Abstract
When chronically silenced, cortical and hippocampal neurons homeostatically upregulate excitatory synaptic function. However, the subcellular position of such changes on the dendritic tree is not clear. We exploited the cable filtering properties of dendrites to derive a parameter, the dendritic filtering index, to map the spatial distribution of synaptic currents. Our analysis indicates that young cortical neurons globally scale AMPA receptor mediated currents, while mature hippocampal neurons do not, revealing distinct homeostatic strategies between brain regions and developmental stages. The dendritic filtering index presents a useful tool for mapping the dendritic origin of synaptic currents and the location of synaptic plasticity changes.
Keywords: homeostatic synaptic plasticity, AMPA receptors, dendritic origin, current shape, dendritic filtering index, mEPSC slope
1
Synapses undergo activity-dependent strengthening or weakening, via processes known as synaptic plasticity. Associative synaptic plasticity produces patterns of synaptic strength (1,2) which are thought to underlie learning and memory (3). A complementary adaptation, homeostatic synaptic plasticity (HSP), constrains synapses via negative feedback (4,5) to stabilize neural networks (6). How can HSP occur without destroying synaptically encoded information (7,8)? The “synaptic scaling” model (4) proposes that all synapses are homeostatically adjusted by the same proportion, thus preserving relative weights. Many studies support this theory, while others are inconsistent with the model (9). The question remains: does HSP scale globally along dendrites? It has been difficult to resolve this question due to technical limitations. Visualizing synaptic proteins permits analysis of the spatial distribution of synaptic change, but is an indirect read-out of synapse function. Conversely, patch-clamp recording AMPAR-mediated miniature excitatory postsynaptic currents (mEPSCs) is a direct readout of synaptic function, but the dendritic origin of each somatically recorded current is unknown. Here, we devised a novel electrophysiological parameter to determine the location of synaptic changes during homeostatic synaptic plasticity.
2. EXPERIMENTAL PROCEDURES
Ethical approval
All animals were treated in accordance with the Georgetown University and Johns Hopkins University Institutional Animal Care and Use Committee guidelines.
Primary cultures and drug treatment
Hippocampal (10,11) and cortical cultures (12) were prepared as described. Briefly, timed-pregnant Sprague Dawley rats or C57BL/6 mice were euthanized with CO2 before cervical dislocation. Embryonic day 18–19 rat or mouse embryos (both sexes) were decapitated into ice cold Hank’s buffered saline solution (Gibco). Hippocampi were dissected, trypsinized, and dissociated for plating (75,000 cells/well, 12-well tissue culture plates) on coverslips previously coated with poly-D-lysine (30 μg/ml) and laminin (2 μg/ml). Cortices were dissected, trypsinized, and dissociated for plating (250,000 cells/well, 12-well tissue culture plates) on coverslips previously coated with poly-D-lysine (30 μg/ml). Dissociated hippocampal neurons were grown in Neurobasal medium (Invitrogen) supplemented with SM1 (Stem Cell Technologies), 10U/mL penicillin/streptomycin, 0.5 mM glutamine and 25 μM glutamate. Neurons were fed once weekly with media lacking glutamate. Dissociated cortical neurons were grown in Neurobasal medium (Invitrogen) supplemented with B-27 (Invitrogen), 50U/mL penicillin/streptomycin, 2 mM Glutamax, and 1% horse serum. Activity was altered via drug application at >21 DIV in hippocampal neurons and >DIV 11 in cortical neurons. TTX (Sigma), stock solution 1 mM in H2O, was diluted in Neurobasal to 1 μM final concentration.
Antibodies and immunolabeling
Hippocampal or cortical neurons were fixed as described (11) in 1% paraformaldehyde/4% sucrose/PBS for 7 min at RT and methanol at −20°C for 7 min. Neurons were permeabilized with 0.01% Triton X-100 in PBS for 30 min at RT and then immunostained with antibodies in GDB buffer (0.1% gelatin, 0.3% Triton X-100, 16 mM sodium phosphate pH 7.4, 450 mM NaCl) at the following concentrations: rabbit GluA1 N-terminal antibody (EMD Millipore Cat# PC246-100UG RRID:AB_564636, 1:100); mouse GluA2 N-terminal antibody (Millipore); mouse monoclonal PSD-95 K28/43 (NeuroMab Cat# 75-028 RRID:AB_2307331, 1:200-400), or rabbit polyclonal PSD-95 D27E11 (Cell Signaling Technology Cat# 3450S RRID:AB_2292883, 1:200). For surface staining (11,13), neurons were incubated for 10 min at 37°C with N-terminal specific anti-GluA1 antibody diluted in conditioned Neurobasal media. Neurons were then fixed, washed and treated with saturating concentrations of fluorescence-tagged secondary antibody all under non-permeabilizing conditions. Cells were then permeabilized and stained for other proteins as needed.
Quantification of immunostaining
Images were acquired using an Axiovert 200M (Zeiss) for conventional epifluorescence and analyzed using MetaMorph software (Molecular Devices). The 3 strongest dendrites of each neuron were traced and divided into 20 μm segments, starting from the soma. Puncta were defined by thresholding images to exclude staining within the dendritic shaft. Integrated intensity was measured for each 20 μm segment and averaged per neuron; neuronal means were then averaged to obtain population means.
