Abstract
Histone deacetylase (HDAC) inhibitors are promising antitumor agents, but they have not been extensively explored in B-cell lymphomas. Many of these lymphomas have the t(14;18) translocation, which results in increased bcl-2 expression and resistance to apoptosis. In this study, we examined the effects of two structurally different HDAC inhibitors, trichostatin A (TSA) and sodium butyrate (NaB), on the cell cycle, apoptosis, and bcl-2 expression in t(14;18) lymphoma cells. We found that in addition to potent cell cycle arrest, TSA and NaB also dramatically induced apoptosis and down-regulated bcl-2 expression, and overexpression of bcl-2 inhibited TSA-induced apoptosis. The repression of bcl-2 by TSA occurred at the transcriptional level. Western blot analysis and quantitative chromatin immunoprecipitation (ChIP) assay showed that even though HDAC inhibitors increased overall acetylation of histones, localized histone H3 deacetylation occurred at both bcl-2 promoters. TSA treatment increased the acetylation of the transcription factors Sp1 and C/EBPα and decreased their binding as well as the binding of CBP and HDAC2 to the bcl-2 promoters. Mutation of Sp1 and C/EBPα binding sites reduced the TSA-induced repression of bcl-2 promoter activity. This study provides a mechanistic rationale for the use of HDAC inhibitors in the treatment of human t(14;18) lymphomas.
The cytogenetic hallmark of most follicular B-cell lymphomas is the chromosomal translocation of the antiapoptotic bcl-2 gene from 18q21 to the immunoglobulin heavy chain (IgH) locus at 14q32 (9, 54, 55). This t(14;18)(q32;q21) translocation constitutes the most common chromosomal translocation in human lymphoid malignancies. Approximately 85% of follicular and 20% of diffuse B-cell lymphomas possess this translocation. The t(14;18) translocation places bcl-2 in the same transcriptional orientation as IgH and results in deregulated overexpression of bcl-2 (15). Increased cell survival due to bcl-2 overexpression has been shown to contribute to the development of many B-cell lymphomas and confer resistance to a variety of anticancer therapies (12, 26, 43, 50).
Two promoters mediate transcriptional control of the bcl-2 gene (52). The 5′ promoter (P1) is located 1,386 to 1,423 bp upstream of the bcl-2 translational start site, and it is GC-rich with multiple Sp1 sites. The start sites of the 3′ promoter (P2) are located 1.3 kb downstream of the P1 promoter. P2 has a classic TATA and CAAT box and a simian virus 40 (SV40) decamer/Ig octamer motif. Important cis elements and associated trans-acting factors participating in the deregulation of bcl-2 have been characterized within the promoter regions. A major positive regulator of P1 activity is a cyclic AMP (cAMP) response element (CRE). CREB (CRE-binding protein) binds to this site and is essential for bcl-2 expression during B-cell development and for bcl-2 deregulation in t(14;18) lymphomas (27, 58). In addition, NF-κB activates bcl-2 in t(14;18) lymphoma cells through interactions with the CRE and Sp1 binding sites (21). C/EBPα (CCAAT/enhancer binding protein-alpha) and A-Myb are activators of P2 promoter activity in t(14;18) lymphoma cells and act through the binding site for the homeodomain protein Cdx (22, 23). WT-1 and p53 have been reported to be negative regulators of bcl-2 expression in t(14;18) lymphoma cells through the P1 and P2 promoters, respectively (19, 59).
Four murine B-cell-specific and cell stage-dependent DNase I hypersensitive sites, MHS1 to MHS4, which are located 10 to 35 kb 3′ of the Cα gene, have been shown to function as enhancers for IgH gene expression (31, 36, 40, 47), and they also up-regulate bcl-2 expression (20). Similar enhancers are located downstream of two human Cα genes, and these regions share some homology with the murine enhancers, although they are not as well characterized (7, 37, 41).
It is becoming clear that posttranslational modifications of histones play important roles in the regulation of gene transcription (4). Among the various histone modifications, the acetylation of specific lysine residues in the N-terminal tails of histones has been correlated with transcriptional activity (42). Two enzyme classes, histone acetyltransferase (HAT) and histone deacetylase (HDAC), catalyze the acetylation and deacetylation of histones, respectively (16, 17). Although the mechanisms involved are complex, the presence of an acetyl residue is believed to neutralize the positive charge of histones and decrease their interactions with negatively charged DNA, while the removal of an acetyl group leads to condensation of nucleosome structure (16, 17). Histone acetylation status is assumed to be an important factor that controls the accessibility of transcription factors to DNA and subsequent gene transcription (17). The functional connection between histone acetylation and transcription has been strengthened by the identification of HAT and HDAC activity within transcriptional coactivators and corepressors, respectively (1, 6).
Altered HAT or HDAC activity has been identified in several cancers (32). HDAC inhibitors are being investigated as a new therapeutic approach to many solid and hematological malignancies (34, 46). The antitumor effects of HDAC inhibitors have been correlated with the transcriptional alteration of specific cancer-related genes, including some critical regulators of cell cycle, apoptosis, differentiation, angiogenesis, and invasion (30, 33, 38). However, these effects of HDAC inhibitors in B-cell lymphomas have not been explored. In this study, we report that HDAC inhibitors are potent antitumor agents in t(14;18) B-cell lymphomas due to cell cycle arrest and induction of apoptosis. Moreover, HDAC inhibitors down-regulate both endogenous bcl-2 expression and bcl-2 promoter activity in an episomal bcl-2 promoter-reporter gene system. We also demonstrate that the repression of bcl-2 expression by HDAC inhibitors occurs at the transcriptional level. While HDAC inhibitors increase the overall histone acetylation level in treated cells, localized histone deacetylation of the bcl-2 promoters and decreased binding of the sequence-specific transcription factors Sp1 and C/EBPα, as well as the coactivator CBP (CREB-binding protein) and the class I histone deacetylase HDAC2, are correlated with the transcriptional repression of bcl-2 by HDAC inhibitors.
MATERIALS AND METHODS
Cell lines and drug treatment.
The human t(14;18) lymphoma cell lines DHL-4 and DHL-6 have been described previously (13, 27). They were maintained in RPMI medium supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine. To confirm that the results were not unique to a single cell line, most of the experiments were performed with both DHL-4 and DHL-6 cells with similar results.
Cells were treated with trichostatin A (TSA) (Sigma) or sodium butyrate (NaB) (Sigma) for 18 h or the time indicated in the figure legend. Actinomycin D (Sigma) was used at 10 μg/ml for mRNA stability analysis. Cells were pretreated with actinomycin D for 30 min before the addition of TSA.
Plasmid constructs and transfections.
For the generation of episomal bcl-2 promoter-luciferase reporter gene constructs, the sequence between the two SalI sites of the pREP4 plasmid (Invitrogen) was replaced with a polylinker that possessed sequential SfiI, AflII, SnaBI, NheI, BamHI, HindIII, and KpnI restriction sites. The full-length bcl-2 promoter, bcl-2 P1 promoter, bcl-2 P2 promoter, Sp1-mutant P1 promoter, and Cdx-mutant P2 promoter were PCR amplified from previously described plasmids (21, 22) and cloned into BamHI and HindIII sites. Luciferase cDNA was cloned into HindIII and KpnI sites. The four murine IgH enhancers, MHS1, MHS2, MHS3, and MHS4, were cloned into SfiI/AflII, AflII/SnaBI, and SnaBI/NheI sites, respectively. MHS1 and MHS2 were cloned together (MHS12) due to their close proximity to each other. To make the episomal NF-κB transcription reporter gene construct, five tandem copies of the NF-κB consensus sequence (TGGGGACTTTCCGC) fused to a TATA-like promoter was cloned into the AflII and HindIII sites. The episomal c-myc promoter-luciferase reporter gene construct was described previously (28). The bcl-2 expression plasmid was generated by cloning the full-length bcl-2 cDNA into the NheI and XhoI sites of pREP4. pBJ5-FLAG-HDAC1, pME18S-FLAG-HDAC2, and pREP4-FLAG-HDAC3, encoding C-terminal FLAG epitope-tagged HDACs, have been described previously (63). All constructs were confirmed by sequencing.
For stable transfections of bcl-2 cDNA and reporter gene constructs or transfections of expression plasmids encoding HDACs, 1 to 5 μg of DNA was transfected into 5 × 106 cells using Kit R from Amaxa according to the manufacturer's protocol and program O-17 on Amaxa's Nucleofector device. Hygromycin B (400 μg/ml) was added 24 h after transfection, and selection for stably transfected cells was performed for 1 week. Stable transfectants were maintained in 100 μg of hygromycin B/ml. The copy numbers of transgenes were determined by Southern blot analysis. An enhanced green fluorescent protein expression vector was cotransfected with the HDAC plasmids, and transfected cells were selected based on GFP signal.
For short-term stable transfections, 2 × 107 cells were transfected with 10 μg of plasmid DNA by electroporation as previously described (20). The transfected cells were placed in 25 ml of supplemented RPMI medium and allowed to recover for 24 h before selection with 400 μg of hygromycin B/ml. Reporter gene activity was determined at day 5 after transfection.
