bouts. Torpor was characterized by upregulated Txnip both in the liver and the heart. The only other condition that exhibited elevated Txnip mRNA was FH in the liver. In contrast, c-Fos and Egr1 were induced in the liver and heart of mice in all hypothermic conditions tested except during natural torpor. Hif1a was only modestly induced in the liver of FH, while in the heart, Hif1a was modestly decreased only in CHA. None of the other mRNAs on the PCR array were significantly different among the groups. A full listing of those mRNAs can be found at this website (https://www.qiagen.com/ch/shop/pcr/primer-sets/rt2-profiler-pcr-arrays/?catno=PAMM-032Z#geneglobe).
Figure 6. Torpor, FH, isoflurane-anesthesia, and CHA exhibit different gene expression patterns.
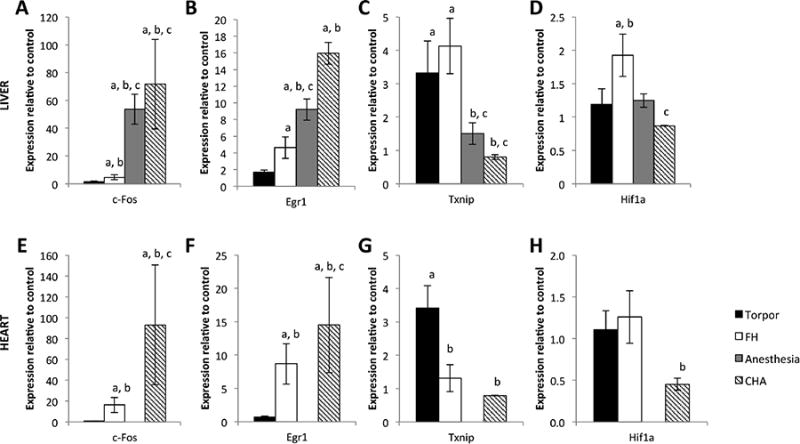
A-D hepatic gene expression. E-H cardiac gene expression (no data for isoflurane-anesthesia). Steady state mRNA levels for the genes c-Fos and Egr1 are upregulated relative to euthermic control mice in all hypothermic states except torpor. Txnip is upregulated in torpor (heart and liver) and FH (liver only) relative to euthermic controls. Hif1a is only modestly induced in the FH liver, and only modestly reduced in the CHA-treated heart.
a = p<0.05 vs. control
b = p<0.05 vs. torpor
c = p<0.05 vs. FH
d = p<0.05 vs. isoflurane-anesthesia
Discussion
We had hypothesized that administration of an A1 receptor agonist would recapitulate the physiological changes that occur during a bout of caloric-restriction induced torpor in the mouse based on the conclusions of previous experiments, including 1) central administration of CHA in the rat (Tupone, Madden et al. 2013) or the arctic ground squirrel (Jinka, Tøien et al. 2011) causes a fall in HR, metabolic rate and Tb, and 2) antagonizing the adenosine receptor before (Iliff and Swoap 2012) or during a bout of torpor (Tamura, Shintani et al. 2005, Iliff and Swoap 2012) prevents torpor entry or induces arousal, respectively. However, the data in the current study do not support the initial hypothesis that central administration of CHA induces a natural bout of daily torpor in mice. Similar to previous reports in the arctic ground squirrel and rat (Jinka, Tøien et al. 2011, Tupone, Madden et al. 2013), Tb and HR decreased significantly in the mouse due to injection of CHA in the lateral ventricle (Figure 1). The mode of CHA administration in the current set of experiments is important. Because cells outside of the brain express adenosine receptors, it is important to acknowledge the possibility that the CHA injected ICV could cross the blood brain barrier into the periphery. However, the dosage that we used here (50 pmol) is vanishingly small relative to the dosage of peripheral adenosine that influences Tb in mice (∼0.0005 mg/kg vs. 100 mg/kg, respectively). Based on multiple lines of evidence, the hypothermia induced by CHA injection into the lateral ventricle has physiological characteristics that are distinct from the hypothermia observed during a natural bout of torpor.
