Abstract
The medial temporal lobe, including the hippocampus, has been implicated in social memory. However, it remains unknown which parts of these brain regions and their circuits hold social memory. Here, we show that ventral hippocampal CA1 (vCA1) neurons of a mouse and their projections to nucleus accumbens (NAc) shell play a necessary and sufficient role in social memory. Both the proportion of activated vCA1 cells and the strength and stability of the responding cells are greater in response to a familiar mouse than to a novel mouse. Optogenetic reactivation of vCA1 neurons that respond to the familiar mouse enabled memory retrieval and the association of these neurons with unconditioned stimuli. Thus, vCA1 neurons and their NAc shell projections are a component of the storage site of social memory.
The ability to recognize and memorize familiar conspecifics (social memory) is crucial for animals that exhibit social interactions (1, 2). Lesion and recording studies in humans and monkeys have suggested that the medial temporal lobe or the hippocampus play an essential role in social memory (3–6). In mice, most early lesion or recording studies concluded that the hippocampus is dispensable for recognizing a familiar conspecific (7–9), while a few recent studies suggested the contrary (10–12). Although these human and animal studies identified brain areas important for social memory, the precise cellular populations storing this type of memory and their essential circuits are unknown.
The intrinsic pattern of connectivity in the hippocampus is fairly invariable along the longitudinal axis (13) across many species (14). However, the afferent and efferent connectivity along this axis changes from one end to the other, suggesting that dorsal and ventral hippocampus of rodents (corresponding to posterior and anterior hippocampus, respectively, of primates) may have distinct functions (14, 15). It is well established that the rodent dorsal hippocampus (dHPC) plays an essential role in episodic memory (15). In contrast, the memory function of the ventral hippocampus (vHPC) is poorly known. In this study, we generated a transgenic mouse line, Trpc4-Cre, to investigate the potential role and physiological characteristics of the pyramidal neurons in the CA1 subfield of the ventral hippocampus (vCA1) in social memory.
Mice naturally spend more time interacting with a novel mouse rather than a familiar one (16); social memory can be quantified by measuring the relative interaction durations with a novel and a familiar mouse under free choice conditions (social discrimination test or SDT) (Fig. 1, A and B). This ability to socially discriminate between the novel and familiar mice persisted for 30 min following a familiarization session, but disappeared by 24 h (Fig. 1C). In order to investigate the potential role of vHPC and dHPC in social memory, we targeted bilateral injections of AAV8-CaMKII:eArchT-EYFP and optic fiber implants to vCA1 or dorsal CA1 (dCA1) of wild-type mice (Fig. 1D). Expression of eArchT-EYFP was abundant in vCA1 or dCA1, although it was also observed to a lesser extent in CA3 and the dentate gyrus (DG) (Fig. 1, E and F). Optogenetic cell body inhibition of vHPC but not dHPC resulted in a deficit in SDT (Fig. 1, G, H, O, and P). In the resident-intruder test (Fig. 1I and fig. S1), optogenetic inhibition of vHPC but not dHPC increased the sniffing duration towards a familiar intruder with no effect when a novel intruder was used (Fig. 1, J and K).
Fig. 1. vHPC in social memory.