Reporter gene activity was assessed using the Luciferase Assay System (Promega). Results were normalized to the amount of protein in each sample. All transfection results are presented as the average from at least six independent transfection experiments.
Flow cytometric analysis.
Apoptosis was determined with the APO-DIRECT kit according to the manufacturer's protocol (BD Pharmingen). Briefly, 2 × 106 cells were sequentially fixed in 1 ml of 1% paraformaldehyde for 15 min and then in 5 ml of 70% cold ethanol overnight. The 3′-hydroxyl ends of double- and single-stranded DNA breaks were labeled with fluorescein isothiocyanate (FITC)-dUTP staining solution for 4 h, followed by phosphatidylinositol (PI) staining of total DNA for 30 min. Flow cytometric data acquisition and analysis were performed with a FACScan equipped with a FACStation running CellQuest software (Becton Dickinson). Cell cycle data was acquired after PI staining and was further analyzed by ModFit LT software (Verity Software House). Debris was eliminated from analysis by using a forward angle light scatter threshold trigger. Cell doublets and other clumps were removed using doublet discrimination gating.
Real-time reverse transcriptase PCR (qRT-PCR) analysis.
A cell-to-cDNA II kit (Ambion) was used for the generation of cDNA from 10,000 cells according to the manufacturer's protocol. Real-time PCR of cDNA was performed on the ABI Prism 7900-HT Sequence Detection System using the Universal PCR Master Mix (Applied Biosystems) and Assay-on-Demand primer/probe sets for bcl-2 and GAPDH (Applied Biosystems). All quantitative real-time PCR results are presented as the averages from at least three independent experiments with duplicate PCR analysis.
Immunoprecipitation and Western blot analysis.
To monitor the interaction of Sp1 or C/EBPα with HDACs, lysates from DHL-4 cells were precleared by incubation with protein G-Sepharose (Zymed) for 1 h at 4°C. The precleared lysates were then incubated with 2 μg of Sp1 or C/EBPα antibodies overnight. Twenty-five microliters of protein G-Sepharose was added to the lysates, and after incubation for 1 h at 4°C the protein G-Sepharose was centrifuged and washed twice prior to preparation for electrophoresis. This was followed by Western blotting with antibodies against HDACs. To monitor the acetylation of Sp1, the Sp-antibody-immunoprecipitated lysate was analyzed by Western blotting using an antibody against acetyl-lysine. To monitor the acetylation of C/EBPα, nuclear extract from DHL-4 cells was first immunoprecipitated with an antibody against acetyl-lysine followed by Western blotting with an antibody against C/EBPα.
For Western blot analysis, whole-cell lysates or nuclear extracts were boiled with sample loading buffer and electrophoresed in a sodium dodecyl sulfate-10% polyacrylamide gel. Antibodies for bcl-2 (sc-492), Sp1 (sc-59x), C/EBPα (sc-9315), HDAC1 (sc-7872), and HDAC2 (sc-7899) were from Santa Cruz Biotechnology. Antibodies for acetyl-lysine (05-515), acetyl-histone H3 (06-599), and acetyl-histone H4 (06-866) were from Upstate Biotechnologies. The anti-FLAG antibody was from Sigma (F3165). Detection of β-actin expression was performed to ensure equivalent protein loading. When stripping was needed, membranes were incubated in buffer (62.5 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate, 0.1 M 2-mercaptoethanol) at 60°C for 30 min and washed extensively with 20 mM Tris-HCl (pH 7.6)-0.8% sodium chloride-0.1% Tween 20 before reblocking and probing.
Quantative ChIP assay.
The chromatin immunoprecipitation (ChIP) assay was performed as outlined previously with primers that are specific for the mutated translocated allele (20, 23). The antibody for CBP (sc-583) was from Santa Cruz Biotechnology. Real-time PCR was performed to quantitate the amount of immunoprecipitated DNA. The TaqMan primers and probe for bcl-2 P1 promoter detection were the following: forward primer, 5′-GGCTCAGAGGAGGGCTCTTT-3′; reverse primer, 5′-GTGCCTGTCCTCTTACTTCATTCTC-3′; probe,5′-[6-FAM]TT-GAATGAACCGTGTGACGTTACGCAC[TAMRA-Q]-3′.Primers and probe for the detection of the bcl-2 P2 promoter (TATA site) were the following: forward primer, 5′-GTGTTCCGCGTGATTGAAGAC-3′; reverse primer, 5′-CAGAGAAAGAAGAGGAGTTATAA-3′; probe, 5′-[6-FAM]-CCCTCGTCCAAGAATGCAAAGCACAT[TAMRA-Q]-3′. The primers and probe for the detection of the bcl-2 P2 promoter (Cdx site) were the following: forward primer, 5′-CCAGGCAGCTTAATACATTCTTTTTAG-3′; reverse primer, 5′-TGATGCTGAAAGGTTAAAGAAAAAAC-3′; probe, 5′-[6-FAM]CGTGTTACTTGTAGTGTGTATGCCCTGCTTTCAC[TAMRA-Q]-3′.
All quantitative ChIP assay results are presented as the averages from at least three independent immunoprecipitations followed by duplicate real-time PCR analysis.
RESULTS
TSA and NaB induce apoptosis in t(14;18) lymphoma cells.
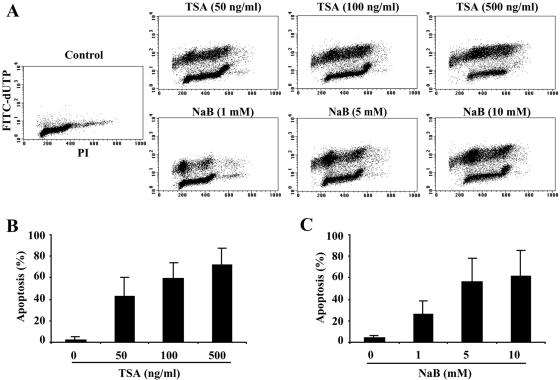
HDAC inhibitors induce apoptotic cell death in a wide variety of transformed cells, including cells from breast and prostate cancers, as well as neuroblastoma, hepatoma, and some types of hematologic malignancies (24, 25, 34). However, this effect of HDAC inhibitors has not been explored in B-cell lymphomas. Increased resistance to apoptosis is observed in many B-cell lymphomas, so we examined the effect of HDAC inhibitors on this type of malignancy. DHL-4 cells, a t(14;18) lymphoma line, were treated with two structurally different HDAC inhibitors: trichostatin A, a hydroxamic acid, and sodium butyrate, a short-chain fatty acid. After treatment for 18 h, apoptotic cell death was determined by measuring DNA strand breaks by flow cytometric analysis. As shown in Fig. 1A, a dose-dependent induction of apoptosis (40 to 75%) was observed in the TSA (50 to 500 ng/ml)-treated cells, compared to a low level of apoptosis (4%) in untreated DHL-4 cells. A similar induction of apoptosis (25 to 60%) was also observed in cells treated with NaB (Fig. 1A) but with higher doses at the millimolar level (1 to 10 mM). It appeared that apoptotic cell death occurred across all phases of the cell cycle, but relatively more was observed at the G0/G1 phase. This was particularly obvious in NaB-treated cells, where about 55 to 65% of the apoptotic cells were in G0/G1. Quantification of the amount of apoptosis induced by different concentrations of TSA and NaB is shown in Fig. 1B and C, respectively. The induction of apoptosis by TSA and NaB was also observed in another t(14;18) lymphoma cell line, DHL-6 (data not shown). These results suggest that HDAC inhibitors are potent inducers of apoptosis in t(14;18) B-cell lymphomas.
FIG. 1.
TSA and NaB induce apoptosis in DHL-4 cells. (A) Mid-log-phase cells were treated with TSA or NaB at the indicated concentrations for 18 h. Apoptotic DNA breaks were labeled with FITC-dUTP in the presence of TdT enzyme. Cells were then stained with propidium iodide (PI) and analyzed on a Becton Dickinson FACScan. (B) Quantitative analysis of TSA-induced apoptosis in DHL-4 cells from three independent experiments. (C) Quantitative analysis of NaB-induced apoptosis in DHL-4 cells from three independent experiments.
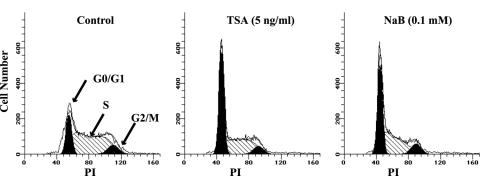
TSA and NaB cause cell cycle arrest at G0/G1 in t(14;18) lymphoma cells.
