Abstract
Coping styles theory provides a framework for understanding individual variation in how animals respond to environmental change, and predicts how individual differences in stress responsiveness and behavior might relate to cognitive differences. According to coping styles theory, proactive individuals are bolder, less reactive to stressors, and more routinized than their reactive counterparts. A key tenet of coping styles theory is that variation in coping styles is maintained by tradeoffs with behavioral flexibility: proactive individuals excel in stable environments while more flexible, reactive individuals perform better in variable environments. Here, we assess evidence for coping styles within a natural population of threespined sticklebacks (Gasterosteus aculeatus). We developed a criterion-based learning paradigm to evaluate individual variation in initial and reversal learning. We observed strong individual differences in boldness, cortisol production, and learning performance. Consistent with coping styles, fish that released more cortisol were more timid in response to a predator attack and slower to learn a color discrimination task. However, there was no evidence that reactive individuals performed better when the environment changed (when the rewarded color was reversed). The failure to detect trade-offs between behavioral routinization and flexibility prompts other explanations for the maintenance of differing coping styles.
Keywords: Animal personality, Coping styles, Threespined stickleback, Learning, Cortisol, Individual differences
1. Introduction
Interest in individual variation in animal cognition is booming (e.g., Bensky et al., 2013; Chittka et al., 2009; Matzel et al., 2003; Niemela et al., 2013; Thornton and Lukas 2012). For example, a growing number of studies are showing that there is substantial intra-specific variation in performance on different cognitive tasks in a wide range of taxa (e.g., birds: Guillette et al., 2015; dogs: Nippak and Milgram 2005; primates: Herrmann et al., 2009; rodents: Galsworthy et al., 2002). Additionally, there has been increased interest in how individual variation in cognition may correspond with consistent individual differences in behavior, or animal personalities (Budaev and Brown, 2011; Carere and Locurto, 2011; Locurto, 2007; Sih and Del Giudice, 2012). As a result, studies exploring the nature of the correlations between various animal personality axes and cognitive differences are on the rise (e.g., Bebus et al., 2016; Ruiz-Gomez et al., 2011; Sneddon 2003; also see Guillette et al. 2017), and more attention is being called to developing best-practices for examining cognitive differences to address these types of questions (Griffen et al., 2015). However, the proximate and ultimate causes of correlations between cognition and behavior are presently unknown.
Coping styles theory offers a framework for understanding individual variation in cognition and behavior. According to this theory, variation in cognition is a part of a suite of correlated traits adapted for coping with changes in the environment, with individuals varying along the proactive-reactive axis (Koolhaas et al., 1999). At one end of a continuous distribution are proactive individuals, which are relatively exploratory, bold, and aggressive relative to their reactive counterparts at the other extreme. A key tenet of coping styles theory is that a cost of being proactive is reduced behavioral flexibility: proactive individuals establish initial learning contingencies quickly, largely due to their exploratory nature, but then become routinized and perform poorly when the reward is reversed (Koolhaas et al., 1999). Reactive individuals, on the other hand, are more stress reactive and timid, and may be slow initial learners but then quickly respond to environmental change. Following this reasoning, it has been suggested that variation along the proactive-reactive axis is maintained by fluctuating selection pressures (Thornton and Lukas, 2012). Specifically, proactive, inflexible individuals might have higher fitness in stable environments while reactive, flexible individuals may be better suited for environments that experience moderate change (Benus et al., 1990; Guillette et al., 2011; Niemela et al., 2013; Ruiz-Gomez et al., 2011; Verbeek et al., 1994). Additionally, such variation may be driven by frequency-dependent benefits (Wolf et al., 2008) or differences in dispersal states (Canestrelli et al., 2016).
Given that different coping styles are expected to reflect variation in how individuals respond to challenging conditions or stressors, one of the proximate mechanisms underlying coping styles is thought to involve the hypothalamic-pituitary-adrenal (HPA) axis (Koolhaas et al., 2010). Indeed, some studies suggest that proactive individuals have relatively low stress reactivity and higher sympathetic activity (Carere et al., 2010; Sorensen et al., 2013), but some authors have suggested that stress responsiveness is an independent axis of variation (Koolhaas et al., 2010). Upon perceiving a stimulus as stressful, the hypothalamus initiates a cascade that culminates in an increase in circulating glucocorticoid levels (Sapolsky et al., 2000). Once the stimulus is removed or no longer perceived as a threat, feedback loops are triggered to shut down the production of glucocorticoids (Lupien et al., 2009). Several studies in diverse organisms have shown that individuals consistently vary in stress responsiveness (repeatability of glucocorticoid hormone levels: Boulton et al., 2015; Cockrem et al., 2009; Cook et al., 2012; Fürtbauer et al., 2015; Ouyang et al., 2011; selection on glucocorticoid responsiveness: Øverli et al., 2005; Pottinger and Carrick, 1999; Roberts et al., 2007), therefore individual variation in the transient production of glucocorticoids has been hypothesized to underlie variation in the behavioral response to stress (Carere et al., 2010; Sorensen et al., 2013). These physiological mechanisms have also tied coping styles to potential fitness differences in terms of stress-related health and disease susceptibility (Costantini et al., 2012; de Boer et al., 2016).
Individual variation in stress responsiveness has also been linked to variation in cognitive performance (Lupien and McEwen, 1997; Kosten et al., 2012). For example, lines of trout that had been selected for divergent post-stressor cortisol levels differed in cognitive performance: trout that produced high levels of cortisol in response to a stressor were quicker to alter their behavior in response to food relocation during reversal trials (Ruiz-Gomez et al., 2011). These reactive fish were also faster to stop a conditioned response during extinction trials (Moreira et al., 2004). Overall, there is a plethora of data (e.g. McEwen and Sapolsky, 1995; Øverli et al., 2005, 2007; Schoech et al., 2011) demonstrating that glucocorticoids are related to behavioral responses to stress and cognitive performance. However very few studies have characterized HPA axis function, behavior, and cognition simultaneously, and those that have tend to mostly focus on selected lines or domesticated animals (e.g. Benus et al., 1990; Bolhuis et al., 2004; Øverli et al., 2002; but see Bebus et al., 2016; Øverli et al., 2007). Attempts to simultaneously characterize several different facets of coping styles in natural populations are rare due to the time intensive nature of collecting extensive individual-based data on many subjects.