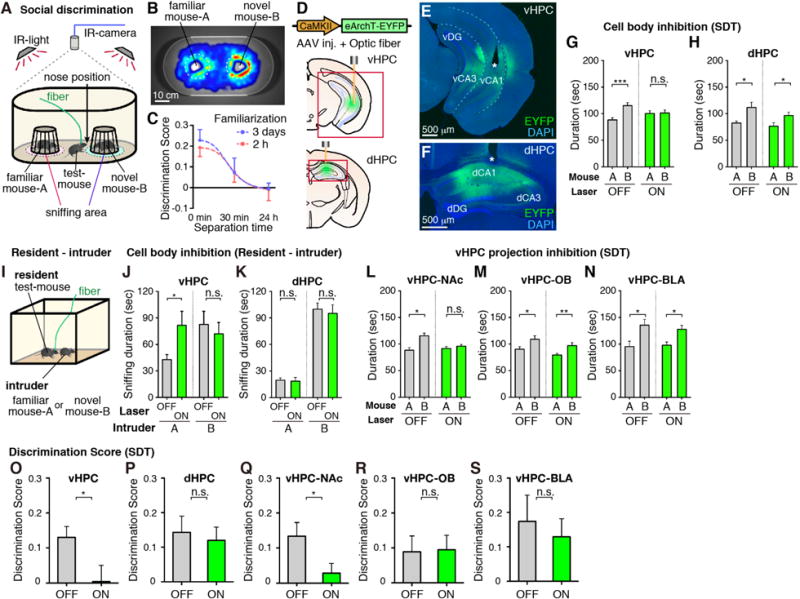
(A) Social discrimination test (SDT). (B) Representative heat map of test-mouse position during SDT. (C) Kinetics of SDT (see Methods). (D to F) Expression of AAV8-CaMKII:eArchT-EYFP (green) in vHPC or dHPC of wild-type mice. Asterisk indicates optic fiber tip. (G, H) Total duration in the sniffing area of familiar mouse-A or novel mouse-B during SDT with inhibition of vHPC (G, O, n = 17) or dHPC (H, P, n = 14). (I) Resident-intruder test. (J, K) Total sniffing duration by the resident to intruder (J, vHPC inhibition; K, dHPC inhibition) in familiar intruder group (left) and novel intruder group (right) (n = 7, each group). (L to N) Total duration in sniffing area during SDT observed in wild-type mice bilaterally injected with AAV8-CaMKII-eArchT-EYFP into vHPC and implanted with optical fibers targeting NAc (L, Q, n = 23), OB (M, R, n = 16), or BLA (N, S, n = 13). (O to S) Comparison of discrimination scores. Green bars, laser-ON; Grey bars, laser-OFF. Significance for multiple comparisons: paired t-test, *p < 0.05; **p < 0.01; ***p < 0.001, n.s., not significant. Data presented as mean ± S.E.M.
To identify downstream brain region(s) involved in social memory, AAV9-TRE:channelrhodopsin-2 (ChR2)-EYFP was injected into vCA1 of c-fos:tTA mice to label neurons activated by social interaction (17, 18). NAc shell, olfactory bulb (OB), and basolateral amygdala (BLA) were the major targets of the social interaction-specific vCA1 neuronal projections (fig. S2). Moreover, retrograde tracer cholera toxin subunit B (CTB)-Alexa555 injection into NAc, OB, or BLA labeled vCA1 but not dCA1 neurons (fig. S3). We then examined whether any of these projections are necessary for social memory by bilaterally injecting AAV8-CaMKII:eArchT-EYFP into vHPC and then optogenetically inhibiting axonal terminals of the vCA1 neurons in the respective target areas while the mice went through SDT (Fig. 1, L to N, Q to S, and fig. S4). vHPC-NAc projections, but not vHPC-OB or vHPC-BLA, were essential for social discrimination behavior.
To more rigorously establish the functional role of the vCA1-NAc connection, we generated a CA1 pyramidal cell-specific Cre mouse line, Trpc4-Cre that covers both vCA1 and dCA1 (fig. S5, and Methods). Using Trpc4-Cre mice, vCA1 excitatory pyramidal neurons were selectively targeted by injecting AAV9-hsyn:DIO-eArchT-EYFP into vCA1 (Fig. 2, A and B, and fig. S6). We confirmed that the vCA1 neurons specifically project to the NAc shell but not to the NAc core, as identified by tyrosine hydroxylase (TH) staining (Fig. 2C). Further, CTB injection into NAc labeled the Trpc4-expressing deep layer (vCA1d), but not the superficial layer (vCA1s) pyramidal cells in vCA1 (Fig. 2B). Bilateral injections of AAV9-hsyn:DIO-eArchT-EYFP into the vCA1 of Trpc4-Cre mice and optogenetic inhibition of vCA1 cell body (Fig. 2, D and H) or its axonal terminals in NAc (Fig. 2, F, G, and J) resulted in a SDT deficit similar to that observed by vHPC-NAc inhibition (compare Fig. 2G with Fig. 1L). Optogenetic inhibition of vCA1 cell body during the familiarization period also led to a similar SDT defect (fig. S8). The possibility that these deficits are due to inhibition of dorsal CA2 (dCA2) activity is excluded because dCA2 is unlabeled with eArchT-EYFP in these mice (fig. S6C). dCA1 cell body inhibition was carried out using Wfs1-Cre mouse line expressing Cre in dCA1 but not in vCA1 or dCA2 (fig. S9) (19), which showed no deficit in SDT (Fig. 2, E and I).
