The impact of filamentous fungi on human welfare has never been greater. Fungi are acknowledged as the most economically devastating plant pathogens (1) and are attaining increasing notoriety for their ability to cause life-threatening infections in humans (57, 71), and fungal products sustain a billion-dollar manufacturing industry (70). The tools available to study filamentous fungi are more sophisticated than ever and include the complete annotated genome sequences of multiple filamentous fungi (12), resources being made available through various functional genomics projects, and advanced bioimaging methods, including high-resolution live-cell imaging (20, 32) and electron tomography (19, 50). The increasing impact of filamentous fungi, along with the rediscovery of pseudohyphal growth in yeast (22), has focused attention on the molecular mechanisms underlying hyphal morphogenesis.
Attempts to understand hyphal morphogenesis have historically followed two different lines of investigation. Microscopists have defined, with increasing detail, the subcellular organization of the hyphal tip. This led to the description of the Spitzenkörper, an apical cluster of vesicles, cytoskeletal elements, and other proteins, which plays a crucial role in hyphal extension (4). Geneticists have identified gene products required for hyphal morphogenesis by characterizing morphological mutants (51, 52). Initial studies in the laboratories of Beadle, Tatum, and colleagues attempted to link morphogenesis to specific biochemical pathways. More recent screens have identified a multitude of signaling and cytoskeletal functions required for hyphal extension (62, 72).
In the past few years, comparative genomics efforts have allowed fungal biologists interested in hyphal morphogenesis to exploit the wealth of knowledge about polarized growth in the yeast Saccharomyces cerevisiae. Many informative homologies between filamentous fungi and yeast have been uncovered. Notably, this includes several components of a multiprotein complex termed the polarisome (28), which regulates microfilament formation at polarized growth sites in yeast (61). Perhaps more importantly, several gene products involved in hyphal morphogenesis have been shown to have no homologue outside of the filamentous fungi. This emphasizes the potential novelty of the mechanisms underlying hyphal morphogenesis. In this review, we summarize past efforts to understand hyphal morphogenesis and pose a series of questions designed to focus future efforts in this area.
HYPHAL MORPHOGENESIS: A BRIEF OVERVIEW
Fungal hyphae originate from either a germinating spore or another hypha (i.e., during branch formation). Initially, an axis of polarity is established from a symmetrically expanding spore or hyphal compartment. Subsequently, cell surface expansion is restricted to the specified axis, thereby leading to the formation of a polarized hypha that displays a gradient of expansion that peaks at the tip (2, 15, 25, 30, 52). Maintenance of the polarity axis allows hyphae to achieve a linear extension rate that can approach 7.5 mm/h (45). Successive hyphal branching, together with hyphal fusion in the colony interior (24), results in a complex coenocytic mycelial network in which nutrients acquired at the colony periphery can be effectively distributed to distal regions and used to support reproductive development.
MICROSCOPY
The pioneering work of Girbardt (23) laid the foundation of research on the biology of the Spitzenkörper (= apical body). Using phase-contrast light microscopy, Girbardt provided the first description of the Spitzenkörper as a phase-dark structure located in tips of growing hyphae of higher fungi. His meticulous observations of living hyphae demonstrated that the Spitzenkörper (i) is present only in growing vegetative hyphal tips, (ii) forms at sites of spore germination and branch formation, and (iii) is located at a position within the hyphal tip that correlates with the direction of hyphal growth. Girbardt was thus the first to show the intimate association that exists between Spitzenkörper behavior and hyphal morphogenesis.