HDAC inhibitors have been shown to inhibit the cell cycle in various cell types through arrest in G0/G1 or G2/M (48). The effect of TSA or NaB on the cell cycle of DHL-4 cells was assessed. Due to the extensive apoptosis of cells across the cell cycle as shown in Fig. 1, we used smaller doses of TSA and NaB for this study. As shown in Fig. 2, significant G0/G1 arrest was induced after treatment for 24 h. Approximately 21% of the untreated cells were in G0/G1, and this increased to 55 to 57% of the cells after treatment with 5 ng of TSA/ml or 0.1 mM NaB. Correspondingly decreased cell numbers in the S and G2/M phases were observed after treatment with both drugs. A consistent induction of G0/G1 arrest was also observed in cells treated with even smaller doses of TSA (0.05 to 0.5 ng/ml) and NaB (0.001 to 0.01 mM). The quantitative analyses of cell cycle distribution in cells treated with different doses of TSA and NaB are shown in Table 1 and Table 2. We speculate that cell cycle arrest at G0/G1 phase by HDAC inhibitors may contribute to the preferential apoptotic cell death at this phase that is depicted in Fig. 1.
FIG. 2.
TSA and NaB induce cell cycle arrest at G0/G1 phase in DHL-4 cells. Cells were left untreated or were treated with 5 ng of TSA/ml or 0.1 mM NaB for 24 h. Cell cycle data were acquired following PI staining and further modulated by ModFit software. The cells in G0/G1, S, and G2/M phases are labeled.
TABLE 1.
TSA effect on cell cycle in DHL-4 cellsa
| TSA (ng/ml) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| 0 | 21.8 ± 1.6 | 65.1 ± 2.2 | 13.2 ± 2.7 |
| 0.05 | 45.0 ± 0.5 | 43.2 ± 1.9 | 11.9 ± 1.4 |
| 0.5 | 47.7 ± 1.2 | 41.3 ± 1.3 | 11.3 ± 0.8 |
| 5 | 56.8 ± 2.8 | 38.0 ± 1.5 | 5.2 ± 0.6 |
Cells were treated with or without TSA for 24 h. DNA content at different phases of the cell cycle was determined following PI staining. Results are means ± standard deviations of the percentage of cells in each phase.
TABLE 2.
NaB effect on cell cycle in DHL-4 cellsa
| NaB (mM) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| 0 | 21.8 ± 1.6 | 65.1 ± 2.2 | 13.2 ± 2.7 |
| 0.001 | 48.6 ± 2.0 | 37.8 ± 2.9 | 13.7 ± 1.7 |
| 0.01 | 52.6 ± 2.3 | 36.9 ± 1.7 | 10.6 ± 1.1 |
| 0.1 | 54.8 ± 1.6 | 38.6 ± 1.9 | 8.3 ± 0.9 |
Cells were treated with or without NaB for 24 h. DNA content at different phases of the cell cycle was determined following PI staining. Results are means ± standard deviations of the percentage of cells in each phase.
TSA and NaB repress endogenous bcl-2 expression at the mRNA and protein levels, and overexpression of Bcl-2 decreases TSA-induced apoptosis.
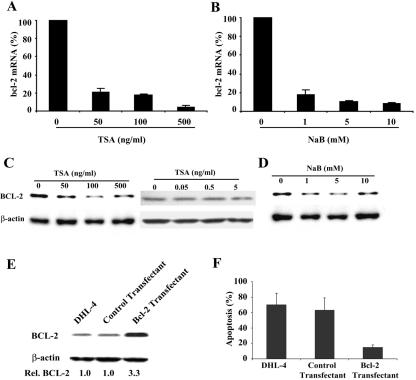
Overexpression of bcl-2 contributes to the resistance to apoptosis of t(14;18) lymphoma cells. Because of the profound apoptosis induced by TSA and NaB, we hypothesized that these drugs might influence bcl-2 expression as well. To determine the effect of TSA and NaB on bcl-2 mRNA expression, DHL-4 cells were treated with TSA or NaB for 18 h at the same doses that induced apoptosis. The amount of bcl-2 mRNA expression in each sample was determined by qRT-PCR. As shown in Fig. 3A and B, TSA decreased bcl-2 mRNA expression to 5% of the level of untreated cells, and NaB decreased bcl-2 mRNA expression to 10% of the level of untreated cells.
FIG. 3.
TSA and NaB decrease bcl-2 expression in DHL-4 cells, and overexpression of bcl-2 blocks TSA-induced apoptosis. (A) Cells were left untreated or were treated with different doses of TSA for 18 h. Bcl-2 mRNA expression was determined by real-time RT-PCR and normalized with GAPDH expression in each sample. (B) Bcl-2 mRNA determined by real-time RT-PCR after treatment with NaB for 18 h. (C) Bcl-2 protein expression was determined by Western blot analysis after treatment of DHL-4 cells with TSA for 18 h. The same blot was stripped and reprobed with a β-actin antibody, which was used as loading control. (D) Western analysis of Bcl-2 protein after treatment with NaB for 18 h. (E) Western analysis of Bcl-2 protein in cells transfected with an empty expression vector (control) or a bcl-2 expression vector (Bcl-2). (F) Untransfected DHL-4 cells and cells stably transfected with either an empty vector or bcl-2 expression plasmid were treated with 500 ng of TSA/ml for 18 h. Apoptotic DNA breaks were determined by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay.
To determine if down-regulation of bcl-2 mRNA expression resulted in decreased protein levels, Western blot analysis was performed. A 70% decrease in Bcl-2 protein was observed in TSA (50 to 500 ng/ml)- and NaB (1 to 10 mM)-treated cells (Fig. 3C and D). As shown in the right panel of Fig. 3C, smaller doses of TSA (0.05 to 5 ng/ml) had much less of an effect on Bcl-2 protein expression, with an 11% reduction in cells treated with 5 ng of TSA/ml. Neither drug had an effect on β-actin expression. These results suggest that the effect of HDAC inhibitors on the expression of bcl-2 was gene specific and not due to the cell death that is depicted in Fig. 1.
To further address the role of Bcl-2 down-regulation in HDAC inhibitor-induced apoptosis, we examined whether Bcl-2 overexpression could block apoptosis induced by HDAC inhibitors. A bcl-2 expression vector was generated and stably expressed in DHL-4 cells. As shown in Fig. 3E, a 3.3-fold increase of Bcl-2 protein expression was observed in this stable cell line. Expression of an empty pREP4 vector did not change Bcl-2 expression. While TSA treatment induced apoptosis as expected in the control stable cells, overexpression of Bcl-2 eliminated approximately 75% of the apoptosis induced by TSA (Fig. 3F). In contrast to the down-regulation of endogenous Bcl-2 protein expression in DHL-4 cells with TSA, a persistent high level of Bcl-2 protein was observed in the TSA-treated cells that overexpress Bcl-2 (data not shown). These results suggest that Bcl-2 efficiently blocks TSA-induced apoptosis and that it plays a pivotal role in the apoptotic signaling induced by HDAC inhibitors.
Down-regulation of bcl-2 expression by HDAC inhibitors occurs at the transcriptional level.
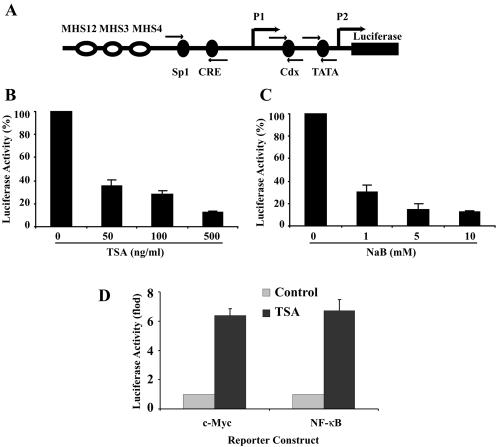
To investigate whether the repression of bcl-2 expression by HDAC inhibitors was through direct down-regulation of bcl-2 promoter transcriptional activity, an episomal bcl-2 promoter-reporter gene construct was generated. To mimic the endogenous bcl-2 promoter activity in t(14;18) cells, the four IgH enhancers (MHS1234) were cloned with the bcl-2 promoter, which was linked to a luciferase reporter gene (Fig. 4A). This reporter gene construct was transfected into DHL-4 cells, and pooled stable transfectants were subjected to reporter gene activity studies. The cells were treated with TSA or NaB for 18 h, and the luciferase activity was determined and normalized to the amount of protein in each sample. As shown in Fig. 4B and C, both TSA and NaB induced dose-dependent down-regulation of bcl-2 promoter activity in the stable cell lines. The repression of the bcl-2 promoter by TSA was specific and not an artifact of the episomal construct. TSA up-regulated the c-myc promoter and a promoter consisting of NF-κB consensus sequences linked to a TATA site in episomal constructs (Fig. 4D). Based on these results, it is likely that transcriptional down-regulation of bcl-2 promoter activity is involved in the repression of bcl-2 expression by HDAC inhibitors.
FIG. 4.