Fig. 2. vCA1-NAc circuit in SDT.
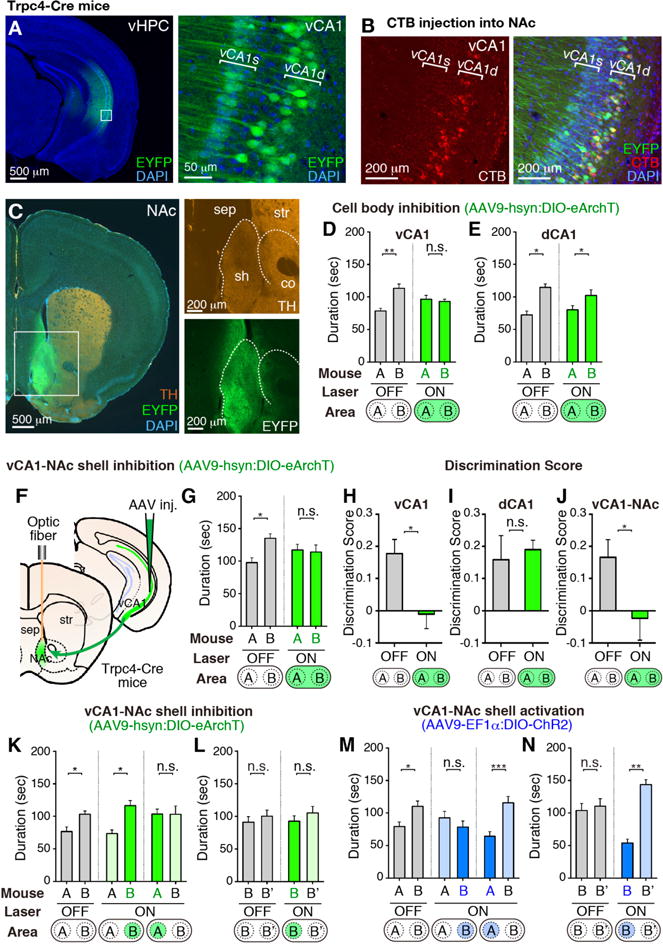
(A) Coronal vHPC sections of a Trpc4-Cre mouse injected with AAV9-hsyn:DIO-eArchT-EYFP into vCA1, stained with anti-GFP (green) and DAPI (blue). (B) CTB-Alexa555 (red) injection into NAc and stained with anti-GFP (green) and DAPI (blue). (C) Coronal NAc sections of a Trpc4-Cre mouse injected with AAV9-hsyn:DIO-eArchT-EYFP into vCA1, stained with anti-GFP (green), anti-TH (orange), and DAPI (blue). NAc shell (sh); NAc core (co); Septum (sep); Striatum (str). Boxed areas in (A, C) are magnified. (D, E) Cell body inhibition of vCA1 and dCA1 during SDT in Trpc4-Cre and Wfs1-Cre mice, respectively. (F) Manipulation of vCA1-NAc shell projections. (G, K to N) SDT in Trpc4-Cre mice during vCA1-NAc manipulation. vCA1 injections of AAV9-hsyn:DIO-eArchT-EYFP (G, n =14; K and L, each n = 12) and AAV9-EF1α:DIO-ChR2-EYFP (M and N, each n = 14). (H to J) Comparison of discrimination scores. Bottom, targeting area for the laser-stimulation. mouse-A, familiar mouse; mouse-B and -B′, novel mouse. Green bars, green laser-ON; blue bars, blue laser-ON; grey bars, laser-OFF. Significance for multiple comparisons: paired t-test, *p < 0.05; **p < 0.01; n.s., not significant. Data presented as mean ± S.E.M.
Inhibition of vCA1-NAc shell projections in Trpc4-Cre mice only during the interaction with a novel mouse did not affect SDT (Fig. 2K, middle, and fig. S7A), whereas inhibition only during interaction with a familiar mouse disrupted social discrimination between the two mice (Fig. 2K, right, and fig. S7A). In contrast, when a pair of novel mice (Fig. 2L and fig. S7B), novel and familiar objects (fig. S10A), or novel and familiar contexts (fig. S10B) were used, there was no effect of vCA1-NAc shell inhibition.