Electrophysiology
DIV 11–12 cortical neurons and DIV 21–27 hippocampal neurons were incubated for 24 hr in normal growth media in the absence or presence of TTX (1 μM). For recording, neurons were transferred into room temperature (22–25°C) extracellular solution containing (in mM, all from Fisher Scientific): 145 NaCl, 2.5 KCl, 2 MgCl2, 1 CaCl2, 5 HEPES, 5 glucose, and 25 sucrose (330 mOsm, pH 7.4). Whole-cell patch-clamp recordings were made with 3–6 MΩ (Narishige) borosilicate pipettes containing (in mM, all from Fisher): 145 KGluconate, 5 EGTA, 5 MgCl2, 10 HEPES, 5 NaATP, 0.2 NaGTP (285 mOsm, pH 7.2). A potassium-based intracellular solution was selected because we wished to ensure that neuronal health and function were intact and restored after chronic TTX treatment and washout, and to avoid pooling of recordings from heterogeneous cell types. Thus, we verified a healthy resting membrane potential and then recorded the current responses to a series of hyperpolarizing and depolarizing steps, including for analysis only cells with firing patterns characteristic of excitatory pyramidal neurons. Data were collected with an Axiopatch-ID or Multiclamp 700B amplifier, Bessel filtered at 2 kHz and digitized at 10 kHz using a Digidata 1440A and Clampex10 software (all from Molecular Devices). Miniature EPSCs were recorded from neurons voltage-clamped at −70 mV in 1 μM TTX with 25 μM bicuculline methobromide (Ascent Scientific) or 50 μM picrotoxin (Sigma) to block GABAA receptor-mediated currents. Input and access resistances were regularly monitored by 10 mV hyperpolarizing steps. Cells were excluded for the following additional criteria: holding currents >200 pA, input resistance <200 MΩ or >500 MΩ, access resistance >25 MΩ, resting Vm more depolarized than −50 mV, or unstable series resistance (>10% change). Synaptic currents were detected offline from 60s voltage-clamp traces using MiniAnalysis (Synaptosoft) or Clampfit. Events were detected automatically using a template search and verified manually.
Local synaptic manipulation
For local synaptic manipulation experiments, 2–5 MΩ (Narishige) borosilicate pipettes were positioned directly above an isolated dendrite at proximal (<30 μm) or distal (>100 μm) locations. Positive pressure was applied to release a plume of solution (visible with DIC optics) locally onto the dendrite (14,15). Pipettes were filled with extracellular solution supplemented with either 2 mM Ca2+ and 500 mM sucrose (for stimulation) or 10 μM NBQX (for inhibition). Miniature EPSCs were identified from 5 sec recordings extracted immediately before and after local drug application. Recordings were continuously monitored for changes in access resistance and discarded if resistances changed >20%.
Dendritic filtering index
The shapes of individual mEPSCs were described using the dendritic filtering index (DFI), defined as the reciprocal of the slope (i.e., rise time/amplitude). The slope captures the “steepness” of the current, and the reciprocal is taken so that more proximal currents have smaller DFI values. For each neuron, the DFI was calculated for each individual mEPSC. Miniature EPSCs were then binned into groups according to filtering index (0.2 ms/pA bins for hippocampal, 0.02 ms/pA for cortical). Total number and average amplitude of mEPSCs for each bin was calculated per neuron, which were then averaged to yield group distributions. In the retrospective analysis of data from Bekkers & Stevens (1996) (14), the amplitude and rise time of individual mEPSCs were obtained by overlying graphs of the original data with a grid, and these extracted values used to compute DFIs for each current. Relative filtering indices were obtained by grouping values by condition (control and TTX-treated). For each condition, a linear relationship between DFI and dendritic distance was observed (NT index = 0.0031*distance + 0.8856, R2=0.344; TTX index = 0.0018*distance + 0.5062, R2=0.316). The relative change in DFI versus distance was then calculated by substitution.
Statistical analysis
All data represent mean±SEM unless otherwise indicated. Group data were analyzed for normality (D’Agostino-Pearson omnibus test) prior to statistical comparison. Non-normally distributed data was analyzed using non-parametric equivalent tests. Immunolabeling or synaptic activity as a function of distance was examined via 2-way ANOVA (treatment & distance effects). When treatment effects were confirmed, post hoc Bonferroni tests (NT vs. TTX) were performed. Synaptic current amplitude, DFI, or resistances were analyzed via unpaired Student’s t-test. Paired comparisons within neurons were made using Student’s paired t-test or nonparametric equivalent (Wilcoxon signed rank test). Cumulative probability distributions were compared using Kolmogorov-Smirnoff tests.
