Abstract
Purpose
Choroidal thickness increases linearly with intraocular pressure (IOP) lowering. We studied the relationship between the change in size of the choroidal vasculature and IOP lowering after glaucoma procedures.
Methods
Thirty eyes of twenty-nine patients were examined pre- and postoperatively for up to 6 months with standard clinical assessment, enhanced depth imaging spectral-domain optical coherence tomography (OCT), and axial length measurement. Each enhanced depth imaging spectral-domain OCT image was analyzed using three separate methods to determine the choroidal thickness, choroidal vessel thickness, choroidal interstitial thickness, large choroidal vessel layer thickness, medium choroidal vessel layer thickness, and light-dark ratio. Bivariate linear regression analysis was completed with largest change in IOP as the independent variable. The dependent variables included choroidal thickness, choroidal vessel thickness, and choroidal interstitial thickness, at the largest change in IOP. Multivariable regression analysis using a generalized estimating equation to account for multiple measurements per eye was also completed.
Results
Mean choroidal vessel thickness increases 1.5 μm for every 1 mm Hg decrease in IOP (P < 0.0001; 95% confidence interval [CI], 0.8, 2.1) and choroidal interstitial thickness increases 1.3 μm for every 1 mm Hg change in IOP (P < 0.0001; 95% CI, 0.8, 1.8). There was no significant association between change in IOP and change in large choroidal vessel layer temporally (P = 0.13), nasally (P = 0.20), or subfoveally (P = 0.18). There was also no association between IOP and the light-dark ratio (P = 0.16).
Conclusions
The increase in choroidal thickness at lower IOP is associated with approximately equal increases in its intravascular and extravascular compartments.
Keywords: glaucoma, choroidal thickness, choroidal vessel, OCT
The choroid is responsible for providing nutrition to the retina, regulating ocular temperature, and contributing to growth of the sclera.1 It is composed of the choriocapillaris, the medium diameter choroidal vessel layer known as Sattler's layer, and the large diameter choroidal vessel layer known as Haller's layer.2 Abnormal choroidal blood volume or compromised flow has been implicated in various ocular diseases such as AMD,3 diabetic retinopathy,4 polypoidal choroidal vasculopathy,5 central serous chorioretinopathy,6 panuveitis,7 and punctate inner choroidopathy.8 Although there is controversy over the role of abnormal choroidal blood flow in glaucoma,9,10 eyes with angle closure glaucoma have been shown to have a greater change in choroidal thickness with water drinking compared with eyes with open angle glaucoma.11 Longer axial length, increased myopia, older age, and thicker central corneal thickness are associated with a thinner choroid.12–16 Conversely, higher diastolic perfusion pressure, a lower intraocular pressure (IOP), and male sex are associated with a thicker choroid.13,14 Choroidal thickness (CT) also varies on a diurnal basis.17,18
Although indocyanine green angiography has traditionally been used to visualize choroidal vasculature,6,19 enhanced depth imaging spectral-domain optical coherence tomography (EDI SD-OCT) allows for rapid and precise measurement of CT and visualization of the anatomy of choroidal vasculature in vivo.20,21
IOP reduction due to trabeculectomy is associated with a corresponding increase in CT.12,22–25 The current investigation aims to determine whether the increase in CT after trabeculectomy is due to an increase in area of large choroidal vessels and whether this change can be localized to the large choroidal vessel layer (LCVL) or the medium choroidal vessel layer (MCVL).
Materials and Methods
Subjects
We conducted a prospective longitudinal study of 30 eyes of 29 patients who were undergoing either trabeculectomy or laser trabeculoplasty at the Glaucoma Center of Excellence in the Wilmer Eye Institute. Informed consent was obtained for each subject, the study adhered to the tenets of the Declaration of Helsinki, and approval was obtained from the Institutional Review Board of Johns Hopkins University. All subjects were older than 18 and had no significant ocular media opacities.
Study Procedure
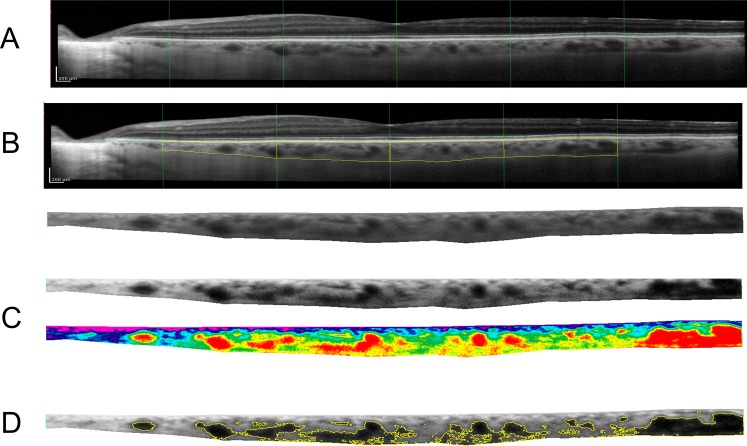
A longitudinal cohort of patients was examined preoperatively and at scheduled postoperative visits with standard clinical assessment including Goldmann applanation tonometry, mean arterial pressure, axial length as measured with the IOLMaster 500 (Carl Zeiss Meditec, Dublin, CA, USA), and EDI SD-OCT using the Heidelberg Spectralis (Heidelberg Instruments, Heidelberg, Germany).22 All measurements were completed on presentation for routine office visit prior to suture lysis or any intervention. EDI SD-OCT images of the posterior 6 mm of choroid centered on the fovea were obtained to visualize the choroidal−scleral interface (Fig. 1). EDI SD-OCT scans were focused on the macula using a single 30° linear B-scan made of 1536 A-scans on the fovea averaged 75 times. Images were registered to provide consistency between the images taken per patient. Six images where the choroidal−scleral interface could not be visualized were excluded.