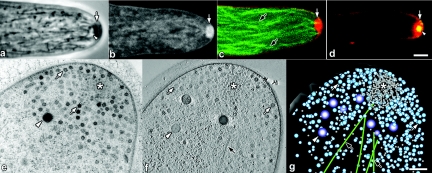
With the extraordinary insights into fungal subcellular structure made possible by the use of transmission electron microscopy (TEM) during the 1960s, various research groups focused on trying to correlate what Girbardt had observed in living hyphae with what could be detected in chemically fixed hyphae at the ultrastructural level. Of these early studies, that of Grove and Bracker (26) stands out in providing the most detailed analysis of the ultrastructural organization of hyphal tips, as well as illustrating the variable morphology of the Spitzenkörper among different groups of fungi. Ultrastructural preservation took a quantum leap forward with the development and use of rapid cryofixation and freeze-substitution protocols to prepare hyphal cells for TEM (36, 37, 38), which set the benchmark for subsequent ultrastructural studies (33, 34, 49, 58, 59, 60, 68). All studies of hyphal tip ultrastructure by TEM have demonstrated that the Spitzenkörper is a complex, multicomponent structure dominated by vesicles (Fig. 1e). These studies have also shown that vesicles are organized around a prominent core region that is enriched in a dense meshwork of microfilaments (36; R. Roberson, unpublished results). Interestingly, polysomes are often closely associated with the posterior boundary of the Spitzenkörper core (36; Roberson, unpublished). In addition, microtubules extend into and often through the Spitzenkörper (Fig. 1c and g) (36, 60). Woronin bodies are also found in the apical region near the Spitzenkörper (Fig. 1e and f), although these peroxisome-related structures are not thought to have any role in hyphal growth (39, 48, 54). Vesicles of the Spitzenkörper can be divided into two populations on the basis of size (Fig. 1e to g), the large so-called apical vesicles (70 to 90 nm in diameter) and the small microvesicles (30 to 40 nm in diameter). Although the concept that many, if not all, of these vesicles are secretory vesicles involved in delivering materials required for hyphal elongation is entrenched in the literature, little is known regarding vesicle composition and whether there are biochemically distinct populations of vesicles that contribute specifically to tip growth. In support of the latter possibility, Bracker, Ruiz-Herrera, and Bartnicki-Garcia (5, 10) isolated a population of microvesicles termed chitosomes from fungal hyphae and showed that they possessed chitin synthase activity. It is not clear if these vesicles correspond to the population of chitosomes of endocytic origin that were subsequently characterized in yeast (75).
FIG. 1.
(a to d) Live-cell imaging of growing hyphal tips of N. crassa (a to c) and A. nidulans (d). Scale bars = 2.5 μm. (a) Digitally enhanced phase-contrast image of an unstained hypha showing Spk composed of a phase-dark cloud of secretory vesicles (arrow) and a phase-bright core (arrowhead) (M. Uchida and R. W. Roberson, unpublished data). (b) Confocal image of a hypha stained with FM4-64 showing pronounced staining of vesicles (arrow) within the Spk (P. C. Hickey and N. D. Read, unpublished data). (c) Confocal image colabeled with β-tubulin-GFP (green, black arrow) and FM4-64 (red, white arrow) showing microtubules extending into Spk. (d) Confocal image colabeled with SepA-GFP and FM4-64 (red, arrow) showing colocalization of SepA (yellow, arrowhead). In the central region of Spk is the region occupied by microvesicles (see panels e to g) (E. R. Kalkman and N. D. Read, unpublished data). (e to g) TEM of a hyphal tip of A. nidulans. A cluster of microvesicles within the Spk core (asterisks) surrounded by apical vesicles (white arrows), Woronin bodies (white arrowheads), and microtubules (black arrows) are indicated in these images. (e) Standard transmission electron micrograph (60-nm-thick section). (f) Electron tomographic section (∼3-nm z-axis resolution) of a dual-axis tomogram taken at 200 kV. (g) Model of panel f showing the 3D distribution of apical cytoplasm through an ∼170-nm-thick section (Uchida and Roberson, unpublished). Scale bar = 250 nm.
Beginning in the 1990s, there has been a significant return to live-cell analysis (Fig. 1a to d). Using video-enhanced light microscopy, Lopez-Franco et al. (45, 46, 47) described the diversity of the Spitzenkörper among the fungi and discovered a structure referred to as the “satellite Spitzenkörper.” This is a small cluster of vesicles and associated proteins that form de novo at the plasma membrane near the hyphal apex that, upon fusing with the Spitzenkörper, may be involved in generating growth pulses. Further work in Bracker's laboratory on living hyphae provided the most compelling evidence that the Spitzenkörper is responsible for growth directionality and hyphal tip morphogenesis through the use of laser tweezers (11; see also reference 4). In more recent years, live-cell analysis has profited from the use of fluorescent probes that label the Spitzenkörper. An important breakthrough was the observation that the endocytosis marker dye FM4-64 rapidly stains vesicles within the Spitzenkörper (Fig. 1b to d) (18, 32, 35), not only providing evidence for the interconnection of endocytic and secretory pathways but demonstrating the dye's utility for visualizing and analyzing Spitzenkörper behavior in wild-type and mutant strains (31, 32).