HR dynamics differed between conditions at two levels: 1) the relationship between HR and Tb, and 2) HR pauses. The Tb/HR loop observed in natural torpor has been seen previously (Morhardt 1970, Milsom, Zimmer et al. 1999, Swoap and Gutilla 2009), and similar loops have been observed between Tb/metabolic rate and Tb/QT interval of the ECG (Mertens, Stiedl et al. 2008, Geiser, Currie et al. 2014). The breadth of the Tb/HR loop is driven by the autonomic nervous system, with the parasympathetic nervous system (PNS) dominating during entry into torpor and the sympathetic nervous system (SNS) active during exit from torpor (Milsom, Zimmer et al. 1999). Tb/HR loop in FH mice has almost no breadth, suggesting that the autonomic influence on HR is nearly the same during entry and exit into hypothermia. The Tb/HR loops in isoflurane-anesthetized mice were intermediate in their breadth as has been seen previously (Morhardt 1970), consistent with the finding that isoflurane depresses the SNS but has little effect on the PNS (Constantinides, Mean et al. 2011). We show here that CHA causes a drop in HR before a drop in Tb, but the resulting Tb/HR loop has diminished breadth as compared to natural torpor. Others have shown similar relationships with oxygen consumption, carbon dioxide production, and HR, as these all fall before Tb when arctic ground squirrels or rats are injected ICV with CHA (Jinka, Tøien et al. 2011, Tupone, Madden et al. 2013). The diminished breadth in the CHA condition (Figure 1) shown here is important because it suggests that injection of CHA did not fully involve the ANS for HR control. Specifically, entry into hypothermia did not reduce HR in the CHA condition as deeply as in the natural torpor condition, resulting in a smaller difference in HR during entry and exit from hypothermia (Figure 1C). Because the slowed HR during entry into torpor is mediated through elevated PNS activity, it seems that the PNS is not fully engaged during entry into hypothermia induced by CHA. However, this hypothesis requires further study with pharmacological methods to manipulate ANS control of HR during induced hypothermia.
One distinctive cardiovascular feature of natural torpor in mammals is the appearance of skipped beats, particularly during entry into torpor (Lyman and O'Brien 1963, Milsom, Zimmer et al. 1999). Administration of atropine, a muscarinic antagonist, causes the disappearance of the skipped beats during a natural bout of torpor, suggesting that the skipped beats are mediated via the PNS (Milsom, Zimmer et al. 1999). While the current study observed skipped beats in natural torpor, no HR pauses or skipped beats were observed in either FH or isoflurane-anesthesia, demonstrating a lack of the torpor-specific PNS action in these two models of hypothermia. Mice given CHA exhibited long, asymmetric HR pauses that required a 2-3 beat recovery period to return to the HR before the pause (see Figure 3B tracing and open arrow in Figure 5). Rats also exhibit HR pauses/skipped beats in response to central CHA administration that then disappear with the administration of atropine after CHA (Tupone, Madden et al. 2013). This suggests that the long pauses between beats in rats in response to central CHA are mediated by the PNS. Administration of atropine during CHA-induced hypothermia in mice in the current study was not performed so the mechanism of sinus pauses herein remains unknown. However, the HR of CHA-treated mice is higher than the HR of torpid mice at all Tbs during descent into hypothermia, suggesting that if the PNS activity is elevated in CHA-treatment (lowering heart rate), it is not elevated nearly as much as it is during a torpor bout. Hence, the mechanism of the sinus pauses in the mouse given ICV CHA is unknown, and we speculate that they may be a result of altered autonomic activity.
The CHA-induced hypothermic bout had a faster fall in Tb during entrance into hypothermia compared to natural torpor (Figure 2). This elevated rate of descent into hypothermia could be a result of faster metabolic inhibition by CHA relative to natural torpor, increased heat loss with CHA relative to natural torpor (e.g. the tail artery vasoconstriction that occurs in natural torpor may be impaired or absent with CHA) or some combination of the two. In addition, the CHA-treated mice recovered from hypothermia more slowly than the other groups (Figure 2). CHA-treated mice rarely shivered and this lack of shivering likely contributed to the slower Tb recovery (Figure 3). The lack of shivering with central CHA administration has also been seen in the rat (Tupone, Madden et al. 2013). The mechanism of shivering inhibition by CHA is not clear, although this could be an advantage for this compound in targeted temperature management, where current protocols for humans utilize anti-shivering agents (Group 2002, Scirica 2013). Further, the slowed rate of rewarming from CHA administration may be due to the inability to sympathetically activate brown fat pads. Indeed, mice deficient in UCP1 recover from torpor bouts at about half the rate of wild type mice (Oelkrug, Heldmaier et al. 2011).