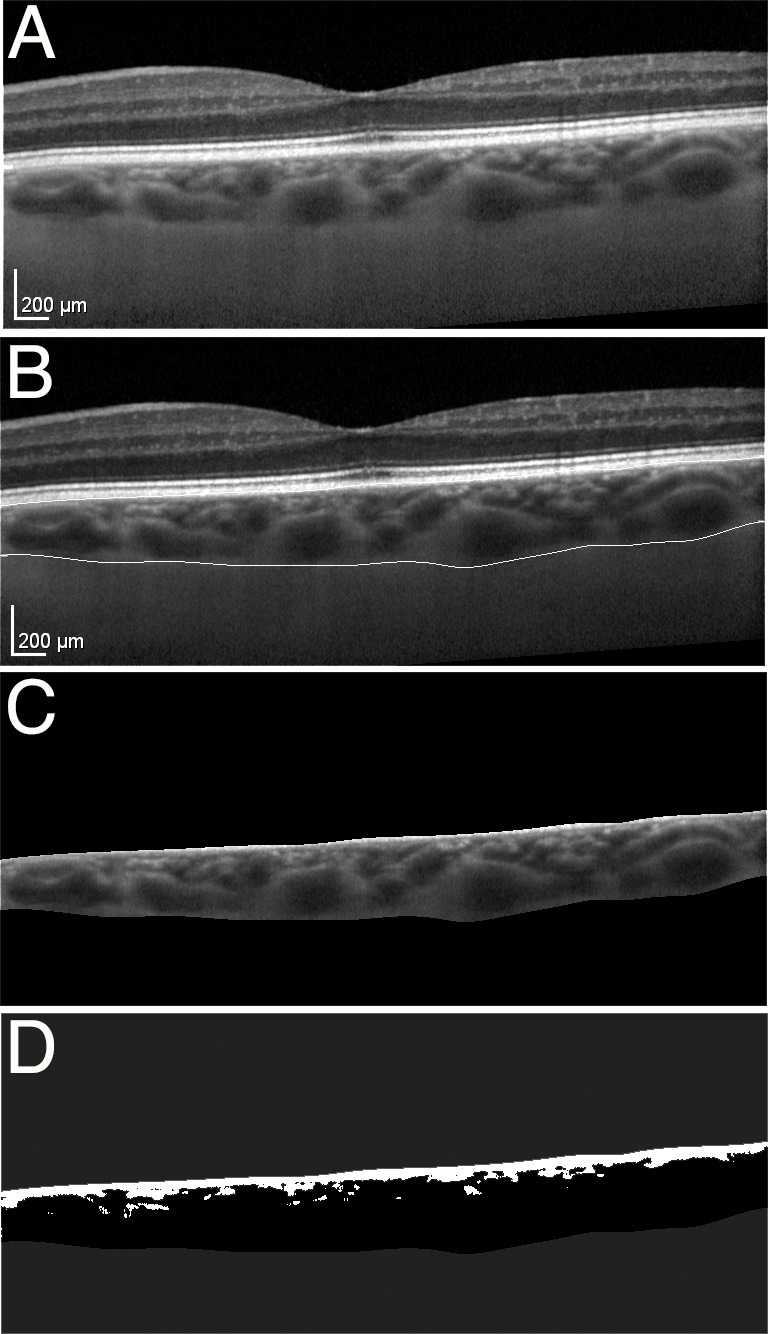
Figure 1.
(A) Sample enhanced depth SD-OCT on the posterior 6 mm surrounding the fovea. (B) Using ImageJ software (National Institutes of Health), the choroid was manually outlined extending from the retinal pigment epithelium to the choroidal–scleral interface and isolated. (C) The image was contrast enhanced with histogram equalization (a) and compared with a color gradient-map (b) to determine the extent of the choroidal vessels. (D) A threshold value was set, above which the large choroidal vessels were outlined. The outlined vessel area was then averaged over the 6 mm choroid to obtain CVT. Interstitial thickness outside of the major vessels was calculated by subtracting the CVT from the CT.
