Abstract
Key metabolic hormones, such as insulin, leptin, and adiponectin, have been studied extensively in obesity, however the pathophysiologic relevance of the calcitonin family of peptides remains unclear. This family includes calcitonin (CT), its precursor procalcitonin (PCT), and alpha calcitonin-gene related peptide (αCGRP), which are all encoded by the gene Calca. Here, we studied the role of Calca-derived peptides in diet-induced obesity (DIO) by challenging Calcr−/− (encoding the calcitonin receptor, CTR), Calca−/−, and αCGRP−/− mice and their respective littermates with high-fat diet (HFD) feeding for 16 weeks. HFD-induced pathologies were assessed by glucose tolerance, plasma cytokine and lipid markers, expression studies and histology. We found that DIO in mice lacking the CTR resulted in impaired glucose tolerance, features of enhanced nonalcoholic steatohepatitis (NASH) and adipose tissue inflammation compared to wildtype littermates. Furthermore, CTR-deficient mice were characterized by dyslipidemia and elevated HDL levels. In contrast, mice lacking Calca were protected from DIO, NASH and adipose tissue inflammation, and displayed improved glucose tolerance. Mice exclusively lacking αCGRP displayed a significantly less improved DIO phenotype compared to Calca-deficient mice. In summary, we demonstrate that the CT/CTR axis is involved in regulating plasma cholesterol levels while Calca, presumably through PCT, seems to have a detrimental effect in the context of metabolic disease. Our study provides the first comparative analyses of the roles of Calca-derived peptides and the CTR in metabolic disease.
Introduction
It is well established that obesity is linked to components of the metabolic syndrome including insulin resistance, dyslipidemia and a low-grade pro-inflammatory state [1,2,3,4]. Aberrant lipid metabolism in obese adipose and liver tissue is linked to insulin resistance and lipotoxicity. In this context, inflammatory and lipid metabolism gene expression profiles in liver and adipose tissue are strong predictors of metabolic health in humans [5]. Accumulation of excess cholesterol in plasma, in particular as LDL or lipoprotein remnants is linked to the development of atherosclerosis [6]. In the investigation of molecules that may be involved in the regulation of metabolic health in obesity, there is still uncertainty regarding the roles of several circulating hormones such as the calcitonin family of peptides. These include calcitonin (CT) and its precursor procalcitonin (PCT), as well as calcitonin-gene related peptide (αCGRP) all of which are encoded by the Calca gene in mice and have been linked to metabolic regulation in mice and humans. Under normal conditions, the primary transcript of the Calca gene is subjected to extensive post-transcriptional and post-translational modifications. It is processed into two different mRNAs by alternative splicing, resulting in the synthesis of αCGRP in the central and peripheral nervous system, and PCT in the thyroid gland [7]. Thyroidal PCT is further processed into mature calcitonin (CT) by proteolytic cleavage. Importantly, systemic inflammation as observed in sepsis annihilates tissue specificity and results in ubiquitous Calca expression, leading to PCT release from many cell types, including adipocytes and hepatocytes [8,9].
CT is primarily known for its regulatory effects on osteoclast function, while αCGRP was shown to control vascular tone and the activity of bone forming osteoblasts. In contrast, PCT was shown to modulate leukocyte function and survival in experimental sepsis [8,9]. All three peptides derived from the Calca gene bind to either the calcitonin receptor (Calcr, CTR) or the calcitonin receptor-like receptor (CTRL), two G-protein coupled receptors whose ligand specificity is determined by complexing with three different receptor-activity modifying proteins (RAMPs). Whereas the CTR mediates the biological effects of CT and Amylin (AMY), a peptide co-secreted with insulin and involved in glucose handling, αCGRP and PCT have been shown to exert their biological effects through the CTRL, displaying high levels of expression in the lung and the gastrointestinal tract [10,11]. Despite their pleiotropic effects within the organism, Calca-derived peptides have recently been linked to glucose, fat and lipid metabolism: First, salmon CT, exhibiting a much higher pharmacologic potency than mammalian CT, was reported to decrease cholesterol and triglyceride levels, improve energy and glucose homeostasis and attenuate diabetic progression in obese rats [12,13,14]. Second, αCGRP-deficient mice were reported to display increased energy expenditure [15], which is supported by clinical findings showing circulating αCGRP levels to positively correlate with obesity [16,17]. Finally, PCT and αCGRP were both found to be expressed in human adipocytes under various conditions including lipopolysaccharide and glucose-dependent insulinotropic polypeptide stimulation [18,19,20,21]. In this context, a hitherto unknown association of PCT, representing one of the most specific and sensitive markers of bacterial infection and sepsis, with body mass index (BMI), waist circumference, and indices of lipid and glucose metabolism was recently reported [22].
Taken together, these observations point towards a significant role of Calca-derived peptides in metabolic regulation. In order to test whether Calca-derived peptides are involved in the pathogenesis of obesity and metabolic dysfunction, we performed a comparative study employing three different mouse models that display global CTR-, αCGRP-, or Calca-deficiency. These mice were subjected to high-fat diet (HFD) feeding for 16 weeks, followed by the analyses of dynamic glucose tolerance, plasma cytokine and lipid markers, expression studies and histology for assessing metabolic disease.
Methods
Mouse studies
Calca-, αCGRP- and CTR-deficient mice were generated and genotyped as described previously [23,24,25]. All mice were kept on a C57BL/6 background (backcrossed at least 6 times) and housed in the animal facility of the University Medical Center Hamburg-Eppendorf at 22°C with ad libitum access to water and standard laboratory chow diet (Lasvendi). Diet-induced obesity (DIO) was induced in single-caged male mice by feeding a high-fat diet (HFD; Bio-Serv F3282, 35 wt. % lard) ad libitum, beginning at 4 weeks of age as described previously [26,27]. For each strain, respective WT (wildtype, C57BL/6 background) littermates were fed at the same time. Standardized necropsies were performed after 4 h fasting around noon. Mice were anesthetized with a lethal dose of Ketamine/Xylazine, blood was withdrawn by cardiac puncture and animals were perfused with PBS (phosphate-buffered saline). Organs were harvested and immediately conserved in TRIzol (Invitrogen), formalin or snap-frozen in liquid N2 and stored at -80°C. Throughout all experiments, 8–10 mice were analyzed per group. All experiments were approved by the institutional board at the University Medical Center Hamburg-Eppendorf.
