Abstract
Mutations affecting the RNA sequence of the first 10 codons of the Saccharomyces cerevisiae mitochondrial gene COX2 strongly reduce translation of the mRNA, which encodes the precursor of cytochrome c oxidase subunit II. A dominant chromosomal mutation that suppresses these defects is an internal in-frame deletion of 67 codons from the gene YDR494w. Wild-type YDR494w encodes a 361-residue polypeptide with no similarity to proteins of known function. The epitope-tagged product of this gene, now named RSM28, is both peripherally associated with the inner surface of the inner mitochondrial membrane and soluble in the matrix. Epitope-tagged Rsm28p from Triton X-100-solubilized mitochondria sedimented with the small subunit of mitochondrial ribosomes in a sucrose gradient containing 500 mM NH4Cl. Complete deletion of RSM28 caused only a modest decrease in growth on nonfermentable carbon sources in otherwise wild-type strains and enhanced the respiratory defect of the suppressible cox2 mutations. The rsm28 null mutation also reduced translation of an ARG8m reporter sequence inserted at the COX1, COX2, and COX3 mitochondrial loci. We tested the ability of RSM28-1 to suppress a variety of cox2 and cox3 mutations and found that initiation codon mutations in both genes were suppressed. We conclude that Rsm28p is a dispensable small-subunit mitochondrial ribosomal protein previously undetected in systematic investigations of these ribosomes, with a positive role in translation of several mitochondrial mRNAs.
Virtually all Saccharomyces cerevisiae mitochondrial ribosomal proteins are coded by nuclear genes, synthesized in the cytoplasm, and imported into the organelle, where they are assembled with a single protein and two rRNAs encoded in the mitochondrial DNA (23). Proteomic investigations of yeast mitochondrial ribosomal proteins have revealed that they contain a substantially larger number of polypeptides than bacterial ribosomes (19, 53). Mammalian mitochondrial ribosomes also contain a large number of proteins, all of which are encoded in the nucleus (36, 37, 45, 59, 60). Only about half of the proteins identified in mitochondrial ribosomes are detectably homologous to bacterial ribosomal proteins. This suggests that the remaining proteins could carry out novel ribosomal functions in mitochondrial translation systems.
The critical role of mitochondrial translation systems in cellular metabolism is to translate a handful of hydrophobic polypeptides from mitochondrially encoded mRNAs. These proteins must be inserted into the inner mitochondrial membrane and assembled into respiratory chain complexes (1). In S. cerevisiae, one adaptation for this specialized role of the translation system appears to be membrane-bound mRNA-specific translational activators that recognize targets in mRNA 5′ untranslated leaders (5′-UTLs) (16). The translational activators appear to both limit (24, 58) and localize (13, 16, 33, 38, 44, 50, 52) translation of mitochondrial mRNAs. Genetic evidence suggests that mitochondrial ribosomal proteins of the small subunit interact both with translational activators (27, 28, 40) and with targets in the mitochondrial mRNA 5′-UTLs (25). Biochemical and genetic evidence suggests that interaction between mitochondrial ribosomes and the conserved inner membrane protein Oxa1p facilitates insertion of mitochondrially coded proteins into the membrane (34, 61). A large-subunit ribosomal protein homologous to bacterial L23 can be cross-linked to Oxa1p (34). All of the ribosomal proteins identified in these functional studies are essential for mitochondrial translation.
In addition to the translational activator target in its 5′-UTL, the COX2 mRNA contains antagonistic sequence elements with the pre-Cox2p coding sequence that control its translation: a positively acting element in the RNA sequence immediately downstream of the initiation codon, and negative elements further downstream in the coding sequence (3, 66). The mechanisms by which these elements function remain unknown. However, mutations within the first 10 codons of COX2 that reduce translation can be suppressed in cis by nearby mutations that weaken a predicted stem structure (3). They can also be suppressed in trans by overexpression of the COX2 mRNA-specific activator protein Pet111p as well as a mitochondrial ribosomal large-subunit protein, MrpL36p (3), which contains an essential core domain homologous to bacterial L31 and additional nonessential domains that cause suppression when overexpressed (67). Finally, dominant nuclear suppressors of these cox2 mutations were isolated (3).
In this study we describe an as-yet-undetected yeast mitochondrial ribosomal small-subunit protein, Rsm28p, as the product of a gene identified by one of the previously isolated dominant nuclear suppressor mutations. Interestingly, the suppressor mutation is an internal in-frame deletion that alters the structure and function of Rsm28p. Furthermore, Rsm28p is not essential for mitochondrial translation, unlike other mitochondrial ribosomal proteins identified genetically by functional screens (25, 27, 28, 40).
MATERIALS AND METHODS
Strains, media, and genetic techniques.
The S. cerevisiae strains used in this study are listed in Table 1. They are isogenic or congenic to strain D273-10B (ATCC 25627). Fermentable complete medium was YPAD or YPAGal containing 1% yeast extract, 2% Bacto-Peptone, 100 mg of adenine/liter, and either 2% glucose or 2% galactose supplemented with 0.1% glucose. Nonfermentable medium was YPEG containing 1% yeast extract, 2% Bacto-Peptone, 100 mg of adenine/liter, 3% ethanol, and 3% glycerol. Minimal medium (0.67% yeast nitrogen base without amino acids) was supplemented with specific amino acids, uracil, and adenine as required, and either 2% glucose or 3% ethanol and 3% glycerol for nonfermentable medium. Standard genetic methods were as previously described (17, 26, 56). S. cerevisiae nuclear transformation was carried out with the Frozen-EZ Yeast Transformation II kit (Zymo Research) or lithium acetate (9).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotypea | Source or reference |
|---|---|---|
| DL2 | MATalys2 [rho+] | 15 |
| DUL1 | MATα lys2 ura3Δ [rho+] | 15 |
| EHW292 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 rsm28Δ::URA3 [rho+, cox2(1-91)::ARG8m] | This study |
| EHW385 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 rsm28Δ::URA3 [rho+, cox2Δ::ARG8m] | This study |
| EHW424 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 rsm28Δ::URA3 [rho+, cox2-22] | This study |
| EHW463 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox1Δ::ARG8m] | This study |
| EHW464 | MATaarg8Δ::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 rsm28Δ::URA3 [rho+, cox1Δ::ARG8m] | This study |
| EHW465 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox3Δ::ARG8m] | This study |
| EHW466 | MATaarg8Δ::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 rsm28Δ::URA3 [rho+, cox3Δ::ARG8m] | This study |
| HMD22 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2Δ::ARG8m] | 4 |
| LSF74 | MATalys2 ura3Δ [rho+, cox3-1] | 14 |
| NAB86 | MATα ade2-101 ade3-24 rsm28Δ::URA3 leu2-3,112 ura3-52 [rho+] | This study |
| NAB93 | MATaade2-101 ade3-24 arg8::hisG rsm28Δ::URA3 leu2-3 leu2-112 ura3-52 [rho+, cox2-22] | This study |
| NAB97 | MATaarg8::hisG RSM28-HA his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-22] | This study |
| NAB106 | MATaarg8::hisG RSM28-1-HA his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-22] | This study |
| NAB108 | MATα ade2-101 ade3-24 rsm28Δ::URA3 leu2-3,112 ura3-52 [rho+, cox2-27] | This study |
| NAB109 | MATaarg8Δ::hisG rsm28Δ::URA3 his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-22::ARG8m] | This study |
| NAB111 | MATaarg8::hisG rsm28Δ::URA3 his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2::ARG8m] | This study |
| NAB112 | MATaarg8::hisG rsm28Δ::URA3 his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-27::ARG8m] | This study |
| NAB119 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2::ARG8m] | This study |
| NAB120 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-22] | This study |
| NAB121 | MATaarg8::hisG RSM28-1 his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-22] | This study |
| NAB122 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-27] | This study |
| NAB123 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-22::ARG8m] | This study |
| NAB124 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-27::ARG8m] | This study |
| NAB125 | MATα ade2-101 ade3-24 leu2-3,112 ura3-52 pDB20 [rho+] | This study |
| NAB126 | MATaarg8::hisG RSM28-1 his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+] | This study |
| NAB127 | MATaarg8::hisG RSM28-1 his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2::ARG8m] | This study |
| NAB128 | MATaarg8::hisG RSM28-1 his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-22::ARG8m] | This study |
| NAB129 | MATaarg8::hisG RSM28-1 his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-27::ARG8m] | This study |
| NAB130 | MATaarg8::hisG RSM28-1 his3ΔHindIII leu2-3,112 lys2 ura3-52 pDB20 [rho+, cox2-27] | This study |
| NB40-3C | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-62] | 4 |
| NB43 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2(1-91)::ARG8m] | 4 |
| NB60 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-10] | 4 |
| NB64 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-22] | 3 |
| NB64-S7c | MATaarg8::hisG RMS28-1 his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-22] | This study |
| NB80 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+] | 4 |
| NB110 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-10(1-91)::ARG8m] | 4 |
| NB134 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-34(1-91)::ARG8m] | 4 |
| NB164 | MATaarg8::hisG his3ΔHindIII leu2-3,112 lys2 ura3-52 [rho+, cox2-34] | 4 |
Mitochondrial genotypes are in brackets.