We performed SDT using Trpc4-Cre mice expressing AAV9-EF1α:DIO-ChR2-EYFP in vCA1 while stimulating vCA1-NAc shell projections (Fig. 2, M and N, and fig. S7). Optogenetic activation of vCA1-NAc shell terminals during social interaction with a novel mouse disrupted SDT (Fig. 2M, middle, and fig. S7C). With a pair of novel mice as the targets, stimulating vCA1-NAc shell projections during interactions with one of the novel mice greatly reduced the sniffing duration of that mouse compared to the other novel mouse (Fig. 2N, and fig. S7D). Optogenetic activation of vCA1-NAc shell terminal during interaction with a familiar mouse had no effect in SDT (Fig. 2M, right, and fig. S7C). Light activation did not affect SDT in the EYFP control groups (fig. S11, A and B). With novel objects in place of mice, interactions of the test-mouse were not affected by activation of the vCA1-NAc shell projections (fig. S11C). These results suggest that increased activation of the vCA1-NAc shell projections while the test mouse was in the novel mouse domain disrupted the discriminatory social behavior by making the test mouse perceive the novel mouse as familiar.
In order to monitor the activity of vCA1 cells before and after the familiarization of a conspecific mouse, AAV5-hsyn:DIO-GCaMP6f was injected into the vCA1 of Trpc4-Cre mice and implanted a microprism grin lens targeting the pyramidal cell layer in vCA1 (Fig. 3, A and B, and Methods) (20, 21). Ca2+ events in vCA1 neurons (Fig. 3, D and E) were recorded during exposure to two novel mice (A and B) in two consecutive 5 min sessions with mouse-A and mouse-B in counterbalanced positions, followed by a 5 min control session with no mice (Before Group). The test mice were subjected to 3 day-long or 2 h-long familiarization with mouse-A and the recording sequence was repeated after 30 min or 24 h separation (After-1 Group) (Fig. 3F). For each neuron, we calculated a “Preference Score” based on the head position of the test-mouse during each recorded Ca2+ event, and identified vCA1 cells that exhibited selective activation by mouse-A or mouse-B (Fig. 3G and fig. S12). There was a significant increase in the proportion of mouse-A neurons after 3 days or 2 h familiarization to mouse-A (Fig. 3H). In contrast, there was no effect of familiarization on the proportion of cells active around mouse sampling locations in control sessions (No mouse). Similarly, familiarization had no effect on the proportion of mouse-A neurons in dCA1 of Wfs1-Cre transgenic mice (Fig. 3, C and H).
Fig. 3. Ca 2+ events in vCA1.
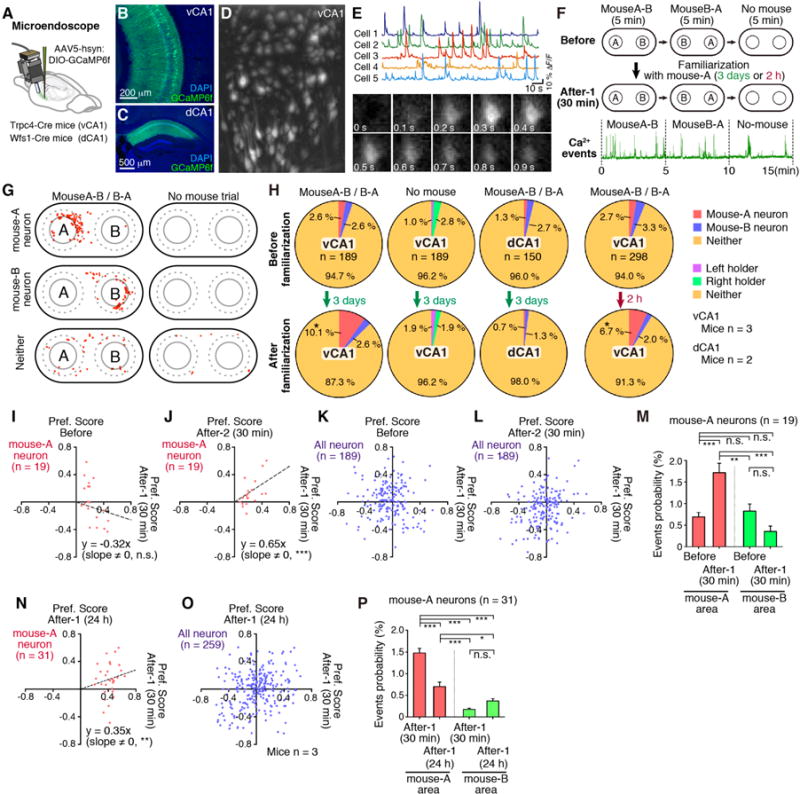