The central importance of the cytoskeleton in hyphal morphogenesis is well established (4). It is thought that microtubules are primarily responsible for the long-distance transport of secretory vesicles to the Spitzenkörper, while actin microfilaments primarily control vesicle organization within the Spitzenkörper and transport to the plasma membrane. If this is correct, then the Spitzenkörper may be viewed as a switching station from microtubule-based to microfilament-based vesicle transport. These concepts emerged in part from an insightful vesicle-based mathematical model of fungal morphogenesis, which predicts that the Spitzenkörper acts as a vesicle supply center (i.e., a moveable distribution center for vesicles involved in cell surface expansion) (3, 4). Immunolocalization studies and phalloidin staining have emphasized the presence of actin in the Spitzenkörper and hyphal apex (8, 66, 69), supporting TEM data and suggesting that the Spitzenkörper may function as a microfilament-organizing center. γ-Tubulin, which is normally associated with microtubule organizing centers (MTOCs) such as spindle pole bodies, was immunolocalized within the Spitzenkörper of the chytrid Allomyces macrogynus (49). The idea that the Spitzenkörper might be an MTOC is a very attractive one (4), but attempts to localize γ-tubulin within the Spitzenkörper of higher fungi have failed (Roberson, unpublished). Furthermore, results of recent live-cell studies of microtubule dynamics after tagging α- or β-tubulin with green fluorescent protein (GFP) have provided no indication that microtubules emanate from the Spitzenkörper (20, 27), which would be expected from an MTOC.
GENETICS
The first attempt at using a genetic approach to understand hyphal morphogenesis evolved directly from the Neurospora crassa mutagenesis program initiated by Beadle and Tatum. Because N. crassa hyphae extend at such a rapid rate, morphogenetic mutants typically form compact colonies that can be easily distinguished from the wild type. Accordingly, a few obvious morphogenetic mutants were recovered and described (6). Later, Garnjobst and Tatum (21) undertook the first systematic attempt to identify and characterize morphological mutants, which resulted in the identification of 90 mutants that defined at least 58 loci. These mutants were sorted into phenotypic classes (i.e., cot [temperature-sensitive colonial], col [colonial], spco [spreading colonial], ro [ropy], etc.) on the basis of colony morphology. As a result of these and related efforts (summarized in references 13 and 51), several key mutations such as cot-1, mcb, and cr-1 were initially characterized. Using the tools that were then available, attempts were made to link the new morphological mutations to specific biochemical defects. This approach was successful in some cases, such as linking cr-1 to a severe defect in adenylyl cyclase activity (67). More generally, however, these studies highlighted the contribution of the cell wall to the maintenance of normal hyphal morphology.
With the advent of molecular genetics, it became possible to clone and characterize several genes identified in the earlier genetic screens, including cot-1 (73) and mcb (14), both of which encode protein kinases. In addition, Plamann and colleagues showed that the ro genes encode components of the cytoplasmic dynein motor complex (56). Simultaneously, reverse genetic approaches were used to characterize the morphogenetic functions played by diverse components of the cytoskeleton, cell wall, and signaling pathways (reviewed in references 52 and 71). Notably, these studies demonstrated that the establishment and maintenance of hyphal polarity are compromised by mutations affecting, among other functions, microtubule- and microfilament-based motor proteins, chitin deposition, and both cyclic AMP and mitogen-activated protein kinase signaling. Although these studies have only provided fragmented insights into the molecular mechanisms underlying hyphal morphogenesis, they have revealed sufficient differences from the well-characterized yeast morphogenetic model system to justify additional mutation hunts.
More recently, systematic attempts to identify a large fraction of the genes involved in hyphal morphogenesis have been initiated by using N. crassa and Aspergillus nidulans. The most thorough screen identified 45 genes involved in diverse aspects of morphogenesis in N. crassa (62). Remarkably, this collection included several genes not previously implicated in polarized growth on the basis of studies with yeast, as well as another group of novel hypothetical proteins with morphogenetic functions. Multiple temperature-sensitive mutations affecting polarity establishment and maintenance have also been recovered in A. nidulans (29, 40, 53). Consistent with the results obtained with N. crassa, characterization of the affected genes has identified several functions whose involvement in hyphal morphogenesis was not previously suspected (43, 44, 64, 65). Collectively, these systematic studies underscore the complexity of hyphal morphogenesis and emphasize several features that distinguish it from the well-characterized yeast model.
