Abstract
Objective
To determine the relationship of lifestyle factors and neurocognitive functioning in older adults with vascular risk factors and cognitive impairment, without dementia (CIND).
Methods
One hundred sixty adults (Mean = 65.4±6.8 years) with CIND completed neurocognitive assessments of executive function, processing speed, and memory. Objective measures of physical activity using accelerometry, aerobic capacity determined by exercise testing, and dietary habits quantified by the Food Frequency Questionnaire and 4-Day Food Diary to assess adherence to the Mediterranean and DASH diets were obtained to assess direct effects with neurocognition. Potential indirect associations of high sensitivity C-Reactive Protein (hsCRP) and the Framingham Stroke Risk Profile (FSRP) also were examined.
Results
Greater aerobic capacity (β =0.24) and daily physical activity (β = 0.15) were associated with better Executive Functioning/Processing Speed and Verbal Memory (βs = 0.24; 0.16). Adherence to the DASH diet was associated with better Verbal Memory (β = 0.17). Greater hsCRP (βs = −0.14; −0.21) and FSRP (β= −0.18; −0.18) were associated with poorer Executive Functioning/Processing Speed and Verbal Memory. Greater stroke risk partially mediated the association of aerobic capacity with Executive Functioning/Processing Speed, and Verbal Memory, and greater inflammation partially mediated the association of physical activity and aerobic fitness, with Verbal Memory.
Conclusions
Higher levels of physical activity, aerobic fitness, and adherence to the DASH diet are associated with better neurocognitive performance in adults with CIND. These findings suggest that the adoption of healthy lifestyle habits could reduce the risk of neurocognitive decline in vulnerable older adults.
Trial Registration
NCT0157354
Keywords: DASH diet, Exercise, Neurocognition, cognitive impairment, CIND
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death and disability in the United States, affecting more than 80 million Americans.(1) It is well established that CVD risk factors such as hypertension(2), diabetes,(3) and hyperlipidemia(4) place individuals at risk not only for stroke and ischemic heart disease, but also for neurocognitive impairment and dementia,(3, 5, 6) and that the effects of these CVD risk factors on the brain may be additive.(7) CVD risk factors also are associated with cognitive decline in patients without dementia,(8) a condition referred to as CIND (Cognitive Impairment, No Dementia).(9)
Current medical therapies for CIND offer limited benefit and appear to have no effect on the underlying disease pathophysiology.(10) With no medical treatments currently available to effectively halt or reverse the neuropathology of CIND, recent attention has shifted to prevention strategies in vulnerable individuals. Lifestyle behaviors, including physical activity and healthy dietary habits have been shown to improve CVD risk factors and may aid in the prevention of neurocognitive decline. Physically active individuals are less likely to develop dementia(11, 12) and studies have suggested that aerobic exercise may improve neurocognition in healthy adults,(13, 14) although few studies have included individuals with cognitive impairments.(15) Dietary habits also have been shown to be associated with neurocognition.(16) Higher CVD risk(17) and greater inflammation(18) have been linked to impaired neurocognition (19, 20) and may mediate the relationship of lifestyle habits and neurocognition.(21–24)
Two dietary patterns that have received the most attention are the Dietary Approaches to Stop Hypertension (DASH) eating plan and the Mediterranean Diet (MediDiet). Both diets recommend high consumption of fruits, vegetables, legumes and nuts, and low amounts of red or processed meat. The Dietary Approaches to Stop Hypertension (DASH) eating plan places greater emphasis on low fat dairy products and is part of current national recommendations for the prevention and treatment of hypertension, (25, 26) while the MediDiet emphasizes greater consumption of olive oil and red wine. Observational studies have shown that the MediDiet is associated with slower cognitive decline, improved cognitive function, and decreased risk of dementia (27–29), while the DASH diet also has been shown to be associated with better cognitive function in older adults and with less cognitive decline over time. (30) In a large prospective study of 16,144 women participating in the Nurses’ Health Study, Berendsen and colleagues(31) reported that greater adherence to the DASH diet was associated with better cognitive performance and verbal memory, which was equivalent to being one year younger compared to those women who were non-adherent to the DASH diet.
The purpose of the present report is to examine the relationship of key lifestyle behaviors--physical activity and dietary habits (including examination of both the DASH diet and MediDiet) on neurocognitive functioning in a sample of sedentary older adults with CIND and CVD risk factors. Because inflammation also has been shown to be associated with increased risk for adverse CVD events(32, 33) and impaired cognitive function, (34) we also sought to determine whether the relation between lifestyle factors and neurocognitive function is explained by C-reactive protein and CVD risk factors.
METHODS
Participants
One hundred sixty older adults were recruited from newspaper and television advertisements, flyers posted throughout the community, and physician referrals as part of the Exercise and Nutritional Interventions for Neurocognitive Health Enhancement (ENLIGHTEN) trial (NCT01573546).(35) The protocol was approved by Duke University and written informed consent was provided by each participant. Data were collected between December 2011 and April 2016.
Inclusion criteria included sedentary adults age ≥55 years with subjective cognitive complaints and a score of 19–25 on the Montreal Cognitive Assessment (MoCA)(36, 37) or letter fluency < 13 or animal fluency < 15,(38) and either the presence of ischemic heart disease or at least one CVD risk factor (in addition to physical inactivity). The MoCA has been well-validated as a screening measure of cognitive decline, with scores <26 suggesting the presence of mild cognitive impairment.
Procedures
Assessment of Neurocognitive Functioning
Neurocognitive functioning was assessed using a 45–60 minute test battery recommended by the Neuropsychological Working Group for vascular cognitive disorders:(39) A more complete description of the instruments along with normative data are available for the interested reader.(40–42)
Verbal Memory
Hopkins Verbal Learning Test- Revised (HVLT-R)(43)
The HVLT-R requires participants to learn and recall a 12-item word list.
Animal Naming Test(44)
This test requires participants to rapidly generate words in a specified category (in this case, animals) within a 60-second time period.