(A) Microendoscope. AAV5-hsyn:DIO-GCaMP6f injection into vCA1 of Trpc4-Cre mice or dCA1 of Wfs1-Cre mice. (B, C) Coronal sections of vCA1 and dCA1 stained with anti-GFP (green, for GCaMP6f) and DAPI (blue). (D) Stacked image acquired during a 15 min microendocope recording. (E) Top, Relative fluorescence changes (ΔF/F) for five vCA1 pyramidal neurons. Bottom, Time-lapse image sequence of GCaMP6f fluorescence in an individual neuron. (F) Top, Experimental protocol for microendoscope recording. Bottom, Relative fluorescence changes during 15 min recording. (G) Representative mouse-A neuron, mouse-B neuron, and neither neuron. Head-position at each Ca2+ event (red dots). (H) Proportion of mouse-A, mouse-B, or neither neurons in vCA1 and dCA1, before and after familiarization (3 days or 2 h) (chi-square test, *p < 0.05). (I to L, N, O) Comparison of the preference scores of mouse-A neurons (I, J, N, red dots) and all recorded neurons (K, L, O, blue dots) between After-1 (30 min) and Before sessions (I, K; linear regression, p = 0.208), between After-1 (30 min) and After-2 (30 min) (J, L, linear regression, p = 0.0002; Spearman rank correlation test, r = 0.65), and between After-1 (30 min) and After-1 (24 h) (N, O, linear regression, p = 0.0019; Spearman rank correlation test, r = 0.23). (M, P) Comparison of Ca2+ event probabilities of mouse-A neurons (ANOVA; post-hoc, Scheffe, ***p < 0.001, **p < 0.01). Data presented as mean ± S.E.M.
We determined the Preference Score of mouse-A neurons in Before Group and After-1 Group as well as After-2 Group that had a second 3 day familiarization. The Preference Scores of individual mouse-A neurons were not correlated between Before Group and After-1 Group while these score were correlated between After-1 Group and After-2 Group (Fig. 3, I to L). In addition, mouse-A neurons of After-1 Group showed an increase in Ca2+ event probability around the mouse-A sniffing area while mouse-A neurons did not show such an increase in Ca2+ probability in the mouse B sniffing area. (Fig. 3M). Although the individual mouse-A neurons were still significantly activated by mouse-A (Fig. 3, N and O), Ca2+ event probability of these neurons in mouse-A area was reduced when the recordings were conducted after 24 h separation following familiarization (Fig. 3P).
The c-fos-tTA/TRE system permits labeling and manipulations of memory engram cells (17, 24). We used this technology to characterize mouse-A neurons. First, we injected AAV9-TRE:fluorescent timer (FT)-Slow into the vCA1 of c-fos:tTA mice and induced expression of FT-Slow in neurons activated by social interaction (Fig. 4A). The fluorescence of FT-Slow naturally changes over time (22), from blue (pseudo-green) 12 h after induction to red 72 h after induction (Fig. 4, A and B, and fig. S13). Test-mice interacted with mouse-A twice, or with mouse-A and then mouse-B, with a 72 h separation (Fig. 4, C and D). The proportions of reactivated cells with an overlap of red and blue signal was significantly higher in test-mice exposed to the same mouse-A twice, versus those exposed to mouse-A followed by mouse-B (Fig. 4E, and fig. S13). A similar quantitative analysis of vCA1 reactivation using H2B-EGFP (nuclear localized EGFP) and c-Fos expression showed comparable results (fig. S14).
Fig. 4. Social memory engrams.