The use of genetic approaches has provided initial insight into the molecular composition of the Spitzenkörper. Notably, Sharpless and Harris (63) demonstrated that the formin SepA is a component of the Spitzenkörper of A. nidulans (Fig. 1d). SepA is a homologue of yeast Bni1p, which is a key part of the multiprotein polarisome complex responsible for nucleation of microfilaments. Homologues of another polarisome protein, Spa2p, localize to hyphal tips in Ashbya gosypii, Candida albicans, and A. nidulans (41, 74; A. Virag and S. Harris, unpublished results). Because the Spitzenkörper may represent a microfilament-organizing center, these results are consistent with the idea that the polarisome is a component of the Spitzenkörper. However, the full extent of the relationship between the Spitzenkörper and the polarisome remains to be determined. One attractive possibility is that the Spitzenkörper is a dynamic structure composed of many interacting protein complexes, one of which is the polarisome.
GENOMICS: LESSONS FROM YEAST AND ANIMALS
The mechanisms that generate polarity in yeast cells have been characterized in considerable detail. These studies have led to the development of a model whereby divergent positional landmarks locally activate conserved signaling modules that subsequently function via scaffold proteins to organize the cytoskeleton and regulate protein transport (55). With this model as a guide, it is reasonable to predict that a similar hierarchy of functions underlies the establishment and maintenance of hyphal polarity (28). For example, as in yeast, positional landmarks presumably designate polarization sites within hyphae. These landmarks may include internal cues such as cell wall proteins or cell surface receptors that respond to external factors. Notably, most of the landmarks characterized in yeast do not appear to be conserved in filamentous fungi (7, 28). Once polarization sites are specified, the positional information is likely to be transduced by conserved Cdc42 and Rho-related GTPase modules. As in animal cells, a Rac GTPase module may also mediate this step in filamentous fungi (9, 16). Effectors of the GTPases regulate organization of the cytoskeleton and vesicle trafficking and are generally well conserved in the genomes of filamentous fungi (7, 28). This model provides a useful framework for assessing the function of those morphogenetic proteins that are highly conserved among animals, yeast, and filamentous fungi. However, the functional role of the growing number of novel, filamentous-fungus-specific morphogenetic proteins remains unclear. These proteins may contribute to the far greater complexity of hyphal morphogenesis relative to that of yeast.
Hyphae have morphological features in common with other highly polarized cells, such as animal neurons and plant pollen tubes. While the mechanisms are not fully known, it is clear that some of the same signaling molecules important in neurotransmitter activity are up-regulated in the tip growth of pollen tubes and that these molecules affect the actin cytoskeleton (17). It seems likely that animals, plants, and fungi use the same core cellular machinery for polar growth but that they organize this common polarity machinery in different ways. It will be of great interest to see which Spitzenkörper components represent conserved core polarity machinery and which are unique to filamentous fungi.
HYPHAL MORPHOGENESIS: THE KEY PROBLEMS
The significant impact of fungi on human welfare has helped to focus attention on the role that hyphae play in fungal biology. Because of this, and the recent development of genomic and imaging tools that greatly enhance our ability to manipulate fungi, we propose that now is the time to initiate a systematic analysis of the molecular mechanisms underlying hyphal morphogenesis. Below, we propose several critical questions whose answers will likely provide unparalleled insight into hyphal morphogenesis and, in a more general sense, fungal biology.
(i) What is the composition of the Spitzenkörper?
The Spitzenkörper is the organizing center for hyphal growth and morphogenesis. Moreover, its behavior has been the subject of extensive microscopic characterization and mathematical modeling. Nevertheless, the molecular composition of the Spitzenkörper remains largely unclear. To fill this void, it will first be necessary to identify and characterize its component parts. This should include (i) characterization of the different populations of vesicles found within the Spitzenkörper and the identification of their contents, (ii) characterization of the resident proteins that underlie Spitzenkörper function, and (iii) identification of the mRNA species that are transported to ribosomes associated with the Spitzenkörper.