Plasma parameters
Plasma triglycerides and cholesterol were determined using commercial kits (Roche) that were adapted to microtiter plates. ADM, PCT and CT levels were determined by ELISA (abbexa, Cusabio and Phoenix pharmaceuticals, respectively). For fast performance liquid chromatography (FPLC), pooled plasma was separated using S6-superose columns (GE Healthcare) and lipid levels were analyzed in each fraction as described above. Leptin (R&D), adiponectin (R&D) and insulin (CrystalChem) ELISAs were conducted according to the manufacturer’s instructions. Oral glucose tolerance was assessed after a 4 h fasting period by a gavage of 1 g/kg glucose (Sigma) diluted in 0.9% NaCl (Braun). Blood glucose levels were measured using AccuCheck Aviva sticks (Roche).
Expression analysis
Tissues in TRIzol® (Invitrogen) were disrupted using a TissueLyser (Qiagen). Total RNA was isolated using NucleoSpin RNA II kit (Macherey & Nagel). Complementary DNA was synthesized using SuperScript® III Reverse Transcriptase (Invitrogen). Quantitative real-time PCR reactions were performed on a 7900HT sequence detection system (Applied Biosystems) using TaqMan Assay-on-Demand primer sets supplied by Applied Biosystems (Adipoq: Mm00456425_m1, Cd68: Mm03047340_m1, Emr1: Mm00802530_m1, Fasn: Mm00662319_m1, Hmgcr: Mm01282499_m1, Hmgcs1: Mm00524111_m1, Hmgcs2: Mm00550050_m1, Il6: Mm00446190_m1, Scd1: Mm00772290_m1, Srebf2: Mm01306292_m1, Tbp: Mm00446973_m1, Tnfa: Mm00443258_m1). Gene expression was calculated as copy number per housekeeper gene TATA box-binding protein (Tbp) by the ΔΔCT method and expressed as relative expression to wild-type controls.
Histology
After sacrifice, mouse organs were fixed in 4% buffered formaldehyde for 24 h, rinsed with PBS, dehydrated in a series of graded ethanol and embedded in paraffin. Sections of 5 μm thickness were cut and stained with haematoxylin and eosin. For immunohistochemistry 5 μm thick sections were cut, dewaxed, microwaved in Target Retrieval Solution (DAKO) for 2 x 4 min and cooled down to room temperature for 40 min. After washing with Tris-buffered saline (TBS), non-specific binding was blocked by incubating sections in 10% normal swine serum (DAKO) for 30 min at room temperature. Slides were incubated with anti-CD68 antibody (ABCAM ab955) at a dilution of 1 μg/ml for 60 min (RT), followed by a biotinylated rabbit anti mouse antibody (DakoCytomation) at a dilution of 1:200 for 30 min. After careful washes in TBS, an incubation with an avidin-alkaline phosphatase complex (ABC kit, Vectastain, Vector) for 30 min followed and thereafter, additional washes in TBS were performed. Alkaline phosphatase activity was visualized using Liquid Permanent Red (LPR) Substrate-Chromogen (DAKO) for 15 min. After washing with water, slides were counterstained with Mayer's hemalum diluted 1:1 in water for ten seconds, blued under water and mounted with Eukitt® (Sigma).
Liver lipids
For lipid quantification, 50 mg pieces of frozen liver were homogenized in lysis buffer (2 mM CaCl2, 80 mM NaCl, 1% TritonX-100, 50 mM Tris/HCl, pH 8.0). Triglycerides and cholesterol were determined using commercial kits (Roche/Hitachi, Mannheim, Germany). Protein concentrations were measured by a Lowry method, which was modified for lipid containing samples by addition of 0.1% SDS.
Statistics
Two-tailed, unpaired Student’s T-test was used for comparison of groups except in experiments with multiple groups, which were assessed by one-way ANOVA. P<0.05 was considered statistically significant, as indicated by asterisks.
Results
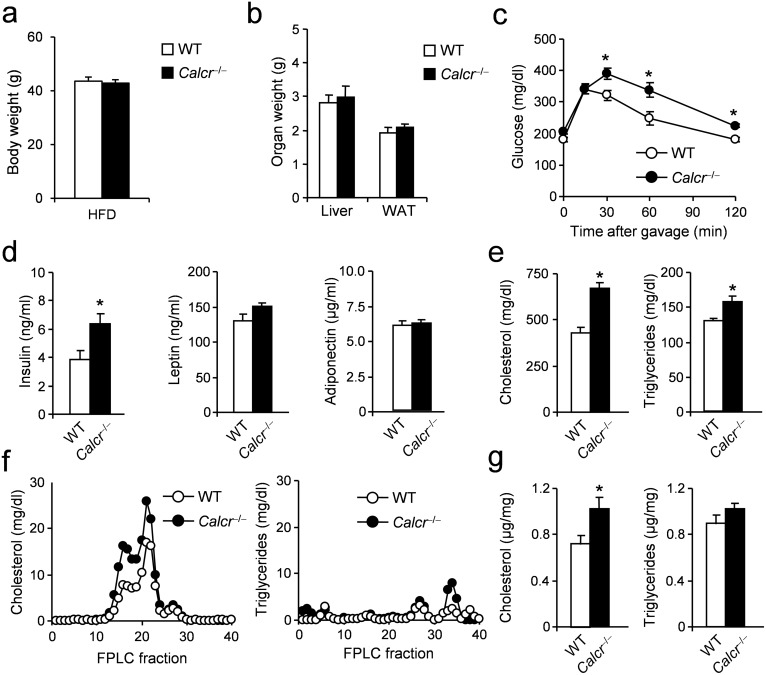
In order to study the role of Calca-derived peptides in metabolic dysfunction, we first investigated mice lacking the CTR globally. As basic metabolic parameters including body weight, blood glucose, and total cholesterol levels are not altered in CTR-deficient mice under standard conditions [23], we fed CTR-deficient mice and controls a HFD for 16 weeks, starting at the age of 4 weeks in order to induce obesity and associated metabolic disturbances. After the feeding regimen, mice lacking CTR displayed no alteration in final body weight (Fig 1a). Analyses of organ weights at the end of the study revealed no alteration in the weights of liver and epididymal white adipose tissue (WAT) (Fig 1b). Although CTR-deficient mice showed normal glucose levels at baseline, they demonstrated a significantly impaired glucose tolerance beginning 30 minutes after glucose challenge (Fig 1c). The impaired glucose handling was accompanied by increased insulin levels in CTR-deficient animals while no alterations in leptin or adiponectin concentrations were found (Fig 1d). Furthermore, total cholesterol and triglyceride levels were significantly elevated in CTR-deficient animals (Fig 1e). FPLC of plasma samples revealed a peak in the concentration of HDL-cholesterol in CTR-deficient mice, whereas analysis of triglyceride fractions did not reveal any major abnormalities (Fig 1f). To analyze these differences on the tissue level, cholesterol and triglycerides were measured in livers after 16 weeks of feeding. Here, while no alterations in triglyceride concentrations were found, CTR-deficient mice exhibited increased hepatic cholesterol content (Fig 1g).