Method 1: Choroidal Vessel Area Image Analysis
EDI SD-OCT images from each time point for each patient were graded by a certified masked grader (AP, XZ). OCT images were processed as previously detailed by Maul et al.13 OCT images were exported and rescaled to correct for magnification using Photoshop (CS5; Adobe Systems, San Jose, CA, USA). A grid extending 3 mm on either side was overlaid on the images, centered on the fovea (Fig. 1A). Poor-quality images,13 as well as images in which the choroidal−scleral interface was not visible or could not be determined, were excluded from the study. The determination of which images were poor used a previously published criterion outlined in Maul et al.13
Using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA), the manually outlined choroid was isolated from the background OCT (Fig. 1B). CT was then calculated by measuring the choroidal area using ImageJ and averaging it over a 6-mm length of choroid using ImageJ as described in Maul et al.13,22
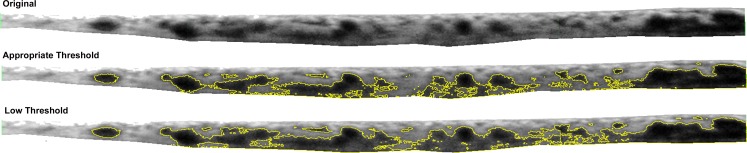
The ImageJ enhance contrast feature was then used on the isolated choroid to provide histogram equalization contrast enhancement. This function uses a monotonic nonlinear mapping to reassign the intensity values of pixels. This feature enables detail enhancement by creating an output image with uniform distribution of intensities. The result is darker intravascular choroidal vessels and lighter extravascular tissue (Fig. 1C). The masked grader set an individualized threshold for each image, above which choroidal vessel area can be isolated. This was set to best approximate the vessel walls such that the entire intravascular area is highlighted, without introducing extravascular noise (Fig. 2). This resulted in a value for the total choroidal vessel area, which was then averaged over a 6-mm choroid to calculate average choroidal vessel thickness (CVT) for ease of comparison with CT values. The area outside the major vessels was defined as the interstitial area, and the average choroidal interstitial thickness (CIT) was calculated by subtracting the CVT from the CT.
Figure 2.
Example of using appropriate threshold to best approximate vessel walls and minimize extravascular noise. The total choroidal area shown was isolated manually from the RPE to the choroidal–scleral interface and subsequently processed through the ImageJ Enhance Contrast feature (National Institutes of Health) as detailed in Figure 1.
To form an artificially color-overlaid image, the contrast-enhanced isolated choroid was then imported into Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The Photoshop Gradient Map tool was used to overlay the transparent rainbow function, allowing for the black and white grayscale image to be spread over a 256 RGB spectrum. This gradient shifts the darkest pixels toward the red spectrum and the lightest pixels toward the blue spectrum. The result is a red highlighting of the intravascular portion of the choroidal vessels (Fig. 1C).
Method 2: Large Choroidal Vessel Area
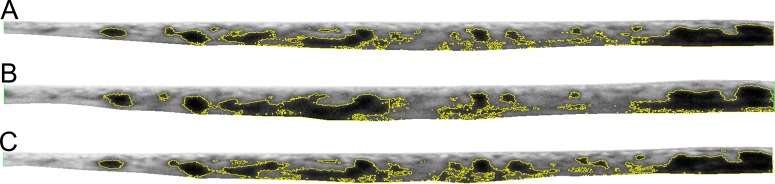
EDI SD-OCT images from each time point for each patient were graded by a certified masked grader (EDC) using a method described previously.26 Using the Spectralis linear measurement tool with the scale set in micrometers, the linear CT was measured perpendicularly from the outer edge of the RPE to the inner sclera at these three locations: fovea, 750 μm temporal to the fovea, and 750 μm nasal to the fovea. The large vessels that were most proximal to those three locations were selected for measurements, measured from the inner point of the large choroidal vessel to the inner border of the sclera. Medium choroidal vessel layer was calculated as difference between LCVL and CT (Fig. 3). Choroidal images that did not have a clear choroidal−scleral interface or did not have a clear large choroidal vessel lumen at any of the three measurement points were excluded.
Figure 3.
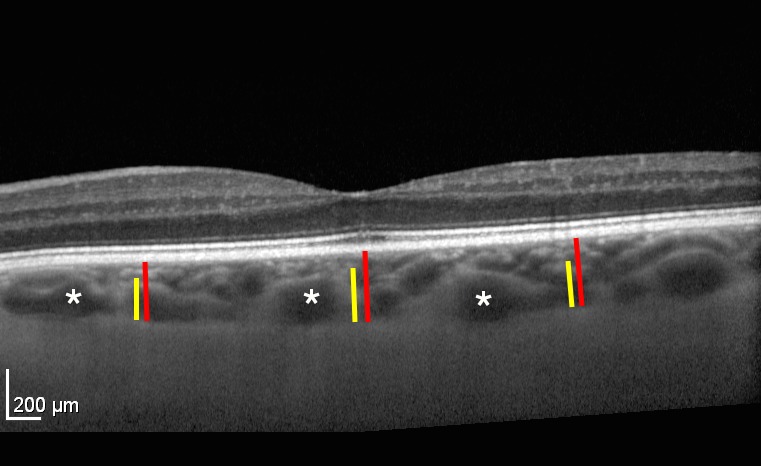
Choroidal vasculature measurements. The vertical red bars delineate the CT from the RPE to the choroidal–scleral interface. The temporal and nasal measurements are located 750 μm from the subfoveal CT measurement. The yellow bars delineate the large choroidal vessel layer. In this measurement, any vessel larger than 100 μm was considered part of the LCVL. The MCVL/choriocapillaris layer is the difference between the red and yellow lines. Asterisks are several examples of large choroidal vessels.
Method 3: Light-Dark Ratio
Each choroidal area was then evaluated for the ratio between the choroidal stroma (light pixels) and choroidal vessel lumen (dark pixels) in the image, otherwise known as the light-dark ratio (LDR).26 Analysis of images using Otsu's method and custom software in Matlab (Mathworks, Natick, MA, USA) was performed (EDC, ML) to determine the LDR26 (Fig. 4).