As discussed above, the autonomic nervous system is important at several stages of the torpor bout. The PNS is active during entrance into torpor which slows HR and leads to asystoles. Vasoconstriction of vessels feeding peripheral tissues like the tail is induced via the SNS during the torpor bout, slowing the rate of heat loss. SNS activity increases HR and heat production from brown fat in a UCP1 dependent way during arousal, influencing the rate of heat gain during arousal. Further, we (Swoap, Gutilla et al. 2006, Swoap and Weinshenker 2008) and others (Braulke and Heldmaier 2010) have shown the absolute requirement for a functional SNS for entry into torpor, whereas blocking PNS activity with atropine does not impact the likelihood of torpor bouts in the hamster (Braulke and Heldmaier 2010). Given that CHA-induced hypothermia resulted in 1) an elevated hypothermic HR relative to natural torpor, 2) entrance into hypothermia at a faster rate than natural torpor, and 3) arousal from hypothermia at a slower rate than natural torpor, it seems that CHA administered ICV at this dose does not fully capture the modulation of the autonomic nervous system throughout a natural torpor bout.
The third line of evidence utilized for assessment of hypothermia was circulating lactate. Elevated circulating lactate is traditionally believed to be caused by respiring tissues that turn to anaerobic metabolism during hypoxia and hypoperfusion. Hypoxia in the mouse is associated with elevated levels of circulating adenosine (Dunwiddie and Masino 2001), and thus there may be a link between hypoxia, adenosine, and hypothermia. In both natural torpor and CHA-induced hypothermia, however, the lactate levels were lower than baseline (Figure 4), suggesting that neither of these models evoked global hypoxia. Indeed, the low circulating lactate level in the CHA condition indicates either an active suppression of metabolism or increased utilization of lactate, or both. The lack of shivering observed in this study in mice injected with CHA, combined with previous data showing that CHA in the rat decreases brown adipose heat production, EMG activity, and CO2 production, suggest a suppression of metabolic rate as the reason for low lactate levels (Tupone, Madden et al. 2013). The high lactate levels during FH may be at least partially explained by the vigorous shivering exhibited by these mice due to SNS activation during descent into hypothermia.
The fourth level of interrogation of the hypothermic state was analysis of hepatic and cardiac steady state mRNA levels using a “mouse-hypoxia” PCR array. The vast majority of the analyzed hypoxia-related mRNAs were unchanged among any of the states (data not shown). Hypoxia inducible factor 1-alpha (Hif1a) is a transcription factor involved in the cellular response to hypoxic conditions that plays an important role in the metabolic switch between oxidative phosphorylation and anaerobic metabolism. It was expected that Hif1a would be upregulated in hypothermic states due to the decreased blood flow to tissues, particularly with isoflurane-anesthesia and FH as both of these states lead to elevated circulating lactate levels. However, Hif1a was only moderately induced in the liver of FH mice. Two genes that distinguished torpid from non-torpid hypothermic states were c-Fos and Egr1. These transcription factors are both early response genes, where the former is a proto-oncogene and the latter is part of the MAP kinase pathway, and are markers of increased cellular activity. The mRNAs for both of these genes were robustly activated in the heart and liver of FH, isoflurane anesthesia, and CHA-treated mice, yet not induced in natural torpor (Figure 6). In contrast, thioredoxin interacting protein (Txnip) was upregulated only in the liver and heart of torpid mice and the liver of FH mice. Txnip regulates cellular redox by binding and inhibiting the action of the thioredoxin protein, which results in the accumulation of reactive oxygen species. This gene was previously found to be induced in the hypothalamus, white and brown adipose tissue, and liver of torpid mice and Siberian hamsters (Hand, Saer et al. 2013). Based on their results, these authors suggested that Txnip is involved in regulating energy expenditure during torpor.