3. RESULTS
3.1
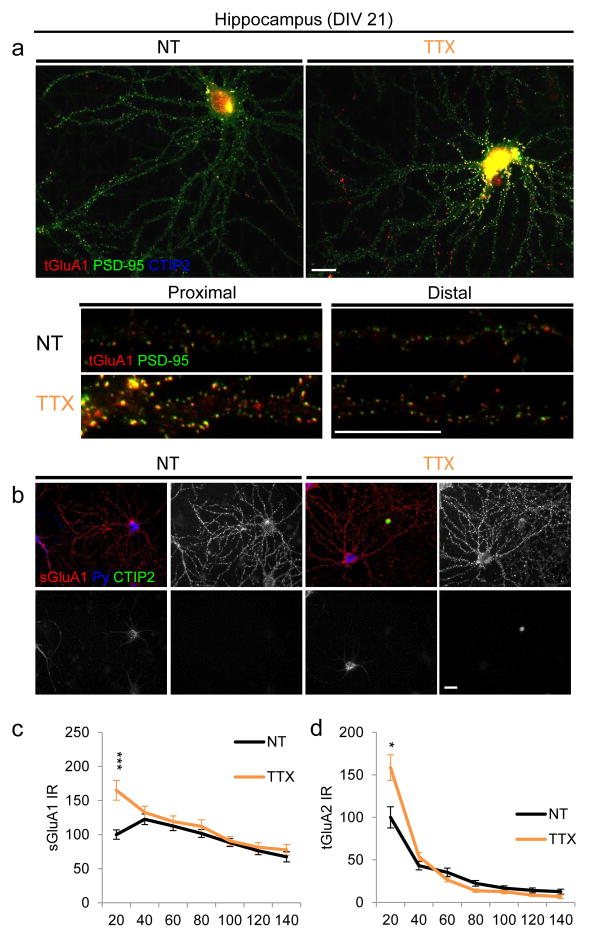
To investigate the spatial localization of HSP, we chronically silenced hippocampal and cortical neurons via action potential blockade with tetrodotoxin (TTX) (4,11) and recorded synaptic currents (Fig. 1a–b). As reported (4,11), we observed comparable increases in mEPSC amplitude in cells from both regions (Fig. 1c–d, Table 1). To visualize the location of these synaptic changes, we immunostained for the excitatory scaffolding protein PSD-95 and AMPA receptor subunits GluA1 and GluA2. As we previously described (11), in mature (>21 days in vitro (DIV)) hippocampal neurons, homeostatic increases in PSD-95 and total GluA1 were confined to proximal (<40 μm from soma) dendrites of CA3 cells (Fig. 1e, g, Table 1), with no changes in distal (100–140 μm) dendritic regions. A similar phenomenon was observed in young (DIV 12) hippocampal neurons as well (data not shown). In contrast, young (DIV 12) cortical neurons displayed no changes in either PSD-95 or AMPAR immunostaining (Fig. 1f, h, Table 1). Consistent with these data, hippocampal CA3 neurons also increased proximal dendritic surface GluA1 and total GluA2 with TTX (Fig. 2), implicating this dendritic region morphologically as the predominant homeostatic domain in cultured hippocampal excitatory neurons.
Figure 1. Activity bidirectionally alters proximal excitatory synapses.
(a, b) Representative mEPSCs recorded from control (non-treatment, NT) and silenced (24 hr TTX) hippocampal (a) and cortical (b) neurons. (c, d) Absolute (c) and relative (d) amplitude of recorded mEPSCs. *P=0.043, **P=0.0026, Mann-Whitney test (N=10–15 neurons/group). (e, f) Representative images (top) and linearized dendrites (bottom) of hippocampal (e) and cortical (f) neurons immunostained for PSD-95 after 24 hrs of normal activity (NT) or inactivity (TTX). Scale bar, 20 μm. (g, h) PSD-95 intensity as a function of distance from soma in hippocampal (g, N=36–40) and cortical neurons (h, N=19–24). Inset, total GluA1 intensity (N=86–95 hippocampal, 19–24 cortical). Data are mean±SEM from neuronal averages (3 dendrites/neuron). *P<0.05, **P<0.01, ***P<0.001, two-way ANOVA and Bonferroni post hoc test. See Tables 1, 2.
Table 1. Effects of inactivity on synaptic function and protein accumulation.
| Cell type | Hpc | Ctx | ||||
|---|---|---|---|---|---|---|
| Parameter | NT | TTX | P | NT | TTX | P |
| Functional | ||||||
| Amplitude (pA) | 13.4±0.804 (N=14) | 19.1±1.5 (N=10) | **P=0.0026, Mann-Whitney test | 21.9±3.6 (N=10) | 31.6±2.8 (N=10) | *P=0.0429, Mann-Whitney test |
| Dendritic filtering index (ms/pA) | 0.942±0.006 | 0.644±0.050 | ***P=0.0002, two-tailed t-test | 0.050±0.016 | 0.056±0.007 | P=0.696, two-tailed t-test |
| Biochemical: Immunoreactivity was quantified in 20 μm bins. Proximal indicates <40μm, distal indicates 100–140 μm. Distributions were analyzed via 2-way ANOVA (see Table 2) to account for the effects of distance and treatment | ||||||
| Proximal PSD-95 | 100.0±7.6 | 477.4±37.6 | ***P<0.001‡ | 100.0±11.7 | 110.5±11.1 | P=0.326, no treatment effect |
| Distal PSD-95 | 35.0±5.2 | 37.9±4.5 | P>0.05‡ | 55.0±9.2 | 60.5±11.4 | |
| Proximal tGluA1 | 100.0±15.1 | 708.4±66.9 | ***P<0.001‡ | 100±2.5 | 97.5±1.8 | P=0.100, no treatment effect |
| Distal tGluA1 | 29.9±10.8 | 47.3±10.3 | P>0.05‡ | 95.6±3.2 | 95.3±2.1 | |
| Proximal sGluA1 | 100.0±6.8 | 165.0±14.5 | ***P<0.001‡ | nd | nd | |
| Distal sGluA1 | 77.3±6.3 | 83.2±7.0 | P>0.05‡ | nd | nd | |
Post hoc Bonferroni test after treatment effect was confirmed via 2-way ANOVA (treatment & distance). See Table 2 for results of 2-way ANOVA.