Figure 4.
Light-dark ratio analysis. (A) Original image. (B) Manually segmented image with the upper bound at the RPE and the lower bound at the choroidal–scleral interface. (C) Choroidal vasculature segmentation, which is the region of interest. (D) Otsu thresholded image used to generate the final light-dark ratio. The light pixels or white area correspond with the choroidal stroma and the dark pixels or black area correspond with the choroidal vessel area.
Statistical Analysis
Variables recorded from image analysis included IOP, CT, CVT, CIT, linear choroidal thickness, LCVL, MCVL, and LDR for each patient at all time points. Mean and SD for each of these measurements was calculated. We first determined the change in each of these variables at the largest difference in IOP. Then, to take advantage of all the follow-up data, we specified repeated-measures regression models in which the dependent variable was one of the variables recorded from the image analysis and the predictor was change in IOP. These models were fit using generalized estimating equations. We added additional covariates to subsequent models to adjust for potential confounders. For the subset of 12 patients who had blood pressure measured at all visits, we used the same approach to assess the association between mean arterial pressure and choroidal measurements.
Predictors of change in CT, CVT, and CIT were determined using a multivariable model with independent variables that included age, race, sex, postoperative day, operation type, baseline mean arterial pressure (MAP), and change in IOP. Interclass coefficients were determined for CT, CVT, MCVL, and LDR. All statistical analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Thirty eyes of 29 patients were deemed of adequate quality for analysis to ascertain overall choroidal vessel thickness, and 27 eyes of 27 patients were deemed sufficient for analysis to determine the difference in LCVL and MCVL thickness. The average age was 69.8 ± 9.2 years. Eleven patients (38%) were male, 24 patients (83%) were white, 5 (17%) were black, and 1 (3%) was Asian. Twenty-four (83%) eyes had primary open-angle glaucoma (POAG), 1 (3%) had primary angle-closure glaucoma POAG, 1 (3%) had congenital glaucoma, 2 (6%) had pigmentary glaucoma, and 1 (3%) had pseudoexfoliation glaucoma. The average baseline axial length was 24.6 ± 1.7 mm, and the average baseline IOP was 23.8 ± 8.3 mm Hg. Nineteen (62%) patients underwent a trabeculectomy, 8 (28%) underwent a phacoemulsification-trabeculectomy, 2 (7%) underwent an argon laser trabeculoplasty, and 1 (3%) underwent a selective laser trabeculoplasty followed by trabeculectomy.
Ninety-nine images of 30 eyes of 29 patients were analyzed to obtain CT, IOP, CVT, CIT, and LDR at different time points for each patient using the choroidal vessel image area analysis and LDR methods. Twelve images were then excluded for the analysis of LCVL and MCVL using the exclusion criteria for that methodology. This left 87 images of 27 eyes of 27 patients that were analyzed to obtain temporal, subfoveal, and nasal CT, LCVL, and MCVL at different time points. Three eyes, two with primary open angle closure and one with pseudoexfoliation glaucoma that underwent trabeculectomy, were excluded from the LCVL analysis.
The average baseline CT was 213.4 ± 66.1 μm. The average baseline CVT and CIT were 74.7 ± 61.7 and 141.9 ± 83.9 μm, respectively.
Reproducibility
For each method, each image was graded by one grader who was certified in a predetermined rigorous fashion in that specific methodology. The CT measurements were performed by two graders (AP, XZ), but only one grader graded each image using a previously published technique.13 The MCVL measurements were performed by one grader (EDC) using a measurement technique that was previously validated.26 The LDR was performed by two graders (EDC, ML), with one grader per image. To evaluate the reproducibility of the methods, a 10% subset of the MCVL and LDR measurements was repeated by the same reader (EDC). Interclass correlation coefficient was calculated as 0.78 for MCVL and 0.95 for LDR. To evaluate reproducibility of CT and CVT, a 10% subset of measurements graded by both readers (AP, XZ) was analyzed. Interclass correlation coefficient was 0.97 for CT and 0.94 for CVT.
Correlations at Largest Change in IOP
The average change in CVT and CIT for each patient at the largest change in IOP was 26.2 ± 38.8 and 29.1 ± 37.9 μm, respectively. The Table shows the change in CVT and CIT for each eye studied at the largest change in IOP. Bivariate linear regression analysis was completed, with the largest change in IOP as the independent variable. Under these parameters, an increase in CVT was correlated with a decrease in IOP (P = 0.009, r2 = 0.22). Similarly, CIT increased with decrease in IOP at the largest change in IOP (P = 0.040, r2 = 0.15). There was also an increase in CT with a decrease in IOP at the largest change in IOP (P < 0.0001, r2 = 0.40). Figure 5 illustrates the change in CVT with change in IOP for one patient. The patient has an IOP of 19 mm Hg before trabeculectomy. When this drops to 6 mm Hg at postoperative month 1, both CT and CVT increase. When the IOP then increases back to 16 mm Hg at postoperative month 4, both CT and CVT increase.
Table.