Here, we took a high-resolution approach to test the generalizability of whether individual differences in cognitive performance are part of a larger suite of behaviors, or coping styles, in a natural population. Again, much of the work on coping styles has been focused on mammalian and avian species (Carere et al., 2010), and while significant work has also been done looking at fish, particularly salmonids (Øverli et al., 2005), we were interested in further expanding the research applied to this taxa with a focus on natural variation. Here we tested the threespined stickleback (Gasterosteus aculeatus), a species renowned for their natural intraspecific variation in behavior (e.g., Bell 2005; Dingemanse et al., 2007; Girvan and Braithwaite, 1998; Huntingford, 1976; Mamuneas et al., 2015; Odling-Smee and Braithwaite, 2003; Stein and Bell, 2012), and a system that was recently used to investigate behavioral and endocrine associations in coping with increased predatory threat (Fürtbauer et al., 2015). Additionally, this species has been established as being conducive to fish cognition studies (Girvan and Braithwaite, 1998; Odling-Smee and Braithwaite, 2003). We repeatedly measured stress reactivity and ‘boldness’ towards a model predator on the same individuals and then asked whether those traits were related to variation in cognitive performance. We developed a criterion-based method to quantify the distribution of individual variation in cognitive performance in a discrimination-reversal learning paradigm. Subjects were trained to associate a food reward with one of two colors. Then, after a success criterion was reached, the stimuli and reward association were reversed. We interpret performance on the reversal task as behavioral flexibility (as in Pintor et al., 2014; see Stamps, 2015), where persistent (proactive, routinized) individuals continue to approach the previously learned cue while more flexible (reactive) individuals more quickly modify their behavior and learn the new association. According to coping styles theory, we predicted that: 1) bolder (proactive) individuals are less stress reactive; 2) bolder individuals learn faster; 3) bolder individuals are less flexible; 4) individuals that are more stress reactive are more flexible.
2. Materials & methods
2.1. Overview of the experiment
We measured stress reactivity, “boldness”, learning and behavioral flexibility on the same individual sticklebacks in a fixed order. Individuals were measured for stress responsiveness on two occasions. We used a non-invasive method that samples cortisol released from the gills into the water following confinement stress for 4.5 h (Sebire et al., 2007; Sebire et al., 2009; Aubin-Horth et al., 2012). Individual differences in “boldness” were measured by testing the same individuals for their response to a model predator on four occasions. Finally, the same subjects were tested for learning performance in a color discrimination task and for behavioral flexibility in a reversal-learning task.
2.2. Subjects
Adult sticklebacks were collected from Putah Creek, CA in April 2014 prior to the onset of the breeding season. Fish were transported to the University of Illinois Urbana-Champaign where they were housed in 37 L tanks in groups of five for approximately four weeks until experiments began. Fish were housed in 20° Celsius water and were placed on a summer (16L: 8D) photoperiod. A recirculating flow-through system consisting of a series of particulate, biological, and UV filters (Aquaneering, San Diego, USA) was used to clean the water. 10% of the water volume in the tanks was replaced each day. The fish were fed a mixed diet consisting of frozen bloodworm, frozen brine shrimp, and frozen Mysis shrimp ad lib each day except during the learning trials (see below). Ten individuals were randomly selected for this experiment (females: n = 6, average length = 43.8 mm, average mass = 1.02 g; males: n = 4, average length = 42.5 mm, average mass = 0.87 g). Females were not gravid during the experiment and males showed no evidence of nuptial coloration. Each individual was marked by clipping a unique combination of their dorsal and/or pelvic spines.
2.3. Cortisol release rate
The purpose of this experiment was to determine whether there were consistent individual differences in stress reactivity. The hypothalamic-pituitary-interrenal (HPI) response of each fish was characterized by measuring the amount of cortisol released into water at five time points over the course of 4.5 h. This process was repeated one week later in order to determine whether individual differences in cortisol release rate were stable over a one-week period.
Fish were transferred to individual static 100 mL tanks and left undisturbed for 30 min. Then, a 50 mL water sample was collected from a release valve at the bottom of the tank. The water supply was turned on and allowed to run for 30 min to flush the cortisol-laden water from the tank. Preliminary trials confirmed that turning on the water supply for 30 min flushed the tank sufficiently such that cortisol could no longer be detected in the water. Following the 30-min flush, the water supply was turned back off to allow another 30 min of static water to collect cortisol. This process was repeated so that we generated water samples from minutes 0–30, 60–90, 120–150, 180–210, and 240–270. Fish were returned to their home tank once sample collection was complete.
Water samples (10 fish × 5 samples/trial × 2 trials = 100 samples) were frozen at −20 °C until steroid quantification. Freeze storage of water samples does not influence steroid concentrations (Ellis et al., 2004). Steroids were extracted from water samples by pulling water through C18 Sep-Pak cartridges (Waters Ltd.) that had been primed with 5 mL methanol followed by 5 mL distilled water. After the sample had dripped through at a rate of approximately 2 mL/min, the cartridge was washed with 5 mL of distilled water and the steroids were then eluted with 5 mL of diethyl ether. The ether was dried under nitrogen and submitted to Metabolomics Center, Roy J. Carver Biotechnology Center, University of Illinois at Urbana-Champaign.
Samples were analyzed via mass spectrometry with the 5500 QTRAP LC/MS/MS system (AB Sciex, Foster City, CA). The 1200 series HPLC system (Agilent Technologies, Santa Clara, CA) includes a degasser, an autosampler, and a binary pump. The LC separation was performed on a Phenomenex C6 Phenyl column (2.0 × 100 mm, 3 μm.) with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile). The flow rate was 0.25 mL/min. The linear gradient was as follows: 0–1 min, 80%A; 10 min, 65%A; 15 min, 50%A; 20 min, 40%A; 25 min, 30%A; 30 min, 20%A; 30.5–38 min, 80%A. The autosampler was set at 5 °C. The injection volume was 5 μL. Mass spectra were acquired under positive electrospray ionization (ESI) with the ion spray voltage of 5500 V. The source temperature was 500 °C. The curtain gas, ion source gas 1, and ion source gas 2 were 36 psi, 50 psi, and 65 psi, respectively. Multiple reaction monitoring (MRM) was used to measure cortisol with the Q1–Q3 transition of 363.1–121.0 (m/z). In order to control for possible size effects, cortisol release rates were calculated as the amount of released cortisol per gram of body weight per hour (ng/g/hr).
2.4. Boldness assays
Three weeks after the steroid measures were complete, each individual was observed in a standardized behavioral assay designed to measure boldness in response to a predatory threat. The response of each individual fish to a model predator was recorded. Each individual was tested on four consecutive days in order to determine whether individual differences in behavior were consistent over time.
The testing arena was a circular plastic pool (150 cm diameter) filled to a depth of 10 cm of water (Fig. 1a). An opaque refuge (10 cm diameter, 10 cm height) was placed in the center of the pool. The pool was divided into nine equally-sized sections (one centered, eight around the perimeter), with drawn lines demarking each of these sections. A rock was placed into each of the perimeter sections, providing objects to “explore” or additional refuges.