Figure 2. Hippocampal neurons upregulate proximal but not distal synaptic proteins.
(a) Top: Representative images of mature cultured hippocampal CA3 neurons immunostained for total levels of the AMPAR subunit GluA1 (red) and the excitatory scaffolding protein PSD-95 (green). CTIP2 (blue) is a cell-type specific marker used to exclude dentate gyrus (DG) and CA1 neurons, which do not demonstrate homeostatic responses in vitro (11). Bottom: Representative proximal and distal dendrites from control and silenced neurons. Scale bar, 20 μm. (b) Representative images of surface GluA1 (red). Neurons were stained with Py (blue) to label CA3 neurons (11,21) and CTIP2 (green) to exclude DG and CA1 neurons (11,21). (c, d) Quantification of surface GluA1 (c) and total GluA2 (d) as a function of dendritic distance from the soma. *P<0.05, ***P<0.0001, 2-way ANOVA and post hoc Bonferroni test.
3.2
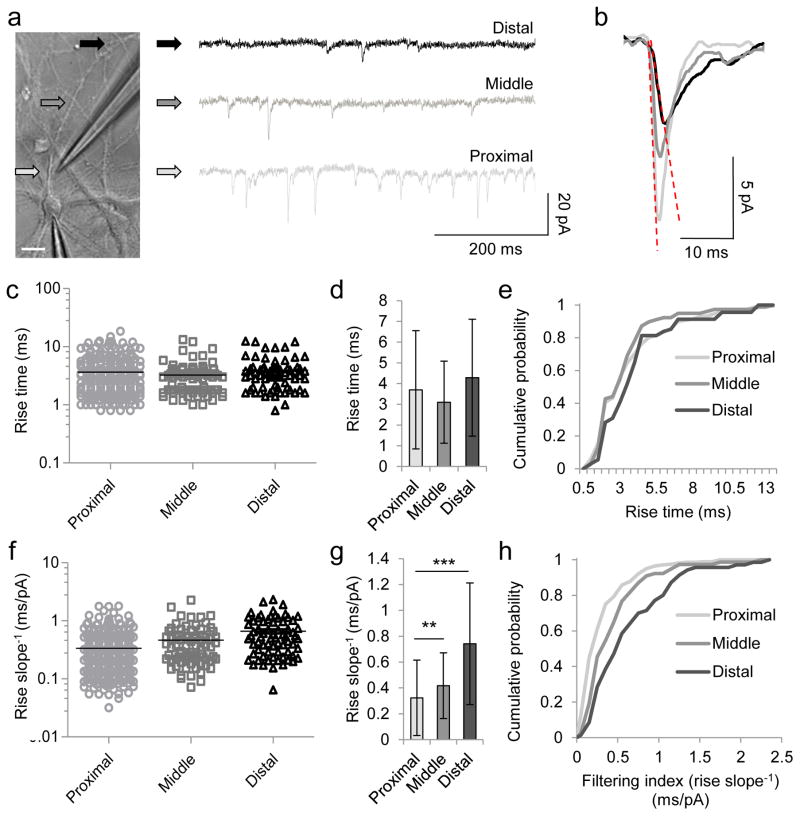
Because of the discrepancy between the synaptic staining and electrophysiological results in young cortical neurons, we investigated whether the spatial distribution of synaptic activity could be mapped functionally. We noticed that currents evoked at proximal locations appeared “sharper”-looking than currents evoked distally on the same dendrite (Fig. 3a–b). Dendrites act as cables (14,16), filtering waves or currents traveling along them. Like ripples emanating from a splash, currents that begin tall and sharp (small rise times) become smaller and broader (reduced amplitude, longer rise times) proportional to distance traveled (17). We therefore examined whether current shape could be used to estimate the dendritic origin (18). We created a new parameter termed the dendritic filtering index (DFI), based on the mEPSC slope to reflect the “steepness” of each current (Fig. 3b). The DFI is then defined as the inverse of the slope (rise time/amplitude), so that smaller values correspond to more (putatively) proximal dendritic distances from the soma. Indeed, unlike mEPSC rise times (or amplitudes) alone (Fig. 3c–e and ref. (14,15)), which cannot discriminate the synaptic origin of currents evoked with local application of high osmolar sucrose at different distances along the dendrite, the DFI of proximally evoked currents was significantly smaller than those evoked distally on the same dendrite (Fig. 3f–h).
Figure 3. Current shape varies with dendritic origin.
(a) Miniature EPSCs recorded during sucrose stimulation of a selected dendrite at locations indicated (arrows). Scale bar, 20 μm. (b) Averaged mEPSCs evoked during a single proximal (light gray), middle (dark gray), or distal (black) stimulation of neuron in a. Dotted lines denote dendritic filtering index (DFI), defined as rise time/amplitude (i.e. rise slope−1). (c, f) Individual rise times (c) and rise slopes−1 (f) of mEPSCs evoked from neuron in a at proximal, middle, and distal locations. (d, g) Means±SD of mEPSC rise times (d) and rise slopes−1 (g) from c and f, respectively. (e, h) Cumulative distributions of rise times (e) and rise slopes−1 (h) from c and f, respectively. **P<0.01, ***P<0.001, Kruskal-Wallis and post-hoc Dunn’s multiple comparison test.