Visual Memory
Medical College of Georgia Complex Figure Test (CFT)(45)
The CFT requires participants to copy and then recall a complex geometrical shape..
Executive Function/Processing Speed
Stroop Test(46)
This test requires participants to view color names presented in various ink colors and name the color of the ink. In incongruent stimuli, color names and ink colors are non-matching.
Digit Span Forward (DST)(47)
This subtest from the Wechsler Adult Memory Scale requires participants to repeat digits of increasing length in the forward and then reverse direction as they have heard the numbers.
Controlled Oral Word Association Test (COWA)(44)
This test requires participants to rapidly generate as many words as possible beginning with a particular letter (i.e. P-R-W or C-F-L) in 60 seconds.
Trail Making Test (TMT)(48)
This test consists of two parts: For Part A of the test, participants draw lines to connect consecutively numbered circles (1,2,3, etc.); for Part B, these items are both numbers and letters (1,2,3, A,B,C etc.) and the order is determined by a combination of increasing numbers AND letters (e.g., 1 A 2 B 3 C..) requiring participants to alternate between letters and numbers.
Digit Symbol Substitution Test (DSST)(47)
This subtest from the Wechsler Adult Intelligence Scale requires participants to draw symbols that match one of 10 digits copied from a key. Scores on this task are the number of correct symbols drawn in 120 seconds.
Ruff 2 & 7 Test(49)
This requires participants to cross out all instances of the numbers ‘2’ and ‘7’ under two conditions: one in which they are embedded among other digits and in the second in which they are embedded among letters. The score is the total number of correct cancellations within a five-minute time period.
Assessment of Lifestyle Habits
Daily Activity and Aerobic Fitness
We performed two objective measures of functional capacity: a measure of routine, physical activity during daily life and a measure of aerobic fitness using standardized exercise stress testing.
Accelerometry
Physical activity during daily life was quantified using the Kenz Lifecorder Plus accelerometer (Model NL-2160; Suzuken Co. Ltd., Nagoya, Japan). Patients wore the accelerometer during waking hours for 2 successive days on the waist in a horizontal position, placed between the navel and hip. The accelerometer was removed at bedtime and was programmed for each individual participant and personalized with age, sex, height, and weight. We utilized the Physical Activity Analysis Software compatible with the accelerometer and all data were downloaded to a secure PC via a USB cable after each participant’s use. This device accurately counts more than 85% of manually observed steps in older adults at walking speeds as low as 54 m/min and greater than 95% of observed steps at speeds of at least 67 m/min.(50) The Kenz Lifecorder has been shown to be exceptionally accurate, having 95% prediction intervals that are within ± 17 steps from zero.(51) The average number of steps per day was used for the current analysis.
Exercise testing
Participants underwent a maximal graded exercise treadmill test in which workloads were increased at a rate of 1 metabolic equivalent per minute. Expired air was collected by mouthpiece for quantification of minute ventilation, oxygen consumption, and carbon dioxide production with the Parvo Medics TrueOne measurement system (model 2400; Parvo Medics, Sandy, Utah).
Assessment of Inflammation
High-Sensitivity C-Reactive Protein (hsCRP)
hsCRP was quantified by Lab Corp using commercial enzyme-linked immunosorbent assay kits (R&D Systems). Values exceeding 10 mg/dL were truncated at 10 mg/dL, consistent with current guidelines for the analysis of inflammatory markers.(52)
Assessment of Stroke Risk
The Framingham Stroke Risk Profile (FSRP) was used as a marker of stroke risk
The FSRP is a clinical assessment tool used to quantify the risk of incident stroke (53) and has previously been shown to be associated with neurocognitive performance.(53, 54) Stroke risk is quantified separately for men and women and includes the following CVD risk factors: systolic blood pressure (SBP), use of antihypertensive therapy, diabetes mellitus, cigarette smoking, ischemic heart disease, atrial fibrillation, and left ventricular hypertrophy. Because age was modeled separately, age was not included in the stroke risk factor calculation, which is consistent with our prior work.(54)
Assessment of Dietary Habits
Block Food Frequency Questionnaire (FFQ) and 4-day food diary
We sought to quantify the degree of concordance between participants’ daily dietary intake and the DASH and Mediterranean diets. To quantify the DASH eating pattern, we used a modified DASH scoring algorithm adopted from Folsom and colleagues.(55, 56) Each dietary component is scored as 0, 0.5 or 1.0, with a total range of scores of 0 to 10 with higher scores indicating greater adherence to the DASH eating plan. Because the FFQ may be more susceptible to inaccurate recall among older adults with memory impairment,(57) dietary intake entered for each meal for four days was utilized to compute dietary components for which a score could be derived (fruits, vegetables, dairy, grains, fat calories, saturated fat calories, and sodium), and the remaining categories that could not be quantified by the diary being generated from the FFQ (meats, nuts, seeds, legumes, and sweets).
Similarly, a Mediterranean diet (MediDiet) score was derived from weekly reported frequency of dietary consumption of the following food groups: non-refined cereals, potatoes, fruits, vegetables, legumes, fish, red meat, poultry, full fat dairy products, use of olive oil for cooking, and alcohol consumption. Scores of 0 to 5 were derived for each dietary component for a total score range of 0 to 55, with higher scores indicating better MediDiet adherence.(58)
Data Analysis
Data analyses were performed using SAS 9.3 (Cary, NC) and R 3.3.1 (https://cran.r-project.org). General linear models were used to examine the association between physical activity, aerobic fitness, diet, and neurocognitive functioning. Within each model we adjusted for age, education, sex, and ethnicity, which were previously set forth as a priori covariates,(35) as well as family history of dementia and chronic use of anti-inflammatory medications (inflammation analyses only). In models examining the DASH and MediDiet we also adjusted for total caloric intake. In response to a request from an anonymous reviewer, neurocognitive subtests were grouped into factors based on Principal Axis Factor analysis (PROC FACTOR) using a Promax rotation (see Table S1, Supplemental Digital Content 1). Results yielded a three-factor solution: 1) Executive Functioning/Processing Speed (Stroop Test, DST, Ruff, COWA, TMT, and DSST), Verbal Memory (HVLT-R immediate, HVLT-R delayed recall, and Animal Naming), and Visual Memory (CFT copy, immediate, and delayed recall). All subtests were transformed to z-scores and a mean of all subtests was generated for each factor.(59) In order to examine the independent and additive associations of the diet and physical activity/aerobic fitness, we conducted a series of regression models. We first tested the association between 1) the DASH diet (model 1), 2) total step count (model 2), and peak VO2 (model 3) with neurocognitive performance. We then examined two combined models in which DASH diet score was modeled simultaneously with total step count (model 4) or peak VO2 (model 5). In a final step, we examined whether the relationship between total step count, peak VO2, and/or DASH score was attenuated after accounting for hsCRP and/or the FSRP by examining the bootstrapped procedures available using the PROCESS macro in SAS (60).