TSA and NaB down-regulate bcl-2 promoter activity in DHL-4 cells. (A) Schematic representation of the bcl-2 promoters and the IgH enhancers cloned into the pREP4 episomal vector. The arrows indicate the location of the primers for ChIP analysis. (B) DHL-4 cells stably expressing the episomal bcl-2 promoter-IgH enhancer luciferase reporter gene construct were treated with TSA for 18 h. The luciferase activity was determined and normalized to protein content in each sample. The relative bcl-2 promoter activity is represented as the percentage of the normalized luciferase activity in the untreated cells. (C) Relative bcl-2 promoter activity after treatment with NaB for 18 h. (D) As a control for the effect of TSA on promoter activity in the episomal vector, DHL-4 cells were transfected with episomal luciferase reporter gene constructs driven by the c-myc promoter or NF-κB-responsive sequences fused to a TATA-like promoter and selected with hygromycin. The cells were left untreated or were treated with 500 ng of TSA/ml for 18 h, and the luciferase activity was determined and normalized to protein content in each sample. The reporter gene activity is plotted relative to the activity of the reporter gene construct without TSA treatment.
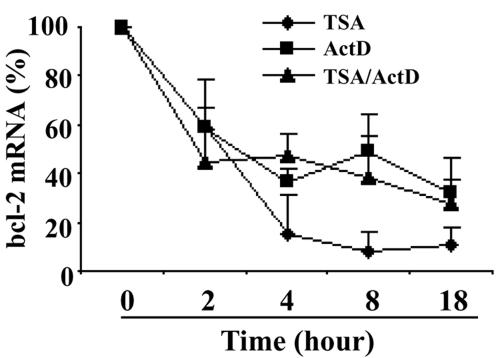
To further determine if HDAC inhibitors also influence bcl-2 mRNA stability in DHL-4 cells, actinomycin D was used to block de novo mRNA transcription. The level of mRNA at different time points was determined by qRT-PCR. As shown in Fig. 5, when cells were treated with actinomycin D, the half-life of bcl-2 mRNA was between 2 to 4 h, which was consistent with a previous report of bcl-2 mRNA half-life in t(14;18) lymphoma cells (52). When TSA was added 30 min after actinomycin D, the half-life of bcl-2 mRNA did not change significantly and was similar to that in the cells without TSA treatment. These results indicate that TSA did not influence bcl-2 mRNA stability. We also found that the repression of bcl-2 mRNA expression by TSA was a very early event, with 40% repression at 2 h of treatment, 85% at 4 h, and the maximum repression of 90 to 95% observed at 8 to 18 h (Fig. 5). Thus, the regulatory effect of TSA on bcl-2 expression was most likely direct rather than a secondary effect.
FIG. 5.
Repression of bcl-2 by TSA is an early event and occurs at the transcriptional level. DHL-4 cells were treated with TSA (500 ng/ml), actinomycin D (ActD; 10 μg/ml), or TSA plus ActD for different time periods. Bcl-2 mRNA expression was determined by real-time RT-PCR and normalized to GAPDH expression.
TSA and NaB increase the overall acetylation of histones but decrease the binding of acetyl-histone H3 to the bcl-2 promoters.
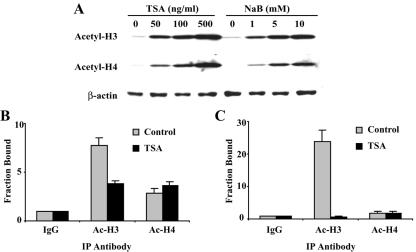
The biological consequence of HDAC inhibition is the accumulation of acetylated histones. To verify the activity of the HDAC inhibitors in our studies, DHL-4 cells were treated with TSA or NaB for 18 h, and the acetyl-histone H3 and acetyl-histone H4 protein levels were determined by Western blot analysis using antibodies specific to acetyl-histone H3 (lys 9, 14) and acetyl-histone H4 (lys 5, 8, 12, 16). Consistent with previous observations in other cell lines, we found that TSA and NaB increased the acetyl-histone H3 and H4 levels in DHL-4 cells (Fig. 6A).
FIG. 6.
TSA decreases histone acetylation of the bcl-2 promoters. (A) Western analysis of the expression of acetyl-histone H3 (Ac-H3) and acetyl-histone H4 (Ac-H4) in DHL-4 cells after treatment with different doses of TSA or NaB for 18 h. β-Actin expression was used as the loading control. (B) Quantitative ChIP assay of acetyl-histone H3 and acetyl-histone H4 binding to the bcl-2 P1 promoter. DHL-4 cells were left untreated or were treated with 500 ng of TSA/ml for 18 h. Chromatin proteins and DNA were cross-linked by formaldehyde. The cross-linked chromatin was sheared and then fractionated using specific antibodies as indicated. Purified immunoprecipitated DNA was quantified using primer/probe sets corresponding to the bcl-2 P1 promoter. The fraction bound represents the fold increase of the amount of the bcl-2 P1 promoter in the acetyl-histone antibody immunoprecipitated (IP) DNA compared to the level of nonspecific IgG antibody immunoprecipitated DNA. (C) Quantitative ChIP assay of acetyl-histone H3 and acetyl-histone H4 binding to the bcl-2 P2 promoter.
To better understand the mechanisms underlying the generalized increased cellular histone acetylation and the repression of bcl-2 transcription by HDAC inhibitors in DHL-4 cells, we performed quantitative ChIP assays to examine the binding of acetylated histones to the bcl-2 promoter regions. In this assay, chromatin isolated from formaldehyde-fixed DHL-4 cells was subjected to immunoprecipitation reactions using antibodies specific for acetyl-histone H3 and H4. An anti-IgG antibody was used as a nonspecific immunoprecipitation control. The immunoprecipitated DNA was phenol-chloroform purified and quantitated by real-time PCR using TaqMan primers and probes corresponding to the bcl-2 P1 (Sp1/CRE sites) and P2 core (TATA) promoter regions (Fig. 4A).
Consistent with the TSA-induced bcl-2 transcriptional repression, ChIP analysis showed a significant reduction of acetyl-histone H3 binding to both bcl-2 promoters. There was a 50% reduction at the P1 promoter (Fig. 6B) and a greater than 90% reduction at the P2 promoter (Fig. 6C). TSA treatment did not change the binding of acetyl-histone H4 to either promoter. This may be due to the lower basal level of acetyl-histone H4 bound to the bcl-2 promoters. Primer/probe sets corresponding to bcl-2 coding sequences that are 500 bp and 8 kb downstream of the bcl-2 promoter regions, as well as a primer/probe set corresponding to the GAPDH coding region, were also used to quantitate the DNA immunoprecipitated by acetyl-histone H3 and acetyl-histone H4 antibodies. No significant change was observed in the untreated and TSA-treated samples (data not shown). These results suggest that the transcriptional repression of bcl-2 by TSA was associated with deacetylation of histone H3 localized at both bcl-2 promoters.
The Sp1 site within the bcl-2 P1 promoter and the Cdx site within the bcl-2 P2 promoter are involved in TSA-induced bcl-2 transcriptional repression.
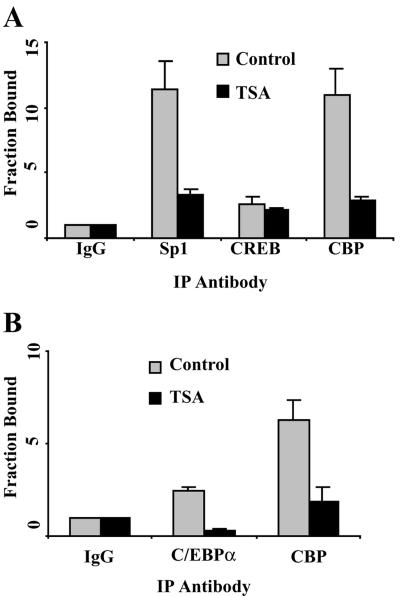
To further characterize the involvement of particular cis elements within the bcl-2 promoter regions in TSA-mediated repression, quantitative ChIP assays were performed to determine the binding of several transcription factors and cofactors that are essential for bcl-2 expression. As we showed previously, Sp1 and CRE sites are major positive regulatory elements for bcl-2 P1 promoter activity (21, 58). A schematic representation of the P1 promoter and the location of the primers used for real-time PCR are shown in Fig. 4A. ChIP assay revealed that Sp1 and CREB as well as the coactivator CBP bound to the P1 promoter in vivo (Fig. 7A). TSA (500 ng/ml) treatment significantly down-regulated Sp1 and CBP binding to this region (Fig. 7A). No significant change in the binding of CREB to this region was observed. We found previously that CBP physically associated with both the CRE and Sp1 binding sites, most likely by direct interaction with the CREB and Sp1 transcription factors (21, 58). Our previous results, together with those presented here, suggest that the HDAC inhibitor-induced decrease in CBP binding to the bcl-2 promoter may be secondary to decreased Sp1 binding.
FIG. 7.