3.3
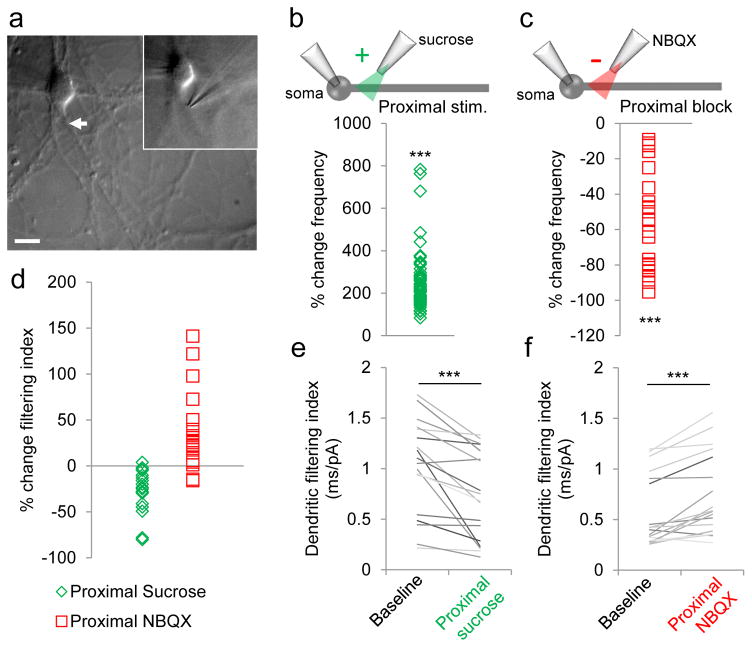
To further validate that proximal currents were indeed “sharper” (exhibited smaller DFI) than distal currents when recorded at the soma, we bidirectionally manipulated proximal synapses selectively (Fig. 4a). Locally stimulating proximal dendrites with sucrose increased the total number of detected mEPSCs (Fig. 4b), while locally blocking proximal synapses with AMPA receptor antagonist NBQX significantly decreased mEPSC number (Fig. 4c), as expected. Concurrently, proximal stimulation significantly decreased the DFI (Fig. 4d, e), while proximal blockade significantly increased this parameter (Fig. 4d, f). Furthermore, the change in DFI was proportional to the recruitment of proximal events: the higher the current frequency during stimulation, the larger the decrease in DFI (R2 = 0.339). These results confirmed that proximal mEPSCs have smaller DFI than distal ones.
Figure 4. Validation of DFI with local dendritic manipulation.
(a) Representative micrograph of micropipette used for local synaptic manipulation of proximal dendrites. (b–d) Change in mEPSC frequency (b, c) and filtering index (d) induced by proximal sucrose stimulation (b, d, n=18) or proximal NBQX blockade (c, d, n=34). (e, f) Average DFI of mEPSCs recorded before (left) or after (right) proximal sucrose stimulation (e) or NBQX blockade (f). ***P<0.0001, Wilcoxon rank sum test. See Table 3.
3.4
Finally, we used the DFI to map the dendritic distribution of homeostatic synaptic plasticity. We recorded AMPAR mEPSCs from hippocampal and cortical neurons under basal conditions or silenced with TTX. Compared to controls, the DFI revealed that chronically inactive mature hippocampal neurons had significantly more synaptic currents (Fig. 5a) and increased current amplitudes (Fig. 5c) originating selectively from proximal dendrites. In contrast, young cortical neurons showed increases in both mEPSC number (Fig. 5b) and amplitude (Fig. 5d) occurring more globally throughout the dendritic tree. Importantly, local stimulation of dendrites under both basal and TTX conditions showed that DFI behaved as a highly linear function of dendritic distance (Fig. 5e, f). Changes in DFI could not be explained by an AMPAR subunit switch to higher conductance GluA1 homomeric receptors with faster kinetics (19,20), because addition of philanthotoxin-433, a selective antagonist of GluA2-lacking AMPA receptors, had no effect on the decrease in DFI observed in hippocampal neurons with chronic inactivity (Fig. 6a, b). There was also no difference in input or access resistance (Fig. 6c–h), suggesting that changes in DFI were likely not due to variations in recording and seal quality. Therefore, the DFI analysis suggests that homeostatic global scaling operates in cortical neurons, whereas proximal dendritic homeostatic plasticity is the preferred mechanism in hippocampal neurons.
Figure 5. Dendritic filtering index maps the location of homeostatic synaptic plasticity.
(a–d) Number (a, b, mean±SD) and amplitude (c, d, mean±SEM) of mEPSCs recorded from hippocampal (a, c) and cortical (b, d) neurons (N=10–15/condition), plotted as a function of DFI. *P<0.05, P<0.01, ***P<0.001, two-way ANOVA and post hoc Bonferroni test (NT vs. TTX). See Tables 4,5. (e) Average filtering indices of mEPSCs evoked from individual neurons stimulated along isolated dendrites at the indicated distances. Control neurons in black/gray, TTX neurons in orange. (f) Relative filtering index as a function of distance. For both control (NT) and TTX-treated neurons, data from neurons in (e) were plotted by group (NT vs. TTX) to yield linear trendlines. The absolute DFI values for each cell were then normalized to the DFI of the neuron’s most proximal currents (at 20 μm) in order to quantify the change in current shape that occurs as a function of dendritic distance. This shape-invariant analysis showed that the relative relationship of proximal versus distal events was preserved across neurons and treatment groups (i.e., the relative change in normalized DFI was identical): under all conditions, currents were attenuated by one-fourth at ~100 μm and one-half by at 170 μm (corresponding to a 25% and 50% increase in DFI compared to mEPSCs evoked at 20 μm).