Cloning of the suppressor gene.
A library of DNA from the suppressor strain NB64-S7c was prepared by partially digesting genomic DNA with Sau3AI. Restriction fragments were fractionated on sucrose gradients (10 to 40%) (51), and the 10-kb population was cloned into the BclI-cut vector pNB34. pNB34 is a low-copy-number shuttle plasmid with URA3 and ampicillin resistance selections in S. cerevisiae and Escherichia coli, respectively. Inserts are cloned into the λ cI gene, which regulates expression of a kanamycin resistance cassette, allowing selection of insert-containing plasmids (30). The resulting library was used to transform NB64. Transformants (≈7,500) were screened for suppression of the respiration defect at 30 and 16°C. Respiring colonies that appeared at both temperatures were isolated and tested for linkage of respiration to the plasmid.
Plasmids were isolated from four strains, and their suppression phenotype was confirmed by transformation of NB64. Two plasmids were sequenced from both ends, revealing a stretch of DNA sequence (≈14 kb) from chromosome IV with 11 possible open reading frames (ORFs). To determine which of these ORFs was responsible for the suppression phenotype, the GPS-1 Genome Priming System (New England Biolabs, Inc., Beverly, Mass.) for in vitro Tn7 mutagenesis was used. The Tn7-mutagenized plasmid library was transformed into NB64 and screened for the loss of suppression of respiration. Five plasmids were isolated and sequenced from both arms of Tn7, revealing insertion of Tn7 in all plasmids into different positions in the same ORF, YDR494w, which we have named RSM28.
YDR494w/RSM28 sequence correction, disruption, and hemagglutinin tagging.
YDR494w/RSM28 was PCR amplified from both NB64 and NB64-S7c to determine the nature of the suppressor mutation. The sequences of the PCR products lack one copy of a 16-base tandem repeat (TGATCCTCCTTGATTT) reported in the original published sequence for wild-type YDR494w (22), extending the protein from a putative size of 288 to 361 amino acids. We confirmed this sequence correction in DNA from both FY23 and strains in the D273-10B (ATCC 25657) background used here (GenBank accession no. AF459095). This has been corrected in the Saccharomyces Genome Database reference sequence. The suppressor allele, termed RSM28-1 (GenBank accession no. AF459096), has an internal in-frame deletion of 67 amino acids (amino acids 120 to 186), which are flanked by a GCAGC direct repeat.
For functional testing, genomic fragments carrying RSM28 and RSM28-1 were amplified by PCR from genomic DNA, cut with DraI, and subcloned into the SmaI site of the multicopy 2μm vector YEp352 (32) to yield pEHW217 (RSM28) and pEHW218 (RSM28-1). The clones were sequenced to confirm the absence of PCR-induced mutations. The RSM28 and RSM28-1 fragments from pEHW217 and pEHW218 then were subcloned as SacI-XbaI fragments into the same sites of the CEN vector pRS316 (57) to yield plasmids pEHW219 (RSM28) and pEHW220 (RSM28-1), respectively.
RSM28 was disrupted by replacement of nucleotides −16 to +854 (with respect to the translation initiation codon) with a PCR-derived URA3 cassette introduced into the genome by homologous recombination and checked by PCR. To tag the products of RSM28 and RSM28-1 with three hemagglutinin (HA) epitopes, an HA-URA3-HA cassette (54) flanked by RSM28 C-terminal coding sequence was amplified by PCR and transformed into NB64 and NB64-S7c. Due to the design of the tagging cassette, the tagged proteins bear 12 additional amino acids after the epitope. Following pop-out of the URA3 marker, the tag junction and coding sequence were confirmed by PCR and sequencing.
Northern and Western analyses.
RNA isolation and Northern blotting procedures have been described previously (66). Hybridization probes were, for COX2, a 1.6-kb PacI fragment from plasmid pJM2 (42) and, for 15S rRNA, XhoI-linearized plasmid pT82 (55). Total cellular protein extracts were prepared as described previously (68). Proteins of interest were visualized by electrophoresis of 50 to 100 μg of total protein per sample on sodium dodecyl sulfate-12% acrylamide gels, followed by Western blotting. The following antisera were used: mouse monoclonal anti-Cox2p (47); rabbit polyclonal anti-Arg8p (58); rabbit polyclonal anti-glucose-6-phosphate dehydrogenase (G6PDH) (Sigma-Aldrich); mouse monoclonal anti-Mrp7p (11); mouse monoclonal anti-Mrp13p (46); rabbit polyclonal anti-Hsp60p (29); rabbit polyclonal anti-Yme1p (62); rabbit polyclonal anti-citrate synthase, rabbit polyclonal anti-cytochrome b2, and horseradish peroxidase-conjugated mouse monoclonal anti-HA (Roche Molecular Biochemicals). For non-horseradish peroxidase-conjugated mouse antisera, secondary detection utilized 1:5,000 to 1:10,000 (Bio-Rad Laboratories) or 1:10,000 (Sigma-Aldrich) horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G; for rabbit antisera, 1:10,000 to 1:20,000 horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Invitrogen or Bio-Rad Laboratories). Chemiluminescent signal was visualized with the ECL or ECL Plus detection kit (Amersham Pharmacia Biotech).
Mitochondrial isolation, subfractionation, and protein analyses.
Mitochondrial isolation and purification, mitochondrial membrane fractionation, and alkaline extraction, mitoplasting, proteinase K protection assay, in vivo pulse-labeling and Western blot analysis were as previously described (3, 18, 20, 21, 31). Mitochondrial ribosomes were extracted from purified mitochondria (1 mg of protein) resuspended in 1 ml of 10 mM magnesium acetate-0.1 M NaCl-20 mM HEPES-KOH (pH 7.4)-1 mM phenylmethylsulfonyl fluoride by the addition of Triton X-100 to 0.5% and incubation on ice for 30 min. The lysate was clarified by centrifugation at 34,000 rpm for 20 min at 4°C in a Beckman TLA100.3 rotor (40,000 × g) and layered onto a 39-ml continuous 15 to 30% sucrose gradient containing 500 mM NH4Cl, 10 mM Tris, 10 mM magnesium acetate, pH 7.4, 7 mM beta-mercaptoethanol, 0.2% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and one complete protease inhibitor minitablet without EDTA (Roche). Gradients were centrifuged at 20,000 rpm for 17 h at 4°C in a Beckman SW28 rotor, and 1-ml fractions were collected; 0.4 ml of each fraction analyzed was concentrated by trichloroacetic acid precipitation and subjected to SDS gel electrophoresis and Western blotting.
RESULTS
A dominant nuclear mutation suppresses mitochondrial mutations that alter the pre-Cox2p leader peptide coding sequence and reduce COX2 mRNA translation.
Two mutations, cox2-22 and cox2-27, alter the pre-Cox2p leader peptide coding region of the mitochondrially coded COX2 mRNA and reduce its translation (3). cox2-22 is a compound allele with a deletion of codons 7 to 10 and the translationally silent change of codon 6 from AGA to CGT (3). cox2-27 is a deletion of codons 2 to 6 (3). We previously isolated spontaneous pseudorevertants of the cox2-22 mutant strain NB64 with improved respiratory growth and found that roughly half contained dominant nuclear suppressor mutations that were not characterized (3).