The Sp1 site within the bcl-2 P1 promoter and the Cdx site within the bcl-2 P2 promoter are involved in TSA-induced bcl-2 transcriptional repression. (A) Quantitative ChIP assays of the binding of Sp1, CREB, and CBP to the bcl-2 P1 promoter. DHL-4 cells were treated with 500 ng of TSA/ml for 18 h. ChIP assays were performed as described in the legend to Fig. 6B, except that antibodies specific to Sp1, CREB, and CBP were used. The primers for the bcl-2 P1 promoter are shown in Fig. 4A. The fraction bound represents the fold increase of the amount of the bcl-2 P1 promoter in the specific antibody immunoprecipitated (IP) DNA compared to the level of nonspecific IgG antibody-immunoprecipitated DNA. (B) Quantitative ChIP assays of the binding of C/EBPα and CBP to the bcl-2 P2 promoter. The assays were performed as described for panel A with antibodies specific to C/EBPα and CBP. The primers for the bcl-2 P2 promoter are shown in Fig. 4A.
Although the P1 promoter is the most active bcl-2 promoter in normal B cells, we have found that the P2 promoter is also active in t(14;18) lymphoma cells (59). The bcl-2 P2 promoter is a classic TATA- and CAAT-containing promoter, with a major positive regulatory Cdx site that is −307 to −312 bp upstream of the translation start site (Fig. 4A). Two primer/probe sets specific to the TATA element and the Cdx site were used to study the binding of transcription factors and cofactors to these regions (Fig. 4A). We have shown that p53 binds to the TATA region and mediates transcriptional repression in t(14;18) lymphoma cells (59). However, we did not observe significant changes in the binding of p53, acetyl-p53, HDAC1, mSin3A, or TBP to the bcl-2 TATA region with TSA treatment (data not shown). Our previous studies have shown that C/EBPα, which is not expressed in normal B cells, binds to the Cdx site in t(14;18) lymphoma cells and plays an important role in bcl-2 transactivation in these cells (23). Thus, we further characterized the binding of the transcription factor C/EBPα and its cofactor CBP to the Cdx site. Decreased binding of C/EBPα and CBP was observed in the TSA-treated cells compared to binding in untreated cells (Fig. 7B). Therefore, bcl-2 repression by TSA correlated with decreased binding of specific transcription factors and the cofactor CBP to both promoter regions without any significant change in the level of expression of these factors (data not shown).
Mutation of the Sp1 site in the P1 promoter and the Cdx site in the P2 promoter reduces the repression of bcl-2 by TSA.
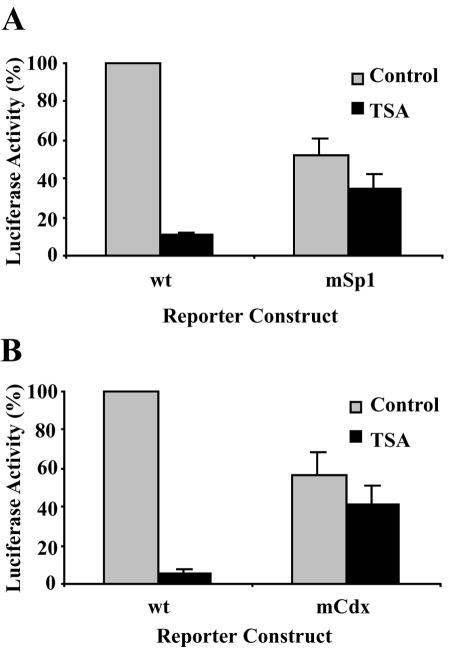
To further verify the involvement of the Sp1 and Cdx sites in TSA-mediated bcl-2 transcriptional repression, wild-type and mutant reporter gene constructs of the bcl-2 P1 and P2 promoters were generated. The wild-type or Sp1 and Cdx mutant bcl-2 P1 and P2 promoters replaced the full-length bcl-2 promoter as shown in Fig. 4A. As shown in Fig. 8A, mutation of the Sp1 site resulted in decreased basal bcl-2 promoter activity. While TSA significantly down-regulated wild-type bcl-2 P1 promoter activity, mutation of the Sp1 site significantly attenuated TSA-mediated repression (Fig. 8A). Similarly, TSA repressed wild-type bcl-2 P2 promoter activity. Mutation of the Cdx site significantly relieved repression of the P2 promoter by TSA (Fig. 8B). These results suggest that the Sp1 and Cdx sites are important elements that are involved in TSA-mediated repression of bcl-2 transcription.
FIG. 8.
Mutation of the Sp1 site in the P1 promoter and the Cdx site in the P2 promoter reduces the repression of bcl-2 by TSA. (A) Effect of Sp1 mutation on the TSA-induced bcl-2 P1 promoter repression. Wild-type (wt) or Sp1 mutant bcl-2 P1 promoter-reporter gene constructs were transfected into DHL-4 cells. Hygromycin B (400 μg/ml) was added 24 h after transfection to select for transfected cells. Four days after transfection, cells were left untreated or were treated with 500 ng of TSA/ml, and 18 h later luciferase activity was determined and normalized to protein content in each sample. The relative bcl-2 promoter activity is represented as the percentage of normalized luciferase activity in cells transfected with the wild-type P1 promoter-reporter gene construct. (B) Effect of Cdx mutation on TSA-induced bcl-2 P2 promoter repression. Wild-type or Cdx mutant bcl-2 P2 promoter-reporter gene constructs were transfected into DHL-4 cells as described for panel A. The relative bcl-2 promoter activity is represented as the percentage of normalized luciferase activity in cells transfected with the wild-type P2 promoter-reporter gene construct.
TSA treatment increases the acetylation of Sp1 and C/EBPα and decreases the binding of HDAC2 to Sp1 and C/EBPα and to the bcl-2 promoters.
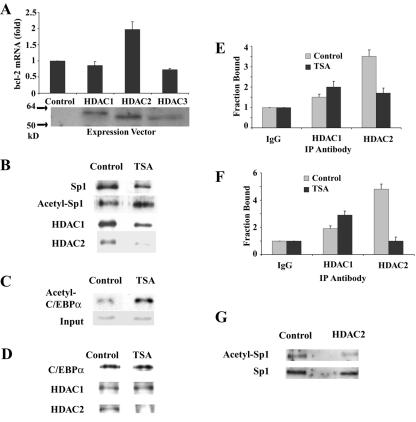
To examine the role of HDACs in bcl-2 transcription, expression vectors for FLAG epitope-tagged HDAC1 to -3 were transiently transfected into DHL-4 cells. Expression of HDAC1, HDAC2, and HDAC3 was verified by Western blot analysis with a FLAG antibody (Fig. 9A). HDAC1 and HDAC3 had very little effect on endogenous bcl-2 mRNA levels as determined by real-time RT-PCR, but HDAC2 resulted in a reproducible twofold increase in bcl-2 mRNA expression (Fig. 9A). Although FLAG-tagged HDAC2 was clearly visible in the transfected cells, there was only a 1.5- to 2-fold increase in total HDAC2 protein (data not shown). The reason for this is not clear, although there may be autoregulation of HDAC2 expression, as has been reported for HDAC1 (51). A similar stimulatory effect on bcl-2 promoter activity was observed with the bcl-2 promoter-IgH enhancer stable cell lines after transfection of HDAC2 (data not shown). These studies suggest that HDAC2 may be involved in the transcriptional regulation of bcl-2 expression.
FIG. 9.
HDAC2 is involved in bcl-2 transcription, less HDAC2 is bound to acetylated Sp1 and C/EBPα, and TSA treatment decreases HDAC2 binding to the bcl-2 promoter regions. (A) DHL-4 cells were cotransfected with 1 μg of a green fluorescent protein (GFP) expression plasmid and 2.5 μg of plasmids encoding different FLAG epitope-tagged HDACs or an empty expression plasmid. The cells were sorted based on GFP signal 48 h after transfection. Bcl-2 mRNA expression in the sorted cells was determined by qRT-PCR. The results shown are the mean and standard deviation from two independent experiments. The expression of the HDACs in the sorted samples was determined by Western blot analysis using an anti-FLAG antibody. (B) DHL-4 cells were left untreated or were treated with 500 ng of TSA/ml for 18 h, and lysates from the treated and untreated cells were analyzed after immunoprecipitation (IP) with an antibody against Sp1. Western blotting was performed with antibodies against Sp1, acetyl-lysine, HDAC1, and HDAC2. (C) DHL-4 cells were left untreated or were treated with 500 ng of TSA/ml for 18 h. Input nuclear extracts and immunoprecipitates of an acetyl-lysine antibody from nuclear extracts of each sample were analyzed by Western blotting with antibody against C/EBPα. (D) DHL-4 cells left untreated or were treated with 500 ng of TSA/ml for 18 h, and lysates from the treated and untreated cells were analyzed after immunoprecipitation with an antibody against C/EBPα. Western blotting was performed with antibodies against C/EBPα, HDAC1, and HDAC2. (E) Quantitative ChIP assays of the binding of HDAC1 and HDAC2 to the bcl-2 P1 promoter. DHL-4 cells were treated with 500 ng of TSA/ml for 18 h. ChIP assays were performed as described in the legend to Fig. 6B, except that antibodies specific to HDAC1 and HDAC2 were used. The primers for the bcl-2 P1 promoter are shown in Fig. 4A. (F) Quantitative ChIP assays of the binding of HDAC1 and HDAC2 to the bcl-2 P2 promoter. (G) DHL-4 cells were transfected with an empty vector or an HDAC2 expression vector and with the GFP expression vector as described for panel A. Immunoprecipitation was performed with an Sp1 antibody followed by Western blotting with an antibody against acetyl-lysine.