In conclusion, this study robustly replicates and extends to mice the observation that CHA induces hypothermia, while further investigating cardiovascular and biochemical characteristics during this induced hypothermic state. The data herein suggest that CHA-induced hypothermia in fed mice captured some of the aspects of natural torpor, but missed others, notably the HR dynamics of natural torpor. However, we tested only a single dose of CHA, 50 pmol, which was based on two previous publications (weight adjusted for rats and arctic ground squirrels), that also published only a single dose of CHA ICV (Jinka, Tøien et al. 2011, Tupone, Madden et al. 2013). A more comprehensive dose response study might help to fully illuminate the influence that CHA might have on the suite of coordinated physiological responses in the mouse. Also, only female mice were tested in the current study, and thus extrapolation of our findings to male mice should be taken with caution. Administering CHA to a mouse with a genotype that is unable to undergo daily torpor, such as a dbh-/- mouse (Swoap, Gutilla et al. 2006) or a mouse with an ablated ARC (Gluck, Stephens et al. 2006), might definitively distinguish between the two states. Further, we recognize the potential for off-target effects of CHA, such as such as phosphorylation, and subsequent de-sensitization, of delta-opioid receptors (Cheng, Tao et al. 2010), which may be relevant as delta opioid receptors may be involved in hypothermia/torpor (Borlongan, Hayashi et al. 2009). Future experiments should use specific knock-out mice or combine CHA with a specific A1-antagonist to rule out effects mediated by targets other than the A1-receptor. It is important to note that CHA was administered to mice in the fed condition in the current study whereas natural torpor was achieved through caloric restriction. Others have shown an increased sensitivity to CHA in the fasted state in rats (Jinka, Carlson et al. 2010). Hence, it may be possible that CHA requires another signal induced by caloric restriction to fully recapitulate the physiological and behavioral aspects of natural torpor. The mode of administration, namely through an indwelling catheter in the lateral ventricle of the brain and not peripherally, suggests that adenosine receptor agonists can function at regions within the brain. Given the dosage used in the current study, 50 pmol, and the mode of administration (ICV) it is highly unlikely that CHA was acting at peripheral sites (i.e. heart, vasculature).
It is very likely that the central signal that initiates fasting-induced torpor is actually a combination of signals that arise from a calorically restricted state. Indeed, circulating satiety hormone levels, such as leptin, have been implicated in the torpor pathway as torpor can be inhibited in ob/ob mice through leptin administration (Gavrilova, Leon et al. 1999). While CHA injection appears not to induce a natural torpor state, it is important to note that 1) despite the arrhythmias in CHA-injected mice, no mice died as a result of the CHA; and 2) administration of this compound may have therapeutic potential, as rats subjected to cardiac arrest showed decreased neuronal cell death and increased survival after CHA IP injection (Miller and Hsu 1992, Jinka, Combs et al. 2015).