Change in CT, CVT, and CIT per Change in IOP in Each Patient (Linear Regression Model)
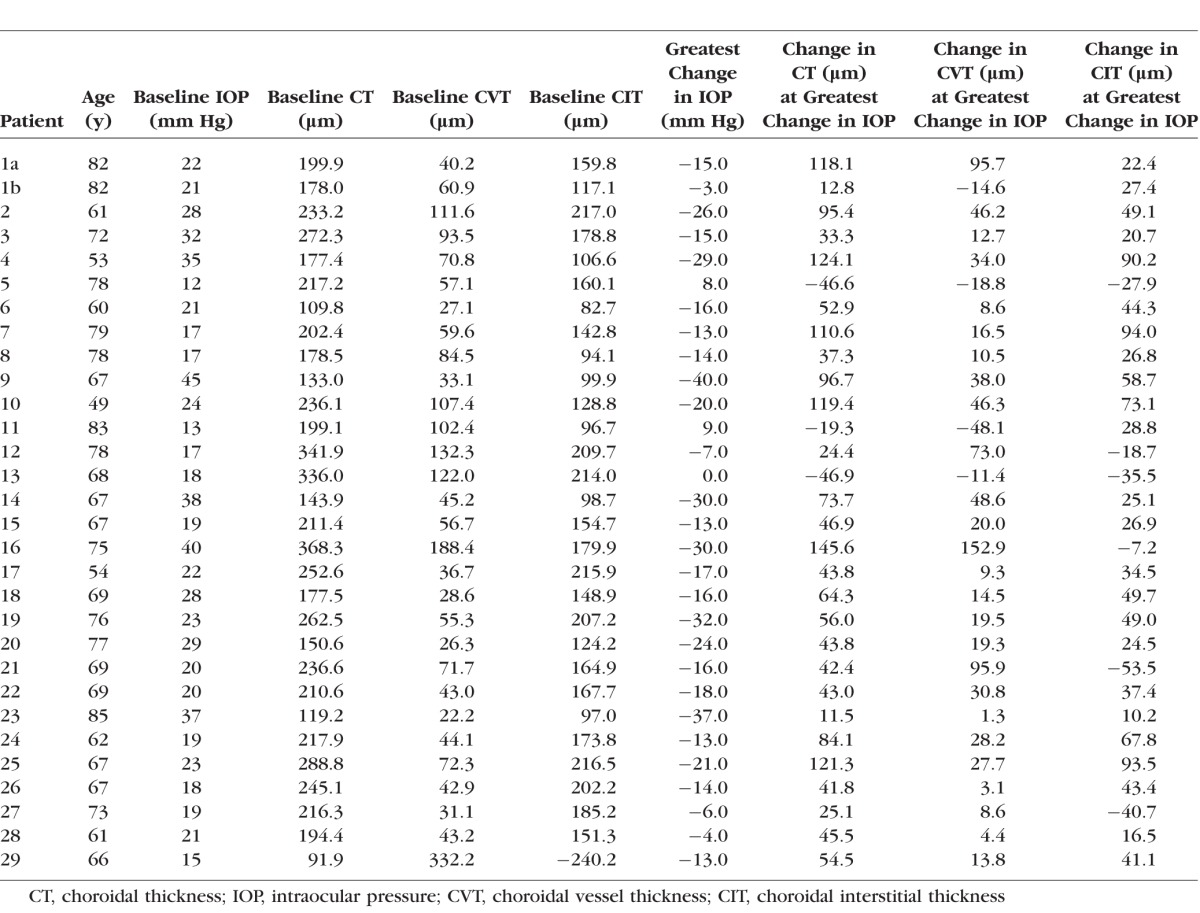
Figure 5.
Longitudinal follow-up of patient using processed SD-OCT images with CVT visibly increasing with increase in CT and decrease in IOP. Choroidal area was isolated from the RPE to the choroidal–scleral interface as seen in Figure 1. (A) Preoperative processed SD-OCT image. IOP of 19 mm Hg, CT = 211.4 μm, and choroidal vessel thickness of 56.74 μm. (B) One month after trabeculectomy for patient. IOP of 6 mm Hg, CT of 258.26 μm, and choroidal vessel thickness of 75.83 μm. (C) Four months after trabeculectomy. IOP of 16 mm Hg, CT of 218.88 μm, and choroidal vessel thickness of 69.11 μm.
There was no evidence of correlation between change in LDR and change in IOP (P = 0.16, r2 = 0.07). There was also no evidence of correlation between change in IOP and change in temporal LCVL (P = 0.13, r2 = 0.09), subfoveal LCVL (P = 0.18, r2 = 0.07), or nasal LCVL (P = 0.19, r2 = 0.06).
Comparing the change in CVT and CIT to overall change in CT, we found that both CVT (P ≤ 0.0001, r2 = 0.43; 95% confidence interval [CI], 0.29, 0.77) and CIT (P < 0.0001, r2 = 0.38; 95% CI, 0.25, 0.73) change linearly with change in overall CT.