RESULTS
Sample Characteristics
Background and demographic characteristics are provided in Table 1. The average age of the study sample was 65 years, two-thirds of study participants were women, and the sample was nearly equally distributed between white and African-American participants. Participants tended to be well-educated and approximately half were retired at the time of enrollment.
Table 1.
Background and clinical characteristics of the sample.
| Variable | Total Cohort (n = 160) |
|---|---|
|
| |
| Age (Mean±SD) | 65.4 (6.8) |
|
| |
| Male Sex, n (%) | 53 (33%) |
|
| |
| Ethnicity, n (%) | |
| White | 83 (52%) |
| African-American | 74 (46%) |
| Other | 1 (1%) |
|
| |
| Married, n (%) | |
| Married | 86 (54%) |
| Divorced/Widowed | 63 (39%) |
| Single, Never Married | 11 (7%) |
|
| |
| Level of Education, n (%) | |
| < High School | 1 (1%) |
| High School Graduate | 14 (9%) |
| Some College | 48 (30%) |
| College Degree | 50 (31%) |
| Graduate School + | 47 (29%) |
|
| |
| Annual Household Income, n (%) | |
| <$30k | 23 (15%) |
| $30–60k | 66 (46%) |
| $61–100k | 44 (29%) |
| $100k+ | 17 (11%) |
|
| |
| Employment Status, n (%) | |
| Full-Time | 38 (24%) |
| Part-Time | 21 (13%) |
| Retired | 82 (51%) |
| Not Employed | 19 (12%) |
|
| |
| Montreal Cognitive Assessment Battery (Mean ± SD) | 24.7 (2.6) |
|
| |
| Cardiovascular Risk Factors and Inflammation | |
|
| |
| Hypertension, n (%) | 118 (74%) |
|
| |
| Hyperlipidemia, n (%) | 93 (58%) |
|
| |
| Diabetes, n (%) | 35 (22%) |
|
| |
| Obesity, n (%) | 104 (65%) |
|
| |
| Body Mass Index (BMI), kg/m2 | 32.5 (4.8) |
|
| |
| Current Smoker, n (%) | 6 (4%) |
|
| |
| History of Cardiovascular disease (CVD), n (%) | 27 (17%) |
|
| |
| Family history of CVD, n (%) | 50 (31%) |
|
| |
| Charlson Comorbidity Index, n (%) | 0.8 (1.1) |
|
| |
| Framingham Stroke Risk Score | 6.1 (3.1) |
|
| |
| C-Reactive Protein, mg/dL (Mean ± SD) | 3.5 (2.6) |
|
| |
| History of Cardiac Rehabilitation, n (%) | 15 (9%) |
|
| |
| Dietary, Physical Activity, and Fitness Variables | |
|
| |
| DASH Score (Mean ± SD) | 3.49 (1.12) |
| Vegetables, servings/day | 4.3 (3.1) |
| Fruits, servings/day | 2.3 (2.1) |
| Total Grains, servings/day | 4.9 (2.1) |
| Dairy, servings/day | 0.9 (0.7) |
| Meat, servings/day | 1.9 (1.0) |
| Nuts/Beans/Legumes, servings/day | 1.1 (0.9) |
| Sweets, servings/week | 15.6 (12.1) |
| % Calories from Fat | 39.2 (6.1) |
| % Calories from Saturated Fat | 11.2 (3.1) |
| Dietary Sodium, mg/day | 2949 (1029) |
|
| |
| Calories, kcal/day | 1844 (566) |
|
| |
| Peak VO2, ml/kg/min (Mean ± SD) | 18.3 (4.5) |
|
| |
| Total Actigraphy Steps per Day, (Mean ± SD) | 5273 (2577) |
|
| |
| Neurocognitive Test Performance | |
|
| |
| Stroop Word | 86.3 (13.9) |
|
| |
| Stroop Color | 63.2 (10.5) |
|
| |
| Stroop Color-Word | 31.4 (8.9) |
|
| |
| Digit Span | 15.7 (3.8) |
|
| |
| Ruff 2 & 7 Test | 242.2 (42.1) |
|
| |
| Controlled Oral Word Association Test | 38.2 (10.9) |
|
| |
| Digit Symbol Substitution Test | 59.9 (11.3) |
|
| |
| Trail Making Test B, seconds to completion | 89.1 (38.4) |
|
| |
| Trail Making Test A, seconds to completion | 36.8 (13.3) |
|
| |
| Hopkins Verbal Learning Test Delay | 8.6 (2.1) |
|
| |
| Hopkins Verbal Learning Test Total Learning (Trials 1–3) | 24.8 (4.1) |
|
| |
| Animal Naming | 18.0 (4.5) |
|
| |
| Medical College of Georgia Short Delay | 19.2 (6.9) |
|
| |
| Medical College of Georgia Long Delay | 19.1 (6.9) |
|
| |
| Medical College of Georgia Copy | 29.7 (5.0) |
N for income does not sum to 160 because some individuals did not respond to this item.