(ii) How is the Spitzenkörper assembled and disassembled?
The mechanisms underlying the de novo formation and division of the Spitzenkörper must be addressed. This includes (i) investigating the possible role of landmark proteins in regulating the location and timing of assembly, (ii) characterizing the interactions between proteins and complexes that underlie the assembly pathway, (iii) determining how a satellite Spitzenkörper is incorporated into a preexisting structure, and (iv) understanding the mechanisms that regulate Spitzenkörper division.
(iii) How is Spitzenkörper function regulated during hyphal morphogenesis?
The mechanisms that regulate Spitzenkörper behavior must be characterized. This includes (i) characterizing the dynamic interactions between proteins involved in Spitzenkörper function, (ii) determining the nature of the physiological and environmental signals that regulate Spitzenkörper function, and (iv) understanding how apical dominance, which normally constrains hyphal branching to subapical regions, is controlled.
(iv) How is hyphal morphogenesis modified during pathogenesis and development?
Fungal hyphae can differentiate elaborate structures that facilitate pathogenesis or the dissemination of spores. The signaling pathways that regulate differentiation are beginning to be understood (42). Nevertheless, the mechanisms by which these signals affect Spitzenkörper function during infection structure differentiation and reproductive development are not known.
REFERENCES
- 1.Agrios, G. N. 1997. Plant Pathology, 4th ed. Academic Press, London, United Kingdom.
- 2.Bartnicki-García, S., and E. Lippman. 1969. Fungal morphogenesis: cell wall construction in Mucor rouxii. Science 165:302-304. [DOI] [PubMed] [Google Scholar]
- 3.Bartnicki-Garcia, S., F. Hergert, and G. Gierz. 1989. Computer simulation of fungal morphogenesis and the mathematical basis for hyphal tip growth. Protoplasma 153:46-57. [Google Scholar]
- 4.Bartnicki-García, S. 2002. Hyphal tip growth: outstanding questions, p. 29-58 In H. D. Osiewacz (ed.), Molecular biology of fungal development. Marcel Dekker, Inc., New York, N.Y.
- 5.Bartnicki-Garcia, S., C. E. Bracker, E. Reyes, and J. Ruiz-Herrera. 1978. Isolation of chitosomes from taxonomically diverse fungi and synthesis of chitin microfibrils in vitro. Exp. Mycol. 2:173-192. [Google Scholar]
- 6.Beadle, G. W., and E. L. Tatum. 1945. Neurospora. II. Methods of producing and detecting mutations concerned with nutritional requirements. Am. J. Bot. 32:678-686. [Google Scholar]
- 7.Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner, N. D. Read, S. Seiler, D. Bell-Pedersen, J. Paietta, N. Plesofsky, M. Plamann, M. Goodrich-Tanrikulu, U. Schulte, G. Mannhaupt, F. E. Nargang, A. Radford, C. Selitrennikoff, J. E. Galagan, J. C. Dunlap, J. J. Loros, D. Catcheside, H. Inoue, R. Aramayo, M. Polymenis, E. U. Selker, M. S. Sachs, G. A. Marzluf, I. Paulsen, R. Davis, D. J. Ebbole, A. Zelter, E. R. Kalkman, R. O'Rourke, F. Bowring, J. Yeadon, C. Ishii, K. Suzuki, W. Sakai, and R. Pratt. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68:1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourett, T. M., and R. J. Howard. 1991. Ultrastructural immunolocalization of actin in a fungus. Protoplasma 163:199-202. [Google Scholar]
- 9.Boyce, K. J., M. J. Hynes, and A. Andrianopoulus. 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 116:1249-1260. [DOI] [PubMed] [Google Scholar]
- 10.Bracker, C. E., J. Ruiz-Herrera, and S. Bartnicki-García. 1976. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril formation in vitro. Proc. Natl. Acad. Sci. USA 73:4570-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bracker, C. E., D. J. Murphy, and R. Lopez-Franco. 1997. Laser microbeam manipulation of cell morphogenesis in growing fungal hyphae. p. 67-80. In D. L. Farkas and B. J. Tromberg (ed.), Functional imaging of optical manipulation of living cells. Proceedings of SPIE, vol. 2983. International Society for Optical Engineering, Bellingham, Wash. [Google Scholar]
- 12.The Broad Institute. 2004. The Fungal Genome Initiative, The Broad Institute, Cambridge, Mass. (http://www.broad.mit.edu/annotation/fungi/fgi/).