Fig 1. Effects of DIO on metabolic parameters in mice lacking CTR (Calcr).
(a) Body weight in CTR-deficient mice and controls fed HFD for 16 weeks. (b) Organ weights (epididymal WAT, white adipose tissue) after 16 weeks of feeding. (c) Plasma glucose concentrations during OGTT (1 g/kg) following a 4h fasting period in CTR-deficient mice and controls after 16 weeks of feeding. (d) Plasma levels of insulin, leptin and adiponectin in the same mice. (e) Total plasma cholesterol and triglycerides concentrations in the same mice. (f) Cholesterol and triglycerides FPLC profile from pooled plasma (n>8) in CTR-deficient mice and controls fed HFD for 16 weeks. (g) Total hepatic cholesterol and triglycerides concentrations in CTR-deficient mice and controls fed HFD for 16 weeks. Results are shown as means ± SEM (n = 8–10). *P < 0.05.
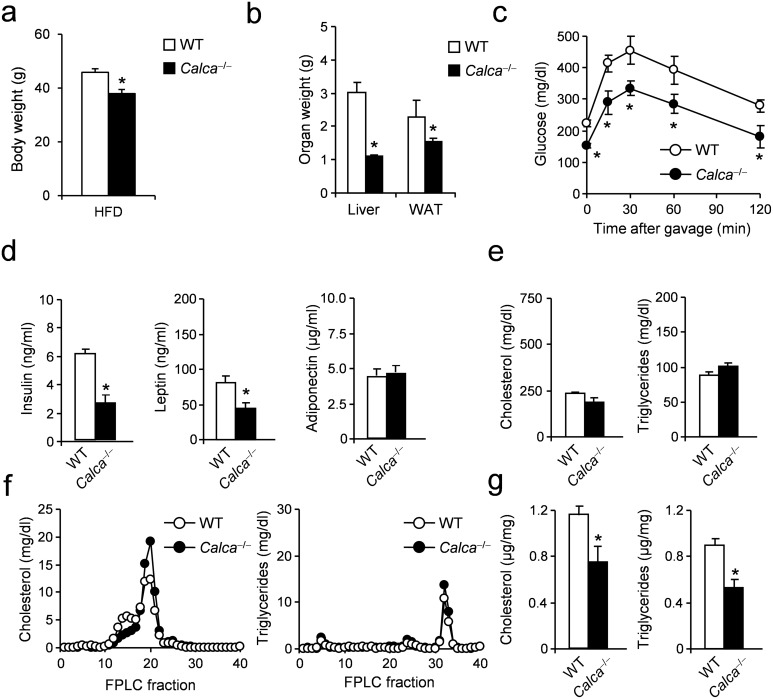
In order to investigate whether these effects can be observed not only in the case of CTR-, but also CT-deficiency, mice lacking Calca, encoding CT as well as PCT and αCGRP and exhibiting normal body weights under standard conditions [24], were fed a HFD and analyzed for phenotypic differences following the same experimental protocol as for CTR-deficient mice. At the end of the feeding regimen, Calca-deficient mice displayed significantly reduced body weight compared to WT controls, resulting in a weight difference of 7.9 g (WT 45.9 g vs. Calca 38.0 g, Fig 2a). Furthermore, a significant reduction in the weights of liver and WAT in Calca-deficient mice compared to WT controls was detected at the end of the feeding period (Fig 2b). In sharp contrast to CTR-deficient mice, Calca-deficient mice did not only show significantly reduced blood glucose at baseline, they also displayed a significantly improved glucose tolerance at all time points after oral glucose challenge (Fig 2c). Analyses of plasma parameters revealed significantly reduced insulin and leptin levels, while adiponectin levels were unchanged (Fig 2d). Furthermore, total plasma cholesterol and triglyceride levels were unaltered in Calca-deficient mice (Fig 2e). Interestingly, the increase in HDL cholesterol observed in CTR-deficient mice was also present in mice lacking Calca (Fig 2f). In contrast, hepatic cholesterol and triglyceride content was significantly reduced compared to controls (Fig 2g).
Fig 2. Effects of DIO on metabolic parameters in mice lacking Calca.
(a) Body weight in Calca-deficient mice and controls fed HFD for 16 weeks. (b) Organ weights (epididymal WAT, white adipose tissue) after 16 weeks of feeding. (c) Plasma glucose concentrations during OGTT (1 g/kg) following a 4h fasting period in Calca-deficient mice and controls after 16 weeks of feeding. (d) Plasma levels of insulin, leptin and adiponectin in the same mice. (e) Total plasma cholesterol and triglycerides concentrations in the same mice. (f) Cholesterol and triglycerides FPLC profile from pooled plasma (n>8) in Calca-deficient mice and controls fed HFD for 16 weeks. (g) Total hepatic cholesterol and triglycerides concentrations in Calca-deficient mice and controls fed HFD for 16 weeks. Results are shown as means ± SEM (n = 8–10). *P < 0.05.