References
- Ahmad FU, Wang MY, Levi AD. Hypothermia for acute spinal cord injury–a review. World Neurosurg. 2014;82(1-2):207–214. doi: 10.1016/j.wneu.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Anderson R, Sheehan MJ, Strong P. Characterization of the Adenosine Receptors Mediating Hypothermia in the Concious Mouse. British Journal of Pharmacology. 1994;113(4):1386–1390. doi: 10.1111/j.1476-5381.1994.tb17151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol. 2009;7(3):238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Hayashi T, Oeltgen PR, Su TP, Wang Y. Hibernation-like state induced by an opioid peptide protects against experimental stroke. BMC Biol. 2009;7:31. doi: 10.1186/1741-7007-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma HR, Verhaag EM, Otis JP, Heldmaier G, Swoap SJ, Strijkstra AM, Henning RH, Carey HV. Induction of torpor: Mimicking natural metabolic suppression for biomedical applications. J Cell Physiol. 2011 doi: 10.1002/jcp.22850. [DOI] [PubMed] [Google Scholar]
- Braulke LJ, Heldmaier G. Torpor and ultradian rhythms require an intact signalling of the sympathetic nervous system. Cryobiology. 2010;60(2):198–203. doi: 10.1016/j.cryobiol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Daly JW, Snyder SH. Adenosine Receptor Binding: Structure–Activity Analysis Generates Extremely Potent Xanthine Antagonists. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(7):2077–2080. doi: 10.1073/pnas.80.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, van den Pol AN. Adenosine modulation of calcium currents and presynaptic inhibition of GABA release in suprachiasmatic and arcuate nucleus neurons. Journal of neurophysiology. 1997;77(6):3035–3047. doi: 10.1152/jn.1997.77.6.3035. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Tao Ym, Sun Jf, Wang Yh, Xu Xj, Chen J, Chi Zq, Liu Jg. Adenosine A1 receptor agonist N6-cyclohexyl-adenosine induced phosphorylation of delta opioid receptor and desensitization of its signaling. Acta Pharmacol Sin. 2010;31(7):784–790. doi: 10.1038/aps.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides C, Mean R, Janssen BJ. Effects of Isoflurane Anesthesia on the Cardiovascular Function of the C57BL/6 Mouse. ILAR journal/National Research Council, Institute of Laboratory Animal Resources. 2011;52:e21–e31. [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The Role and Regulation of Adenosine in the Central Nervous System. Annual Review of Neuroscience. 2001;24(1):31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Johansson S, Wang YQ. Adenosine and the Regulation of Metabolism and Body Temperature. Advances in Pharmacology A J Kenneth and L Joel, Academic Press Volume. 2011;61:77–94. doi: 10.1016/B978-0-12-385526-8.00003-5. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, Vinson C, Reitman ML. Torpor in mice is induced by both leptin-dependent and-independent mechanisms. Proceedings of the National Academy of Sciences. 1999;96(25):14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F, Currie SE, O'Shea KA, Hiebert SM. Torpor and hypothermia: reversed hysteresis of metabolic rate and body temperature. 2014;307(11):R1324–R1329. doi: 10.1152/ajpregu.00214.2014. [DOI] [PubMed] [Google Scholar]
- Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1303–1309. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. The therapeutic potential of regulated hypothermia. Emergency Medicine Journal. 2001;18(2):81–89. doi: 10.1136/emj.18.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group T. H. a. C. A. S. Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. New England Journal of Medicine. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Hand LE, Saer BRC, Hui ST, Jinnah HA, Steinlechner S, Loudon ASI, Bechtold DA. Induction of the Metabolic Regulator Txnip in Fasting-Induced and Natural Torpor. Endocrinology. 2013;154(6):2081–2091. doi: 10.1210/en.2012-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller HC, Ruby NF. Sleep and Circadian Rhythms in Mammalian Torpor. Annual Review of Physiology. 2004;66(1):275–289. doi: 10.1146/annurev.physiol.66.032102.115313. [DOI] [PubMed] [Google Scholar]
- Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012;303(5):R477–R484. doi: 10.1152/ajpregu.00081.2012. [DOI] [PubMed] [Google Scholar]