In this study, we focused on the revertant containing the strongest of these nuclear suppressors, NB64-S7c. Its suppressor mutation, termed RSM28-1 for reasons described below, improved respiratory growth on nonfermentable carbon sources of strains bearing either the cox2-22 or cox2-27 mutation (Fig. 1), and greatly increased the steady-state level of Cox2p in strains bearing the cox2-22 mutation (Fig. 2).
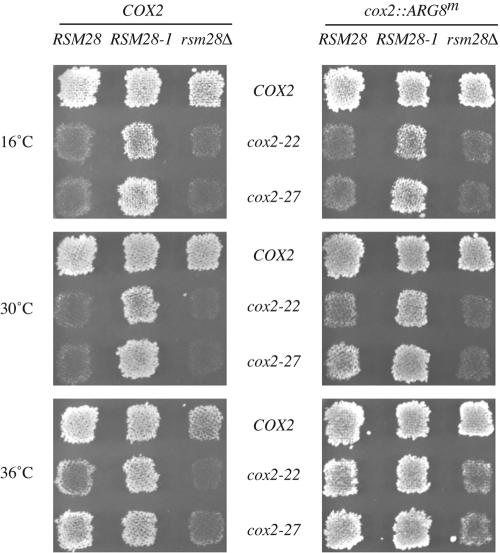
FIG. 1.
RSM28 alleles affect respiratory growth and mitochondrial reporter activity in cox2-22 and cox2-27 strains. Strains with the indicated nuclear wild-type RSM28, suppressor RSM28-1, or deletion rsm28Δ::URA3 alleles and the indicated mitochondrial alleles in an intact COX2 locus or the cox2::ARG8m reporter fusion were grown as patches on minimal glucose medium and then replica plated to nonfermentable minimal medium for the COX2 alleles (left panels) or minimal glucose medium lacking arginine for the cox2::ARG8m alleles (right panels). Plates were incubated at 16, 30, or 36°C as indicated and were photographed after 2 to 6 days. Strains used (from left to right): COX2; NAB125, NAB126, and NAB86; cox2-22; NAB120, NAB121, and NAB93; cox2-27; NAB122, NAB130, and NAB108; cox2::ARG8m; NAB119, NAB127, and NAB111; cox2-22::ARG8m; NAB123, NAB128, and NAB109; and cox2-27::ARG8m; NAB124, NAB129, and NAB112. As indicated in Table 1, strains lacking a chromosomal URA3 gene were transformed with the URA3 plasmid pDB20 (12).
FIG. 2.
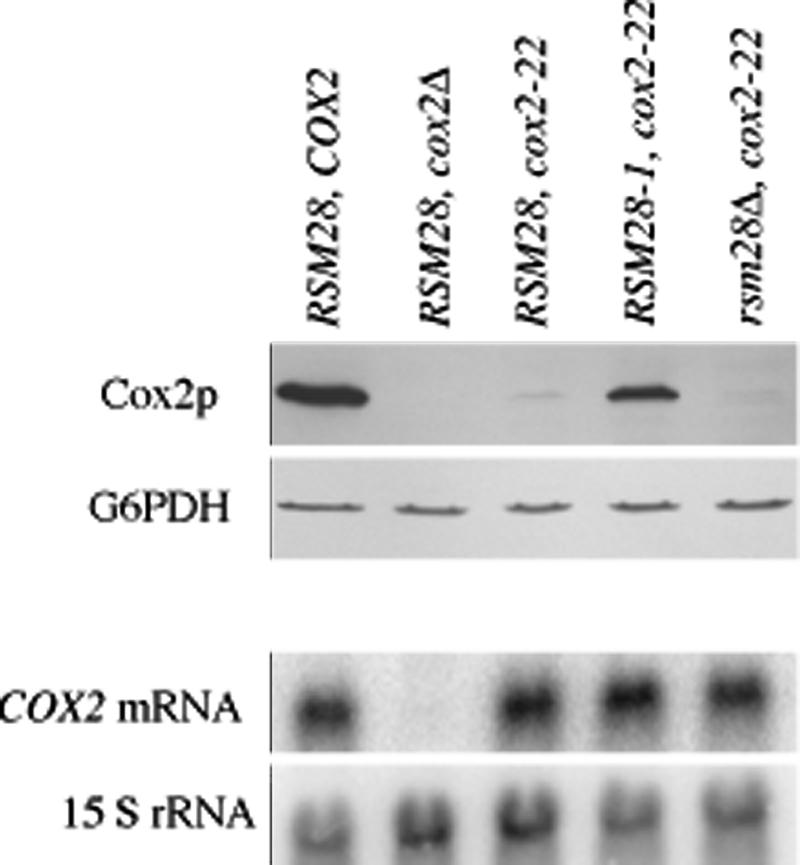
RSM28-1 increases Cox2p accumulation in a cox2-22 mutant without affecting COX2 mRNA levels. Strains containing wild-type RSM28, suppressor RSM28-1, or deletion rsm28Δ::URA3 genomic alleles and wild-type COX2, a cox2 deletion, or cox2-22 mitochondrial alleles were grown to mid-log phase in complete medium containing galactose (YPAGal), and protein and RNA were extracted (from separate cultures). The top two panels show a Western blot of 100 μg of total cellular protein probed with anti-Cox2p antibody (top panel), and anti-glucose-6-phosphate dehydrogenase (G6PDH) as a loading control (second panel). The bottom two panels show a Northern blot of 10 μg of total cellular RNA probed with a genomic fragment containing COX2 (third panel) and a probe for 15S rRNA as a loading control (bottom panel). Relevant genotypes are indicated above the lanes. Strains are described in Table 1: RSM28 COX2 (NB80); RSM28 cox2Δ (NB40-3C); RSM28 cox2-22 (NB64); RSM28-1 cox2-22 (NB64-S7c); and rsm28Δ cox2-22 (EHW424).
To ask whether the suppressor affects the level of gene expression from this locus, as opposed to a downstream step in cytochrome oxidase assembly, we tested its effect on strains bearing the cox2-22 and cox2-27 mitochondrial mutations in a cox2::ARG8m reporter gene (3). This reporter expresses the arginine biosynthetic enzyme Arg8p from the COX2 locus as a fusion to the 91st residue of pre-Cox2p and reliably reports COX2 mRNA translation (3, 4, 66). The RSM28-1 suppressor improved the growth of cox2-22::ARG8m and cox2-27::ARG8m strains on glucose medium lacking arginine (Fig. 1), indicating that it increased gene expression per se. The cox2-22 mutation has no significant effect on the steady-state level of COX2 mRNA relative to that of wild-type (3), and the presence of the suppressor mutation RSM28-1 did not increase the level of cox2-22 mRNA dramatically, if at all (Fig. 2). Thus, RSM28-1 appears to improve translation of the mutant mRNAs.
Identification of the suppressor mutation, RSM28-1, as an in-frame deletion within YDR494w.
To identify the suppressor, we first tested for genetic linkage of the suppressor mutation to the candidate genes PET111, MRP21, and MRP51 (25, 41), but no linkage was observed. We therefore constructed a genomic library of DNA isolated from the suppressed mutant strain NB64-S7c in the CEN vector pNB34 (Materials and Methods) and screened it for plasmids that could suppress the Pet− phenotype of the cox2-22 mutant strain NB64. One suppressing plasmid with 11 putative genes from chromosome IV was obtained. To identify the gene responsible for suppression, this plasmid was mutagenized in vitro with the transposon Tn7 (Materials and Methods). Five mutagenized plasmids that had lost the ability to suppress cox2-22 were isolated and sequenced, revealing distinct Tn7 insertions into ORF YDR494w, now termed RSM28. RSM28/YDR494w is a unique gene predicted to encode a 361-residue protein that exhibits no significant sequence similarity to any other known or predicted protein other than its homologues in closely related fungi (5, 6, 35). (The YDR494w ORF in the original reference genomic sequence of strain S288c [22] predicted a 288-amino-acid protein; see Materials and Methods.)