It is possible that HDAC2 exerts its effect on bcl-2 expression through modifications of Sp1 or C/EBPα. To examine whether Sp1 was acetylated or bound to HDAC1 or HDAC2 and what effect TSA had, immunoprecipitation of cell lysates with an Sp1 antibody followed by Western blot analysis with antibodies for Sp1, acetyl-lysine, HDAC1, and HDAC2 were performed. As shown in Fig. 9B, Sp1 acetylation was observed in untreated cells, and TSA treatment resulted in a 2.0- to 2.5-fold increase of acetylated Sp1. After normalization for the amount of protein present, there was very little effect of TSA on the binding of HDAC1 to Sp1, but it decreased the binding of HDAC2 to Sp1 by 50%. Increased acetylation (threefold) of C/EBPα with TSA treatment was also observed by immunoprecipitation of nuclear extract with an antibody against acetyl-lysine followed by Western blot analysis using an antibody against C/EBPα (Fig. 9C). Furthermore, TSA treatment did not change the binding of HDAC1 to C/EBPα, but it nearly eliminated the binding of HDAC2 to C/EBPα (Fig. 9D).
To determine if the effect of TSA on the interaction of HDAC2 with Sp1 and C/EBPα influenced their binding to the bcl-2 promoters, quantitative ChIP assays were performed. As shown in Fig. 9E and F, the binding of HDAC1 to both bcl-2 promoters was only slightly higher than the level of nonspecific IgG, and TSA treatment slightly increased the binding of HDAC1. A higher level of HDAC2 was observed at both bcl-2 promoters in the untreated cells. TSA decreased the binding of HDAC2 to the P1 promoter by 50% and decreased HDAC2 binding to the P2 promoter by 80%. These results suggest that inhibition of HDAC2 activity by TSA plays a role in TSA-induced bcl-2 repression.
To further investigate the role of HDAC2 in Sp1 deacetylation, the acetylation status of Sp1 was examined in cells transfected with an HDAC2 expression vector or a control vector. Immunoprecipitation with an Sp1 antibody was performed, followed by Western blot analysis with an antibody to acetyl-lysine. After normalization for the amount of Sp1 present, there was a 35% decrease in acetylated Sp1 in the HDAC2-transfected cells (Fig. 9G). These results demonstrate that HDAC2 overexpression results in deacetylation of Sp1 (Fig. 9G) and an increase in the expression of bcl-2 (Fig. 9A).
DISCUSSION
In this study, we showed that the HDAC inhibitors TSA and NaB caused cell cycle arrest and induced apoptosis in t(14;18) lymphoma cells. At low concentrations of TSA, cell cycle arrest was prominent, and there was only a slight decrease in bcl-2 levels. This finding suggests that different HDACs may be involved in the mediation of cell cycle arrest and apoptosis induction. HDAC inhibitor-induced cell cycle arrest has been correlated with the up-regulation of the cyclin-dependent kinase inhibitor p21waf1/cip1 in various transformed cells through effects on HDAC1 (18, 33). We showed that Bcl-2 levels are important in the response of DHL-4 cells to apoptosis induction by HDAC inhibitors and that HDAC2 played a role in the regulation of Bcl-2 expression in DHL-4 cells.
The mechanisms involved in HDAC inhibitor-induced apoptosis are complex and differ among cell types. In some cells, activation of caspase 2, caspase 3, caspase 7, and caspase 8 has been observed with treatment with TSA and suberoylanilide hydroxamic acid (SAHA), another member of the hydroxamic acid family of HDAC inhibitors (24, 25). The altered expression of several pro- and antiapoptotic genes has also been reported. While proapoptotic Bax and Bad were shown to be up-regulated (25, 49), antiapoptotic Mcl-1 and XIAP were down-regulated by HDAC inhibitors (44). Cell-type-specific regulation of Bcl-2 by HDAC inhibitors was reported in two studies: TSA decreased Bcl-2 protein expression in hepatoma cells (25), but no change in Bcl-2 expression was found in glioma cells with either TSA or NaB treatment (49). The mechanism involved in the down-regulation of bcl-2 expression in hepatoma cells was not determined. It is not clear why HDAC inhibitors affect bcl-2 expression in dissimilar ways in different cell types. Several of the transcription factors that regulate the bcl-2 P2 promoter in B cells, such as Cdx and A-Myb as well as C/EBPα, are not ubiquitously expressed, and effects of HDAC inhibition on these factors may be involved.
To better understand the mechanism of bcl-2 gene regulation by HDAC inhibitors, we investigated their effects on the expression of the endogenous bcl-2 gene in vivo and bcl-2 promoter activity in transfection studies. We showed that TSA and NaB repressed steady-state bcl-2 expression at both the mRNA and protein levels in DHL-4 cells. To mimic the endogenous bcl-2 gene promoter function in t(14;18) lymphoma cells, we generated an episomal reporter gene construct with the bcl-2 promoters and the IgH enhancers. We found that both TSA and NaB dramatically down-regulated bcl-2 promoter activity in this reporter gene context. Moreover, the repression of bcl-2 mRNA expression was an early event, and TSA did not change the half-life of bcl-2 mRNA. These results suggest that repression of bcl-2 gene expression by HDAC inhibitors occurs at the transcriptional level and is most likely a direct effect.
We found that even though HDAC inhibitors increased the global accumulation of acetylated histones, decreased acetyl-histone H3 binding to the bcl-2 promoter regions was correlated with the transcriptional repression of bcl-2, while acetyl-histone H4 binding did not change significantly. At the bcl-2 P2 promoter, there was very little acetylated histone H4 bound even in the absence of TSA treatment. Changes in the acetylation of specific histones in response to extracellular signals is not a unique attribute of the bcl-2 promoter. Indeed, several studies have demonstrated differential association of acetylated histone H3 or H4 with gene promoters under conditions of transcriptional alteration. ChIP analysis of the p21 gene promoter revealed increased histone H3 acetylation, whereas H4 acetylation did not change after treatment with trapoxin (TPX), an epoxyketone-containing cyclic tetrapeptide HDAC inhibitor (48). Similarly, the low-density lipoprotein receptor 3-hydroxy-3-methylglutaryl-coenzyme A reductase and steroidogenic acute regulatory protein gene promoters also exhibited preferential acetylation of histone H3 under conditions known to activate these genes (3, 8). In contrast to these observations, changes in both H3 and H4 acetylation have been observed in many gene promoters following treatment with HDAC inhibitors.
While numerous studies have correlated gene transactivation with increased histone acetylation after inhibition of HDAC activity (33), emerging evidence has shown that HDAC inhibitors can also induce localized promoter histone deacetylation (14). In addition, recent data suggest that HDAC activity is required for STAT transcriptional activation of some genes. STAT1 and STAT2 associate with HDAC1, and treatment with alpha interferon results in deacetylation of histone H4 (39). The histone H4 deacetylation and alpha interferon-induced transcription are inhibited by treatment with TSA. HDAC inhibitors prevent transcriptional activation of the Id-1 gene (60). It was shown that during transcriptional activation, STAT5 interacts with HDAC1 at the enhancer and deacetylates histones and C/EBPβ, increasing the binding of C/EBPβ to the enhancer region. While our study suggests a requirement for HDAC2 in transcription of the bcl-2 gene, HDAC inhibitor-induced preferential histone H3 deacetylation during transcriptional repression rather than during activation of bcl-2 expression is observed. Both HDAC2 and CBP are bound to the bcl-2 promoter regions during transcription of the bcl-2 gene. HDAC2 may exert its influence on the transcription factors Sp1 and C/EBPα rather than on histone H3, and thus acetylation of histone H3 is observed.
As noted above, in addition to histones, other nuclear proteins are also targets of acetylation events. Acetylation of transcription factors has been described for Sp1, Sp3, p53, NF-κB, E2F1, and C/EBPβ (2, 5, 29, 45, 60). The functional consequences of posttranslational modification by acetylation of these transcription factors appear to be quite different. In some cases acetylation occurs near the DNA-binding domain and influences DNA binding (35). Acetylation can also modulate transcriptional potency and influence protein-protein interactions (57, 61). Sp1 is acetylated by CBP/p300 (53), and HDAC inhibitors augment acetylation (45). At least in neuronal cells, acetylation of Sp1 increases DNA binding and Sp1-dependent gene expression (45). We showed that TSA increased the acetylation of Sp1 and dramatically decreased binding of Sp1 to the bcl-2 promoter without affecting CREB binding. Decreased binding of Sp1 and Sp3 to the high-mobility group A2 protein promoter was also observed with TSA treatment of NIH 3T3 cells (14). These findings suggest that there are tissue-specific effects of HDAC inhibitors on Sp1 function. Because Sp1 interacts with HDAC2 and both are bound to the bcl-2 promoter, we believe that the effects of TSA are mediated through HDAC2 inhibition. In support of the role of HDAC2, we showed that overexpression of HDAC2 increased bcl-2 mRNA levels and decreased the acetylation of Sp1.