- 13.Brody, S. 1973. Metabolism, cell walls, and morphogenesis. p. 107-154 In S. Coward (ed.), Developmental regulation. Academic Press, New York, N.Y.
- 14.Bruno, K. S., R. Aramayo, P. F. Minke, R. L. Metzenberg, and M. Plamann. 1996. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 15:5772-5782. [PMC free article] [PubMed] [Google Scholar]
- 15.Castle, E. S. 1958. Problems of oriented growth and structure in Phycomyces. Q. Rev. Biol. 28:364-372. [DOI] [PubMed] [Google Scholar]
- 16.Chen, C., and M. B. Dickman. 2004. Dominant active Rac and dominant negative Racrevert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 51:1493-1507. [DOI] [PubMed] [Google Scholar]
- 17.Feijo, J. A., S. S. Costa, A. M. Prado, J. D. Becker, and A. C. Certal. 2004. Signaling by tips. Curr. Opin. Plant Biol. 7:589-598. [DOI] [PubMed] [Google Scholar]
- 18.Fischer-Parton, S., R. M. Parton, P. C. Hickey, J. Dijksterhuis, H. A. Atkinson, and N. D. Read. 2000. Confocal microscopy of FM 4-64 as a tool for analyzing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 198:246-259. [DOI] [PubMed] [Google Scholar]
- 19.Frank, J., T. Wagenknecht, B. F. McEwen, M. Marko, C. Hsieh, and C. A. Mannella. 2002. Three-dimensional imaging of biological complexity. J. Struct. Biol. 138:85-91. [DOI] [PubMed] [Google Scholar]
- 20.Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker, and N. D. Read. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41:907-920. [DOI] [PubMed] [Google Scholar]
- 21.Garnjobst, L., and E. L. Tatum. 1967. A survey of new morphological mutants in Neurospora crassa. Genetics 57:579-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 23.Girbardt, M. 1957. Der Spitzenkörper von Polystictus versicolor. Planta 50:47-59. [Google Scholar]
- 24.Glass, N. L., C. Rasmussen, M. G. Roca, and N. D. Read. 2004. Hyphal homing, fusion, and mycelial interconnectedness. Trends Microbiol. 12:135-141. [DOI] [PubMed] [Google Scholar]
- 25.Gooday, G. W. 1971. An autoradiographic study of hyphal growth of some fungi. J. Gen. Microbiol. 67:125-133. [Google Scholar]
- 26.Grove, S. N., and C. E. Bracker. 1970. Protoplasmic organization of hyphal tips among fungi: Vesicles and Spitzenkörper. J. Bacteriol. 104:989-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han, G., B. Liu, J. Zhang, W. Zuo, N. R. Morris, and X. Xiang. 2001. The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics. Curr. Biol. 11:719-724. [DOI] [PubMed] [Google Scholar]
- 28.Harris, S. D., and M. Momany. 2004. Polarity in filamentous fungi: moving beyond the yeast paradigm. Fungal Genet. Biol. 41:391-400. [DOI] [PubMed] [Google Scholar]
- 29.Harris, S. D., A. F. Hofmann, H. W. Tedford, and M. P. Lee. 1999. Identification and characterization of genes required for hyphal morphogenesis in the filamentous fungus Aspergillus nidulans. Genetics 151:1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heath, I. B. 1990. Tip growth in plant and fungal cells. Academic Press, San Diego, Calif.
- 31.Hickey, P. C., and N. D. Read. 2003. Biology of living fungi. British Mycological Society, Stevenage, United Kingdom.
- 32.Hickey, P. C., S. R. Swift, M. G. Roca, and N. D. Read. Live-cell imaging of filamentous fungi using vital fluorescent dyes and confocal microscopy. Methods Microbiol., in press.