DNA sequence analysis of RSM28-1, the dominant suppressor mutation, revealed an internal in-frame deletion of 67 codons specifying amino acids 120 to 186 (GenBank accession no. AF459096). This result confirmed that YDR494w corresponds to the suppressor gene RSM28 and indicates that the shortened protein, Rsm28-1p, apparently acts to suppress the mitochondrial cox2-22 and cox2-27 mutations.
Rsm28p is a small-subunit mitochondrial ribosomal protein.
To examine wild-type Rsm28p and the suppressor form of the protein, we tagged the C termini of both by inserting DNA encoding three HA epitopes at the ends of the chromosomal ORFs (Materials and Methods). The epitopes did not abolish Rsm28p function, since expression of the HA-tagged suppressor protein Rsm28-1p-HA suppressed the cox2-22 mutation. Expression of the wild-type tagged protein Rsm28p-HA did not diminish the leaky growth phenotype caused by cox2-22, in contrast to the rsm28 null mutation. Indeed, the Rsm28p-HA protein caused slightly improved respiratory growth of cox2-22 strains compared to strains expressing the unmodified Rsm28p (unpublished data). Thus, both tagged proteins appear to be functional.
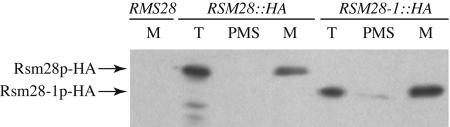
A total extract of cells expressing wild-type Rsm28p-HA contained an immunoreactive protein of the appropriate size (Fig. 3). As expected, the corresponding immunoreactive protein in a total extract of cells expressing the suppressor protein, Rsm28-1p-HA, was shorter due to the internal in-frame deletion (Fig. 3). Highly purified mitochondria prepared from both the wild-type and suppressor strains bearing the HA tag were enriched for these proteins, while the postmitochondrial supernatants contained very little (Fig. 3). Purified mitochondria from a wild-type strain lacking any HA tag did not contain this immunoreactive species (Fig. 3).
FIG. 3.
Rsm28p cofractionates with mitochondria. Cells were grown in YPAGal at 30°C to late log phase, disrupted to yield total cell extracts (T), and fractionated into crude mitochondrial pellets and postmitochondrial supernatants (PMS), and crude mitochondria were then purified on Nycodenz gradients (M). Extracts from untagged wild-type RSM28 (DL2), RSM28::HA (NAB97), and RSM28-1::HA (NAB106) strains were resolved on sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis, immunoblotted, and probed with anti-HA monoclonal antibody. Arrows indicate immunoreactive bands representing epitope-tagged Rsm28p-HA and the suppressor Rsm28-1p-HA.
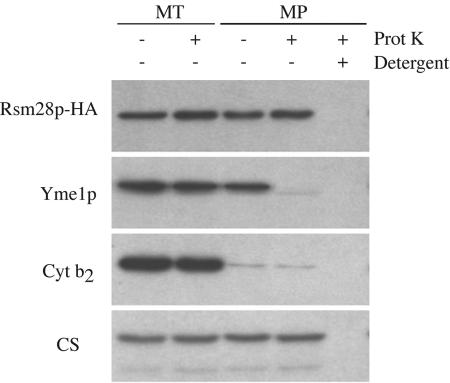
To determine the submitochondrial location of Rsm28p-HA, we first examined its sensitivity to digestion by exogenous protease added to mitochondria and to mitoplasts lacking the outer membrane. Rsm28p-HA was resistant to protease in intact mitochondria and in mitoplasts, while detergent solubilization of mitoplasts rendered Rsm28p-HA sensitive to protease (Fig. 4). This behavior was similar to that of the matrix marker protein citrate synthase (Fig. 4). In contrast, Yme1p, an inner membrane protein exposed on the outer surface, was susceptible to protease degradation in mitoplasts (Fig. 4). Thus, Rsm28p-HA appears to reside inside the inner membrane.
FIG. 4.
Rsm28p is within the inner membrane of mitoplasts. Mitochondria (MT) were purified from strain NAB97 (RSM28::HA) and converted to mitoplasts (MP) by osmotic shock in the absence or presence of 20 μg of proteinase K (Prot K) per ml, as indicated. Mitoplasts also were treated with proteinase K in the presence of 1% octylglucoside (detergent) to solubilize the inner membrane. Treated samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotted, and probed with anti-HA (Rsm28p-HA), anti-Yme1p as an integral inner membrane protein marker (Yme1p), anti-cytochrome b2 as an intermembrane space marker (Cyt b2), and anti-citrate synthase as a matrix marker (CS).
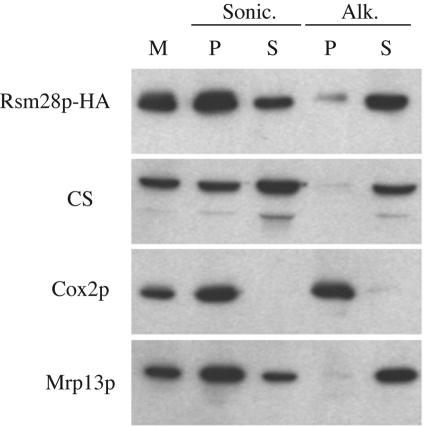
We next asked whether Rsm28p-HA is soluble or membrane bound by sonicating purified mitochondria and separating membrane and soluble fractions by centrifugation. Rsm28p-HA was detected primarily in the membrane pellet but also in the soluble supernatant (Fig. 5). Extraction of the pelleted membranes with alkaline sodium carbonate (18) solubilized almost all the Rsm28p-HA, indicating that it is peripherally associated with membranes. We obtained similar results with the tagged suppressor protein Rsm28-1p-HA (not shown). The behavior of Rsm28p-HA in these experiments closely paralleled that of Mrp13p (Fig. 5), a known mitochondrial small ribosomal subunit protein (46).
FIG. 5.
Rsm28p is peripherally associated with mitochondrial membranes. Mitochondria (M) purified from a strain expressing Rsm28p-HA (NAB97) were sonicated (Sonic.) and separated into a membrane pellet (P) and soluble supernatant (S) by centrifugation. An aliquot of this membrane fraction was extracted with sodium carbonate (pH 11.5) (Alk.), followed by centrifugation to separate pelleted integral membrane proteins (P) from solubilized proteins in the supernatant (S). Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotted, and probed with anti-HA antibody (Rsm28p-HA), anti-citrate synthase as a matrix marker (CS), anti-Cox2p as an integral inner membrane marker (Cox2p), and anti-Mrp13p (Mrp13p) as a mitochondrial ribosomal marker.
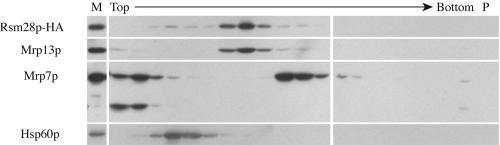
To test whether Rsm28p-HA is a component of mitochondrial ribosomes, purified mitochondria containing Rsm28p-HA were solubilized with Triton X-100, and the resulting lysate was sedimented in the presence of the detergent into a sucrose gradient containing 0.5 M NH4Cl (Materials and Methods). The gradient fractions were analyzed by probing Western blots with anti-HA and antisera against control proteins (Fig. 6). The sedimentation behavior of Rsm28p-HA was essentially identical to that of the mitochondrial ribosomal small-subunit protein Mrp13p, sedimenting ahead of the Hsp60 complex (48) and behind the mitochondrial ribosomal large-subunit protein Mrp7p (11). (A significant proportion of Mrp7p and an apparent degradation product remained near the top of these gradients.) Similar results were obtained when Rsm28p and Rsm28-1p were labeled with a twin affinity purification (49) tag (unpublished results).
FIG. 6.
Rsm28p cosediments with the mitochondrial ribosomal small subunit. Mitochondria were isolated from a strain expressing Rsm28p-HA (NAB97). Mitochondrial ribosomes were extracted by treatment with Triton X-100 and sedimented through continuous 15 to 30% sucrose gradients containing 0.5 M NH4Cl. Alternate fractions of the gradient were precipitated, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotted, and probed with anti-HA antibody (Rsm28p-HA), anti-Mrp13p (Mrp13p) as a small-subunit standard, anti-Mrp7p (Mrp7p) as a large-subunit standard, and anti-Hsp60p (Hsp60p). M, total mitochondria; P, solubilized sucrose gradient pellet.