Dietary Patterns and Neurocognition
Examination of dietary intake revealed that most participants did not consume a diet consistent with the DASH eating plan (mean DASH score = 3.5 points [SD = 1.12; range 1.5 to 7; possible scores range from 0 to 10]). MediDiet scores across the sample suggested modest agreement between usual food consumption and the MediDiet, with a mean score of 30.7 points (SD = 4.6; range = 14 to 44) out of a possible 55. Higher DASH diet scores were associated with better Verbal Memory (Table 2, Model 1: β = 0.18, P = .018), but were not associated with Executive Functioning/Processing Speed (Table 3, Model 1: β = 0.04, P = .569) or Visual Memory (β = 0.09, P = .248). In exploratory analyses of individual DASH diet components, we observed that the relationship between Verbal Memory and DASH diet tended to be most strongly influenced by the association with lower total dietary fat intake (β = 0.15, P = .053). In contrast, MediDiet scores were unrelated to any cognitive domain: Executive Functioning/Processing Speed (β = −0.01, P = .901), Verbal Memory (β = 0.11, P = .167), or Visual Memory (β = 0.01, P = .978).
Table 2.
Physical activity, aerobic fitness, DASH diet, and Verbal Memory.
| Predictor | Model 1: DASH Diet | Model 2: Physical Activity | Model 3: Fitness | Model 4: DASH + PA | Model 5: DASH + Fitness |
|---|---|---|---|---|---|
| Age | −0.22* | −0.19* | −0.16† | −0.18* | −0.15† |
| Education | 0.22* | 0.23* | 0.20* | 0.22* | 0.19* |
| Sex | −0.06 | −0.08 | −0.13 | −0.10 | −0.14 |
| Ethnicity | −0.17* | −0.21* | −0.13 | −0.16* | −0.10 |
| Family History of Dementia | 0.02 | 0.00 | −0.01 | 0.01 | 0.01 |
| Total Caloric Intake | 0.11 | ------- | ------- | 0.11 | 0.11 |
| DASH Diet Score | 0.17* | ------- | ------- | 0.19* | 0.16* |
| Total Accelerometry Steps | 0.16* | ------- | 0.17* | ------- | |
| Peak VO2, ml/kg/min | 0.24* | 0.20* |
P ≤ .01;
P ≤ .05;
P ≤ .10
Results are from separate regression models examining the independent and combined associations between predictors and a z-score composite measure of Verbal Memory. Values represent standardized beta weights.
Table 3.
Physical activity, aerobic fitness, DASH diet, and Executive Function/Processing Speed
| Predictor | Model 1: DASH Diet | Model 2: Physical Activity | Model 3: Fitness | Model 4: DASH + PA | Model 5: DASH + Fitness |
|---|---|---|---|---|---|
| Age | −0.44** | −0.41** | −0.36* | −0.41** | −0.31** |
| Education | 0.12 | 0.14† | 0.09 | 0.14† | 0.09 |
| Sex | 0.06 | 0.02 | −0.03 | 0.01 | −0.03 |
| Ethnicity | −0.11 | −0.13 | −0.05 | −0.12 | −0.04 |
| Family History of Dementia | 0.08 | 0.06 | 0.06 | 0.06 | 0.06 |
| Total Caloric Intake | 0.02 | ------- | ------- | 0.03 | 0.02 |
| DASH Diet Score | 0.04 | ------- | ------- | 0.07 | 0.02 |
| Total Accelerometry Steps | 0.15† | ------- | 0.15† | ------- | |
| Peak VO2, ml/kg/min | 0.24** | 0.23* |
P ≤ .01;
P ≤ .05;
P ≤ .10
Results are from separate regression models examining the independent and combined associations between predictors and a z-score composite measure of Executive Function/Processing Speed. Values represent standardized beta weights.
Physical Activity, Aerobic Fitness, and Neurocognition
Physical activity and aerobic fitness levels varied widely across the sample (Table 1). Participants averaged 5,273 (SD = 2,577) total steps per day, ranging from 898 to 13,553 steps. Most participants were relatively inactive, with a median number of > 10 minute bouts of moderate activity of 5.0 [IQR = 8.5] per day and 0.0 [IQR = 0] bouts of daily vigorous activity. Peak VO2 levels also varied widely, with a mean level of 18.3 ml/kg/min (SD = 4.47). Examination of physical activity levels, defined as the average number of steps per day, revealed that higher levels of physical activity were associated with better Verbal Memory (Table 2, Model 2: β = 0.16, P = .041); physical activity also tended to be associated with better Executive Functioning/Processing Speed (Table 3, Model 2: β = 0.15, P = .058), but was unrelated to Visual Memory (β = −0.03, P = .690). Similarly, higher levels of aerobic fitness were associated with better Executive Functioning/Processing Speed (Table 3, Model 3: β = 0.18, P = .048) and Verbal Memory (Table 2, Model 3: β = 0.22, P = .022), but were not associated with Visual Memory (β = 0.05, P = .613).
Interestingly, the associations between physical activity, aerobic fitness, and Verbal Memory remained significant in a final model in which DASH diet scores were included as an additional predictor (Table 2, Models 4 and 5), suggesting an additive effect. Those individuals demonstrating higher levels of physical activity or aerobic fitness and higher on DASH adherence obtained higher Verbal memory scores compared to those individuals who scored lower on measures of fitness, physical activity or DASH adherence. Unadjusted correlations between dietary habits and physical activity and fitness measures with the neurocognitive tests are provided in Table S2, Supplemental Digital Content 1.