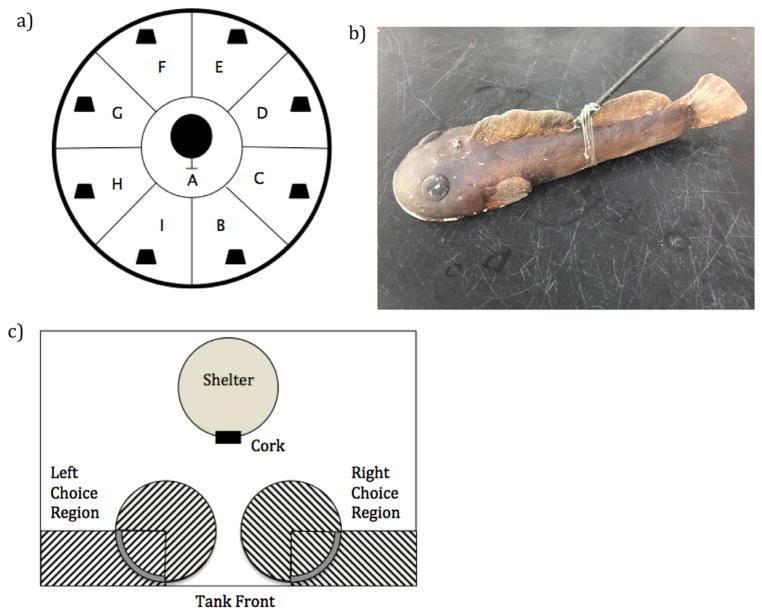
Fig. 1.
Experimental set up. a) Top-down view of tank set up for boldness assay. Kiddie pool was divided into 9 sections and marked A–I. Starting shelter was placed in the middle of section A. Black symbols in each section represent stones that were placed for investigation and additional shelter. Top-down view of the tank set up for training trials. Fish were acclimated to environment by repeatedly being placed in the starting shelter and then allowed to make a choice. The same set up was also used for actual training trials. Striped regions show the choice regions. Once a fish entered either of these regions, its choice was noted. b) Photo of sculpin model used for boldness assay. c) Top-down view of the tank set up for training trials. Fish were acclimated to environment by repeatedly being placed in the starting shelter and then allowed to make a choice. The same set up was also used for actual training trials. Striped regions show the choice regions. Once a fish entered either of these regions, its choice was noted.
At the start of an assay, a randomly selected individual was gently caught from their home group tank, identified via spine clips, and then placed into the refuge at the center of the pool where it was allowed to acclimate for three minutes. Behavioral observations were recorded by an observer positioned behind a blind. After three minutes, the observer opened the side of the refuge remotely and waited for the fish to emerge. After fully emerging, the fish was given three minutes to freely explore the pool. Baseline activity levels were recorded over this time period by recording the number of transitions the fish made between sections (sections before the attack). We then simulated a predator attack by moving a clay predator model (sculpin, 20 cm length; Fig. 1b) from a hidden location into the section of the pool that contained the focal fish. Sculpin (genus: Cottus) are a natural predator to freshwater populations of stickleback (Pressley, 1981). This stimulus elicited a freezing response, which has been found to be an effective antipredator response (McGhee et al., 2012). After the individual resumed movement, we recorded the number of transitions made between sections for three more minutes (sections after the attack). To confirm that the predator attack influenced behavior, and that movement after the predator attack is a reasonable measure of boldness, we compared movement before and after the attack. Locomotion is conspicuous, therefore we predicted that on average the fish should move into fewer sections after the attack if they were exhibiting an antipredator response. After the completion of each assay the individual was placed back into their group tank. One individual died of unknown cause between day 3 and 4 of the behavioral assays; the behavior of this fish was not obviously different from the others. Therefore the data from the deceased fish is included in all analyses except those involving the learning trials since those were carried out after the boldness assays.
2.5. Learning trials
Three weeks after the ‘boldness’ assays were complete, we measured individual differences in performance in an associative learning task. Each fish was simultaneously presented with a yellow and a blue cup, with the yellow cup rewarded with food. We infer that a fish learned the color association task when it reached criterion (two consecutive days of at least 8 out of 10 correct trials).
Each fish was moved into a 36 cm long × 33 cm wide × 24 cm high (26.5 L) tank where they were housed individually. Prior to the learning trials, fish were acclimated to eating out of cups, and to repeatedly being placed in a shelter. Additionally, through these pre-training trials the fish were given the opportunity to learn that food could potentially be available at two different food sites. The fish only obtained food during the learning trials in order to maintain motivation. All the fish readily ate food from both food sites over these pre-training sessions and consistently decreased their latency to emerge from the shelter to approach one of these food sites, which we interpreted as motivation to access these sites. Criteria were used to assure that the fish were sufficiently acclimated to the training environment and motivated to access the food sites before training trials began on the following day. Specifically, individuals were required to eat out of both cups within 10 min on three consecutive days before moving on to the shelter acclimation phase. For this phase, individuals needed to emerge from the shelter, find the available food and eat it within 10 min on two daily trials for three consecutive days before moving on to the training trials.
Yellow/Blue Discrimination Phase
Fish received one learning session per day. Each session consisted of 10 trials. All of the nine fish completed a trial before the next round of trials was started (i.e., all fish received Trial 1 before moving onto Trial 2), so there was an average of 15–20 min between each trial for a single individual. The side that was rewarded during each trial was pseudo-randomized for each individual fish according to the following two rules: 1) half of the trials were rewarded on each side, and 2) a single side could not be rewarded more than two trials in a row. During each trial, the fish was placed in the shelter at the back of the tank. While the fish was in the shelter, two reward cups were placed as they were during the pre-training trials (Fig. 1c). A petri dish was then placed into the base of each cup. One petri dish was designated the “Incorrect Reward Dish,” which contained five bloodworms and was covered with electrical tape to prevent access to the worms. Holes in the tape allowed chemical/odor cues to emanate from the dish, but the holes were too small for the bloodworms to be removed. The other petri dish was designated the “Correct Reward Dish.” This dish had electrical tape lining its rim to limit the odor cues from the tape being used as a discriminatory cue, but nothing obstructed access to the contents of the dish. As seen with many other visual discrimination studies (e.g., Nippak and Milgram, 2005; Ruiz-Gomez et al., 2011), making sure food cues were present in both potential food sites prevented the food cues themselves from directing the animal’s choice. During this phase the yellow cup was always rewarded, so it was placed on the designated rewarded side and the “Correct Reward Dish” was placed in it. Using a dropper, two bloodworms were placed into the “Correct Reward Dish” per trial. The “Incorrect Reward Dish” was then placed into the Blue cup on the opposite side. After the cups were set up, and the fish had been in the shelter at least a minute, the cork of the shelter was removed. Once the fish had exited the shelter, we recorded whether the fish approached the designated choice region of the rewarded (yellow) or unrewarded (blue) cup (see Fig. 1c). If the fish first approached the choice region of the yellow cup, the trial was marked as a “correct choice.” If the fish first approached the choice region of the blue cup, the trial was marked as an “incorrect choice.” Regardless of whether the first choice was correct, the fish was allowed to explore the tank until it found the “Correct Reward Dish,” and was given a chance to eat the worms. This was important because it gave each individual an equal opportunity to associate the rewarded stimulus cues with the food reward, regardless of the first choice. If the fish entered at least one of the choice regions, but did not eat the worms within 5 min of making a choice, the trial ended and it was noted that they did not receive food on that trial. The Yellow/Blue Discrimination phase continued until the fish had met the criterion of two consecutive days of at least 8 correct choices out of 10 trials. This criterion is similar to those used in other published discrimination learning studies (Guillette et al., 2011; Nippak and Milgram, 2005; Tapp et al., 2003), though we decided to base our criterion exclusively on consecutive day performance in order to help ensure long-term retention of the trained contingencies. From a statistical standpoint, this criterion makes it very unlikely that a fish would perform at criterion level simply due to chance (binomial distributon: p = 0.003). Once this criterion was met, the Reversal Learning Phase started during the next session.