TSA also increased the acetylation of C/EBPα and reduced its binding to the Cdx site of the bcl-2 P2 promoter. TSA and NaB have been shown to attenuate the binding of two other C/EBP family members, C/EBPβ and C/EBPδ, to the haptoglobin promoter (11). Studies have also shown that C/EBPα is able to alter histone H3 but not H4 acetylation at large subnuclear domains (62). Our previous studies with the bcl-2 promoters showed that CBP physically associated with the regions containing the P1 promoter Sp1 binding site and the P2 promoter C/EBPα binding site; therefore, it is likely that decreased CBP binding to the bcl-2 promoters was due to decreased Sp1 and C/EBPα binding. The decreased binding of CBP at both bcl-2 promoters most likely contributes to the bcl-2 promoter deacetylation and transcriptional repression by HDAC inhibitors, although another HDAC that is less sensitive to TSA may also play a role. The importance of the Sp1 site and the Cdx site was further supported by the finding that mutation of these two sites could dramatically relieve the repression of the bcl-2 promoter by TSA.
Our results therefore suggest that repression of bcl-2 by HDAC inhibitors involves the inhibition of HDAC2, which leads to increased acetylation of Sp1 and C/EBPα. This results in decreased interaction of each transcription factor with HDAC2 and decreased binding to the bcl-2 promoter. Whether decreased binding to the promoter occurs as a direct effect (acetylated Sp1 and C/EBPα have less affinity for DNA) or as an indirect effect (acetylation of Sp1 and C/EBPα disrupts the interaction with an accessory factor that is necessary for binding to the bcl-2 promoter) is not clear. Although the majority of HDAC inhibitor studies have focused on mechanisms of gene activation, recent investigations have begun to elucidate the mechanisms mediating gene repression (10). Studies such as these, along with the results presented here, will help provide a better model for HDAC inhibitor-induced gene repression.
Our studies suggest that HDAC inhibitors are potential therapeutic agents for human t(14;18) lymphomas and that they act by repressing bcl-2 expression. However, it is likely that bcl-2 is not the only target gene of HDAC inhibitors in t(14;18) lymphoma cells. Previous studies using differential display to examine the differences in gene expression between untreated and treated lymphoid cell lines showed that approximately 2% of cellular genes changed in response to TSA treatment (56). We have found that TSA and NaB increase p21 protein expression in DHL-4 cells, which is consistent with the G0/G1 arrest shown in Fig. 2 (data not shown). The further identification of additional downstream effectors of HDAC inhibition and a better understanding of the underlying mechanisms will help guide the development of more effective agents to treat t(14;18) lymphomas.
Acknowledgments
This work was supported by National Institutes of Health grant CA56764.
We gratefully acknowledge the assistance of Cathy Crumpton and Lusijah Rott of the Stanford University Digestive Disease Center Flow Cytometry Facility.
REFERENCES
- 1.Alland, L., R. Muhle, H. J. Hou, J. Potes, L. Chin, N. Schreiber-Agus, and R. A. DePinho. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49-55. [DOI] [PubMed] [Google Scholar]
- 2.Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, M. K., T. T. Ngo, J. N. Athanikar, J. M. Rosenfeld, and T. F. Osborne. 1999. Costimulation of promoter for low density lipoprotein receptor gene by sterol regulatory element-binding protein and Sp1 is specifically disrupted by the yin yang 1 protein. J. Biol. Chem. 274:13025-13032. [DOI] [PubMed] [Google Scholar]
- 4.Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. [DOI] [PubMed] [Google Scholar]
- 5.Braun, H., R. Koop, A. Ertmer, S. Nacht, and G. Suske. 2001. Transcription factor Sp3 is regulated by acetylation. Nucleic Acids Res. 29:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C., and B. K. Birshtein. 1997. Virtually identical enhancers containing a segment of homology to murine 3′ IgH-E(hs1,2) lie downstream of human Ig Cα1 and Cα2 genes. J. Immunol. 159:1310-1318. [PubMed] [Google Scholar]
- 8.Christenson, L. K., R. L. Stouffer, and J. F. Strauss III. 2001. Quantitative analysis of the hormone-induced hyperacetylation of histone H3 associated with the steroidogenic acute regulatory protein gene promoter. J. Biol. Chem. 276:27392-27399. [DOI] [PubMed] [Google Scholar]
- 9.Cleary, M. L., S. D. Smith, and J. Sklar. 1986. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell 47:19-28. [DOI] [PubMed] [Google Scholar]
- 10.Dehm, S. M., T. L. Hilton, E. H. Wang, and K. Bonham. 2004. SRC proximal and core promoter elements dictate TAF1 dependence and transcriptional repression by histone deacetylase inhibitors. Mol. Cell. Biol. 24:2296-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desilets, A., I. Gheorghiu, S. J. Yu, E. G. Seidman, and C. Asselin. 2000. Inhibition by deacetylase inhibitors of IL-1-dependent induction of haptoglobin involves CCAAT/enhancer-binding protein isoforms in intestinal epithelial cells. Biochem. Biophys. Res. Commun. 276:673-679. [DOI] [PubMed] [Google Scholar]
- 12.Desoize, B. 1994. Anticancer drug resistance and inhibition of apoptosis. Anticancer Res. 14:2291-2294. [PubMed] [Google Scholar]
- 13.Epstein, A. L., R. Levy, H. Kim, W. Henle, G. Henle, and H. S. Kaplan. 1978. Biology of the human malignant lymphomas. IV. Functional characterization of ten diffuse histiocytic lymphoma cell lines. Cancer 42:2379-2391. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson, M., P. A. Henry, and R. A. Currie. 2003. Histone deacetylase inhibition is associated with transcriptional repression of the Hmga2 gene. Nucleic Acids Res. 31:3123-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graninger, W. B., M. Seto, B. Boutain, P. Goldman, and S. J. Korsmeyer. 1987. Expression of bcl-2 and bcl-2-Ig fusion transcripts in normal and neoplastic cells. J. Clin. Investig. 80:1512-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory, P. D., K. Wagner, and W. Horz. 2001. Histone acetylation and chromatin remodeling. Exp. Cell Res. 265:195-202. [DOI] [PubMed] [Google Scholar]
- 17.Grunstein, M. 1997. Histone acetylation in chromatin structure and transcription. Nature 389:349-352. [DOI] [PubMed] [Google Scholar]
- 18.Gui, C.-Y., L. Ngo, W. S. Xu, V. M. Richon, and P. A. Marks. 2004. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl. Acad. Sci. USA 101:1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heckman, C., E. Mochon, M. Arcinas, and L. M. Boxer. 1997. The WT1 protein is a negative regulator of the normal bcl-2 allele in t(14;18) lymphomas. J. Biol. Chem. 272:19609-19614. [DOI] [PubMed] [Google Scholar]
- 20.Heckman, C. A., T. Cao, L. Somsouk, H. Duan, J. W. Mehew, C. Zhang, and L. M. Boxer. 2003. Critical elements of the immunoglobulin heavy chain gene enhancers for deregulated expression of bcl-2. Cancer Res. 63:6666-6673. [PubMed] [Google Scholar]
- 21.Heckman, C. A., J. W. Mehew, and L. M. Boxer. 2002. NF-κB activates bcl-2 expression in t(14;18) lymphoma cells. Oncogene 21:3898-3908. [DOI] [PubMed] [Google Scholar]
- 22.Heckman, C. A., J. W. Mehew, G.-G. Ying, M. Introna, J. Golay, and L. M. Boxer. 2000. A-Myb up-regulates Bcl-2 through a Cdx binding site in t(14;18) lymphoma cells. J. Biol. Chem. 275:6499-6508. [DOI] [PubMed] [Google Scholar]
- 23.Heckman, C. A., M. A. Wheeler, and L. M. Boxer. 2003. Regulation of Bcl-2 expression by C/EBP in t(14;18) lymphoma cells. Oncogene 22:7891-7899. [DOI] [PubMed] [Google Scholar]
- 24.Henderson, C., M. Mizzau, G. Paroni, R. Maestro, C. Schneider, and C. Brancolini. 2003. Role of caspases, Bid, and p53 in the apoptotic response triggered by histone deacetylase inhibitors trichostatin-A (TSA) and suberoylanilide hydroxamic acid (SAHA). J. Biol. Chem. 278:12579-12589. [DOI] [PubMed] [Google Scholar]
- 25.Herold, C., M. Ganslmayer, M. Ocker, M. Hermann, A. Geerts, E. G. Hahn, and D. Schuppan. 2002. The histone-deacetylase inhibitor trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J. Hepatol. 36:233-240. [DOI] [PubMed] [Google Scholar]