Stroke Risk and Neurocognition
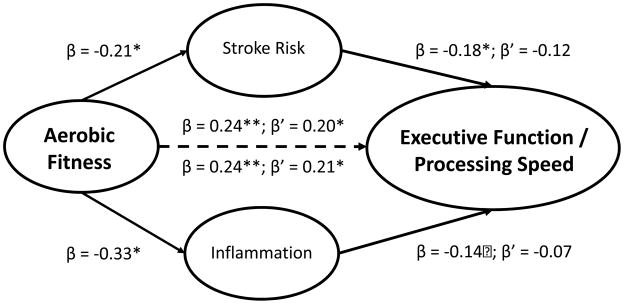
Examination of baseline FSRP levels demonstrated that greater stroke risk was associated with poorer Executive Functioning/Processing Speed (β = −0.18, P = .021), Verbal Memory (β = −0.18, P = .025), and tended to be associated with poorer Visual Memory (β = −0.15, P = .068). Because Visual Memory was not associated with any of our predictors of interest, no further testing of indirect associations was conducted. In order to test the indirect association between stroke risk and neurocognition, we first examined whether stroke risk was associated with the DASH diet, physical activity, or aerobic fitness. We found that lower stroke risk was associated with greater physical activity (β = −0.20, P = .03), but was unrelated to either physical activity (β = −0.05, P = .513) or the DASH diet (β = 0.05, P = .554). In a final model incorporating both aerobic fitness and stroke risk, the association between aerobic fitness and Executive Functioning/Processing Speed approached significance (β = 0.18, P = .061) while the association with stroke risk and Executive Functioning/Processing Speed was attenuated (β = −0.12, P = .128) (Figure 1);bootstrapped estimates of the indirect effects suggested that stroke risk did not completely mediate the observed associations (direct effect = 0.20 [0.02, 0.38]; indirect effects: 0.03 [−0.01, 0.10]).. Similarly, a final model incorporating both aerobic fitness and stroke risk demonstrated that the association between aerobic fitness and Verbal Memory tended to remain significant (β = 0.18, P = .061) while the association with stroke risk and Verbal Memory was attenuated (β = −0.12, P = .128), and bootstrapped estimates of the indirect effects suggested that stroke risk did not completely explain the observed associations (direct effect = 0.14 [−0.01, 0.29]; indirect effect = 0.03 [−0.01, 0.07]).).
Figure 1.
Indirect associations between aerobic fitness, stroke risk, inflammation, and Executive Function and Processing Speed. Values represent the standardized beta coefficients from separate models examining the indirect associations of hsCRP (inflammation) and the Framingham Stroke Risk Profile (stroke risk) on neurocognition. Within each model, parameter estimates from individual predictive models are denoted with β and parameters from the final model including all predictors are denoted with β’. As show, the association between aerobic fitness and Executive Function and Processing Speed remained significant in both models. The association between hsCRP and Executive Function and Processing Speed was attenuated and examination of the bootstrapped estimates of the indirect effect indicated that hsCRP was not a significant mediator of the aerobic fitness and Executive Function and Processing Speed association (direct effect = 0.17 [−0.01, 0.36]; indirect effect = 0.03 [−0.02, 0.10]). The association between stroke risk and Executive Function and Processing Speed tended to be attenuated and examination of the bootstrapped estimates of the indirect effect indicated that stroke risk was not a significant mediator of the aerobic fitness and Executive Function and Processing Speed association (direct effect = 0.20 [0.02, 0.38]; indirect effects: 0.03 [−0.01, 0.10]).). Taken together, the pattern of findings suggested stroke risk partially mediated the association between aerobic fitness and Executive Function and Processing Speed, whereas inflammation did not.
Inflammation and Neurocognition
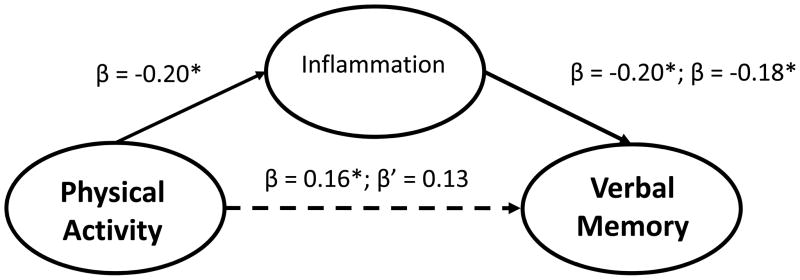
Examination of baseline hsCRP levels revealed that higher inflammation was associated with poorer Verbal Memory (β = −0.21, P = .009) and tended to be associated with poorer Executive Functioning/Processing Speed (β = −0.14, P = .070). In order to test the indirect association between inflammation and neurocognition, we first examined whether hsCRP was associated with the DASH diet, physical activity, or aerobic fitness. hsCRP was associated with greater physical activity (β = −0.17, P = .032) and aerobic fitness (β = −0.33, P < .001), but was not associated with the DASH diet (β = −0.07, P = .349). The association between aerobic fitness and Executive Functioning/Processing Speed was not mediated by hsCRP (hsCRP: β = −0.08, P = .338; VO2: β = 0.21, P = .020; bootstrapped direct effect = 0.17 [−0.01, 0.36]; indirect effect = 0.03 [−0.02, 0.10]). The association between aerobic fitness and Verbal memory appeared to be partially mediated by hsCRP (hsCRP: β = −0.15, P = .073; VO2: β = 0.19, P = .047; bootstrapped direct effect = 0.13 [−0.02, 0.28]; indirect effect = 0.04 [−0.002, 0.10]). hsCRP did not mediate the association between physical activity and Executive Functioning/Processing Speed (hsCRP β = −0.11, P = .155; total steps: β = 0.15, P = .093; bootstrapped direct effect = 0.15 [−0.03, 0.32]; indirect effect = 0.02 [−0.01, 0.06]), but tended to explain the association between physical activity and Verbal Memory (hsCRP β = −0.18, P = .032; steps: β = 0.13, P = .095; bootstrapped direct effect = 0.12 [−0.02, 0.27]; indirect effect = 0.03 [−0.001, 0.07]) (Figure 2).
Figure 2.
Indirect associations between physical activity, inflammation, and Verbal Memory. Values represent standardized beta coefficients. Within each model, parameter estimates from individual predictive models are denoted with β and parameters from the final model including all predictors are denoted with β’. As show, the association between physical activity and verbal memory was modestly attenuated in a final model incorporating inflammation, although the bootstrapped estimates of the indirect effect indicated that hsCRP was not a significant mediator of the physical activity and Verbal Memory association (direct effect = 0.12 [−0.02, 0.27]; indirect effect = 0.03 [−0.001, 0.07]).