2.6. Measuring individual differences in learning flexibility
The purpose of this experiment was to quantify individual differences in reversal learning performance, which we interpret as flexibility (Bond et al., 2007; Stamps, 2015). This experiment was the same as the Yellow/Blue discrimination phase except that now the blue cup was rewarded during each trial. The criterion was again that the fish had to get 8 out of 10 trials correct on two consecutive days.
2.7. Statistical analysis
Given previous findings showing correlations between boldness and sex (e.g., Brown et al., 2007; Harris et al., 2010) and body size (e.g., Brown and Braithwaite, 2004), we tested for differences between the sexes using t-tests, and we tested for an effect of body length on bold behavior using Pearson correlations.
To estimate repeatability, we used Bayesian statistics with Markov Chain Monte Carlo simulations using the MCMCglmm package (Hadfield, 2010) in R 3.1 (http://www.r-project.org/). We computed the repeatability of stress reactivity by computing the average cortisol across the five measures within a week, and estimated the repeatability of average cortisol across the two weeks. Average cortisol was normally distributed. Week and Individual were included as random effects. We estimated the repeatability of boldness (sections after the attack) across four days, while including Day and Individual as random effects and Sex as a fixed factor since there was evidence of a sex effect on behavior (see Results). As our measure of boldness is a count-based measure, a Poisson distribution was used. For all analyses, we used non-informative proper priors (Hadfield, 2010) with 500 000 iterations, thinning of 100 iterations and a burn-in of 1000 iterations. Through these simulations, 95% confidence intervals were generated and reported (in brackets) around our repeatability estimates. To determine the significance of our estimates we evaluated the lower bounds of the 95% confidence interval and looked to see that they were not near zero. To confirm that the fish were responding to the predator attacks, we compared the average sections entered before and after the attacks, and performed a t-test to test to see if there was significant decrease in movement after seeing the predator.
Finally, to test the predicted associations made by coping styles theory, we analyzed pairwise correlations between average cortisol release rate, sections after the attack, sessions to reach criterion on initial learning, and performance on the first reversal session using Pearson correlations. In the case of correlations involving boldness, since there was a trend for an effect of sex, we used partial correlations controlling for sex. All statistical analyses were carried out using R 3.1 (http://www.r-project.org/).
3. Results
3.1. Cortisol release rate
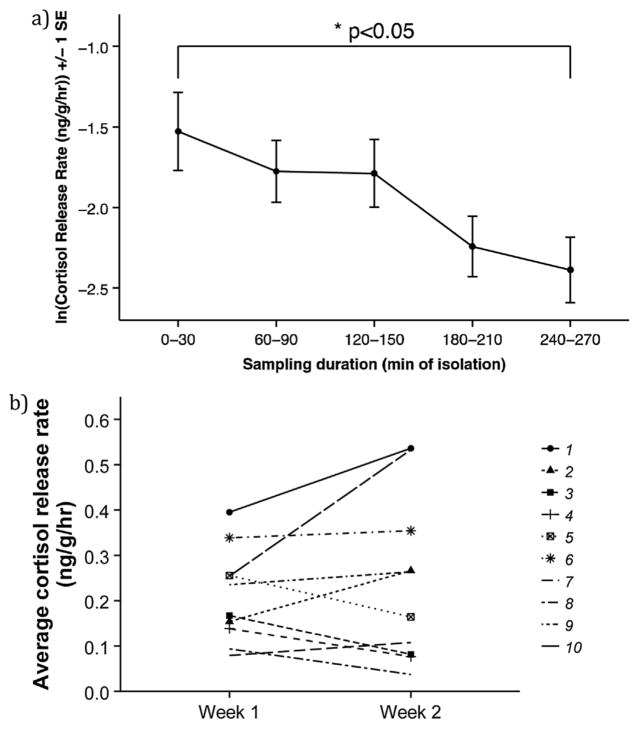
On average, cortisol release rate declined over time within a session, such that cortisol release rate was lower four hours after isolation compared to initially (t9 = 2.37, p = 0.0417, Fig. 2a). Additionally, there were consistent individual differences in cortisol release rate over time, with some individuals consistently releasing more cortisol than others across the two sampling periods (repeatability of sample average, R = 0.6159 [0.2252, 0.9218], n = 10, Fig. 2b).
Fig. 2.
Cortisol repeated measures. a) Average cortisol release rate across the five sampling periods. Cortisol release rate was significantly lower 270 min after the start of isolation compared to 30 min (n = 10). b) Individual differences in average cortisol release rate across the two sampling weeks. Each line represents an individual fish (n = 10).
3.2. Boldness
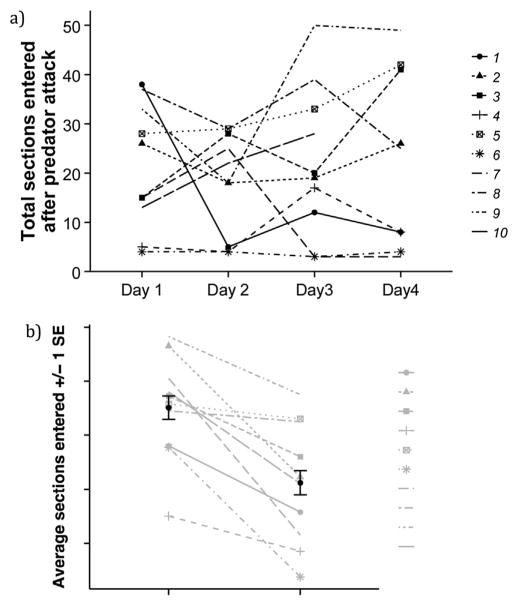
There were consistent individual differences in boldness across the four measurements (R = 0.4980 [0.0817, 0.8244], n = 10, Fig. 3a).