Correlations Using Multiple Data Points for Each Patient
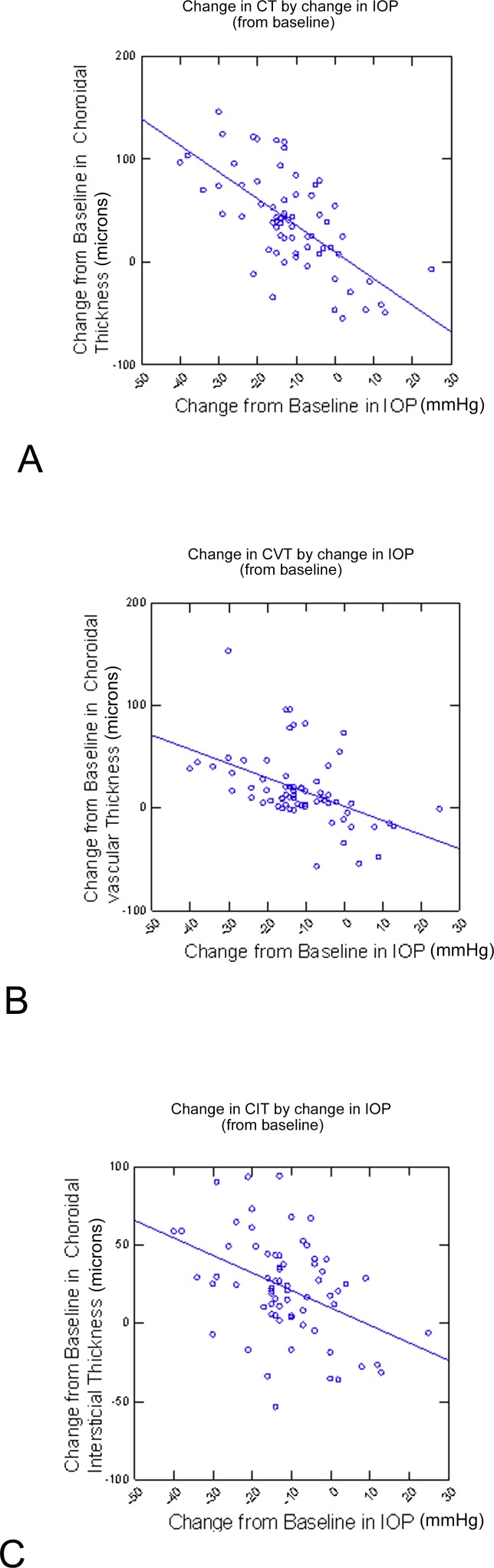
Using a linear regression model that incorporated multiple data points for each individual patient fit using generalized estimating equations, the mean CT was found to increase 3 μm for every 1 mm Hg decrease in IOP (P < 0.0001; 95% CI, 2.3, 3.7). Mean CVT was found to increase 1.5 μm for every 1 mm Hg decrease in IOP (P < 0.0001; 95% CI, 0.8, 2.1), and mean CIT was found to increase 1.3 μm for every 1 mm Hg change in IOP (P < 0.0001; 95% CI, 0.8, 1.8; Fig. 6).
Figure 6.
Change in CT, CIT, and CVT (μm) by change in IOP (mm Hg) from baseline. Each point in the graph represents the results from one postoperative visit from one eye. Each patient contributes multiple points to the graph. (A) Change in CT by change in IOP. (B) Change in CVT by change in IOP. (C) Change in CIT by change in IOP.
Using the same approach to determine the effect of change in blood pressure on CT in this sample, we used data from 12 patients that had blood pressure measured at each visit. For those patients, there was no relationship between the change in mean arterial pressure and CT (P = 0.47), CVT (P = 0.96), or CIT (P = 0.29).
Multivariable Analyses
Accounting for age, race, sex, postoperative day, operation type, baseline MAP, and change in IOP, only change in IOP predicted change in CT (P < 0.0001), CVT (P < 0.0001), and CIT (P < 0.0001). Other variables were not significantly correlated with changes in CT, CVT, or CIT.
Discussion
This is the first study to our knowledge that attempts to assess the relationship between change in IOP and change in choroidal vasculature after IOP lowering due to a glaucoma procedure. We found that the increase in CT seen with decreasing IOP occurs in both the large choroidal vessels and the interstitium of the choroid. Using our newly developed technique to determine CVT over a 6-mm EDI SD-OCT scan, we found that the CVT and CIT change by approximately the same absolute amount with decrease in IOP and with the change in overall CT. The finding that LDR (method 3) does not vary with IOP also indicates that the vessels and interstitium are changing in thickness in proportion with the overall CT and that there is no greater change in one layer than in the other.
When method 2 was used to assess the change in individual layers of the choroid, the LCVL and MCVL did not change with change in IOP. This could potentially be due to limitations in the sensitivity of this methodology. We note that the measurement methodology for LCVL, MCVL, and subfoveal choroidal vessel layer only assesses an approximately 1.5-mm area on a linear scan and focuses only on three separate linear measurements. Although this methodology has been successfully used in assessment of retinal disease,26 the change in CT associated with IOP lowering is global and may be better measured by integrating the entire linear 6-mm scan as described by Maul et al.13 and as we have done using our methodology.
Recent work has shown that in the choroidal vessel, luminal area changes with diurnal variation27 but not with exercise.28 Kinoshita et al.27 found in a study of 38 healthy patients that total choroidal area, central CT, and choroidal vessel luminal area appear to fluctuate diurnally, but not mean stromal area, suggesting that change in the size of large choroidal vessels are largely responsible for the diurnal variation in CT. This is in contrast to our finding that both the choroidal vessel area and the stromal or “interstitial” area change with IOP.