DISCUSSION
Results of this cross-sectional study of baseline data from the ENLIGHTEN trial provide support for the value of the DASH eating plan and physical activity in promoting improved neurocognitive function in a sample of sedentary older adults with CVD risk factors and evidence of cognitive impairments, without dementia (i.e., CIND). Participants who were more active as indicated by higher average daily step count, and who were more physically fit as documented by greater aerobic capacity, demonstrated better performance on cognitive tasks associated with executive function and processing speed, and with verbal memory.
Other observational studies, and several interventional studies, also have examined the relation of physical fitness and aerobic fitness on neurocognition. Observational studies have shown that physically active individuals perform better on neurocognitive tests compared to their less active counterparts.(11, 61) Results from interventional studies also suggest that aerobic exercise may result in improved cognitive functioning,(62–64) although the evidence is inconsistent.(65) The discrepancies in the literature may be in part due to differences in characteristics of the study populations and in the measures used to assess neurocognition. Some studies, for example, have excluded patients with cognitive impairments,(13) while others have suggested that patients with cognitive impairments may be more likely to benefit compared to cognitively intact individuals.(63) Importantly, the present analysis showed that greater aerobic capacity and higher levels of physical activity were associated with better executive function, processing speed, and verbal memory, but not visual memory, providing support for the hypothesis that aerobic fitness may selectively improve some cognitive functions but not others. Kramer and colleagues have maintained that exercise may be particularly beneficial for tasks of executive functioning.(62) More recent work by our group(66) has shown that improvements in aerobic fitness are associated with improved memory and others have shown that aerobic fitness improvements are associated with small volume increases within mesial temporal brain structures preferentially important for memory, but not executive function.(67, 68)
In addition to the association of greater physical activity and greater aerobic fitness with better neurocognitive performance on tasks of executive function, processing speed, and verbal memory, participants with diets high in fruits, vegetables, and low fat dairy products and with reduced fats and sweets, performed better on tasks of verbal memory, but not on executive function and processing speed or verbal memory. This pattern of results raises the possibility that aerobic fitness and the DASH eating plan can be complementary. Indeed, participants who were more physically active, aerobically fit, and more adherent to the DASH diet exhibited better verbal memory compared to those who were adherent to either physical activity or the DASH diet, but not both. Those individuals with both lower levels of physical activity and lower DASH diet scores had the poorest verbal memory performance.
Several studies in non-cognitively impaired adults have examined the combined effects of diet physical activity. In a previous study of cognitively intact adults with high blood pressure, we found that participants on a caloric restricted DASH diet combined with an exercise program exhibited greater improvements in executive function-memory-learning and psychomotor speed compared to controls.(66) In an observational study, Scarmeas and colleagues(69) demonstrated that adherence to a Mediterranean-type diet and greater physical activity were independently and additively associated with lower risk of developing Alzheimer’s Disease. In addition, two randomized trials have examined the effect of the MediDiet on neurocognition (28, 70), one of which found improvements on a global cognitive screening measure(28). The MediDiet was not associated with neurocognitive functioning in our sample of patients with CIND, however. Similarly, a large prospective study of over 130,000 middle aged adults, reported that the DASH diet, but not the MediDiet, was associated with lower risk of colorectal cancer.(71), suggesting that the two diets may not provide the same benefits. Further research is needed to examine the relative merits of these two dietary patterns.
The beneficial effects of lifestyle behaviors on neurocognition are thought to be partly explained by reduced CVD risk factors and inflammation (21–24), although few studies have evaluated this possible mechanism (72, 73). Studies have demonstrated that higher levels of physical activity(74–77), greater aerobic fitness(78, 79), and healthier dietary habits (80–87) are associated with reduced CVD risk and less inflammation. Studies also have shown that CVD risk factors and inflammation are associated with elevated risk of neurocognitive decline(88–90), including Alzheimer’s disease (AD) (6, 19, 20, 91) and dementia(22, 92–94). For example, in one prospective study, Gu and colleagues(72) found that better MediDiet adherence was associated with lower CRP and reduced risk of AD, although the association between the MediDiet and AD risk was unchanged when CRP levels were accounted for in the analysis, suggesting that the observed association between the MediDiet and AD was not mediated by inflammation. The present study found that greater inflammation was related to impaired neurocognitive function, but also found that inflammation did not explain the relationship between physical activity or the DASH diet and neurocognitive impairment.
Limitations
Dietary intake was based on self-reported food consumption. We recognize that dietary recall is subject to bias and inaccuracies. There is also some degree of arbitrariness in the scoring algorithms used to determine adherence to the DASH and MediDiet eating plans. However, our quantification of dietary habits was based on established scoring systems and we believe that our system accurately captures the essential elements of the two diets. Because this was a cross-sectional analysis, we cannot infer causation from the observed associations between lifestyle habits and neurocognitive functioning. It is possible that individuals with better cognitive functioning are more likely to adopt healthier lifestyles. The extent to which improving physical activity, aerobic fitness, or dietary habits may result in improved neurocognition in individuals with cognitive impairments must await the results of interventional trials.
Summary and conclusions
Healthy lifestyles, including adherence to the DASH eating plan and higher levels of physical activity and aerobic fitness, were associated with better performance on tasks of executive function, processing speed, and verbal memory. Some evidence of specificity of benefit was noted, with better adherence to the DASH diet associated with better verbal memory and higher levels of physical activity and aerobic fitness associated with better executive function, processing speed, and verbal memory. Greater consumption of the DASH diet combined with greater physical activity or higher aerobic fitness appears to provide added benefit for verbal memory. Randomized clinical trials are needed to determine the value of adopting the DASH diet and increasing physical fitness for improving neurocognitive function in older adults with cognitive impairments, without dementia.
Supplementary Material
Acknowledgments
Funding source: This study was supported by a grant from the National Heart, Lung, and Blood Institute (HL109219).
We wish to thank Molly McLaren, Amelia Hoyle, Kathryn Sommers, Brittany Manobianco, Brendan Wesp, Megan Gurjar, Julie Johnson, PA-C, Cassandra Germain, PhD, Jeanne Schwartz, PA-C, Lawrence Liao, MD, Carola Ekelund, PT, and Kenlyn Young, MS.