Figure 6. Changes in DFI are not due to altered AMPAR subunit composition or recording conditions.
(a) Quantification of dendritic filtering index from cortical and hippocampal neurons normalized to control. TTX-treated neurons had significantly “sharper” currents indicated by smaller DFI (in ms/pA): NT 0.94±0.06, TTX 0.64±0.05; ***P=0.0002, two-tailed t-test). Cortical neurons showed no change in DFI (NT 0.050±0.016 ms/pA, TTX 0.056±0.007 ms/pA; P=0.696, two-tailed t-test). (b) Blockade of homomeric GluA1 receptors using philanthotoxin (PhlTx) did not prevent DFI reduction with TTX (NT 0.860±0.07ms/pA, NT+PhlTx 0.819±0.07, P=0.1006, paired Student’s t-test; TTX 0.537±0.08, TTX+PhlTx 0.529±0.08, P=0.693 paired Student’s t-test). (c, f) No differences were observed in input resistance (P=0.696, one-way ANOVA) (c) or access resistance (P=0.357, one-way ANOVA) (f), (P=0.979, one-way ANOVA) among groups. (d, g) No strong relationship was observed between the DFI and either input resistance (d) (DFI vs. Rinput: R2=0.155, range=200–500 MΩ) or access resistance (g) (DFI vs. Raccess: R2=0.067, median DFI vs. Raccess: R2=0.095; range=10–25 MΩ). (e, h) Expanded analysis to include recordings excluded due to poor seal quality still demonstrated no relationship between DFI and either input resistance (e) (DFI vs. Rinput: R2=0.0045, range = 100–1000 MΩ) or access resistance (h) (DFI vs. Raccess: R2=0.194, median DFI vs. Raccess: R2=0.199; range = 10–55 MΩ). Red bars in d, e and blue bars in g, h denote equivalent ranges.
4. DISCUSSION
In this study, we developed a novel electrophysiological parameter, the DFI, based on the shape of synaptic AMPAR currents. The “sharpness” of the current is described by the inverse slope and we showed that this index varies linearly in proportion to distance along the dendrite from the cell body, consistent with cable properties of dendrites. Because of this property, the DFI can reliably report on the originating location of synaptic currents. Previous attempts to use mEPSC amplitude or rise time alone were unable to discriminate dendritic distance, as currents of varying amplitudes can occur anywhere along the dendritic tree, and rise time is dependent on current amplitude. By combining the rise time and amplitude in the slope, the DFI reflects the shape of the current in a parameter independent of amplitude.
To further confirm the utility of the DFI, we performed a retrospective analysis of currents reported previously by an independent group acquired during local stimulation at three regions of a dendrite (14). The DFI was able to clearly resolve the different mEPSC populations by dendritic distance, whereas amplitude or rise time alone could not (Fig. 7). To apply our method to other experimental paradigms such as slice preparations, it should be possible to use approximately the same approach as dissociated cells. We envision the method would be most useful in slices obtained from brain regions that feature highly laminated inputs, such as the hippocampus. After passively recording mEPSCs from, for example, a hippocampal CA3 neuron, one could ask whether the amplitude and/or frequency of proximal vs. middle vs. distal events was changed. This analysis could provide the first indication that proximal (mossy fiber), middle (CA3 recurrent) or distal (perforant path) inputs were selectively altered under different circumstances (e.g. injury, learning, or development). Clearly, more work would be needed to validate this approach, but our method could easily be applied retrospectively to existing data from cultured neurons or slices, as well as in prospective studies. We expect that the DFI will help to more rapidly determine likely sites of change, greatly improving the ability of researchers to target the appropriate pathways for analyses or stimulation paradigms.
Figure 7. DFI, but not mEPSC amplitude or rise time, discriminates dendritic origin of currents.
(a) Miniature EPSCs evoked in a previous study at three distances along an isolated dendrite as indicated, plotted as a function of amplitude and rise time (data reproduced from ref. (14) with permission from John Bekkers and the Journal of Neurophysiology). (b) Synaptic currents from a replotted as a function of amplitude and log DFI (rise slopes−1). Note the minimal overlap between populations evoked at 44 versus 165 μm along an isolated dendrite (median [25th–75th percentile] in ms/pA: 44 μm stimulation 0.0346 [0.0236–0.0692], 165 μm stimulation 0.1399 [0.1154–0.2313]).
Although practical at the level of synaptic populations, one limitation of the method is that the spatial resolution is relatively broad and the DFI is unable to discriminate changes occurring at sites that are in close proximity. Nevertheless, these findings provide a novel approach for mapping synaptic distributions and we have used the tool to provide evidence that homeostatic scaling may be a neocortical phenomenon, whereas hippocampal excitatory neurons perform selective homeostatic upregulation of proximal synapses. It is somewhat unexpected that changes in mEPSCs and PSD95 levels correlated in hippocampal neurons, but not in cortical neurons, as PSD-95 levels are often used as a readout for excitatory synaptic strength. However, upon close examination, the increase in total PSD-95 staining in proximal dendrites of hippocampal neurons was due mostly to an increase in the number of PSD-95 puncta (i.e., new synaptic sites in the proximal dendrite) rather than increased PSD-95 accumulation at existing sites (11). Thus, if changes in total PSD-95 immunoreactivity are dominated by increases in the number of synaptic sites, the PSD-95 results are in line with our frequency results: hippocampal neurons increase the number of proximal synaptic sites and therefore proximal mEPSC frequency goes up, while cortical neurons do not change the number of synaptic sites and mEPSC frequency is unchanged. Meanwhile, the mEPSC amplitude changes could be due to changes in AMPAR number and/or phosphorylation status. Further work will be required to elucidate the mechanisms underlying these distinct homeostatic strategies in different cell types.