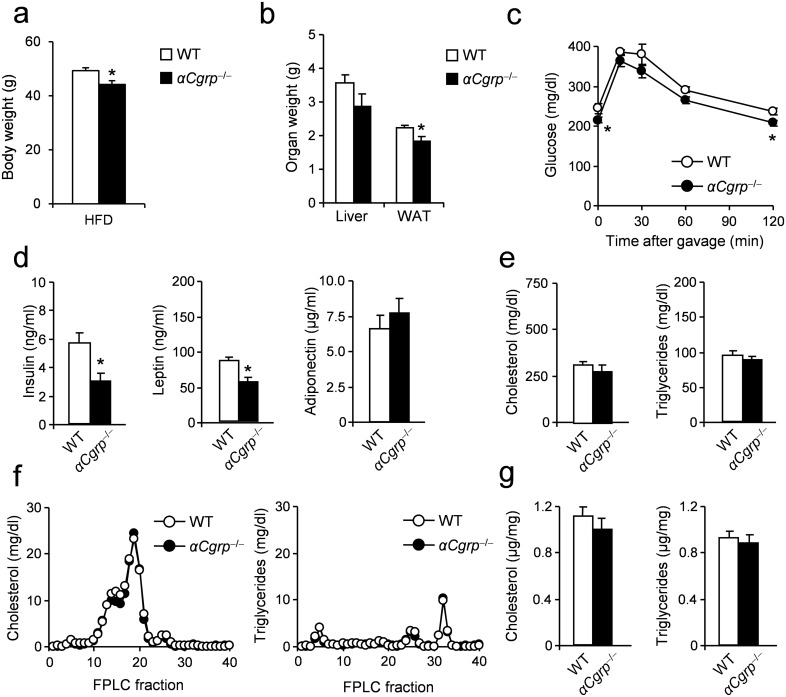
As a previous study suggested a beneficial effect of αCGRP in DIO [15] and a mouse model with exclusive PCT-deficiency is not available to date, we used mice specifically lacking 〈CGRP to investigate any involvement of this peptide in the metabolic phenotype of Calca-deficient mice. In this particular mouse model, a stop codon is placed upstream of the 〈CGRP alternative splice transcript, disabling 〈CGRP synthesis yet allowing intact expression of PCT and CT [25]. The respective mice show no alteration in mean body weight under standard conditions [15]. αCGRP-deficiency was associated with a mild however significant reduction in body weight-gain (Fig 3a), similarly to what was reported previously [15]. Moreover, αCGRP-deficient mice displayed only a slight reduction in the weight of WAT, which was not accompanied by any changes in liver weights, as observed in Calca- or CTR-deficient animals, respectively (Fig 3b). In contrast to the marked improvement in glucose tolerance observed in Calca-deficient mice, αCGRP-deficient mice were characterized by only a slight reduction in blood glucose at baseline and 120 minutes after glucose challenge (Fig 3c). In contrast, αCGRP-deficient mice exhibited similar plasma parameters of glucose metabolism as measured in Calca-deficient mice, including decreased levels of insulin and leptin accompanied by unaltered concentrations of adiponectin (Fig 3d). Again differing from Calca- and CTR-deficient mice, no changes in HDL levels and plasma or hepatic concentrations of cholesterol and triglycerides were detectable (Fig 3e–3g).
Fig 3. Effects of DIO on metabolic parameters in mice lacking αCGRP.
(a) Body weight in αCGRP-deficient mice and controls fed HFD for 16 weeks. (b) Organ weights (epididymal WAT, white adipose tissue) after 16 weeks of feeding. (c) Plasma glucose concentrations during OGTT (1 g/kg) following a 4h fasting period in αCGRP-deficient mice and controls after 16 weeks of feeding. (d) Plasma levels of insulin, leptin and adiponectin in the same mice. (e) Total plasma cholesterol and triglycerides concentrations in the same mice. (f) Cholesterol and triglycerides FPLC profile from pooled plasma (n>8) in αCGRP-deficient mice and controls fed HFD for 16 weeks. (g) Total hepatic cholesterol and triglycerides concentrations in αCGRP-deficient mice and controls fed HFD for 16 weeks. Results are shown as means ± SEM (n = 8–10). *P < 0.05.
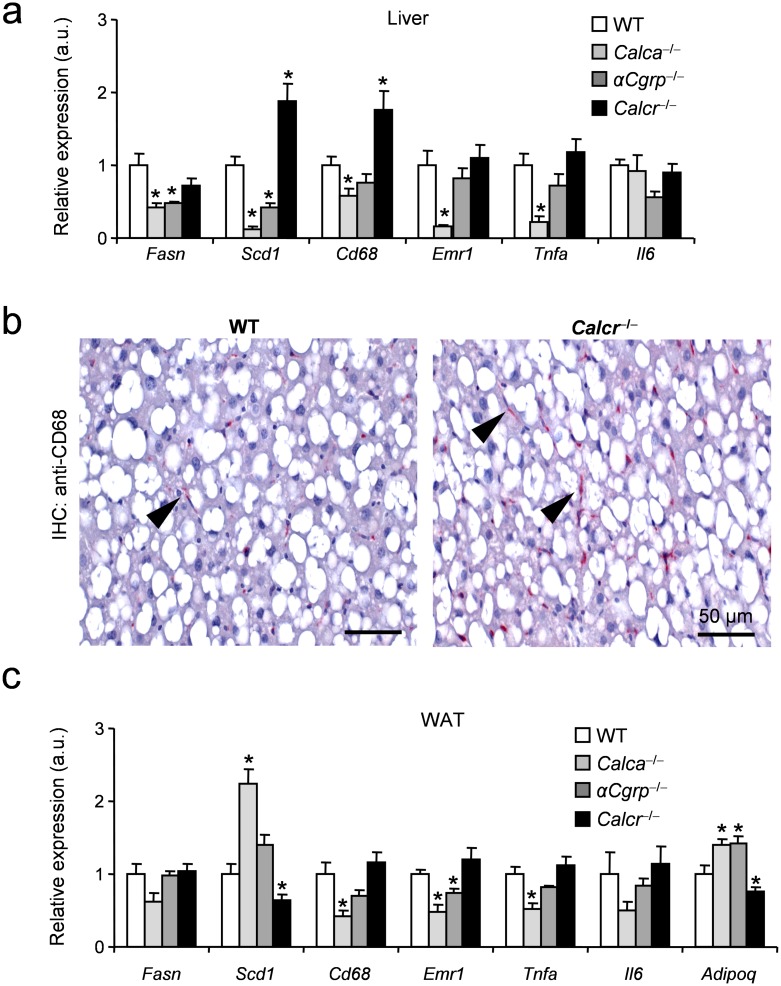
In order to investigate whether these observations could be explained by molecular differences on the tissue level, we performed gene expression analyses of selected surrogate markers for lipid metabolism and inflammation, which correlate tightly with insulin resistance [27,28] using qRT-PCR after 16 weeks of feeding. Here we found decreased expression of Fasn, encoding fatty acid synthase, in the livers of Calca- and αCGRP-deficient mice, which was not the case in mice lacking CTR (Fig 4a). In line with this, a significantly decreased hepatic expression of stearoyl CoA desaturase (Scd1), associated with the metabolic syndrome as well as regulation of inflammation [29,30], was measured in Calca- and αCGRP-deficient mice while it was overexpressed in liver tissue derived from CTR-deficient mice. In agreement, the expression of the macrophage markers Cd68 and EGF-like module-containing mucin-like hormone receptor-like 1 (Emr1) were decreased in Calca- and αCGRP-deficient mice and increased in CTR-deficient mice, indicating a more pronounced steatohepatitis in the latter group. Reduced levels of tumor necrosis factor alpha (Tnf) expression could be identified as a potential mediator of decreased liver inflammation in Calca-deficient mice whereas expression of interleukin-6 (Il6) was not altered in any group. To confirm the increased features of NASH in mice lacking Calcr, we performed Cd68 immunohistochemistry staining of liver samples and found increased numbers of Cd68-positive macrophages in mice lacking CTR (Fig 4b). The gene expression profiles in liver are usually contrasted in adipose tissue, where markers of lipid metabolism are decreased on the context of obesity-induced metabolic disease [27,28]. Gene expression analyses of WAT demonstrated reduced levels of Scd1 in CTR-deficient mice, in line with adipose and liver Scd1 as a strong indicator of metabolic deterioration [28]. In contrast, increased levels of Scd1 and reduced levels of Cd68, Emr1, and Tnf were measured (Fig 4c), overall indicating limited tissue inflammation and retrograde fatty acid transport in Calca-deficient mice. Altogether, our study indicates that loss of CTR has detrimental effects for metabolic homeostasis in the context of obesity and mice lacking Calca are protected from HFD-induced weight gain and metabolic deterioration. αCGRP seems to play only a minor role in this scenario, even though, similar to Calca-deficient mice, mice lacking αCGRP were characterized by a mild improvement of metabolic parameters after HFD feeding.