- 26.Hockenberry, D., G. Nunez, C. Milliman, R. D. Schreiber, and S. J. Korsmeyer. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348:334-336. [DOI] [PubMed] [Google Scholar]
- 27.Ji, L., E. Mochon, M. Arcinas, and L. M. Boxer. 1996. CREB proteins function as positive regulators of the translocated bcl-2 allele in t(14;18) lymphomas. J. Biol. Chem. 271:22687-22691. [DOI] [PubMed] [Google Scholar]
- 28.Kanda, K., H.-M. Hu, L. Zhang, J. Grandchamps, and L. M. Boxer. 2000. NF-κB activity is required for the deregulation of c-myc expression by the immunoglobulin heavy chain enhancer. J. Biol. Chem. 275:32338-32346. [DOI] [PubMed] [Google Scholar]
- 29.Kiernan, R., V. Bres, R. Ng, W. M. P. Coudart, S. El-Messaoudi, C. Sardet, D. Y. Jin, S. Emiliani, and M. Benkirane. 2003. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 278:2758-2766. [DOI] [PubMed] [Google Scholar]
- 30.Kim, M. S., H. J. Kwon, Y. M. Lee, J. H. Baek, J. E. Jang, S. W. Lee, E. J. Moon, H. S. Kim, S. K. Lee, H. Y. Chung, C. W. Kim, and K. W. Kim. 2001. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat. Med. 7:437-443. [DOI] [PubMed] [Google Scholar]
- 31.Madisen, L., and M. Groudine. 1994. Identification of a locus control region in the immunoglobulin heavy-chain locus that deregulates c-myc expression in plasmacytoma and Burkitt's lymphoma cells. Genes Dev. 8:2212-2226. [DOI] [PubMed] [Google Scholar]
- 32.Mahlknecht, U., and D. Hoelzer. 2000. Histone acetylation modifiers in the pathogenesis of malignant disease. Mol. Med. 6:623-644. [PMC free article] [PubMed] [Google Scholar]
- 33.Marks, P., R. A. Rifkind, V. M. Richon, R. Breslow, T. Miller, and W. K. Kelly. 2001. Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer 1:194-202. [DOI] [PubMed] [Google Scholar]
- 34.Marks, P. A., V. M. Richon, and R. A. Rifkind. 2000. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 92:1210-1216. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Balbas, M. A., U. M. Bauer, S. J. Nielsen, A. Brehm, and T. Kouzarides. 2000. Regulation of E2F1 activity by acetylation. EMBO J. 19:662-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaelson, J. S., S. L. Giannini, and B. K. Birshtein. 1995. Identification of 3′α-hs4, a novel Ig heavy chain enhancer element regulated at multiple stages of B cell differentiation. Nucleic Acids Res. 23:975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills, F., N. Harindranath, M. Mitchell, and E. Max. 1997. Enhancer complexes located downstream of both human immunoglobulin C α genes. J. Exp. Med. 186:845-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsiades, C. S., N. S. Mitsiades, C. J. McMullan, V. Poulaki, R. Shringarpure, T. Hideshima, M. Akiyama, D. Chauhan, N. Munshi, X. Gu, C. Bailey, M. Joseph, T. A. Libermann, V. M. Richon, P. A. Marks, and K. C. Anderson. 2004. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc. Natl. Acad. Sci. USA 101:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nusinzon, I., and C. M. Horvath. 2003. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc. Natl. Acad. Sci. USA 100:14742-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersson, S., G. P. Cook, M. Bruggemann, G. T. Williams, and M. S. Neuberger. 1990. A second B cell-specific enhancer 3′ of the immunoglobulin heavy chain locus. Nature 344:165-168. [DOI] [PubMed] [Google Scholar]
- 41.Pinaud, E., C. Aupetit, C. Chauveau, and M. Cogne. 1997. Identification of a homolog of the Cα3′/hs3 enhancer and of an allelic variant of the 3′IgH/hs1,2 enhancer downstream of the human immunoglobulin α1 gene. Eur. J. Immunol. 27:2981-2985. [DOI] [PubMed] [Google Scholar]
- 42.Pogo, B. G., V. G. Allfrey, and A. E. Mirsky. 1966. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc. Natl. Acad. Sci. USA 55:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed, J. C., S. Kitada, S. Takayama, and T. Miyashita. 1994. Regulation of chemoresistance by the bcl-2 oncoprotein in non-Hodgkin's lymphoma and lymphocytic leukemia cell lines. Ann. Oncol. 1(Suppl. 5):S61-S65. [DOI] [PubMed] [Google Scholar]
- 44.Rosato, R. R., J. A. Almenara, and S. Grant. 2003. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 63:3637-3645. [PubMed] [Google Scholar]
- 45.Ryu, H., J. Lee, B. A. Olofsson, A. Mwidau, A. Deodoglu, M. Escudero, E. Flemington, J. Azizkhan-Clifford, R. J. Ferrante, and R. R. Ratan. 2003. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc. Natl. Acad. Sci. USA 100:4281-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakai, E., A. Bottaro, and F. W. Alt. 1999. The Ig heavy chain intronic enhancer core region is necessary and sufficient to promote class switch recombination. Int. Immunol. 11:1709-1713. [DOI] [PubMed] [Google Scholar]
- 47.Saleque, S., M. Singh, R. D. Litter, S. L. Giannini, J. S. Michaelson, and B. K. Birshtein. 1997. Dyad symmetry within the mouse 3′ IgH regulatory region includes two virtually identical enhancers (C alpha3′E and hs3). J. Immunol. 158:4780-4787. [PubMed] [Google Scholar]
- 48.Sambucetti, L. C., D. D. Fischer, S. Zabludoff, P. O. Kwon, H. Chamberlin, N. Trogani, H. Xu, and D. Cohen. 1999. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J. Biol. Chem. 274:34940-34947. [DOI] [PubMed] [Google Scholar]
- 49.Sawa, H., H. Murakami, Y. Ohshima, T. Sugino, T. Nakajyo, T. Kisanuki, Y. Tamura, A. Satone, W. Ide, I. Hashimoto, and H. Kamada. 2001. Histone deacetylase inhibitors such as sodium butyrate and trichostatin A induce apoptosis through an increase of the bcl-2-related protein Bad. Brain Tumor Pathol. 18:109-114. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt, C. A., and S. W. Lowe. 2001. Bcl-2 mediates chemoresistance in matched pairs of primary Eμ-myc lymphomas in vivo. Blood Cells Mol. Dis. 27:206-216. [DOI] [PubMed] [Google Scholar]
- 51.Schuettengruber, B., E. Simboeck, H. Khier, and C. Seiser. 2003. Autoregulation of mouse histone deacetylase 1 expression. Mol. Cell. Biol. 23:6993-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seto, M., U. Jaeger, R. D. Hockett, W. Graninger, S. Bennett, P. Goldman, and S. J. Korsmeyer. 1988. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 7:123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki, T., A. Kimura, R. Nagai, and M. Horikoshi. 2000. Regulation of interaction of the acetyltransferase region of p300 and the DNA-binding domain of Sp1 on and through DNA binding. Genes Cells 5:29-41. [DOI] [PubMed] [Google Scholar]
- 54.Tsujimoto, Y., and C. Croce. 1986. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc. Natl. Acad. Sci. USA 83:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsujimoto, Y., J. Gorham, J. Cossman, E. Jaffe, and C. M. Croce. 1985. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 229:1390-1393. [DOI] [PubMed] [Google Scholar]
- 56.Van Lint, C., S. Emiliani, and E. Verdin. 1996. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Exp. 5:245-253. [PMC free article] [PubMed] [Google Scholar]
- 57.Waltzer, L., and M. Bienz. 1998. Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature 395:521-525. [DOI] [PubMed] [Google Scholar]
- 58.Wilson, B. E., E. Mochon, and L. M. Boxer. 1996. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell. Biol. 16:5546-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, Y., J. W. Mehew, C. A. Heckman, M. Arcinas, and L. M. Boxer. 2001. Negative regulation of bcl-2 expression by p53 in hematopoietic cells. Oncogene 20:240-251. [DOI] [PubMed] [Google Scholar]
- 60.Xu, M., L. Nie, S.-H. Kim, and X.-H. Sun. 2003. STAT5-induced Id-1 transcription involves recruitment of HDAC1 and deacetylation of C/EBPβ. EMBO J. 22:893-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, W., and J. J. Bieker. 1998. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. USA 95:9855-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, W. H., R. Srihari, R. N. Day, and F. Schaufele. 2001. CCAAT/enhancer-binding protein alpha alters histone H3 acetylation at large subnuclear domains. J. Biol. Chem. 276:40373-40376. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, X., W. Wharton, Z. Yuan, S.-C. Tsai, N. Olashaw, and E. Seto. 2004. Activation of the growth-differentiation factor 11 gene by the histone deacetylase (HDAC) inhibitor trichostatin A and repression by HDAC3. Mol. Cell. Biol. 24:5106-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]