Fig. 3.
Boldness measures. a) Repeatability of boldness (total sections entered after predator attack) across four days. Each line represents an individual fish (n = 10), with the same symbols for individuals as in Fig. 2b. One individual (Fish 10) died after day 3. b) Difference in activity before and after predator attack. Fish moved less after the attack suggesting that predator model was perceived as a threat by the fish. The values on the y-axis represent the number of times each fish moved into a different section of the pool over the time observed. Grey lines indicate the average behavioral change in response to the predator attack for each individual fish, with the same symbols for individuals as in Fig. 2b.
On average, sticklebacks were less active after the simulated predator attack compared to before (t9 = 4.026, p = 0.003; Fig. 3b), which suggests that the fish perceived the model predator as a threat. There was no indication that the decline in activity reflects habituation to the testing environment: there was no decline in activity across the 3 min prior to the predator attack (1st minute = 11.23 sections, 2nd minute mean = 12 sections, 3rd minute mean = ll.95 sections). There was a trend for males to be more active than females after the attack (t8 = 2.248, p = 0.055; males: n = 4, mean ± sd = 27.34 ± 7.599 sections; females: n = 6, mean ± sd = 17.42 ± 6.343 sections). We did not detect a difference in body length between male and female fish (t7 = 0.472, p = 0.651; males: n = 4, mean ± sd = 42.5 ± 4.203 mm; females: n = 6, mean ± sd = 43.83 ± 4.622 mm), and did not detect a significant correlation between length and boldness (t8 = 0.6288, p = 0.547). Therefore, variation in activity among individuals or between males and females was not driven by differences in body size.
3.3. Learning
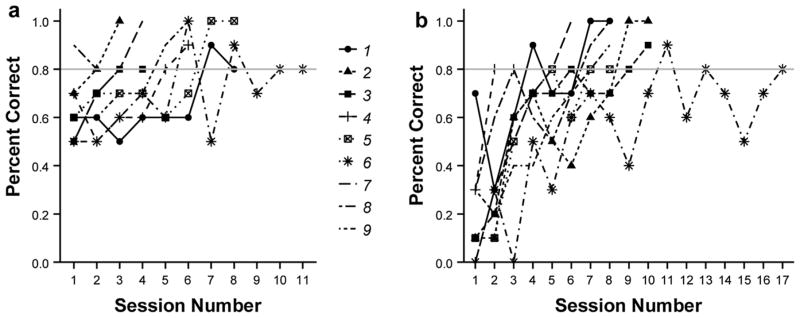
Sticklebacks learned to associate the yellow cup with food. Indeed, all individuals met the criterion (80% correct for two consecutive sessions) in the initial learning phase (Fig. 4a). During the first few sessions, individuals appeared to choose randomly, but average performance improved with subsequent training (Fig. 4a). On average, it took 5.78 sessions (sd = 2.86) to reach the criterion, but individuals differed: the fastest individual met the criterion in two sessions, while the slowest individual took eleven sessions to reach criterion.
Fig. 4.
Individual learning curves during the a) initial learning, and b) reversal learning discrimination phases. Lines represent the performance of individuals across sessions, with the same symbols for individuals as in Fig. 2b. Criterion was reached when the proportion of correct choices was greater than or equal to 0.8 (marked by horizontal grey line) on two consecutive sessions. Note the poor performance during the first and second sessions of the reversal learning phase, suggesting that fish initially relied on the previously-learned association.
3.4. Flexibility
It took longer for fish to learn to associate the blue cup with food after they had been trained to associate the yellow cup with food. Indeed, performance during the first session of reversal training was very poor: on average, sticklebacks went to blue, the previously-learned cue, during 79% of the trials in the first session, which suggests that sticklebacks were relying on a previously-learned association (Fig. 4b). On average, it took 8.67 sessions (sd = 3.77) to reach criterion during the reversal phase (Fig. 4b). The fastest fish during this phase met the criterion in three sessions while the slowest learner never reached criterion, even after 17 sessions. Interestingly, the individual that took the longest to reach criterion during the initial learning phase also took the longest to reach criterion during the reversal learning phase.
3.5. Testing the predictions of coping styles theory
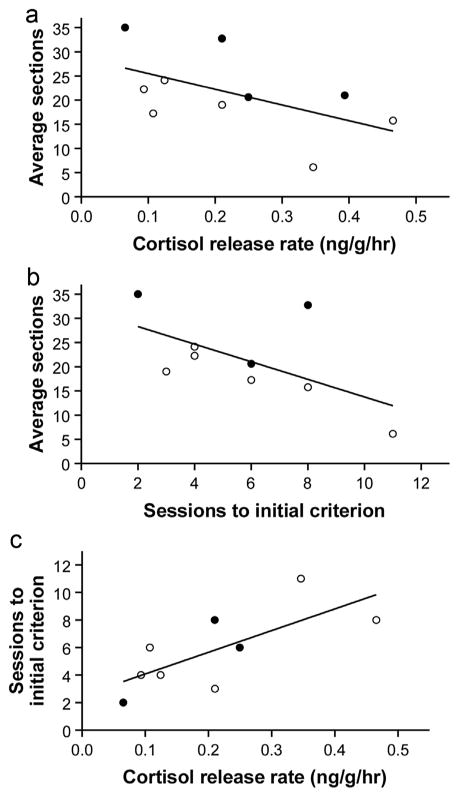
Coping styles theory predicts that bolder individuals are less physiologically responsive to stress. Consistent with this hypothesis, we found that bolder fish had a lower average cortisol release rates across the two weeks sampled (partial r controlling for sex = −0.707, n = 10, p = 0.022, Fig. 5a). We also found support for the prediction that bolder individuals learn faster: bolder fish reached criterion during in the color discrimination task in fewer trials than relatively shy fish (partial r controlling for sex = −0.712, n = 9, p = 0.032, Fig. 5b). Moreover, the individuals that released more cortisol over time were also the ones that took more trials to reach criterion in the color discrimination task (r = 0.722, n = 9, p = 0.028, Fig. 5c).
Fig. 5.
Scatterplots showing results consistent with coping styles theory. Each data point represents a different individual, coded by sex (open circles = females, closed circles = males) a) Bolder individuals (i.e., fish that entered more sections after the predator attack) had lower average cortisol release rates across two weeks. b) Bolder individuals reached criterion in the initial color discrimination task in fewer sessions. c) Individual fish with higher average cortisol release rates took more sessions to reach criterion in the initial color discrimination task.