In conclusion, the DFI presents a novel technique for mapping the loci of currents and synaptic plasticity events to spatially segregated dendritic subdomains, lamina, or inputs under various physiological or pathological conditions.
Table 2. Analysis of PSD-95 and surface GluA1 distributions.
Table accompanies Fig. 1e–h and Fig. 2c–d. We performed a 2-way ANOVA analyzing the distribution of synaptic proteins as a function of treatment (NT vs. TTX) and distance from soma. In almost all cases, we observed strong (P<0.001) effects of distance on synaptic protein levels. We only analyzed the effects of treatment (NT vs. TTX) and interaction (equal or unequal effects as a function of distance).
| Protein | Source of variation | P-value | Interpretation |
|---|---|---|---|
| Hpc (DIV 21) N=86 NT, 95 TTX | |||
| PSD-95 | Treatment | ***P<0.0001 | TTX>NT |
| Interaction | ***P<0.0001 | Unequal effects of TTX at all distances (TTX>NT at 20 & 40 μm) | |
| tGluA1 | Treatment | ***P<0.0001 | TTX>NT |
| Interaction | ***P<0.0001 | Unequal effects of TTX at all distances (TTX>NT at 20 & 40 μm) | |
| sGluA1 | Treatment | **P=0.0023 | TTX>NT |
| Interaction | *P=0.0175 | Unequal effects of TTX at all distances (TTX>NT at 20 μm) | |
| Ctx (DIV 12) N=24 NT, 19 TTX | |||
| PSD-95 | Treatment | P=0.3256 | NT=TTX |
| Interaction | P=0.8077 | Equal effects of TTX at all distances | |
| tGluA1 | Treatment | P=0.1002 | NT=TTX |
| Interaction | P=0.9338 | Equal effects of TTX at all distances | |
| sGluA1 | Treatment | nd | nd |
| Interaction | nd | nd | |
Table 3. Effects of proximal manipulation on DFI.
Table accompanies Fig. 4
| Parameter | Baseline | Sucrose | P | Baseline | NBQX | P |
|---|---|---|---|---|---|---|
| Frequency | 248±137 (SD)% of baseline | ***P<0.0001, Wilcoxon signed rank test | 53±24 (SD)% of baseline | ***P<0.0001, Wilcoxon signed rank test | ||
| DFI | 1.02±0.47 (SD) ms/pA |
0.74±0.43 (SD) ms/pA |
***P=0.0004, Wilcoxon signed rank test | 0.54±0.33 (SD) ms/pA |
0.71±0.39 (SD) ms/pA |
***P<0.0001, Wilcoxon signed rank test |
Table 4. Effects of inactivity on synaptic currents.
| Cell type | Hpc | Ctx | ||||
|---|---|---|---|---|---|---|
| Parameter | NT | TTX | P | NT | TTX | P |
| Total | ||||||
| Amplitude (pA) | 13.4±0.804 (N=14) | 19.1±1.5 (N=10) | **P=0.003 | 21.9±3.6 (N=10) | 31.6±2.8 (N=10) | *P=0.0429, Mann-Whitney test |
| DFI: We performed a 2-way ANOVA analyzing the distribution of synaptic currents as a function of treatment (NT vs. TTX) and distance from soma. In almost all cases, we observed strong (P<0.001) effects of distance on synaptic protein levels. We only analyzed the effects of treatment (NT vs. TTX) and interaction (equal or unequal effects as a function of distance). | ||||||
| Proximal mEPSC # | 33.0±14.5 | 172.9±41.6 | ***P<0.001‡ | 56.9±26.9 | 75.0±15.5 | P>0.05‡ |
| Distal mEPSC # | 8.4±1.5 | 12.0±1.9 | P>0.05‡ | 14.6±3.4 | 27.2±8.2 | P>0.05‡ |
| Proximal mEPSC amplitude | 21.4±1.4 pA |
24.2±1.8 pA |
**P<0.01‡ | 30.8±4.2 | 44.6±2.1 | ***P<0.001‡ |
| Distal mEPSC amplitude | 11.0±0.27 | 11.8±0.43 | P>0.05‡ | 11.9±1.7 | 19.0±1.3 | *P<0.05‡ |
Post hoc Bonferroni test after treatment effect was confirmed via 2-way ANOVA (treatment & distance). See Table 2 for results of 2-way ANOVA.
Table 5. Analysis of synaptic current distributions.