- Jinka T, Carlson Z, Moore J, Drew K. Altered thermoregulation via sensitization of A1 adenosine receptors in dietary-restricted rats. Psychopharmacology. 2010;209(3):217–224. doi: 10.1007/s00213-010-1778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Combs VM, Drew KL. Translating Drug-Induced Hibernation to Therapeutic Hypothermia. ACS Chemical Neuroscience. 2015;6(6):899–904. doi: 10.1021/acschemneuro.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Tøien Ø, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A1 receptors. The Journal of Neuroscience. 2011;31(30):10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman CP, O'Brien RC. Autonomic Control of Circulation During Hibernating Cycle in Ground Squirrels. Journal of Physiology-London. 1963;168(3):477–499. doi: 10.1113/jphysiol.1963.sp007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher RL, Barbash SM, Lynch DV, Swoap SJ. Group housing and nest building only slightly ameliorate the cold stress of typical housing in female C57BL/6J mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2015;308(12):R1070–R1079. doi: 10.1152/ajpregu.00407.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens A, Stiedl O, Steinlechner S, Meyer M. Cardiac dynamics during daily torpor in the Djungarian hamster (Phodopus sungorus) Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R639–650. doi: 10.1152/ajpregu.00496.2007. [DOI] [PubMed] [Google Scholar]
- Miller LP, Hsu C. Therapeutic potential for adenosine receptor activation in ischemic brain injury. J Neurotrauma. 1992;9(2):S563–577. [PubMed] [Google Scholar]
- Milsom WK, Zimmer MB, Harris MB. Regulation of cardiac rhythm in hibernating mammals. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology. 1999;124(4):383–391. doi: 10.1016/s1095-6433(99)00130-0. [DOI] [PubMed] [Google Scholar]
- Minor RK, Chang JW, De Cabo R. Hungry for life: How the arcuate nucleus and neuropeptide Y may play a critical role in mediating the benefits of calorie restriction. Molecular and cellular endocrinology. 2009;299(1):79–88. doi: 10.1016/j.mce.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morhardt JE. Heart Rates, Breathing Rates and Effects of Atropine and Acetylcholine on White-Footed Mice (Peromyscus Sp) During Daily Torpor. Comparative Biochemistry and Physiology. 1970;33(2):441–457. doi: 10.1016/0010-406x(70)90360-9. [DOI] [PubMed] [Google Scholar]
- Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, Pittelli M, Cavone L, Pugliese AM, Moroni F, Chiarugi A. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. Journal of Cerebral Blood Flow and Metabolism. 2013;33(2):183–190. doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkrug R, Heldmaier G, Meyer C. Torpor patterns, arousal rates, and temporal organization of torpor entry in wildtype and UCP1-ablated mice. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2011;181(1):137–145. doi: 10.1007/s00360-010-0503-9. [DOI] [PubMed] [Google Scholar]
- Olson JM, Jinka TR, Larson LK, Danielson JJ, Moore JT, Carpluck J, Drew KL. Circannual Rhythm in Body Temperature, Torpor, and Sensitivity to A1 Adenosine Receptor Agonist in Arctic Ground Squirrels. Journal of Biological Rhythms. 2013;28(3):201–207. doi: 10.1177/0748730413490667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz KM, Routman D, Driscoll JR, Kriegsfeld LJ, Dark J. Monosodium glutamate-induced arcuate nucleus damage affects both natural torpor and 2DG-induced torpor-like hypothermia in Siberian hamsters. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;294(1):R255–R265. doi: 10.1152/ajpregu.00387.2007. [DOI] [PubMed] [Google Scholar]
- Scirica BM. Therapeutic Hypothermia After Cardiac Arrest. Circulation. 2013;127(2):244–250. doi: 10.1161/CIRCULATIONAHA.111.076851. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Gutilla MJ. Cardiovascular changes during daily torpor in the laboratory mouse. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R769–774. doi: 10.1152/ajpregu.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The Full Expression of Fasting-Induced Torpor Requires {beta}3-Adrenergic Receptor Signaling. J Neurosci. 2006;26(1):241–245. doi: 10.1523/JNEUROSCI.3721-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Iliff BW, Le S. Adenosine, AMP, and Daily Torpor. Heidelberg Springer; 2012. [Google Scholar]
- Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;293(1):R468–R473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Weinshenker D. Norepinephrine Controls Both Torpor Initiation and Emergence via Distinct Mechanisms in the Mouse. PLoS ONE. 2008;3(12):e4038. doi: 10.1371/journal.pone.0004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H. Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Research. 2005;1045(1-2):88–96. doi: 10.1016/j.brainres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF. Central Activation of the A1 Adenosine Receptor (A1AR) Induces a Hypothermic, Torpor-Like State in the Rat. Journal of Neuroscience. 2013;33(36):14512–14525. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Glotzbach S, Berger R, Heller H. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1977;233(5):R213–R221. doi: 10.1152/ajpregu.1977.233.5.R213. [DOI] [PubMed] [Google Scholar]
- Yang JN, Tiselius C, Daré E, Johansson B, Valen G, Fredholm BB. Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors. Acta Physiologica. 2007;190(1):63–75. doi: 10.1111/j.1365-201X.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]