Taken together with the submitochondrial localization of Rsm28p-HA, these data strongly indicate that Rsm28p is a small-subunit mitochondrial ribosomal protein. Consistent with this identification, the level of Rsm28p-HA in ρ0 cells, which cannot assemble ribosomal subunits, was dramatically lower than in ρ+ cells (unpublished results) as has been previously observed for the small-subunit proteins Mpr13p (46) and Pet123p (40). Following the systematic nomenclature suggestion of Saveanu et al. (53) for small-subunit mitochondrial ribosomal proteins, we have renamed YDR494w as RSM28.
Rsm28p is required for normal translation but is not essential.
We deleted the first 285 codons (out of 361) of the chromosomal copy of RSM28 by replacing them with URA3 (Materials and Methods). This null mutation, rsm28Δ::URA3, had little effect on the respiratory growth of an otherwise wild-type strain. The only phenotypes observed were a modest reduction in respiratory growth at 36°C (Fig. 1) and a modest increase in sensitivity to H2O2 in mutant cells growing on nonfermentable carbon sources (unpublished results). However, when combined with the cox2-22 and cox2-27 mutations or with the cox2-22::ARG8m and cox2-27::ARG8m reporters, rsm28Δ::URA3 reduced the residual growth caused by these leaky mitochondrial mutations (Fig. 1). As expected, the null allele was recessive to both wild-type RSM28 and the suppressor allele RSM28-1 in heterozygous diploid strains (unpublished data). Tetrad analysis of spores from a diploid heterozygous for rsm28Δ::URA3 and RSM28-1 confirm the linkage of the suppressor to this locus (unpublished data).
The fact that deletion of the gene coding ribosomal protein Rsm28p does not completely block mitochondrial translation is surprising but not unprecedented (10, 19). Despite the subtle respiratory growth defect detectable in rsm28Δ::URA3 COX2 strains on nonfermentable carbon sources (Fig. 1), no significant decrease in steady-state Cox2p accumulation is detectable in stationary-phase cells grown on galactose (unpublished data). However, we have previously observed that steady-state levels of the Arg8p reporter are more sensitive indicators of translational defects than levels of Cox2p (4).
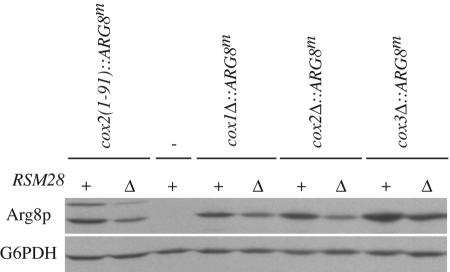
We therefore examined the effect of rsm28Δ::URA3 on the expression of two different cox2::ARG8m reporters: one has ARG8m fused to the 91st COX2 codon, as described above, while the other has no COX2 codons. In both cases, the absence of Rsm28p substantially reduced the level of accumulated Arg8p, indicating a significant reduction in translation of the reporter mRNAs (Fig. 7). (The Arg8p accumulating in these strains is nevertheless sufficient to support normal Arg+ growth [Fig. 1].) Furthermore, expression of the ARG8m reporter inserted in place of the COX1 and COX3 coding sequences was similarly reduced in the absence of Rsm28p (Fig. 7)
FIG. 7.
Rsm28p is required for full expression of the ARG8m reporter inserted at the COX1, COX2, and COX3 loci. Western blots of 50 μg of total cellular protein extracted from cells grown to mid- to late log phase in complete medium containing galactose (YPAGal) were probed with anti-Arg8p. The relevant mitochondrial genotypes of each strain are shown above the horizontal lines: − indicates wild-type mitochondrial DNA lacking ARG8m. The relevant nuclear genotypes are wild-type RSM28 (+) and the null allele rsm28Δ::URA3 (Δ). All strains lack the nuclear ARG8 gene. The blot was reprobed with anti-glucose-6-phosphate dehydrogenase (G6PDH) as a loading control. Strains utilized (from left to right): NB43, EHW292, NB80, EHW463, EHW464, HMD22, EHW385, EHW465, and EHW466.
Allele specificity of suppression by plasmid-borne RSM28-1.
To test the ability of RSM28-1 to suppress other mitochondrial mutations, we first established that it could suppress cox2-22 when borne on a plasmid. Genomic fragments containing RSM28-1 or RSM28 with the endogenous promoter were inserted into centromeric and 2μm plasmids (Materials and Methods) and transformed into a cox2-22 mutant strain. The presence of RSM28-1 on a centromeric plasmid suppressed cox2-22, as would be expected for the dominant suppressor allele (Fig. 8A). Additional copies of RSM28-1 carried on the 2μm plasmid suppressed more strongly (Fig. 8A). Wild-type RSM28 failed to suppress cox2-22 when carried on either the centromeric or 2μm plasmid (Fig. 8A), although this gene did complement the rsm28Δ::URA3 null allele (unpublished data).
FIG. 8.
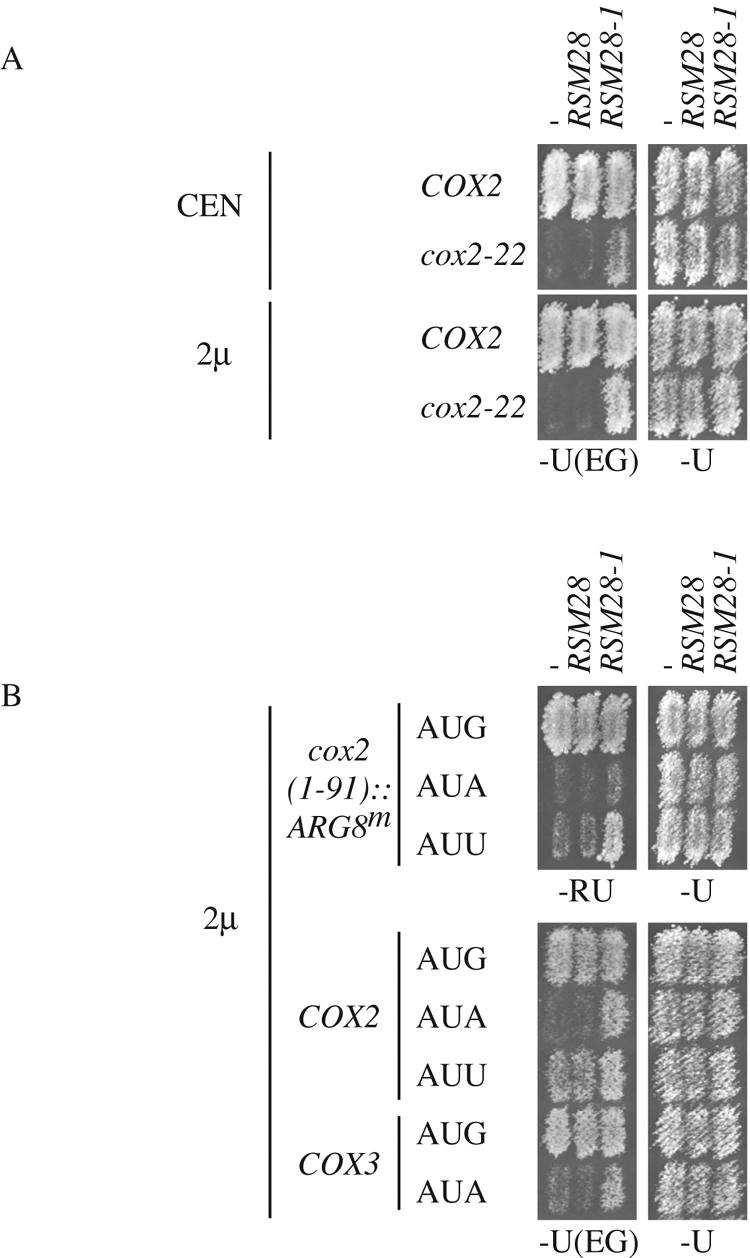
Plasmid-borne RSM28-1 can suppresses cox2-22, as well as cox2 and cox3 initiation codon mutations. (A) COX2 (NB80) and cox2-22 (NB64) strains were transformed with centromeric (CEN) or 2μm multicopy plasmids carrying RSM28 or RSM28-1, as indicated. − indicates empty vector. Transformants were replica plated to nonfermentable medium [−U(EG)] and glucose medium (−U), selecting for the plasmid marker, and incubated for 4 and 2 days at 30°C, respectively. The CEN/ARS plasmids indicated as −, RSM28, and RSM28-1 were pRS316, pEHW219, and pEHW220, respectively (Materials and Methods). The 2μm plasmids indicated as −, RSM28, and RSM28-1 were YEp352, pEHW217, and pEHW218, respectively (Materials and Methods). (B) Wild-type (AUG) and initiation codon mutant (AUA and AUU) strains were transformed with 2μm plasmids expressing RSM28 or RSM28-1. cox2(1-91)::ARG8m strains (top panels) were replica plated to medium selecting for Arg+ growth (−RU) and medium selecting for the plasmids (−U). COX2 and COX3 strains (bottom panels) were replica plated to nonfermentable medium [−U(EG)] and glucose medium, selecting for the plasmid marker (−U). Incubation was for 2 days at 30°C for −U and −U(EG) and 4 days for −RU. Strains used (from top to bottom): NB43, NB110, NB134, NB80, NB60, NB164, DUL1, and LSF74. The 2μm plasmids were as in A.