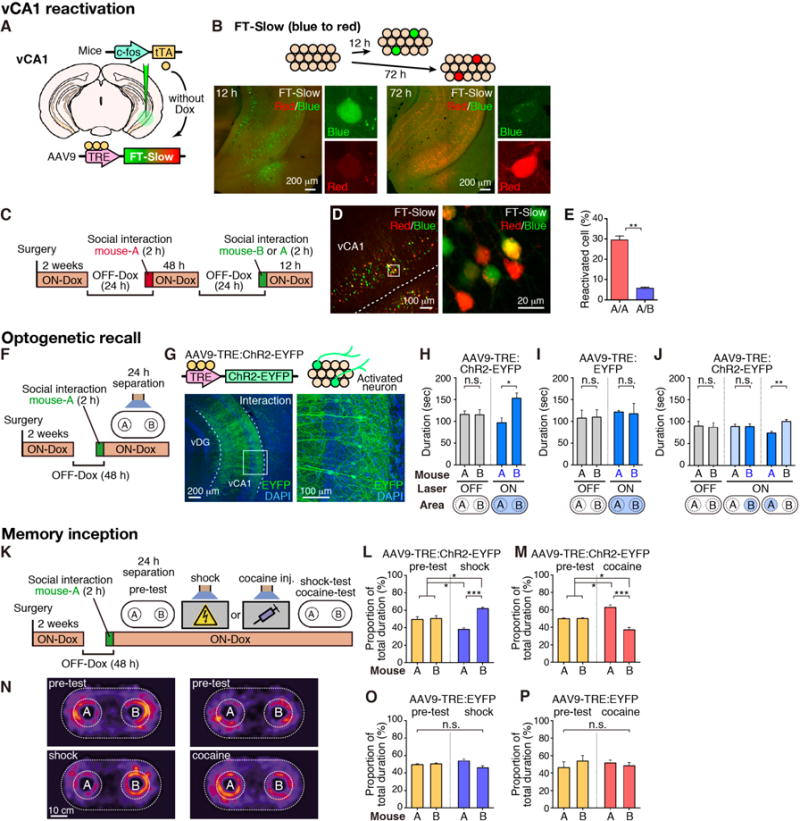
(A) Activity-dependent labeling method. (B) Injection of AAV9-TRE:FT-Slow into vCA1 of c-fos:tTA mice. Top, fluorescent color alteration of FT-Slow. Bottom, coronal vCA1 sections 12 h (left) and 72 h (right) after induction. Representative images of a blue form (pseudo green color)- or red form (red)-expressing cell. (C) Protocol for visualizing two activated neuronal populations. (D) FT-Slow blue form-, red form-, and double (yellow)-positive cells in vCA1 (left) with magnified image (right). (E) Percentage of reactivated cells when the test-mouse was exposed to the same mouse-A twice (A/A) or mouse-A then mouse-B (A/B) (n = 3, each group). (F) Protocol for optogenetic recall of social memory engram. (G) vCA1 section of c-fos:tTA mice injected with AAV9-TRE:ChR2-EYFP showing ChR2-labeling by social interaction. (H to J) SDT with or without activation of engram cells. blue bars, blue laser-ON; grey bars, laser-OFF. Bottom, targeting area for the laser-stimulation. (K) Protocol for memory inception. (L, M, O, P) Proportion of total duration in the sniffing area of mouse-A or mouse-B (yellow bars, pre-test; blue bars, shock-test; red bars, cocaine-test). AAV9-TRE:ChR2-EYFP (H, J, L, M) or AAV9-TRE:EYFP (I, O, P) injected c-fos:tTA mice. (N) Heat map representing nose position of test-mice. H, n = 10; I, n = 6; J, n = 10, L, n = 10; M, n = 14; O, n = 7; P, n = 7; Significance for multiple comparisons: paired t-test (H to I) and ANOVA, post-hoc, Scheffe (L, M, O, P), *p < 0.05; **p < 0.01; n.s., not significant. Data presented as mean ± S.E.M.
Second, we targeted injection of AAV9-TRE:ChR2-EYFP and optic fibers to vCA1 of c-fos:tTA mice and labeled with ChR2 vCA1 cells that were activated by 2 h exposure to a mouse-A while OFF-Dox (Fig. 4, F and G, and fig. S2). As expected, social memory was absent in the SDT conducted after 24 h separation (Fig. 4H, left), but was present when blue light was shone to the whole test box (Fig. 4H, right). Control experiments conducted with no ChR2 mice (Fig. 4I) or a pair of novel mice during SDT (fig. S15A) did not show social memory. Restricting blue light to mouse-A area but not mouse-B area, also caused the restoration of social memory (Fig. 4J). These results suggest that even after social memory cannot be retrieved by natural cues the memory engram cells for the familiar mouse are sufficiently retained and can be reactivated optogenetically for memory retrieval (17, 23). Indeed, the proportion of mouse-A neurons reactivated by blue light is much greater than that reactivated by natural cues (i.e. mouse-A) after 3 day separation (compare fig. S16G and Fig. 4E). We further investigated the parameters that affect social memory, including the post-familiarization separation periods, the proportion of reactivated familiar mouse-A neurons, the nature of recall cues (natural vs. optogenetic) and the strength of the reactivation methods (figs. S14 and S16). The data indicate that optogenetic stimulation is more effective than natural stimulation in re-activating memory engram cells, and that a certain minimum threshold level of reactivation of mouse-A neurons will have to be reached in order for social memory to be expressed in the SDT paradigm.