Acronyms
- AD
Alzheimer’s Disease
- CFT
Medical College of Georgia Complex Figure Test
- CI
Confidence Interval
- CIND
Cognitive impairment, no dementia
- COWA
Controlled Oral Word Association test
- CVD
Cardiovascular disease
- DASH
Dietary Approaches to Stop Hypertension
- DBP
Diastolic Blood Pressure
- DST
Digit Span Test
- DSST
Digit Symbol Substitution Test
- FFQ
Food Frequency Questionnaire
- FSRP
Framingham Stroke Risk Profile
- hsCRP
High Sensitivity C-Reactive Protein
- HVLT-R
Hopkins Verbal Learning Test- Revised
- MediDiet
Mediterranean diet
- MOCA
Montreal Cognitive Assessment
- SBP
Systolic Blood Pressure
- SD
Standard Deviation
- TMT
Trail Making Test
Footnotes
Conflicts of interest: All authors report no conflicts of interest
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Hanon O, Seux ML, Lenoir H, Rigaud AS, Forette F. Hypertension and dementia. Current cardiology reports. 2003;5:435–40. doi: 10.1007/s11886-003-0104-2. [DOI] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. AmJ Epidemiol. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 4.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. AmJ GeriatrPsychiatry. 2008;16:343–54. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 5.Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. AmJEpidemiol. 1993;138:353–64. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 6.Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, DeKosky ST, Kuller LH. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J AmGeriatrSoc. 2005;53:1101–7. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 7.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56:1683–9. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 9.Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–6. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 10.Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J Neurol NeurosurgPsychiatry. 2006;77:429–38. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 12.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychological medicine. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 13.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. CochraneDatabaseSystRev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–9. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 15.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch PhysMed Rehabil. 2004;85:1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004;3:579–87. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 17.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol. 2010;67:87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. The Lancet Neurology. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Annals of neurology. 2013;74:580–91. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 21.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004;35:2620–2. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- 22.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014:9. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sartori AC, Vance DE, Slater LZ, Crowe M. The Impact of Inflammation on Cognitive Function in Older Adults: Implications for Healthcare Practice and Research. Journal of Neuroscience Nursing. 2012;44:206–17. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 26.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 27.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–25. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MA. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013 doi: 10.1136/jnnp-2012-304792. [DOI] [PubMed] [Google Scholar]
- 29.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Advances in nutrition (Bethesda, Md) 2015;6:154–68. doi: 10.3945/an.114.007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tangney CC, Li H, Wang Y, Barnes L, Schneider JA, Bennett DA, Morris MC. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83:1410–6. doi: 10.1212/WNL.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berendsen AAM, Kang JH, van de Rest O, Feskens EJM, de Groot LCPGM, Grodstein F. The Dietary Approaches to Stop Hypertension Diet, Cognitive Function, and Cognitive Decline in American Older Women. Journal of the American Medical Directors Association. 2017 doi: 10.1016/j.jamda.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 33.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, D’Agostino RB, Franzblau C, Wilson PW. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32:2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe Y, Kitamura K, Nakamura K, Sanpei K, Wakasugi M, Yokoseki A, Onodera O, Ikeuchi T, Kuwano R, Momotsu T, Narita I, Endo N. Elevated C-Reactive Protein Is Associated with Cognitive Decline in Outpatients of a General Hospital: The Project in Sado for Total Health (PROST) Dementia and geriatric cognitive disorders extra. 2016;6:10–9. doi: 10.1159/000442585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal JA, Smith PJ, Welsh-Bohmer K, Babyak MA, Browndyke J, Lin PH, Doraiswamy PM, Burke J, Kraus W, Hinderliter A, Sherwood A. Can lifestyle modification improve neurocognition? Rationale and design of the ENLIGHTEN clinical trial. Contemporary clinical trials. 2013;34:60–9. doi: 10.1016/j.cct.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J AmGeriatrSoc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 37.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2007;52:329–32. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 38.Canning SJ, Leach L, Stuss D, Ngo L, Black SE. Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology. 2004;62:556–62. doi: 10.1212/wnl.62.4.556. [DOI] [PubMed] [Google Scholar]
- 39.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 40.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Scoring Program (HRB) Odessa, Florida: Psychological Assessment Resources; 2004. [Google Scholar]
- 41.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford, New York, Auckland, Bangkok, Buenos Aires, Cape Town, Chenna, Dar es Sallam, Delhi, Hong Kong, Istanbul, Karachi, Kolkata, Kuala Lumpur, Madrid, Melbourne, Mexico City, Mumbai, Nairobi, Sao Paulo, Shanghai, Taipei, Tokyo, Toronto: Oxford University Press; 2004. Neuropsychological Assessment Batteries; pp. 647–95. [Google Scholar]
- 42.Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1991. [Google Scholar]
- 43.Brandt J. The Hopkins Verbal Learning Test: Development of a new verbal memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–42. [Google Scholar]
- 44.Benton AL, Sivan A, de Hamsher KS. Multilingual Aphasia Examination. 1994. [Google Scholar]
- 45.Ingram F, Soukup VM, Ingram PT. The Medical College of Georgia Complex Figures: reliability and preliminary normative data using an intentional learning paradigm in older adults. Neuropsychiatry, neuropsychology, and behavioral neurology. 1997;10:144–6. [PubMed] [Google Scholar]
- 46.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychiat. 1935;18:643–62. [Google Scholar]
- 47.Wechsler D. Wechsler Adult Intelligence Scale (WAIS-IV) San Antonio, TX: Harcourt Assessment; 2008. [Google Scholar]
- 48.Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Tucson, AZ: Reitan Neuropsychological Laboratories, Inc; 1979. [Google Scholar]
- 49.Ruff RM, Niemann H, Allen CC. The Ruff 2 and 7 Selective Attention Test: A neuropsychological application. Percept Mot Skills. 1992;75:1311–9. doi: 10.2466/pms.1992.75.3f.1311. [DOI] [PubMed] [Google Scholar]