To investigate the allele specificity of suppression by RSM28-1, we transformed the high-copy-number 2μm plasmid bearing RSM28-1 into a diverse collection of strains with mitochondrial mutations in COX2 and COX3. We observed no suppression of any mutation tested affecting the mRNA 5′-UTL sequences (8, 42, 65) or of any missense or nonsense mutations (unpublished results). Several of these mutations have leaky phenotypes, making them sensitive indicators for a general increase in gene expression. Therefore, the fact that they were not suppressed suggests that RSM28-1 does not globally increase translation.
Interestingly, RSM28-1 did suppress both cox2 and cox3 initiation codon mutations. AUA in place of AUG at either gene reduces but does not eliminate translation (15, 43). AUU in place of AUG at COX2 allows more efficient translation than AUA (4). RSM28-1 on the 2μm plasmid clearly improved the respiratory growth of cox2 and cox3 mutants with AUA initiation codons as well as the cox2 mutant with AUU (Fig. 8B). This suppression was also evident when scored by expression of the cox2::ARG8m reporter containing COX2 codons 1 to 91 and the AUG to AUU mutation (Fig. 8B). Almost no suppression was observed with AUA in the cox2::ARG8m reporter, consistent with previous results showing that this is a tighter mutation (4). We conclude that the suppressor activity of Rsm28-1p appears to be limited to lesions affecting the coding sequence specifying the extreme N-terminal regions of mitochondrial proteins, but it is clearly not gene specific.
DISCUSSION
We have identified a novel yeast mitochondrial ribosomal small-subunit protein, Rsm28p, that had previously gone undetected in systematic proteomic analyses of these ribosomes (19, 53). Interestingly, Rsm28p is partially dispensable for mitochondrial gene expression, although deletion of its gene causes reduced respiratory growth, particularly at elevated temperature, and decreases the expression of a reporter gene inserted into mitochondrial DNA at the COX1, COX2, and COX3 loci. Thus, Rsm28p appears to play a role in normal translation of at least several mitochondrially coded mRNAs. While nonessential ribosomal proteins are unusual, they have previously been described in both cytoplasmic (2, 7) and mitochondrial (10) ribosomes of S. cerevisiae.
We were led to the gene RSM28 (YDR494w) by its functional interaction with the mitochondrially encoded COX2 mRNA. A spontaneous in-frame deletion mutation, RSM28-1, was selected as a dominant suppressor of COX2 mRNA translation defects caused by cox2 mutations that altered the sequence immediately downstream of the COX2 initiation codon (3). This suppression is not simply due to loss of RSM28 function since the complete rsm28 deletion mutation fails to suppress. Indeed, the complete absence of Rsm28p reduces translation of wild-type and mutant COX2 mRNAs as well as cox1Δ::ARG8m and cox3Δ::ARG8m reporter mRNAs. Thus, wild-type Rsm28p enhances the ability of ribosomes to initiate and/or elongate polypeptide synthesis, and the shorter suppressor form, Rsm28-1p, appears to have increased or altered this activity. Interestingly, we found that the RSM28-1 dominant suppressor mutation also weakly suppresses leaky cox2 and cox3 initiation codon mutations. This finding suggests the possibility that Rsm28p could play a general role in translation initiation. In this connection, it is interesting that two proteins with known roles in mitochondrial translation initiation, initiation factor 2, Ifm1p (63, 64), and methionyl-tRNA formyltransferase, Fmt1p (39), are also partially dispensable for yeast mitochondrial translation.
Translation of the COX2 mRNA appears to be controlled in a complex fashion. The COX2 mRNA-specific translational activator Pet111p works through a site in the mRNA 5′-UTL to promote synthesis of downstream coding sequences (4, 8, 41, 42) by an unknown mechanism. Genetic interactions also implicate two small-subunit ribosomal proteins, Mrp21p (a homolog of bacterial S21) and Mrp51p, in recognition of the COX2 mRNA 5′-UTL, although their activity is not mRNA specific and they are essential for global mitochondrial translation (25). Thus, it appears that translational activators and the ribosomes work together to recognize mRNA 5′-UTLs and probably initiation codons.
Additional antagonistic elements controlling COX2 mRNA translation are present within the coding sequence itself. A positive element is embedded in the mRNA sequence of the first 14 codons, which specify the leader peptide of pre-Cox2p (3), while a negative element is embedded in the mRNA sequence of codons 15 to 25 (66). Two other inhibitory sequences are located further downstream (66). It is unknown whether the inhibitory elements affect initiation or elongation or both in the absence of the positive element.
Two mutations within the first 10 COX2 codons that reduce translation are suppressed by the dominant RSM28-1 mutation. The same mutations (cox2-22 and cox2-27) are suppressed by mRNA sequence changes that reduce the stability of a stem structure that begins with the bases of the 10th COX2 codon (3). It is unclear whether the stronger stems reduce initiation, elongation, or both. The same cox2 mutations are also suppressed by overproduction of Pet111p and by overproduction of a large-subunit mitochondrial ribosomal protein, MrpL36p (3). MrpL36p contains an essential domain homologous to the bacterial ribosomal protein L31. It also contains an additional partially dispensable domain that, when overexpressed as a protein lacking the L31 region, suppresses the cox2 mutations but does not detectably associate with ribosomes (67). Interestingly, MrpL36p overexpression also partially suppresses a cox2 initiation codon mutation but not the corresponding cox3 mutation (67).
While the mechanism of yeast mitochondrial initiation codon recognition is not understood, it clearly involves both the initiation codon itself and other features of the mRNA (4, 15, 43). Pet111p and protein components of the ribosome apparently recognize the COX2 initiation site via interactions with the mRNA 5′-UTL, the initiation codon, and possibly sequences within the coding sequence. Rsm28p could clearly play a positive but nonessential role at this step. We propose that, in the absence of the wild-type sequence of the first 10 COX2 codons, this process could be antagonized by elements further downstream within the COX2 coding sequence. Alteration of Rsm28p, increased levels of Pet111p, or increased levels of the non-L31 domain of MrpL36p could at least partially restore translation initiation. (Overexpression of wild-type RSM28 does not cause suppression.) Such a model would not exclude subsequent effects of the downstream negative elements on translation elongation.
Apparent orthologues of Rsm28p are encoded in the sequenced genomes of other budding yeasts (5, 35) and the filamentous fungus Ashbya gossypii (Eremothecium gossypii) (6) but are not clearly detectable in more divergent species. Thus, Rsm28p is not highly conserved in sequence and may play a specialized role in fungal mitochondria. While sequence comparisons do not shed light on its function, it is noteworthy that the homologous proteins of Saccharomyces castellii, Saccharomyces kluyveri, Kluyveromyces lactis, and A. gossypii lack various extents of the 67-amino-acid nonessential sequence that is deleted by the suppressor mutation RSM28-1 of S. cerevisiae.
Acknowledgments
We thank R. L. Hallberg, G. Schatz, B. Glick, T. L. Mason, and C. Koehler for gifts of antibodies.