Coping styles theory also predicts that bold (proactive) individuals are more routinized and less behaviorally flexible. However, we did not detect any statistically significant relationships between the number of sessions to criterion during reversal training and any of the other measures, and indeed the direction of the observed relationships between traits is opposite to our predictions. For example, we predicted a negative relationship between initial color discrimination performance and reversal learning. However, sessions to initial criterion and sessions to reversal criterion were not negatively correlated, and indeed the (non-signficant) pattern was positive (r = 0.467, n = 9, p = 0.206, Suppl. Fig. 1a). Similarly, contrary to our predictions, a negative correlation was not observed between boldness and reversal learning, and indeed the (non-significant) pattern was that bolder individuals performed better during reversal learning (partial r controlling for sex = −0.491, n = 9, p = 0.180, Suppl. Fig. 1b). Finally, again contrary to our original predictions, we observed more stress reactive individuals took longer to reach the reversal criterion, although this was not statistically significant (r = 0.458, n = 9, p = 0.2155, Suppl. Fig. 1c).
It is possible that the number of mistakes during the first reversal session might be a better measure of behavioral flexibility than time to criterion (Bebus et al., 2016; Bond et al., 2007). For example, individual differences in time to reversal criterion might reflect a general difference in learning performance rate, whereas initial performance (number of mistakes) might more effectively capture how individuals immediately respond to a change in the environment. If this is the case, then proactive individuals might have made more mistakes during the first reversal session. However, we found no evidence that proactive individuals made more mistakes; we did not detect any significant correlations between the number of mistakes and the other measures (time to criterion during initial learning (r = 0.064, n = 9, p = 0.870), cortisol (0.483, n = 9, p = 0.188), boldness (partial r controlling for sex = −0.080, n = 9, p = 0.838); Suppl. Fig. 1d–f).
4. Discussion
We observed strong inter-individual variation among wild-caught individual sticklebacks from a natural population. These results are consistent with several studies that have documented extensive variation among individuals within stickleback populations (e.g. Bell, 2005; Dingemanse et al., 2007; Fürtbauer et al., 2015; Huntingford, 1976). For example, some sticklebacks were consistently more bold than others toward a model predator. Stable individual differences in boldness is consistent with previous studies showing dramatic inter-individual differences in boldness in this species (Bell, 2005; Huntingford, 1976; Mamuneas et al., 2015) and with the growing literature on personality in animals generally (Bell et al., 2009). Also consistent with past studies on fish boldness was the trend that males are bolder than females (e.g., Brown et al., 2007; Harris et al., 2010; King et al., 2013). Additionally, we also observed that some individual sticklebacks were consistently more stress reactive than others. This result agrees with previous studies in stickleback (Fürtbauer et al., 2015) and other animals showing intraspecific variation in the responsiveness of the hypothalamic-pituitary-adrenal axis to stressors (e.g. Boulton et al., 2015; Cook et al., 2012; Cockrem et al., 2009; Ouyang et al., 2011). Finally, some individual sticklebacks were also clearly faster at learning a color discrimination task than others, and there was substantial inter-individual variation in performance on a reversal learning task. Examining the correlations of these facets, previous work on stickleback found basal cortisol and anti-predator behavior covaried within individuals across multiple trials (Fürtbauer et al., 2015). In the current study we found evidence that across individuals boldness covaried with stress reactivity and initial learning as predicted by coping styles theory, but we found no support for the prediction that timid, stress reactive individuals are more behaviorally flexible even after controlling for the effect of sex on boldness.
One of the strengths of this study is that our criterion-based learning protocol captured a wide swath of variation in performance on a cognitive task. While our approach required an extended pre-training period and prolonged testing of certain individuals (e.g. it took one individual 11 sessions to finally reach criterion during initial testing), all of the animals in this study eventually learned to associate the yellow cup with the food reward. Further evidence of the effectiveness of our training protocol is revealed by the animals’ poor performance during the first session of reversal testing: whereas individuals appeared to choose randomly between the two colors during the first few sessions of initial testing, they showed a strong preference for yellow (the previously rewarded color) during the first few sessions of reversal testing (Fig. 4b). Yet, again the fish were all eventually able to reach the reversal criterion, suggesting that the use of this paradigm was appropriate for this system.
Consistent with the predictions of coping styles theory and with previous studies (Hennessy et al., 2001; Raoult et al., 2012; Tudorache et al., 2013; but see Boulton et al., 2015; Ferrari et al., 2013), individual sticklebacks that were bolder were also less stress reactive. Moreover, less stress reactive individuals learned faster, and bolder individuals learned faster (similar to Bebus et al., 2016). While the effects of cortisol on learning and memory can be quite dynamic, these findings are consistent with previous research suggesting that long-term baseline elevations in cortisol impede learning (Lupien et al., 1998, 2009). The positive association between boldness and cognitive performance is consistent with other studies (e.g. Dugatkin and Alfieri, 2003; Guillette et al., 2009; Sneddon, 2003; though see Cole and Quinn, 2011), and with previous work on trout that found that bold individuals were more behaviorally labile than their timid counterparts (Frost et al., 2007; Thomson et al., 2012). In some previous work, however, it is difficult to know whether bold individuals learned faster because they were more exploratory and had more opportunities to interact with their environments and therefore faster to learn (DePasquale et al., 2014; Sih and Del Giudice, 2012). Importantly, we ran trials until the fish found the correct cup, even if the correct cup was not their first choice, which standardized the opportunity to gain information from each trial for all individuals in this study (Griffen et al., 2015). Therefore, bold individuals were not able to gather any more information that timid individuals.
Our results suggest that there are many advantages to being bold and proactive: proactive individuals have lower cortisol (chronically high cortisol can be harmful, Sapolsky et al., 1985, 1990) and they are better learners. Therefore what is maintaining timid individuals within this population? A popular idea from coping styles theory is that proactive individuals are better at learning novel contingencies, but become routinized and have a difficult time breaking away from established behavioral patterns when learning contingencies change (e.g., Bolhuis et al., 2004). According to this reasoning, reactive individuals are maintained within populations because they are behaviorally flexible, and perform better when the environment changes.
However, we found no evidence that proactive individuals were less behaviorally flexible: neither of our two measures of behavioral flexibility (number of mistakes and time to criterion during the reversal phase) were significantly correlated with the other traits. One possible explanation for the failure to detect tradeoffs with behavioral flexibility is because we did not have sufficient statistical power to detect them. While the use of a high resolution approach was very successful in producing a rich dataset that allowed us to simultaneously explore multiple facets of coping styles within each individual, it was extremely time intensive and resulted in a limited sample size. However, the observed correlation coefficients between behavioral flexibility and the other traits ranged from 0.4 to 0.46, or ‘medium-large’ effect sizes (Cohen, 1992), and the direction of the relationships were opposite to those predicted.