- 33.Hoch, H. C., and R. J. Howard. 1981. Ultrastructure of freeze substituted hyphae of the basidiomycete Laetisaria arvalis. Protoplasma 103:281-297. [Google Scholar]
- 34.Hoch, H. C., and R. C. Staples. 1983. Ultrastructural organization of non-differentiated uredospore germlings of Uromyces phaseoli variety typical. Mycologia 75:795-824. [Google Scholar]
- 35.Hoffman, J., and K. Mendgen. 1998. Endocytosis and membrane turnover in the germ tube of Uromyces fabae. Fungal Genet. Biol. 24:77-85. [DOI] [PubMed] [Google Scholar]
- 36.Howard, R. J. 1981. Ultrastructural analysis of hyphal tip cell growth in fungi: Spitzenkörper, cytoskeleton and endomembranes after freeze-substitution. J. Cell Sci. 48:89-103. [DOI] [PubMed] [Google Scholar]
- 37.Howard, R. J., and J. R. Aist. 1979. Hyphal tip cell ultrastructure of the fungus Fusarium: improved preservation by freeze-substitution. J. Ultrastruct Res. 66:224-234. [DOI] [PubMed] [Google Scholar]
- 38.Howard, R. J., and J. R. Aist. 1980. Cytoplasmic microtubules and fungal morphogenesis: ultrastructural effects of methyl benzimidazole-2-ylcarbamate determined by freeze-substitution hyphal tip cells. J. Cell Biol. 87:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jedd, G., and N. H. Chua. 2000. A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat. Cell Biol. 2:226-231. [DOI] [PubMed] [Google Scholar]
- 40.Kaminskyj, S. G., and J. E. Hamer. 1998. Hyp loci control cell pattern formation in the vegetative mycelium of Aspergillus nidulans. Genetics 148:669-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knechtle, P., F. Dietrich, and P. Philippsen. 2003. Maximal polar growth potential depends on the polarisome component AgSpa2 in the filamentous fungus Ashbya gosypii. Mol. Biol. Cell 14:4140-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin, X., and M. Momany. 2003. The Aspergillus nidulans swoC1 mutant shows defects in growth and development. Genetics 165:543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin, X., C. Momany, and M. Momany. 2004. SwoHp, a nucleoside diphosphate kinase, is essential in Aspergillus nidulans. Genetics 2:1169-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Franco, R., S. Bartnicki-García, and C. E. Bracker. 1994. Pulsed growth of fungal hyphal tips. Proc. Natl. Acad. Sci. USA 91:12228-12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Franco, R., R. J. Howard, and C. W. Bracker. 1995. Satellite Spitzenkörper in growing hyphal tips. Protoplasma 188:85-103. [Google Scholar]
- 47.Lopez-Franco, R., and C. E. Bracker. 1996. Diversity and dynamics of the Spitzenkörper in growing hyphal tips of higher fungi. Protoplasma 195:90-111. [Google Scholar]
- 48.Markham, P., and A. J. Collinge. 1987. Woronin bodies of filamentous fungi. FEMS Microbiol. Rev. 46:1-11. [Google Scholar]
- 49.McDaniel, D. P., and R. W. Roberson. 2000. Microtubules are required for motility and positioning of vesicles and mitochondria in hyphal tip cells of Allomyces macrogynus. Fungal Genet. Biol. 31:233-244. [DOI] [PubMed] [Google Scholar]
- 50.McIntosh, J. R. 2001. Electron microscopy of cells: a new beginning for a new century. J. Cell Biol. 153:F25-F32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra, N. C. 1977. Genetics and biochemistry of morphogenesis in Neurospora. Adv. Genet. 19:341-405. [DOI] [PubMed] [Google Scholar]
- 52.Momany, M. 2002. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr. Opin. Microbiol. 5:580-585. [DOI] [PubMed] [Google Scholar]
- 53.Momany, M., P. J. Westfall, and G. Abramowsky. 1999. Aspergillus nidulans swo mutants show defects in polarity establishment, polarity maintenance and hyphal morphogenesis. Genetics 151:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Momany, M., E. A. Richardson, C. V. Sickle, and G. Jedd. 2002. Mapping Woronin body position in Aspergillus nidulans. Mycologia 94:260-266. [PubMed] [Google Scholar]