E. H. Williams was a Howard Hughes Medical Institute Predoctoral Fellow. N. Bonnefoy was a Human Frontier Science Program Organization long-term fellow (LT22/96) during the early phases of this investigation. This study was supported by NIH research grant GM29362 to T.D.F.
REFERENCES
- 1.Attardi, G., and G. Schatz. 1988. Biogenesis of mitochondria. Annu. Rev. Cell Biol. 4:289-333. [DOI] [PubMed] [Google Scholar]
- 2.Baronas-Lowell, D. M., and J. R. Warner. 1990. Ribosomal protein L30 is dispensable in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 10:5235-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnefoy, N., N. Bsat, and T. D. Fox. 2001. Mitochondrial translation of Saccharomyces cerevisiae COX2 mRNA is controlled by the nucleotide sequence specifying the pre-Cox2p leader peptide. Mol. Cell. Biol. 21:2359-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnefoy, N., and T. D. Fox. 2000. In vivo analysis of mutated initiation codons in the mitochondrial COX2 gene of Saccharomyces cerevisiae fused to the reporter gene ARG8m reveals lack of downstream reinitiation. Mol. Gen. Genet. 262:1036-1046. [DOI] [PubMed] [Google Scholar]
- 5.Cliften, P., P. Sudarsanam, A. Desikan, L. Fulton, B. Fulton, J. Majors, R. Waterston, B. A. Cohen, and M. Johnston. 2003. Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science 301:71-76. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich, F. S., S. Voegeli, S. Brachat, A. Lerch, K. Gates, S. Steiner, C. Mohr, R. Pohlmann, P. Luedi, S. Choi, R. A. Wing, A. Flavier, T. D. Gaffney, and P. Philippsen. 2004. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science 304:304-307. [DOI] [PubMed] [Google Scholar]
- 7.Dresios, J., P. Panopoulos, K. Suzuki, and D. Synetos. 2003. A dispensable yeast ribosomal protein optimizes peptidyltransferase activity and affects translocation. J. Biol. Chem. 278:3314-3322. [DOI] [PubMed] [Google Scholar]
- 8.Dunstan, H. M., N. S. Green-Willms, and T. D. Fox. 1997. In vivo analysis of Saccharomyces cerevisiae COX2 mRNA 5′-untranslated leader functions in mitochondrial translation initiation and translational activation. Genetics 147:87-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elble, R. 1992. A simple and efficient procedure for transformation of yeast. BioTechniques 13:18-20. [PubMed] [Google Scholar]
- 10.Fearon, K., and T. L. Mason. 1992. Structure and function of MRP20 and MRP49, the nuclear genes for two proteins of the 54 S subunit of the yeast mitochondrial ribosome. J. Biol. Chem. 267:5162-5170. [PubMed] [Google Scholar]
- 11.Fearon, K., and T. L. Mason. 1988. Structure and regulation of a nuclear gene in Saccharomyces cerevisiae that specifies MRP7, a protein of the large subunit of the mitochondrial ribosome. Mol. Cell. Biol. 8:3636-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fikes, J. D., D. M. Becker, F. Winston, and L. Guarente. 1990. Striking conservation of TFIID in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Nature 346:291-294. [DOI] [PubMed] [Google Scholar]
- 13.Fiori, A., T. L. Mason, and T. D. Fox. 2003. Evidence that synthesis of the Saccharomyces cerevisiae mitochondrially-encoded ribosomal protein Var1p may be membrane localized. Eukaryot. Cell. 2:651-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folley, L. S., and T. D. Fox. 1994. Reduced dosage of genes encoding ribosomal protein S18 suppresses a mitochondrial initiation codon mutation in Saccharomyces cerevisiae. Genetics 137:369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folley, L. S., and T. D. Fox. 1991. Site-directed mutagenesis of a Saccharomyces cerevisiae mitochondrial translation initiation codon. Genetics 129:659-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox, T. D. 1996. Genetics of mitochondrial translation, p. 733-758. In J. W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Fox, T. D., L. S. Folley, J. J. Mulero, T. W. McMullin, P. E. Thorsness, L. O. Hedin, and M. C. Costanzo. 1991. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 194:149-165. [DOI] [PubMed] [Google Scholar]
- 18.Fujiki, Y., A. L. Hubbard, S. Fowler, and P. B. Lazarow. 1982. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol. 93:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan, X., M. Kitakawa, K. Yoshino, N. Oshiro, K. Yonezawa, and K. Isono. 2002. Tag-mediated isolation of yeast mitochondrial ribosome and mass spectrometric identification of its new components. Eur. J. Biochem. 269:5203-5214. [DOI] [PubMed] [Google Scholar]
- 20.Glick, B. S. 1995. Pathways and energetics of mitochondrial protein import in Saccharomyces cerevisiae. Methods Enzymol. 260:224-231. [DOI] [PubMed] [Google Scholar]
- 21.Glick, B. S., and L. A. Pon. 1995. Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 260:213-223. [DOI] [PubMed] [Google Scholar]
- 22.Goffeau, A., B. G. Barrell, H. Bussey, R. W. Davis, B. Dujon, H. Feldmann, F. Galibert, J. D. Hoheisel, C. Jacq, M. Johnston, E. J. Louis, H. W. Mewes, Y. Murakami, P. Philippsen, H. Tettelin, and S. G. Oliver. 1996. Life with 6000 genes. Science 274:546, 563-567. [DOI] [PubMed] [Google Scholar]
- 23.Graack, H. R., and B. Wittmann-Liebold. 1998. Mitochondrial ribosomal proteins (MRPs) of yeast. Biochem. J. 329:433-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green-Willms, N. S., C. A. Butler, H. M. Dunstan, and T. D. Fox. 2001. Pet111p, an inner membrane-bound translational activator that limits expression of the Saccharomyces cerevisiae mitochondrial gene COX2. J. Biol. Chem. 276:6392-6397. [DOI] [PubMed] [Google Scholar]
- 25.Green-Willms, N. S., T. D. Fox, and M. C. Costanzo. 1998. Functional interactions between yeast mitochondrial ribosomes and mRNA 5′-untranslated leaders. Mol. Cell. Biol. 18:1826-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthrie, C., and G. R. Fink (ed.). 1991. Guide to yeast genetics and molecular biology. Academic Press, San Diego, Calif.