In another study of 38 healthy subjects, Kinoshita et al.28 used both EDI SD-OCT and laser speckle flowgraphy to measure the choroidal area and choroidal blood velocity before, immediately after, and 10 minutes after mild dynamic exercise. Although choroidal blood velocity changed with dynamic exercise, there were no significant changes in choroidal area or the mean luminal and stromal area in this study, suggesting that exercise does not induce a structural change in the choroid.28 Notably, in the second study, the IOP decreased from 12.9 ± 2.91 mm Hg at baseline to 11.4 ± 2.45 mm Hg 10 minutes after exercise, but this small change in IOP was not associated with any choroidal structural changes.28 This 1.5 mm Hg change in IOPaa may be too small to induce a measurable significant change in CT, as has been shown in prior work,12,22–25 or CVT, as our data show. The different responses of the choroidal vessel area and choroidal interstitial area to different stimuli indicate a potentially complex relationship between choroidal vessel area and overall CT, as well as a possible autoregulatory mechanism in choroidal vasculature.
In the subset of patients that had blood pressure checked at every visit, change in MAP was not correlated with change in CT. Our work adds to prior work showing mixed results on the relationship between blood pressure and CT. Kinoshita et al.27 found no relationship between hemodynamic alterations after exercise and changes in choroidal vessel area and overall CT. Similarly, Alwassia et al.29 found no change in CT when blood pressure was altered dynamically with exercise. Conversely, a study conducted by Tan et al.30 assessing the diurnal variation in CT in healthy subjects found a positive correlation between systolic blood pressure and CT. Patients in our study had large shifts in IOP in each visit, as is common in patients after glaucoma procedures. These large shifts may have overwhelmed relatively smaller changes in CT caused by change in blood pressure.
Suprachoroidal hemorrhage, a devastating and feared complication of intraocular surgery, occurs after glaucoma surgery at a rate between 0.7% and 6.1%.31,32 Although its pathogenesis is not well understood, it is thought to be related to postoperative hypotony.33 Maumenee and Schwartz theorized that “Expulsive hemorrhage may often, if not always begin as a choroidal effusion that turns to hemorrhage as the choroidal vessels are stretched and rupture.”34 Our study is the first to our knowledge to assess the change in the thickness of the choroidal vasculature in vivo with change in IOP. Further study assessing the change in the choroidal vasculature may allow for greater understanding of the pathogenesis and risk factors of suprachoroidal hemorrhage.
There are some limitations to our study. We did not include the choriocapillaris layer separately in our analysis. It was grouped into the MCVL and CIT calculations because of the limitations in accurately isolating the choriocapillaris layer on cross-sectional SD-OCT. Specifically, spectral domain devices are susceptible to sensitivity roll-off beneath the RPE. Additionally, visualization of the individual vessels that comprise the choriocapillaris is limited by the axial resolution of 7 μm and lateral resolution of 14 μm of the Heidelberg device. Images were processed once through ImageJ. Images were processed through Photoshop for artificial coloration to aid with grading with ImageJ. Processing images through two different software has the potential to compound errors. Prior studies have related CT to blood pressure, diurnal variation,17,18,35, sex, axial length, age, and myopia.12–14,16 As each patient is being compared to themselves, patient-specific variables such as sex are accounted for. However, diurnal variation was not accounted for. Another variable that could not be assessed is the potential difference in the dynamic choroidal vascular change between normal subjects and those with glaucoma. We note that there are other methods for determining choroidal vascular area, such as those used by Sonoda and Agrawal, which utilize a binary visualization technique.7 Further work is required to compare these different methodologies.
Acknowledgments
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Seattle, Washington, United States, May 1–5, 2016.
Supported by National Institutes of Health Career Development Award K23 EY025014 (OS). Heidelberg Engineering provided the Heidelberg Spectralis that was used for this study.
Disclosure: X. Zhang, None; E. Cole, None; A. Pillar, None; M. Lane, None; N. Waheed, None; M. Adhi, None; L. Magder, None; Harry Quigley, None; O. Saeedi, None
References
- 1. Nickla DL,, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guyer D,, Schachat A,, Green W. The choroid: structural considerations. : Ryan SJ, Retina. Vol 1. 4th ed. Amsterdam, Netherlands: Elsevier Health Sciences; 2006: 33–42. [Google Scholar]
- 3. McLeod DS,, Taomoto M,, Otsuji T,, Green WR,, Sunness JS,, Lutty GA. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002; 43: 1986–1993. [PubMed] [Google Scholar]