A lack of positive results could also be due to the learning paradigm chosen. Reversal learning is commonly used to measure behavioral flexibility (Stamps, 2015), but the exact behavioral mechanisms driving performance of this task are unclear (Izquierdo and Jentsch, 2012; Izquierdo et al., 2016). Traditionally, performance during reversal learning was largely thought to reflect differences in inhibition control. However, more recent studies on rats of the underlying neural substrates suggest that differences related to sensory perception and awareness of unexpected results are also likely to be involved (Chudasama and Robbins, 2003; Schoenbaum et al., 2009). In other words, there might be multiple independent neural networks underlying reversal learning performance (Jentsch et al., 2014) and the facets linked to proactive/reactive behavioral types may be difficult to isolate with this paradigm. Additionally, tasks aimed at measuring attention sensitivity and behavioral inhibition separately (e.g., 5 choice serial reaction time task [5CSRTT]) in rats find that these behaviors can be manipulated independently (Chudasama and Robbins, 2003; Paine et al., 2007). So perhaps the use of tasks aimed at isolating specific behavioral factors underlying reversal learning performance (e.g., 5CSRTT or barrier tasks) would provide better insights to coping styles predictions.
Another possibility for the failure to detect tradeoffs with behavioral flexibility is that it might not be as costly as is often assumed. While it has been suggested that there are cognitive trade-offs between novel learning and learning flexibility due to physiological and behavioral constraints (Chittka et al., 2009; Koolhaas et al., 1999; Sih and Del Giudice, 2012), there are mixed results in the literature about the relationship between performance during initial and reversal learning (e.g., Bebus et al., 2016; Boogert et al., 2006; Guillette et al., 2015; Head et al., 1998) and some authors have suggested that flexibility and initial learning are independent (Ferrari et al., 2013; Koolhaas et al., 2010; Steimer et al., 1997), or even positively correlated (Galsworthy et al., 2002; Matzel et al., 2003). For example, as in humans (Plomin, 2001), there might be a domain-general intelligence factor (g) in nonhuman animals that causes some individuals to be simply “smarter” relative to others in the population, and some individuals consistently do better on most cognitive tasks (Galsworthy et al., 2002; Matzel et al., 2003). Our findings are consistent with this idea, in that performance during initial and reversal learning were weakly (but nonsignificantly) positively correlated (r ~ 0.4), and the individual that took the longest to reach criterion during the initial learning phase also took the longest to reach criterion during the reversal learning phase. Finally, it is possible that tradeoffs with behavioral flexibility do not help to maintain reactive individuals within populations, and there are other costs of being proactive such as increased vulnerability to predation (Bell and Sih, 2007; Smith and Blumstein, 2008).
There is growing interest in understanding the physiological and cognitive causes of behavioral variation. Testable theory such as coping styles provides a framework for understanding how and why different axes of variation are associated. Coping styles theory surmises that there is an entire suite of traits that distinguish between proactive and reactive individuals. Until recently, coping styles research has largely focused on extreme, discrete variation that results from selective breeding programs. This study adds to the growing number of studies in a wide range of species that have developed paradigms (e.g., criterion-based learning: Guillette et al., 2011, 2015; stress response: Boulton et al., 2015) that can accurately measure natural intraspecific variation of different facets of coping styles in a way that is objective and non-biased.
Compliance with ethical standards
The authors declare that they have no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in this study involving animals were in accordance with the ethical standards of the University of Illinois, Urbana Champaign (IACUC protocol # 15077). This article does not contain any studies with human participants performed by any of the authors.
Acknowledgments
We are grateful to the members of the Bell lab for their help throughout this project, and we would like to specifically thank Wen Li Chen for her help collecting the water samples for the cortisol measures. We would also like to thank Sean Ehlman and Rebecca Halpin of the University of California Davis for their help with fish collection. Financial support was provided the National Science Foundation’s Integrative Graduate Education and Research Traineeship program and by the University of Illinois.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.beproc.2016.12.011.
References
- Aubin-Horth N, Deschênes M, Cloutier S. Natural variation in the molecular stress network correlates with a behavioural syndrome. Horm Behav. 2012;61:140–146. doi: 10.1016/j.yhbeh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Bebus SE, Small TW, Jones BC, Elderbrock EK, Schoech SJ. Associative learning is inversely related to reversal learning and varies with nestling corticosterone exposure. Anim Behav. 2016;111:251–260. [Google Scholar]
- Bell AM, Hankison SJ, Laskowski KL. The repeatability of behaviour: a meta-analysis. Anim Behav. 2009;77:771–783. doi: 10.1016/j.anbehav.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AM. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus) J Evol Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- Bensky MK, Gosling SD, Sinn DL. The world from a dog’s point of view: a review and synthesis of dog cognition research. Adv Study Anim Behav. 2013;45:209–406. [Google Scholar]
- Benus RF, Den Daas S, Koolhaas JM, Van Oortmerssen GA. Routine formation and flexibility in social and non-social behaviour of aggressive and non-aggressive male mice. Behaviour. 1990;112:176–193. [Google Scholar]
- Bolhuis JE, Schouten WG, de Leeuw JA, Schrama JW, Wiegant VM. Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav Brain Res. 2004;152:351–360. doi: 10.1016/j.bbr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica) J Comp Psychol. 2007;121:372–379. doi: 10.1037/0735-7036.121.4.372. [DOI] [PubMed] [Google Scholar]
- Boogert NJ, Reader SM, Laland KN. The relation between social rank, neophobia and individual learning in starlings. Anim Behav. 2006;72:1229–1239. [Google Scholar]
- Boulton K, Couto E, Grimmer AJ, Earley RL, Canario AVM, Wilson AJ, Walling CA. How integrated are behavioral and endocrine stress response traits? A repeated measures approach to testing the stress-coping style model. Ecol Evol. 2015;5:618–633. doi: 10.1002/ece3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Braithwaite VA. Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim Behav. 2004;68:1325–1329. [Google Scholar]
- Brown C, Burgess F, Braithwaite VA. Heritable and experiential effects on boldness in a tropical poeciliid. Behav Ecol Sociobiol. 2007;62:237–243. [Google Scholar]
- Budaev S, Brown C. Personality traits and behaviour. In: Brown CKL, Krause J, editors. Fish Cognition and Behaviour. 2. Blackwell; Cambridge, United Kingdom: 2011. pp. 135–156. [Google Scholar]
- Canestrelli D, Bisconti R, Carere C. Bolder takes all? The behavioral dimension of biogeography. Trends Ecol Evol. 2016;31:35–43. doi: 10.1016/j.tree.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Carere C, Locurto C. Interaction between animal personality and animal cognition. Curr Zool. 2011;57:491–498. [Google Scholar]