Third, we employed the memory inception protocol (Fig. 4K) (24). After 24 h separation ChR2-labeled mouse-A neurons were light-activated simultaneously with foot-shock delivery (i.e. negative US) or cocaine administration (i.e. positive US). The test mouse exhibited avoidance or approach behavior toward mouse-A, respectively, in SDT conducted the following day (Fig. 4, K to N). Negative control groups with no ChR2 (i.e. AAV9-TRE:EYFP alone) (Fig. 4, O and P) or no-US (fig. S15B) did not show any behavioral alterations. No avoidance or approaching behavior was observed when two novel mice (B and B′) were used as the target mice during the test (fig. S15, C and D). AAV9-TRE:ChR2-EYFP injection into dCA1 of c-fos:tTA mice did not lead to memory inception (fig. S17). The possibility that the observed optogenetic recall or memory inception is due to memory held in adjacent dCA2 is excluded because no labeling of dCA2 cells with ChR2 could be observed under our experimental conditions (fig. S17, D to F).
We have established that vCA1 and its projections to NAc shell play a necessary and sufficient role in social memory in the mouse. Furthermore, we have provided evidence supporting that vCA1 pyramidal cells hold the memory of a familiar mouse; a population of vCA1 pyramidal cells are activated by exposure to a familiar mouse, a large fraction of these cells are reactivated by re-exposure to the same mouse, and optogenetic re-activation of the vCA1 cells previously activated by exposure to a familiar mouse elicited recall of the specific social memory as monitored by a social discrimination test. Thus, the vCA1 cells activated by the exposure to a familiar mouse satisfy the criteria to be met by engram cells for a specific memory (25). It is interesting that this recall of the social memory by light can occur even after the mice have fallen into an amnesic state. This indicates that the specific social memory information is retained in the specific vCA1 cell population during at least one day after encoding but that natural recall cues are not strong enough to reactivate these cells for memory recall; in contrast, the 20 Hz blue light is stronger and reactivates the engram cells above the threshold necessary for recall. This interpretation of light-mediated recall of the social memory is supported by the inception experiment; the social memory is retained in the amnesic mice and the light reactivated engram serves as a CS and becomes associated with a high valence US (footshocks or cocaine) to evoke avoidance or preference behavior.
The present study helped resolve the controversy (7–9) regarding necessity of mouse hippocampus for social memory and corroborates previous observations made in primates (4–6). In macaques, a large population of neurons in the anterior hippocampus responded to socially relevant cues such as faces and voices of individuals (6). In human medial temporal lobe, including the anterior hippocampus, there are cells that respond more to famous or personally relevant people than to unfamiliar people (4, 5). The overall results suggest evolutionary conservation of the role of the hippocampal areas as the sites of social memory.
Recent studies have shown that dCA2 is critical for sociocognitive memory processing (10, 11, 26). dCA2 neurons have longitudinal rostro-caudal projections to vCA1 (fig. S18) (27, 28) and connect with the deep layer of CA1 more strongly than with the superficial layer (28). It is thus possible that the dCA2-vCA1d-NAc circuit composes the engram cell ensemble pathway for social memory (25). However, it is also possible that the role of dCA2 in social memory is to convey to vCA1d appropriately processed and socially relevant cues, rather than holding memory information per se.
Compared to other forms of episodic memory, social memory lasts no more than a few hours (Fig. 1C) under laboratory conditions (29, 30), although it can be prolonged to a week by vasopressin release, or to 24 h by group housing (12, 29, 31). We demonstrated by optogenetics that the engram cells for social memory formed in a laboratory environment can be retained in vCA1 for at least two days. Thus, the relatively short duration of social memory is apparently due to inefficient retrieval rather than failed retention of the memory. It would be interesting to test the hypothesis that increased vasopressin and/or group housing prolongs social memory duration by promoting the retrieval process.