Fig 4. Effects of DIO on the tissue level in mice lacking Calca-derived peptides.
(a) Hepatic expression of selected genes (Fasn, fatty acid synthase; Scd1, Stearoyl-CoA desaturase-1; Cd68, cluster of differentiation 68; Emr1, EGF-like module-containing mucin-like hormone receptor-like 1; Tnfa, tumor necrosis factor alpha; Il6, interleukin 6) of the indicated genotypes after 16 weeks of HFD feeding. (b) Representative immunohistochemistry of liver tissue using a Cd68-sepcific monoclonal antibody. Arrows indicate Cd68-positive macrophages. Scale bars 50 m. (c) Epididymal WAT expression of selected genes (Adipoq, adiponectin) of mice of the indicated genotypes after 16 weeks of HFD feeding. Results are shown as means ± SEM (n = 8–10). *P < 0.05.
Discussion
The results of the present study provide genetic evidence for a pathophysiologic role of Calca-derived peptides in metabolic disease. In particular, we found that genetic inactivation of the CTR in DIO results in impaired glucose tolerance, features of enhanced NASH and adipose tissue inflammation. In addition, we show that the CT/CTR axis is involved in regulating cholesterol levels, and Calca, presumably through PCT, may play a deleterious role in metabolic disease.
Although the peptides derived from the Calca gene are known for decades, their roles in different physiologic processes and pathologic conditions, in particular in the context of obesity, which is a medical condition reaching worldwide pandemic magnitude, remains unclear. While the roles of CT and αCGRP in bone remodeling and regulation of vascular tone have been studied extensively, their functions in DIO are still ill defined. Furthermore, although representing the most sensitive and specific marker for bacterial sepsis with widespread clinical use, a potential biologic action of PCT remains unclear to date [31,32]. Although we did not detect significant alterations in serum levels of Calca-derived peptides during DIO (S1a Fig), this study for the first time provides a comparative analysis regarding the roles of PCT, CT and αCGRP in obesity and metabolic disease using mouse models deficient in Calca, CTR, and αCGRP.
With respect to CT, few studies have analyzed its role in DIO so far. This is primarily based on the fact that a suitable mouse model lacking CT signaling has not been available to date. In this study we utilized our recently established mouse model lacking the CTR [23], which does not exhibit the previously reported embryonic lethality of that respective CTR-deficiency model [33,34]. While several recent studies relying on pharmacological approaches demonstrated a beneficial effect of oral salmon CT on body weight, fasting glycaemia and glucose tolerance in rats [12,13,14], our results confirm a potential physiological role of CT in glucose metabolism, as obese CTR-deficient animals displayed features of enhanced NASH, impaired glucose tolerance and hyperinsulinemia in vivo. However, while apparently CTR-deficiency did not influence the rate of body weight gain, a more prominent finding was dyslipidemia due to increased cholesterol and triglyceride concentrations, supporting the concept that signaling through the CTR regulates plasma lipid homeostasis independent of weight gain in DIO. As most of the cholesterol change was observed in the HDL fraction it remains to be evaluated how much of this HDL is actually functional or dysfunctional as it has recently been shown that HDL metabolic flux rather than absolute levels determine reverse cholesterol transport [35]. Interestingly, despite the increased cholesterol levels in the liver of CTR-deficient mice we did not observe changes in gene expression of surrogate markers of the SREBP2 cholesterol-sensing pathway [36], neither in the liver nor in WAT (S1b Fig). As we also observed increased plasma lipids and increased hepatic immune infiltrates in CTR-deficient mice, these data may suggest that Kupffer cells and/or other infiltrating immune cells play a role in the pathophysiology in this model. On the other hand, in line with a decrease in body weight on HFD as observed here, mice deficient in Calca and αCGRP also showed lower expression of hepatic inflammation markers.