- 27.Haffter, P., and T. D. Fox. 1992. Suppression of carboxy-terminal truncations of the yeast mitochondrial mRNA-specific translational activator PET122 by mutations in two new genes, MRP17 and PET127. Mol. Gen. Genet. 235:64-73. [DOI] [PubMed] [Google Scholar]
- 28.Haffter, P., T. W. McMullin, and T. D. Fox. 1991. Functional interactions among two yeast mitochondrial ribosomal proteins and an mRNA-specific translational activator. Genetics 127:319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallberg, E. M., Y. Shu, and R. L. Hallberg. 1993. Loss of mitochondrial hsp60 function: nonequivalent effects on matrix-targeted and intermembrane-targeted proteins. Mol. Biol. Cell 13:3050-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamel, P., C. Lemaire, N. Bonnefoy, P. Brivet-Chevillotte, and G. Dujardin. 1998. Mutations in the membrane anchor of yeast cytochrome c1 compensate for the absence of Oxa1p and generate carbonate-extractable forms of cytochrome c1. Genetics 150:601-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He, S., and T. D. Fox. 1997. Membrane translocation of mitochondrially coded Cox2p: distinct requirements for export of amino- and carboxy-termini, and dependence on the conserved protein Oxa1p. Mol. Biol. Cell 8:1449-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill, J. E., A. M. Myers, T. J. Koerner, and A. Tzagoloff. 1986. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2:163-167. [DOI] [PubMed] [Google Scholar]
- 33.Islas-Osuna, M. A., T. P. Ellis, L. L. Marnell, T. M. Mittelmeier, and C. L. Dieckmann. 2002. Cbp1 Is Required for Translation of the Mitochondrial Cytochrome b mRNA of Saccharomyces cerevisiae. J. Biol. Chem. 277:37987-37990. [DOI] [PubMed] [Google Scholar]
- 34.Jia, L., M. Dienhart, M. Schramp, M. McCauley, K. Hell, and R. A. Stuart. 2003. Yeast Oxa1 interacts with mitochondrial ribosomes: the importance of the C-terminal region of Oxa1. EMBO J. 22:6438-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellis, M., N. Patterson, M. Endrizzi, B. Birren, and E. S. Lander. 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423:241-254. [DOI] [PubMed] [Google Scholar]
- 36.Koc, E. C., W. Burkhart, K. Blackburn, A. Moseley, and L. L. Spremulli. 2001. The small subunit of the mammalian mitochondrial ribosome. Identification of the full complement of ribosomal proteins present. J. Biol. Chem. 276:19363-19374. [DOI] [PubMed] [Google Scholar]
- 37.Koc, E. C., W. Burkhart, K. Blackburn, M. B. Moyer, D. M. Schlatzer, A. Moseley, and L. L. Spremulli. 2001. The large subunit of the mammalian mitochondrial ribosome: Analysis of the complement of ribosomal proteins present. J. Biol. Chem. 276:43958-43969. [DOI] [PubMed] [Google Scholar]
- 38.Krause, K., R. Lopes de Souza, D. G. Roberts, and C. L. Dieckmann. 2004. The mitochondrial message-specific mRNA protectors Cbp1 and Pet309 are associated in a high-molecular weight complex. Mol. Biol. Cell 15:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, Y., W. B. Holmes, D. R. Appling, and U. L. RajBhandary. 2000. Initiation of protein synthesis in Saccharomyces cerevisiae mitochondria without formylation of the initiator tRNA. J. Bacteriol. 182:2886-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMullin, T. W., P. Haffter, and T. D. Fox. 1990. A novel small subunit ribosomal protein of yeast mitochondria that interacts functionally with an mRNA-specific translational activator. Mol. Cell. Biol. 10:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulero, J. J., and T. D. Fox. 1993. Alteration of the Saccharomyces cerevisiae COX2 5′-untranslated leader by mitochondrial gene replacement and functional interaction with the translational activator protein PET111. Mol. Biol. Cell 4:1327-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulero, J. J., and T. D. Fox. 1993. PET111 acts in the 5′-leader of the Saccharomyces cerevisiae mitochondrial COX2 mRNA to promote its translation. Genetics 133:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulero, J. J., and T. D. Fox. 1994. Reduced but accurate translation from a mutant AUA initiation codon in the mitochondrial COX2 mRNA of Saccharomyces cerevisiae. Mol. Gen. Genet. 242:383-390. [DOI] [PubMed] [Google Scholar]
- 44.Naithani, S., S. A. Saracco, C. A. Butler, and T. D. Fox. 2003. Interactions among COX1, COX2 and COX3 mRNA-specific translational activator proteins on the inner surface of the mitochondrial inner membrane of Saccharomyces cerevisiae. Mol. Biol. Cell 14:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Brien, T. W., J. Liu, J. E. Sylvester, E. B. Mougey, N. Fischel-Ghodsian, B. Thiede, B. Wittmann-Liebold, and H. R. Graack. 2000. Mammalian mitochondrial ribosomal proteins (4). Amino acid sequencing, characterization, and identification of corresponding gene sequences. J. Biol. Chem. 275:18153-18159. [DOI] [PubMed] [Google Scholar]
- 46.Partaledis, J. A., and T. L. Mason. 1988. Structure and regulation of a nuclear gene in Saccharomyces cerevisiae that specifies MRP13, a protein of the small subunit of the mitochondrial ribosome. Mol. Cell. Biol. 8:3647-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinkham, J. L., A. M. Dudley, and T. L. Mason. 1994. T7 RNA polymerase-dependent expression of COXII in yeast mitochondria. Mol. Cell. Biol. 14:4643-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reading, D. S., R. L. Hallberg, and A. M. Myers. 1989. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature 337:655-659. [DOI] [PubMed] [Google Scholar]
- 49.Rigaut, G., A. Shevchenko, B. Rutz, M. Wilm, M. Mann, and B. Seraphin. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17:1030-1032. [DOI] [PubMed] [Google Scholar]
- 50.Rödel, G. 1997. Translational activator proteins required for cytochrome b synthesis in Saccharomyces cerevisiae. Curr. Genet. 31:375-379. [DOI] [PubMed] [Google Scholar]
- 51.Rose, M. D., and J. R. Broach. 1991. Cloning genes by complementation in yeast. Methods Enzymol. 194:195-230. [DOI] [PubMed] [Google Scholar]
- 52.Sanchirico, M. E., T. D. Fox, and T. L. Mason. 1998. Accumulation of mitochondrially synthesized Saccharomyces cerevisiae Cox2p and Cox3p depends on targeting information in untranslated portions of their mRNAs. EMBO J. 17:5796-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saveanu, C., M. Fromont-Racine, A. Harington, F. Ricard, A. Namane, and A. Jacquier. 2001. Identification of 12 new yeast mitochondrial ribosomal proteins including 6 that have no prokaryotic homologues. J. Biol. Chem. 276:15861-15867. [DOI] [PubMed] [Google Scholar]
- 54.Schneider, B. L., W. Seufert, B. Steiner, Q. H. Yang, and A. B. Futcher. 1995. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11:1265-1274. [DOI] [PubMed] [Google Scholar]
- 55.Shen, Z., and T. D. Fox. 1989. Substitution of an invariant nucleotide at the base of the highly conserved “530-loop” of 15S rRNA causes suppression of mitochondrial ochre mutations. Nucleic Acids Res. 17:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherman, F., G. R. Fink, and C. W. Lawrence. 1974. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steele, D. F., C. A. Butler, and T. D. Fox. 1996. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc. Natl. Acad. Sci. USA 93:5253-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki, T., M. Terasaki, C. Takemoto-Hori, T. Hanada, T. Ueda, A. Wada, and K. Watanabe. 2001. Proteomic analysis of the mammalian mitochondrial ribosome. Identification of protein components in the 28 S small subunit. J. Biol. Chem. 276:33181-33195. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki, T., M. Terasaki, C. Takemoto-Hori, T. Hanada, T. Ueda, A. Wada, and K. Watanabe. 2001. Structural compensation for the deficit of rRNA with proteins in the mammalian mitochondrial ribosome. Systematic analysis of protein components of the large ribosomal subunit from mammalian mitochondria. J. Biol. Chem. 276:21724-21736. [DOI] [PubMed] [Google Scholar]
- 61.Szyrach, G., M. Ott, N. Bonnefoy, W. Neupert, and J. M. Herrmann. 2003. Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 22:6448-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thorsness, P. E., K. H. White, and T. D. Fox. 1993. Inactivation of YME1, a member of the ftsH-SEC18-PAS1-CDC48 family of putative ATPase-encoding genes, causes increased escape of DNA from mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:5418-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tibbetts, A. S., L. Oesterlin, S. Y. Chan, G. Kramer, B. Hardesty, and D. R. Appling. 2003. Mammalian mitochondrial initiation factor 2 supports yeast mitochondrial translation without formylated initiator tRNA. J. Biol. Chem. [DOI] [PubMed]
- 64.Vambutas, A., S. J. Ackerman, and A. Tzagoloff. 1991. Mitochondrial translational-initiation and elongation factors in Saccharomyces cerevisiae. Eur. J. Biochem. 201:643-652. [DOI] [PubMed] [Google Scholar]
- 65.Wiesenberger, G., M. C. Costanzo, and T. D. Fox. 1995. Analysis of the Saccharomyces cerevisiae mitochondrial COX3 mRNA 5′-untranslated leader: translational activation and mRNA processing. Mol. Cell. Biol. 15:3291-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams, E. H., and T. D. Fox. 2003. Antagonistic signals within the COX2 mRNA coding sequence control its translation in Saccharomyces cerevisiae mitochondria. RNA 9:419-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams, E. H., X. Perez-Martinez, and T. D. Fox. 2004. MrpL36p, a highly diverged L31 ribosomal protein homolog with additional functional domains in Saccharomyces cerevisiae mitochondria. Genetics 167:65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yaffe, M. P. 1991. Analysis of mitochondrial function and assembly. Methods Enzymol. 194:627-643. [DOI] [PubMed] [Google Scholar]