Overall, our study establishes vCA1 and its NAc projections as a site of social memory and provides insights and clues to the neuronal mechanisms underlying this important form of memory (fig. S19).
Supplementary Material
One Sentence Summary.
The ventral CA1 subfield of the mouse hippocampus contributes to social memory by holding information about a familiar mouse.
Acknowledgments
We thank C. Sun, X. Liu, S. LeBlanc, J. Derwin, W. Yu, F. Bushard, S. Huang, G. Das, T. O’Connor, J. Young, and L. Brenner for their help. Supported by the RIKEN Brain Science Institute, Howard Hughes Medical Institute, the JPB Foundation (to S.T.), and JSPS Grant-in-Aid (to T.O.). All data and computer codes necessary to understand and assess the conclusions of this research are available in the Supplementary Materials. AAV9-hSyn:DIO-eArchT3.0-EYFP, AAV9-TRE:FT-Slow, AAV9-TRE:ChR2-EYFP, and AAV9-TRE:EYFP were developed at MIT in the group of S.T.; virus plasmids are available through MTA. The Trpc4-Cre transgenic mouse line was developed at RIKEN BSI by the group of S.I.; it is available through MTA.
Footnotes
References and Notes
- 1.McGraw LA, Young LJ. Trends Neurosci. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okuyama T, et al. Science. 2014;343:91–94. doi: 10.1126/science.1244724. [DOI] [PubMed] [Google Scholar]
- 3.Smith CN, et al. Proc Natl Acad Sci U S A. 2014;111:9935–9940. doi: 10.1073/pnas.1409878111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 5.Viskontas IV, Quiroga RQ, Fried I. Proc Natl Acad Sci U S A. 2009;106:21329–21334. doi: 10.1073/pnas.0902319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sliwa J, Plante A, Duhamel JR, Wirth S. Cereb Cortex. 2016;26:950–966. doi: 10.1093/cercor/bhu257. [DOI] [PubMed] [Google Scholar]
- 7.von Heimendahl M, Rao RP, Brecht M. J Neurosci. 2012;32:2129–2141. doi: 10.1523/JNEUROSCI.3812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squires AS, Peddle R, Milway SJ, Harley CW. Neurobiol Learn Mem. 2006;85:95–101. doi: 10.1016/j.nlm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Bannerman DM, Lemaire M, Beggs S, Rawlins JN, Iversen SD. Exp Brain Res. 2001;138:100–109. doi: 10.1007/s002210100687. [DOI] [PubMed] [Google Scholar]
- 10.Hitti FL, Siegelbaum SA. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson EL, Caldwell HK. Eur J Neurosci. 2014;40:3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogan JH, Frankland PW, Silva AJ. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Andersen P, Bliss TV, Skrede KK. Exp Brain Res. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- 14.Strange BA, Witter MP, Lein ES, Moser EI. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 15.Fanselow MS, Dong HW. Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camats Perna J, Engelmann M. Curr Top Behav Neurosci. 2015 doi: 10.1007/7854_2015_413. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, et al. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura T, et al. Science. 2014;343:896–901. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziv Y, et al. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen TW, et al. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subach FV, et al. Nat Chem Biol. 2009;5:118–126. doi: 10.1038/nchembio.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Science. 2015;348:1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez S, et al. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 25.Tonegawa S, Liu X, Ramirez S, Redondo R. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Caruana DA, Alexander GM, Dudek SM. Learn Mem. 2012;19:391–400. doi: 10.1101/lm.025304.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamamaki N, Abe K, Nojyo Y. Brain Res. 1988;452:255–272. doi: 10.1016/0006-8993(88)90030-3. [DOI] [PubMed] [Google Scholar]
- 28.Kohara K, et al. Nat Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bluthe RM, Gheusi G, Dantzer R. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 30.Thor DH, Holloway WR. J Comp Physiol Psych. 1982;96:1000–1006. [Google Scholar]
- 31.Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, Young WS. Mol Psychiatry. 2016;21:1137–1144. doi: 10.1038/mp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi Y, et al. Frontiers in behavioral neuroscience. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakazawa K, et al. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redondo RL, et al. Nature. 2014;513:426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitamura T, et al. Neuron. 2015;87:1317–1331. doi: 10.1016/j.neuron.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