- 55.Nelson, W. J. 2003. Adaptation of core mechanisms to generate cell polarity. Nature 422:766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plamann, M., P. F. Minke, J. H. Tinsley, and K. S. Bruno. 1994. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J. Cell Biol. 127:139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ponton, J., R. Ruchel, K. V. Clemons, D. C. Coleman, R. Grillot, J. Guarro, D. Aldebert, P. Ambroise-Thomas, J. Cano, A. J. Carrillo-Munoz, J. Gene, C. Pinel, D. A. Stevens, and D. J. Sullivan. 2000. Emerging pathogens. Med. Mycol. 38(Suppl. 1):225-236. [DOI] [PubMed] [Google Scholar]
- 58.Riquelme, M., R. W. Roberson, D. P. McDaniel, and S. Bartnicki-García. 2002. The effect of ropy-1 mutation on cytoplasmic organization in mature hyphae of Neurospora crassa. Fungal Genet. Biol. 37:171-179. [DOI] [PubMed] [Google Scholar]
- 59.Roberson, R. W., and M. S. Fuller. 1988. Ultrastructural aspects of the hyphal tip of Sclerotium rolfsii preserved by freeze substitution. Protoplasma 146:143-149. [Google Scholar]
- 60.Roberson, R. W., and M. S. Fuller. 1990. Effects of the demethylase inhibitor, cyproconazole, on hyphal tip cells of Sclerotium rolfsii. II. An electron microscope study. Exp. Mycol. 14:124-135. [Google Scholar]
- 61.Sagot, I., S. K. Klee, and D. Pellman. 2002. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4:42-50. [DOI] [PubMed] [Google Scholar]
- 62.Seiler, S., and M. Plamamm. 2003. The genetic basis of cellular morphogenesis in the filamentous fungus Neurospora crassa. Mol. Biol. Cell 14:4352-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharpless, K. E., and S. D. Harris. 2002. Functional characterization and localization of the Aspergillus nidulans formin SEPA. Mol. Biol. Cell 13:469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaw, B. D., C. Momany, and M. Momany. 2002. Aspergillus nidulans swoF encodes an N-myristoyl transferase. Eukaryot. Cell 1:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi, X., Y. Sha, and S. Kaminskyj. 2004. Aspergillus nidulans hypA regulates morphogenesis through the secretion pathway. Fungal Genet. Biol. 41:75-88. [DOI] [PubMed] [Google Scholar]
- 66.Srijayanthi, S., M. Vargas, and R. W. Roberson. 1996. Functional, organizational, and biochemical analysis of actin in the hyphal tip cells of Allomyces macrogynus. Mycologia 88:57-70. [Google Scholar]
- 67.Terenzi, H. F., M. M. Flawia, M. T. Tellez-Inon, and H. N. Torres. 1976. Control of Neurospora crassa morphology by cyclic adenosine 3′,5′-monophosphate and dibutyryl cyclic adenosine 3′,5′-monophosphate. J. Bacteriol. 126:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vargas, M., J. M. Aronson, and R. W. Roberson. 1993. Cytological organization of hyphal tip cells of Allomyces macrogynus. Protoplasma 176:43-52. [Google Scholar]
- 69.Virag, A., and A. J. F. Griffiths. 2004. A mutation in the Neurospora crassa actin gene results in multiple defects in tip growth and branching. Fungal Genet. Biol. 41:213-225. [DOI] [PubMed] [Google Scholar]
- 70.Wainright, M. 1992. An introduction to fungal biotechnology. John Wiley, New York, N.Y.
- 71.Walsh, T. J., A. Groll, J. Hiemenz, R. Fleming, E. Roilides, and E. Anaissee. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 10(Suppl. 1):48-66. [DOI] [PubMed] [Google Scholar]
- 72.Wendland, J. 2001. Comparison of morphogenetic networks of filamentous fungi and yeast. Fungal Genet. Biol. 34:63-82. [DOI] [PubMed] [Google Scholar]
- 73.Yarden, O., M. Plamann, D. J. Ebbole, and C. Yanofsky. 1992. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 11:2159-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng, X. D., Y. M. Wang, and Y. Wang. 2003. CaSPA2 is important for polarity establishment and maintenance in Candida albicans. Mol. Microbiol. 49:1391-1405. [DOI] [PubMed] [Google Scholar]
- 75.Ziman, M., J. S. Chuang, and R. W. Scheckman. 1996. Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol. Biol. Cell 7:1909-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]