- 50.Dondzila CJ, Swartz AM, Miller NE, Lenz EK, Strath SJ. Accuracy of uploadable pedometers in laboratory, overground, and free-living conditions in young and older adults. Int J Behav Nutr Phys Act. 2012;9:143. doi: 10.1186/1479-5868-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider PL, Crouter SE, Lukajic O, Bassett DR., Jr Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Medicine and science in sports and exercise. 2003;35:1779–84. doi: 10.1249/01.MSS.0000089342.96098.C4. [DOI] [PubMed] [Google Scholar]
- 52.Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, Kimberly MM, Stein EA, Taubert KA, Warnick GR, Waymack PP Cdc, Aha. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the laboratory science discussion group. Circulation. 2004;110:e545–9. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 53.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–3. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 54.Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, Hinderliter A, Sherwood A. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med. 2007;69:578–86. doi: 10.1097/PSY.0b013e31812f7b8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20:225–32. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epstein DE, Sherwood A, Smith PJ, Craighead L, Caccia C, Lin PH, Babyak MA, Johnson JJ, Hinderliter A, Blumenthal JA. Determinants and Consequences of Adherence to the Dietary Approaches to Stop Hypertension Diet in African-American and White Adults with High Blood Pressure: Results from the ENCORE Trial. J Acad Nutr Diet. 2012;112:1763–73. doi: 10.1016/j.jand.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNeill G, Winter J, Jia X. Diet and cognitive function in later life: a challenge for nutrition epidemiology. Eur J Clin Nutr. 2009;63(Suppl 1):S33–7. doi: 10.1038/ejcn.2008.62. [DOI] [PubMed] [Google Scholar]
- 58.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44:335–40. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–87. [PubMed] [Google Scholar]
- 60.Hayes AF. A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper] 2012 Available from: http://www.afhayes.com/public/process2012.pdf.
- 61.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 62.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 63.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA: The Journal of the American Medical Association. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 65.Gates N, Fiatarone Singh MA, Sachdev PS, Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry. 2013;21:1086–97. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 66.Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55:1331–8. doi: 10.1161/HYPERTENSIONAHA.109.146795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. ProcNatlAcadSci US A. 2007;104:5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical Activity, Diet, and Risk of Alzheimer Disease. JAMA: The Journal of the American Medical Association. 2009;302:627–37. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McMillan L, Owen L, Kras M, Scholey A. Behavioural effects of a 10-day Mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite. 2011;56:143–7. doi: 10.1016/j.appet.2010.11.149. [DOI] [PubMed] [Google Scholar]
- 71.Fung TT, Hu FB, Wu KN, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. American Journal of Clinical Nutrition. 2010;92:1429–35. doi: 10.3945/ajcn.2010.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis. 2010;22:483–92. doi: 10.3233/JAD-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Braskie MN, Boyle CP, Rajagopalan P, Gutman BA, Toga AW, Raji CA, Tracy RP, Kuller LH, Becker JT, Lopez OL, Thompson PM. Physical activity, inflammation, and volume of the aging brain. Neuroscience. 2014;273:199–209. doi: 10.1016/j.neuroscience.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmed HM, Blaha MJ, Nasir K, Rivera JJ, Blumenthal RS. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109:288–95. doi: 10.1016/j.amjcard.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 75.Pattyn N, Cornelissen VA, Eshghi SRT, Vanhees L. The Effect of Exercise on the Cardiovascular Risk Factors Constituting the Metabolic Syndrome A Meta-Analysis of Controlled Trials. Sports Medicine. 2013;43:121–33. doi: 10.1007/s40279-012-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ertek S, Cicero A. Impact of physical activity on inflammation: effects on cardiovascular disease risk and other inflammatory conditions. Arch Med Sci. 2012;8:794–804. doi: 10.5114/aoms.2012.31614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamer M, Sabia S, Batty GD, Shipley MJ, Tabak AG, Singh-Manoux A, Kivimaki M. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation. 2012;126:928–33. doi: 10.1161/CIRCULATIONAHA.112.103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women A Meta-analysis. Jama-J Am Med Assoc. 2009;301:2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 79.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clinica Chimica Acta. 2010;411:785–93. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J Nutr. 2006;136:2588–93. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 81.Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke - A meta-analysis of cohort studies. Neurology. 2005;65:1193–7. doi: 10.1212/01.wnl.0000180600.09719.53. [DOI] [PubMed] [Google Scholar]
- 82.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases-Incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–8. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 83.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean Diet and Incidence of and Mortality From Coronary Heart Disease and Stroke in Women. Circulation. 2009;119:1093–100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 85.Steckhan N, Hohmann CD, Kessler C, Dobos G, Michalsen A, Cramer H. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: A systematic review and meta-analysis. Nutrition. 2016;32:338–48. doi: 10.1016/j.nut.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovas. 2014;24:929–39. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation - Emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–85. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 88.Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging. 2010;31:1894–902. doi: 10.1016/j.neurobiolaging.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gasecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep. 2013;15:547–58. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts RO, Geda YE, Knopman DS, Boeve BF, Christianson TJ, Pankratz VS, Kullo IJ, Tangalos EG, Ivnik RJ, Petersen RC. Association of C-reactive protein with mild cognitive impairment. Alzheimers Dement. 2009;5:398–405. doi: 10.1016/j.jalz.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature Reviews Neuroscience. 2015;16:358–72. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 92.Sharp SI, Aarsland D, Day S, Sonnesyn H, Ballard C, Aarsland D, Brayne C, Gupta S, Keage H, Seshasai SRK, Llewellyn D, McDougall F, Muangpaisan W, Ballard C, Sharp S, Day S Alzheimer’s Society Vascular Dementia Systematic Review G. Hypertension is a potential risk factor for vascular dementia: systematic review. International journal of geriatric psychiatry. 2011;26:661–9. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- 93.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–74. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia aging study. Annals of Neurology. 2002;52:168–74. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.