In general our findings partially confirm a recent study demonstrating a beneficial effect of the dual amylin and calcitonin receptor agonist, KBP-089, on weight loss and metabolic parameters in obese rats [37]. Mice lacking the CTR are characterized by increased bone formation and a subsequent increase in circulating osteocalcin [23], an osteoblast-derived peptide increasing secretion of and sensitivity to insulin [38]. While we cannot exclude a potential influence on the metabolic phenotype in our model, a possible beneficial effect of osteocalcin seems to be overridden by the effects of global CTR-deficiency in DIO.
As the CTR not only serves as a receptor for CT, but reportedly also for AMY, CTR-deficient mice additionally serve as a valuable tool to study the role of endogenous AMY in DIO [39]. Although AMY has been implicated in the pathogenesis of diabetes and obesity, leading to the clinical use of its pharmaceutical analogue, pramlintide, as an FDA-approved anti-diabetic drug lowering body weight and hyperglycemia [39], our findings in mice can only confirm CTR signaling role in glucose handling and moreover extend these observations to plasma lipid levels. In particular the effect on HDL levels was pronounced and needs to be explored in more detail towards HDL function and atherosclerosis development. The lack of effect on the rates of body weight gain is in line with previous studies reporting AMY-deficient mice to display only modest or no alteration in body weight [40,41]. The differences between endogenous AMY and its pharmacological actions might be explained by the fact that pramlintide represents a conjunct of human and rat AMY to avoid the highly amyloidogenic effects of the human form. Likewise, it is possible that, apart from the CTR, AMY binds to another hitherto unrecognized receptor other than CTR, as originally suggested by Daquin et al. [33]. In this context, we explored the levels of adrenomedullin, which is thought to bind CTRL but might also act on CTR and exert beneficial effects on HFD-induced metabolic disease [42,43]. However, neither plasma concentrations in lean compared to obese mice, nor gene expression in liver and WAT of CTR-deficient mice showed any differences (S1a and S1b Fig), making it unlikely that adrenomedullin is implicated here.
Based on the receptor pharmacology of the CTR, we additionally monitored the effects of DIO in mice lacking Calca. Strikingly, these mice showed a markedly reduced rate of weight gain compared to WT controls, which was in sharp contrast to what we observed in CTR-deficient mice. As Calca encodes not only CT, but also PCT and αCGRP, one possibility was that the observed phenotype is caused by the absence of the latter two hormones [7]. To rule out an involvement of αCGRP in the observed phenotype, αCGRP-deficient mice were used as controls. A previous study investigating the role of αCGRP in DIO found αCGRP-deficient mice to exhibit a lower body weight, improved glucose handling and insulin sensitivity which was accompanied by reduced hyperinsulinemia and adiposity compared to controls [15]. Likewise, Riera et al. showed that pharmacologic antagonism of CGRP signaling improved metabolic parameters and potentially inhibits metabolic decline in aged mice (23 months) [44]. In contrast, a recent study demonstrated a beneficial effect of a long acting αCGRP agonist on food intake and body weight in DIO rats [45]. While it is known that pharmacologic agonists may exert different biologic effects, a phenomenon also observed in the case of parathyroid hormone or CT [23] and potentially explaining the findings by Nilsson et al, our study principally confirms the negative effect of endogenous αCGRP on metabolic parameters during DIO. The fact that we observed a less pronounced metabolic phenotype in DIO αCGRP-deficient mice compared to Walker e al. is most likely explained by specific differences in the experimental setup. Walker et al. employed diets with a different fat content 10%, 45%, and 60% compared to 35% in the present study) and fed αCGRP-deficient mice and WT controls for a longer duration (26 weeks of feeding compared to 8 weeks of feeding in the present study). A recent study corroborates the role of αCGRP in obesity as the authors also found that mice lacking αCGRP were partially protected from weight gain on HFD and displayed improved metabolic parameters [46]. In this study, the authors suggest that rather than regulating food intake, αCGRP plays a role in sympathetic output, which warrants further investigation of adaptive thermogenesis and brown adipose tissue activity in this model [47]. As we found αCGRP-deficient mice to exhibit a less pronounced phenotype compared to mice lacking Calca with our study protocol, it is reasonable to speculate that the metabolic phenotype of Calca-deficient mice is, in part, caused by the absence of PCT, pointing towards a hitherto unknown biologic role of PCT in DIO. This is especially interesting in the context of a recent study demonstrating a positive and significant association of PCT with body mass index (BMI), waist circumference, and indices of lipid and glucose metabolism in humans [22]. Although we cannot provide direct evidence for a detrimental role of PCT in metabolic health, our results may indicate an important impact of PCT signaling during DIO, warranting further mechanistic studies of the underlying molecular pathways.
We are aware of the fact that our study exhibits at least one major limitation. In fact, due to the complex regulation of Calca gene expression, we had to use 3 different models including Calca-, CTR-, and αCGRP-deficient mice to provide a first comparative analyses on the roles of Calca-derived peptides in DIO. Thus, additive or interregulatory effects of the respective peptides cannot be completely excluded, and further studies are required to address the question how the observed phenotypes can be explained mechanistically. Specifically, even though we did not find Calca-derived peptides to be regulated in obesity, the individual levels of CT, PCT and αCGRP in the three mouse models studied here might share some mechanistic insight in future. Despite this limitation, our study may prove valuable for future diabetes and atherosclerosis research, as it provides the first comparative characterization of the functions of Calca-derived peptides in DIO and associated metabolic disturbances. Our results demonstrate a critical role for CT-CTR signaling for metabolic health. Moreover, these findings point towards a deleterious role of PCT in the pathogenesis of DIO, suggesting PCT as a potential novel molecular target for the treatment of obesity and associated metabolic disorders.