- 4. Adhi M,, Brewer E,, Waheed NK,, Duker JS. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013; 131: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang LH,, Jonas JB,, Wei WB. Optical coherence tomographic enhanced depth imaging of polypoidal choroidal vasculopathy. Retina. 2013; 33: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 6. Guyer DR,, Yannuzzi LA,, Slakter JS,, Sorenson JA,, Ho A,, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994; 112: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 7. Agrawal R,, Salman M,, Tan KA,, et al. Choroidal vascularity index (CVI): a novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS One. 2016; 11: e0146344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirooka K,, Saito W,, Hashimoto Y,, Saito M,, Ishida S. Increased macular choroidal blood flow velocity and decreased choroidal thickness with regression of punctate inner choroidopathy. BMC Ophthalmol. 2014; 14: 73–2415 –14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ulrich A,, Ulrich C,, Barth T,, Ulrich WD. Detection of disturbed autoregulation of the peripapillary choroid in primary open angle glaucoma. Ophthalmic Surg Lasers. 1996; 27: 746–757. [PubMed] [Google Scholar]
- 10. Yin ZQ, Vaegan, Millar TJ, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997; 6: 23–32. [PubMed] [Google Scholar]
- 11. Arora KS,, Jefferys JL,, Maul EA,, Quigley HA. Choroidal thickness change after water drinking is greater in angle closure than in open angle eyes. Invest Ophthalmol Vis Sci. 2012; 53: 6393–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kara N,, Baz O,, Altan C,, Satana B,, Kurt T,, Demirok A. Changes in choroidal thickness, axial length, and ocular perfusion pressure accompanying successful glaucoma filtration surgery. Eye (Lond). 2013; 27: 940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maul EA,, Friedman DS,, Chang DS,, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011; 118: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ooto S,, Hangai M,, Yoshimura N. Effects of sex and age on the normal retinal and choroidal structures on optical coherence tomography. Curr Eye Res. 2015; 40: 213–225. [DOI] [PubMed] [Google Scholar]
- 15. Mwanza JC,, Hochberg JT,, Banitt MR,, Feuer WJ,, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 3430–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu XS,, Shen LJ,, Chen RR,, Lyu Z. Peripapillary choroidal thickness in Chinese children using enhanced depth imaging optical coherence tomography. Int J Ophthalmol. 2016; 9: 1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chakraborty R,, Read SA,, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011; 52: 5121–5129. [DOI] [PubMed] [Google Scholar]
- 18. Tan CS,, Ouyang Y,, Ruiz H,, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 261–266. [DOI] [PubMed] [Google Scholar]
- 19. Stanga PE,, Lim JI,, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003; 110: 15–21; quiz 22–23. [DOI] [PubMed] [Google Scholar]
- 20. Spaide RF,, Koizumi H,, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496–500. [DOI] [PubMed] [Google Scholar]
- 21. Tanabe H,, Ito Y,, Iguchi Y,, Ozawa S,, Ishikawa K,, Terasaki H. Correlation between cross-sectional shape of choroidal veins and choroidal thickness. Jpn J Ophthalmol. 2011; 55: 614–619. [DOI] [PubMed] [Google Scholar]
- 22. Saeedi O,, Pillar A,, Jefferys J,, Arora K,, Friedman D,, Quigley H. Change in choroidal thickness and axial length with change in intraocular pressure after trabeculectomy. Br J Ophthalmol. 2014; 98: 976–979. [DOI] [PubMed] [Google Scholar]
- 23. Usui S,, Ikuno Y,, Uematsu S,, Morimoto Y,, Yasuno Y,, Otori Y. Changes in axial length and choroidal thickness after intraocular pressure reduction resulting from trabeculectomy. Clin Ophthalmol. 2013; 7: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshikawa M,, Akagi T,, Nakanishi H,, et al. Longitudinal change in choroidal thickness after trabeculectomy in primary open-angle glaucoma patients. Jpn J Ophthalmol. 2016; 61: 105–112. [DOI] [PubMed] [Google Scholar]
- 25. Kadziauskiene A,, Kuoliene K,, Asoklis R,, Lesinskas E,, Schmetterer L. Changes in choroidal thickness after intraocular pressure reduction following trabeculectomy. Acta Ophthalmol. 2016; 94: 586–591. [DOI] [PubMed] [Google Scholar]
- 26. Branchini LA,, Adhi M,, Regatieri CV,, et al. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013; 120: 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinoshita T,, Mitamura Y,, Shinomiya K,, et al. Diurnal variations in luminal and stromal areas of choroid in normal eyes [published online ahead of print June 13, 2016] Br J Ophthalmol. doi:10.1136/bjophthalmol-2016-308594. [DOI] [PMC free article] [PubMed]
- 28. Kinoshita T,, Mori J,, Okuda N,, et al. Effects of exercise on the structure and circulation of choroid in normal eyes. PLoS One. 2016; 11: e0168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alwassia AA,, Adhi M,, Zhang JY,, et al. Exercise-induced acute changes in systolic blood pressure do not alter choroidal thickness as measured by a portable spectral-domain optical coherence tomography device. Retina. 2013; 33: 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan CS,, Ouyang Y,, Ruiz H,, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 261–266. [DOI] [PubMed] [Google Scholar]
- 31. Gedde SJ,, Herndon LW,, Brandt JD,, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012; 153: 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaziri K,, Schwartz SG,, Kishor KS,, et al. Incidence of postoperative suprachoroidal hemorrhage after glaucoma filtration surgeries in the united states. Clin Ophthalmol. 2015; 9: 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tuli SS,, WuDunn D,, Ciulla TA,, Cantor LB. Delayed suprachoroidal hemorrhage after glaucoma filtration procedures. Ophthalmology. 2001; 108: 1808–1811. [DOI] [PubMed] [Google Scholar]
- 34. Maumenee AE,, Schwartz MF. Acute intraoperative choroidal effusion. Am J Ophthalmol. 1985; 100: 147–154. [DOI] [PubMed] [Google Scholar]
- 35. Iwase T,, Yamamoto K,, Ra E,, Murotani K,, Matsui S,, Terasaki H. Diurnal variations in blood flow at optic nerve head and choroid in healthy eyes: diurnal variations in blood flow. Medicine (Balt). 2015; 94: e519. [DOI] [PMC free article] [PubMed] [Google Scholar]