We performed a 2-way ANOVA analyzing the distribution of synaptic currents as a function of treatment (NT vs. TTX) and distance from soma. In all cases, we observed strong (P<0.001) effects of distance on synaptic currents. We only analyzed the effects of treatment (NT v. TTX) and interaction (equal or unequal effects as a function of distance).
| Protein | Source of variation | P-value | Interpretation |
|---|---|---|---|
| Hpc (DIV 21) N=10 NT, 15 TTX | |||
| mEPSC # | Treatment | ***P<0.0001 | TTX>NT |
| Interaction | ***P<0.0001 | Unequal effects of TTX at all distances (TTX>NT at 0.2 and 0.4ms/pA) | |
| mEPSC amplitude | Treatment | ***P=0.0003 | TTX>NT |
| Interaction | P=0.1005 | Equal effects of TTX at all distances | |
| Ctx (DIV 12) N=10 NT, 10 TTX | |||
| mEPSC # | Treatment | **P=0.0090 | TTX>NT |
| Interaction | P=0.8994 | Equal effects of TTX at all distances | |
| mEPSC amplitude | Treatment | ***P<0.0001 | TTX>NT |
| Interaction | P=0.4471 | Equal effects of TTX at all distances | |
HIGHLIGHTS.
We derive a new electrophysiological parameter, the dendritic filtering index (DFI)
The DFI describes the shape of AMPA receptor currents
The DFI allows mapping of the dendritic origin of synaptic currents
The DFI reveals distinct forms of homeostatic plasticity in different neuron types
Acknowledgments
We thank John Bekkers for the use of his data and John Partridge for critical reading of the manuscript. This work was supported by NS041218 and NS080462 (BNQ) and NS075278 (DTSP) from NINDS.
Footnotes
Competing interests: The authors declare no competing financial interests.
Author contributions: BNQ, KJL, and DTSP conceived of and designed, while BNQ and HT performed experiments; BNQ collected and analyzed, while BNQ, RH, SV, and DTSP interpreted data; BNQ and DTSP wrote the article. All authors approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science (80-) 1997 Jan;275(5297):209–13. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 3.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science [Internet] 2006 Aug 25;313(5790):1093–7. doi: 10.1126/science.1128134. [cited 2011 Jun 12] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16931756. [DOI] [PubMed] [Google Scholar]
- 4.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998 Feb;391(6670):892–6. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998 Nov;21(5):1067–78. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 6.Oja E. A simplified neuron model as a principal component analyzer. J Math Biol. 1982;15(3):267–73. doi: 10.1007/BF00275687. [DOI] [PubMed] [Google Scholar]
- 7.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Ann Rev Neurosci. 2000;23(Hebb 1949):649–711. doi: 10.1146/annurev.neuro.23.1.649. Annual Reviews 4139 El Camino Way, PO Box 10139, Palo Alto, CA 94303-0139, USA. [DOI] [PubMed] [Google Scholar]
- 8.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000 Jun;10(3):358–64. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 9.Queenan BN, Lee KJ, Pak DTS. Wherefore art thou, homeo(stasis)? Functional diversity in homeostatic synaptic plasticity. Neural Plast [Internet] 2012 Jan;2012:718203. doi: 10.1155/2012/718203. [cited 2013 Apr 9] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3362963&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sala C, Piëch V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001 Jul;31(1):115–30. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee KJ, Queenan BN, Rozeboom AM, Bellmore R, Lim ST, Vicini S, et al. Mossy fiber-CA3 synapses mediate homeostatic plasticity in mature hippocampal neurons. Neuron [Internet] 2013 Jan 9;77(1):99–114. doi: 10.1016/j.neuron.2012.10.033. [cited 2013 Mar 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23312519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diering GH, Gustina AS, Huganir RL. Neuron. 4. Vol. 84. Elsevier Inc; 2014. Oct, PKA-GluA1 Coupling via AKAP5 Controls AMPA Receptor Phosphorylation and Cell-Surface Targeting during Bidirectional Homeostatic Plasticity; pp. 790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evers DM, Matta JA, Hoe H-S, Zarkowsky D, Lee SH, Isaac JT, et al. Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic overexcitation. Nat Neurosci [Internet] 2010 Oct;13(10):1199–207. doi: 10.1038/nn.2624. [cited 2011 Aug 18] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2947592&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bekkers JM, Stevens CF. Cable properties of cultured hippocampal neurons determined from sucrose-evoked miniature EPSCs. J Neurophysiol. 1996;75(3):1250–5. doi: 10.1152/jn.1996.75.3.1250. [DOI] [PubMed] [Google Scholar]
- 15.Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci. 2000 Sep;3(9):895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- 16.Williams SR, Stuart GJ. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science (80-) 2002 Mar;295(5561):1907–10. doi: 10.1126/science.1067903. [DOI] [PubMed] [Google Scholar]
- 17.Forti L, Bossi M, Bergamaschi A, Villa A, Malgaroli A. Loose-patch recordings of single quanta at individual hippocampal synapses. Nature. 1997 Aug;388(6645):874–8. doi: 10.1038/42251. [DOI] [PubMed] [Google Scholar]
- 18.Rall W. Theoretical significance of dendritic trees for neuronal input-output relations. In: Reiss R, editor. Neural Theory and Modeling. Stanford University Press; 1964. [Google Scholar]
- 19.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005 Sep;47(5):725–37. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 20.Lindskog M, Li L, Groth RD, Poburko D, Thiagarajan TC, Han X, et al. Postsynaptic GluA1 enables acute retrograde enhancement of presynaptic function to coordinate adaptation to synaptic inactivity. Proc Nat Acad Sci. 2010;107(50):21806–11. doi: 10.1073/pnas.1016399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams ME, Wilke SA, Daggett A, Davis E, Otto S, Ravi D, et al. Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron [Internet] 2011 Aug 25;71(4):640–55. doi: 10.1016/j.neuron.2011.06.019. [cited 2013 Feb 28] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3272880&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]