Supporting information
(a) Serum levels of the indicated peptides in WT mice with DIO (16 weeks of feeding) compared to control fed mice (Adm: adrenomedullin). (b) Hepatic and epididymal WAT expression of selected genes (Srebf2, Sterol-regulatory element binding factor 2; Hmgcr, 3-Hydroxy-3-Methylglutaryl-CoA Reductase; Hmgcs1, 3-Hydroxy-3-Methylglutaryl-CoA Synthase 1; Hmgcs2, 3-Hydroxy-3-Methylglutaryl-CoA Synthase 2; Adm, Adrenomedullin) of WT and Calcr-deficient mice after 16 weeks of HFD feeding.
(TIF)
Acknowledgments
We thank Gudrun Arndt for managing the mouse colonies and Mona Neven for performing genotyping of the applied mouse colonies. We thank Walter Tauscher, Birgit Henkel and Sandra Ehret for expert technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by grants from Deutsche Forschungsgemeinschaft to AN (Ni 637/2-3), JH (SFB841) and AM (AM103/15-1,) and Bundesministerium für Bildung und Forschung (Project ANCYLOSS) to AN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.(2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421. [PubMed] [Google Scholar]
- 2.Luna-Luna M, Medina-Urrutia A, Vargas-Alarcon G, Coss-Rovirosa F, Vargas-Barron J, Perez-Mendez O (2015) Adipose Tissue in Metabolic Syndrome: Onset and Progression of Atherosclerosis. Arch Med Res. [DOI] [PubMed] [Google Scholar]
- 3.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. (2013) Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 21: E439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, et al. (2008) Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet 371: 1927–1935. doi: 10.1016/S0140-6736(08)60602-9 [DOI] [PubMed] [Google Scholar]
- 5.Bartelt A, Heeren J (2014) Adipose tissue browning and metabolic health. Nat Rev Endocrinol 10: 24–36. doi: 10.1038/nrendo.2013.204 [DOI] [PubMed] [Google Scholar]
- 6.Berbee JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, et al. (2015) Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6: 6356 doi: 10.1038/ncomms7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huebner AK, Keller J, Catala-Lehnen P, Perkovic S, Streichert T, Emeson RB, et al. (2008) The role of calcitonin and alpha-calcitonin gene-related peptide in bone formation. Arch Biochem Biophys 473: 210–217. doi: 10.1016/j.abb.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Becker KL, Snider R, Nylen ES (2010) Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol 159: 253–264. doi: 10.1111/j.1476-5381.2009.00433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker KL, Nylen ES, White JC, Muller B, Snider RH Jr. (2004) Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 89: 1512–1525. doi: 10.1210/jc.2002-021444 [DOI] [PubMed] [Google Scholar]
- 10.Walker CS, Hay DL (2013) CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol 170: 1293–1307. doi: 10.1111/bph.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sexton PM, Christopoulos G, Christopoulos A, Nylen ES, Snider RH Jr., Becker KL (2008) Procalcitonin has bioactivity at calcitonin receptor family complexes: potential mediator implications in sepsis. Crit Care Med 36: 1637–1640. doi: 10.1097/CCM.0b013e318170a554 [DOI] [PubMed] [Google Scholar]
- 12.Nishizawa Y, Okui Y, Inaba M, Okuno S, Yukioka K, Miki T, et al. (1988) Calcium/calmodulin-mediated action of calcitonin on lipid metabolism in rats. J Clin Invest 82: 1165–1172. doi: 10.1172/JCI113713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigh M, Andreassen KV, Hjuler ST, Nielsen RH, Christiansen C, Henriksen K, et al. (2013) Oral salmon calcitonin protects against impaired fasting glycemia, glucose intolerance, and obesity induced by high-fat diet and ovariectomy in rats. Menopause. [DOI] [PubMed] [Google Scholar]
- 14.Feigh M, Hjuler ST, Andreassen KV, Gydesen S, Ottosen I, Henriksen JE, et al. (2014) Oral salmon calcitonin enhances insulin action and glucose metabolism in diet-induced obese streptozotocin-diabetic rats. Eur J Pharmacol 737: 91–96. doi: 10.1016/j.ejphar.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 15.Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, et al. (2010) Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 151: 4257–4269. doi: 10.1210/en.2010-0284 [DOI] [PubMed] [Google Scholar]
- 16.Recober A, Goadsby PJ (2010) Calcitonin gene-related peptide: A molecular link between obesity and migraine? Drug News Perspect 23: 112–117. doi: 10.1358/dnp.2010.23.2.1475909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelissen PM, Koppeschaar HP, Lips CJ, Hackeng WH (1991) Calcitonin gene-related peptide in human obesity. Peptides 12: 861–863. [DOI] [PubMed] [Google Scholar]
- 18.Linscheid P, Seboek D, Nylen ES, Langer I, Schlatter M, Becker KL, et al. (2003) In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 144: 5578–5584. doi: 10.1210/en.2003-0854 [DOI] [PubMed] [Google Scholar]
- 19.Radimerski TM, Grisouard J, Timper K, Zulewski H, Christ-Crain M, Keller U, et al. (2011) Role of calcium in lipopolysaccharide-induced calcitonin gene expression in human adipocytes. Innate Immun 17: 403–413. doi: 10.1177/1753425910377100 [DOI] [PubMed] [Google Scholar]
- 20.Timper K, Grisouard J, Radimerski T, Dembinski K, Peterli R, Haring A, et al. (2011) Glucose-dependent insulinotropic polypeptide (GIP) induces calcitonin gene-related peptide (CGRP)-I and procalcitonin (Pro-CT) production in human adipocytes. J Clin Endocrinol Metab 96: E297–303. doi: 10.1210/jc.2010-1324 [DOI] [PubMed] [Google Scholar]
- 21.Linscheid P, Seboek D, Zulewski H, Keller U, Muller B (2005) Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology 146: 2699–2708. doi: 10.1210/en.2004-1424 [DOI] [PubMed] [Google Scholar]
- 22.Abbasi A, Corpeleijn E, Postmus D, Gansevoort RT, de Jong PE, Gans RO, et al. (2010) Plasma procalcitonin is associated with obesity, insulin resistance, and the metabolic syndrome. J Clin Endocrinol Metab 95: E26–31. doi: 10.1210/jc.2010-0305 [DOI] [PubMed] [Google Scholar]
- 23.Keller J, Catala-Lehnen P, Huebner AK, Jeschke A, Heckt T, Lueth A, et al. (2014) Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat Commun 5: 5215 doi: 10.1038/ncomms6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoff AO, Catala-Lehnen P, Thomas PM, Priemel M, Rueger JM, Nasonkin I, et al. (2002) Increased bone mass is an unexpected phenotype associated with deletion of the calcitonin gene. J Clin Invest 110: 1849–1857. doi: 10.1172/JCI200214218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schinke T, Liese S, Priemel M, Haberland M, Schilling AF, Catala-Lehnen P, et al. (2004) Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res 19: 2049–2056. doi: 10.1359/JBMR.040915 [DOI] [PubMed] [Google Scholar]
- 26.Bartelt A, Beil FT, Schinke T, Roeser K, Ruether W, Heeren J, et al. (2010) Apolipoprotein E-dependent inverse regulation of vertebral bone and adipose tissue mass in C57Bl/6 mice: modulation by diet-induced obesity. Bone 47: 736–745. doi: 10.1016/j.bone.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 27.Bartelt A, Orlando P, Mele C, Ligresti A, Toedter K, Scheja L, et al. (2011) Altered endocannabinoid signalling after a high-fat diet in Apoe(-/-) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia 54: 2900–2910. doi: 10.1007/s00125-011-2274-6 [DOI] [PubMed] [Google Scholar]
- 28.Eissing L, Scherer T, Todter K, Knippschild U, Greve JW, Buurman WA, et al. (2013) De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun 4: 1528 doi: 10.1038/ncomms2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popeijus HE, Saris WH, Mensink RP (2008) Role of stearoyl-CoA desaturases in obesity and the metabolic syndrome. Int J Obes (Lond) 32: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 30.Sampath H, Ntambi JM (2011) The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann N Y Acad Sci 1243: 47–53. doi: 10.1111/j.1749-6632.2011.06303.x [DOI] [PubMed] [Google Scholar]
- 31.Matwiyoff GN, Prahl JD, Miller RJ, Carmichael JJ, Amundson DE, Seda G, et al. (2012) Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res 61: 401–409. doi: 10.1007/s00011-012-0439-5 [DOI] [PubMed] [Google Scholar]
- 32.Dahaba AA, Metzler H (2009) Procalcitonin's role in the sepsis cascade. Is procalcitonin a sepsis marker or mediator? Minerva Anestesiol 75: 447–452. [PubMed] [Google Scholar]
- 33.Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, et al. (2004) Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol 164: 509–514. doi: 10.1083/jcb.200312135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davey RA, Turner AG, McManus JF, Chiu WS, Tjahyono F, Moore AJ, et al. (2008) Calcitonin receptor plays a physiological role to protect against hypercalcemia in mice. J Bone Miner Res 23: 1182–1193. doi: 10.1359/jbmr.080310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartelt A, John C, Schaltenberg N, Berbee JFP, Worthmann A, Cherradi ML, et al. (2017) Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat Commun 8: 15010 doi: 10.1038/ncomms15010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H (1998) Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest 101: 2331–2339. doi: 10.1172/JCI2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gydesen S, Hjuler ST, Freving Z, Andreassen KV, Sonne N, Hellgren LI, et al. (2017) A novel Dual Amylin and Calcitonin Receptor Agonist (DACRA), KBP-089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karsenty G, Ferron M (2012) The contribution of bone to whole-organism physiology. Nature 481: 314–320. doi: 10.1038/nature10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD (2015) Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev 67: 564–600. doi: 10.1124/pr.115.010629 [DOI] [PubMed] [Google Scholar]
- 40.Gebre-Medhin S, Mulder H, Pekny M, Westermark G, Tornell J, Westermark P, et al. (1998) Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin). Biochem Biophys Res Commun 250: 271–277. doi: 10.1006/bbrc.1998.9308 [DOI] [PubMed] [Google Scholar]
- 41.Gebre-Medhin S, Mulder H, Zhang Y, Sundler F, Betsholtz C (1998) Reduced nociceptive behavior in islet amyloid polypeptide (amylin) knockout mice. Brain Res Mol Brain Res 63: 180–183. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Zhang SY, Jiang C, Li Y, Xu G, Xu MJ, et al. (2016) Intermedin/adrenomedullin 2 polypeptide promotes adipose tissue browning and reduces high-fat diet-induced obesity and insulin resistance in mice. Int J Obes (Lond) 40: 852–860. [DOI] [PubMed] [Google Scholar]
- 43.Zhang SY, Lv Y, Zhang H, Gao S, Wang T, Feng J, et al. (2016) Adrenomedullin 2 Improves Early Obesity-Induced Adipose Insulin Resistance by Inhibiting the Class II MHC in Adipocytes. Diabetes 65: 2342–2355. doi: 10.2337/db15-1626 [DOI] [PubMed] [Google Scholar]
- 44.Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, et al. (2014) TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 157: 1023–1036. doi: 10.1016/j.cell.2014.03.051 [DOI] [PubMed] [Google Scholar]
- 45.Nilsson C, Hansen TK, Rosenquist C, Hartmann B, Kodra JT, Lau JF, et al. (2016) Long acting analogue of the calcitonin gene-related peptide induces positive metabolic effects and secretion of the glucagon-like peptide-1. Eur J Pharmacol 773: 24–31. doi: 10.1016/j.ejphar.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 46.Liu T, Kamiyoshi A, Sakurai T, Ichikawa-Shindo Y, Kawate H, Yang L, et al. (2017) Endogenous calcitonin gene-related peptide regulates lipid metabolism and energy homeostasis in male mice. Endocrinology. [DOI] [PubMed] [Google Scholar]
- 47.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205. doi: 10.1038/nm.2297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Serum levels of the indicated peptides in WT mice with DIO (16 weeks of feeding) compared to control fed mice (Adm: adrenomedullin). (b) Hepatic and epididymal WAT expression of selected genes (Srebf2, Sterol-regulatory element binding factor 2; Hmgcr, 3-Hydroxy-3-Methylglutaryl-CoA Reductase; Hmgcs1, 3-Hydroxy-3-Methylglutaryl-CoA Synthase 1; Hmgcs2, 3-Hydroxy-3-Methylglutaryl-CoA Synthase 2; Adm, Adrenomedullin) of WT and Calcr-deficient mice after 16 weeks of HFD feeding.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.