- Carere C, Caramaschi D, Fawcett TW. Covariation between personalities and individual differences in coping with stress: converging evidence and hypotheses. Curr Zool. 2010;56:728–740. [Google Scholar]
- Chittka L, Skorupski P, Raine NE. Speed accuracy trade-offs in animal decision making. Trends Ecol Evol. 2009;24:400–407. doi: 10.1016/j.tree.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrem JF, Barrett DP, Candy EJ, Potter MA. Corticosterone responses in birds: individual variation and repeatability in Adelie penguins (Pygoscelisadeliae) and other species, and the use of power analysis to determine sample sizes. Gen Comp Endocrinol. 2009;163:158–168. doi: 10.1016/j.ygcen.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cole EF, Quinn JL. Personality and problem-solving performance explain competitive ability in the wild. Proc R Soc B. 2011:rspb20111539. doi: 10.1098/rspb.2011.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KV, O’Connor CM, McConnachie SH, Gilmour KM, Cooke SJ. Condition dependent intra-individual repeatability of stress-induced cortisol in a freshwater fish. Comp Biochem Phys A. 2012;161:337–343. doi: 10.1016/j.cbpa.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Costantini D, Ferrari C, Pasquaretta C, Cavallone E, Carere C, von Hardenberg A, Réale D. Interplay between plasma oxidative status, cortisol and coping styles in wild alpine marmots, Marmota marmota. J Exp Biol. 2012;215:374–383. doi: 10.1242/jeb.062034. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Buwalda B, Koolhaas JM. Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.07.008. [DOI] [PubMed] [Google Scholar]
- DePasquale C, Wagner T, Archard GA, Ferguson B, Braithwaite VA. Learning rate and temperament in a high predation risk environment. Oecologia. 2014;176:661–667. doi: 10.1007/s00442-014-3099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Dugatkin LA, Alfieri MS. Boldness, behavioral inhibition and learning. Ethol Ecol Evol. 2003;15:43–49. [Google Scholar]
- Ellis T, James JD, Stewart C, Scott AP. A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. J Fish Biol. 2004;65:1233–1252. [Google Scholar]
- Fürtbauer I, Pond A, Heistermann M, King AJ. Personality, plasticity and predation: linking endocrine and behavioural reaction norms in stickleback fish. Funct Ecol. 2015;29:931–940. [Google Scholar]
- Ferrari C, Pasquaretta C, Carere C, Cavallone E, von Hardenberg A, Réale D. Testing for the presence of coping styles in a wild mammal. Anim Behav. 2013;85:1385–1396. [Google Scholar]
- Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU. Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc R Soc B. 2007;274:333–339. doi: 10.1098/rspb.2006.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Monleón S, Plomin R. Evidence for general cognitive ability (g) in heterogeneous stock mice and an analysis of potential confounds. Genes Brain Behav. 2002;1:88–95. doi: 10.1034/j.1601-183x.2002.10204.x. [DOI] [PubMed] [Google Scholar]
- Girvan JR, Braithwaite VA. Population differences in spatial learning in three–spined sticklebacks. Proc R Soc B. 1998;265:913–918. [Google Scholar]
- Griffen AS, Guillette LM, Healy SD. Cognition and personality: an analysis of an emerging field. Trends Ecol Evol. 2015;30:207–214. doi: 10.1016/j.tree.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Reddon AR, Hurd PL, Sturdy CB. Exploration of a novel space is associated with individual differences in learning speed in black capped chickadees, Poecile atricapillus. Behav Process. 2009;82:265–270. doi: 10.1016/j.beproc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Reddon AR, Hoeschele M, Sturdy CB. Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc R Soc B. 2011;278:767–773. doi: 10.1098/rspb.2010.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LM, Hahn AH, Hoeschele M, Przyslupski AM, Sturdy CB. Individual differences in learning speed, performance accuracy and exploratory behaviour in black-capped chickadees. Anim Cogn. 2015;18:165–178. doi: 10.1007/s10071-014-0787-3. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Naguib M, Griffen AS. Individual differences in cognition and personality. Behav Process. 2017;134:1–3. doi: 10.1016/j.beproc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw. 2010;33:1–22. [Google Scholar]
- Harris S, Ramnarine IW, Smith HG, Pettersson LB. Picking personalities apart: estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata. Oikos. 2010;119:1711–1718. [Google Scholar]
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and β-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Voith VL, Mazzei SJ, Buttram J, Miller DD, Linden F. Behavior and cortisol levels of dogs in a public animal shelter, and an exploration of the ability of these measures to predict problem behavior after adoption. Appl Anim Behav Sci. 2001;73:217–233. doi: 10.1016/s0168-1591(01)00139-3. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hernandez-Lloreda MV, Call J, Hare B, Tomasello M. The structure of individual differences in the cognitive abilities of children and chimpanzees. Psychol Sci. 2009;21:102–110. doi: 10.1177/0956797609356511. [DOI] [PubMed] [Google Scholar]
- Huntingford FA. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim Behav. 1976;24:245–260. [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: an updated perspective. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Fürtbauer I, Mamuneas D, James C, Manica A. Sex-differences and temporal consistency in stickleback fish boldness. PLoS One. 2013;8:e81116. doi: 10.1371/journal.pone.0081116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress–physiology. Neurosci Biobehav Rev. 1999;23:925–936. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Kim JJ, Lee HJ. Early life manipulations alter learning and memory in rats. Neurosci Biobehav Rev. 2012;36:1985–2006. doi: 10.1016/j.neubiorev.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locurto C. Individual differences and animal personalities. Comp Cogn Behav Rev. 2007;2:67–78. [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, De Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neuro. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mamuneas D, Spence AJ, Manica A, King AJ. Bolder stickleback fish make faster decisions, but they are not less accurate. Behav Ecol. 2015;26:91–96. [Google Scholar]
- Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, et al. Individual differences in the expression of a general learning ability in mice. J Neurosci. 2003;23:6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McGhee KE, Pintor LM, Suhr EL, Bell AM. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Funct Ecol. 2012;26:932–940. doi: 10.1111/j.1365-2435.2012.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PSA, Pulman KG, Pottinger TG. Extinction of a conditioned response in rainbow trout selected for high or low responsiveness to stress. Horm Behav. 2004;46:450–457. doi: 10.1016/j.yhbeh.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Niemela PT, Vainikka A, Forsman JT, Loukola OJ, Kortet R. How does variation in the environment and individual cognition explain the existence of consistent behavioral differences. Ecol Evol. 2013;2:457–464. doi: 10.1002/ece3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nippak PMD, Milgram NW. An investigation of the relationship between response latency across several cognitive tasks in the beagle dog. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:371–377. doi: 10.1016/j.pnpbp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Odling-Smee L, Braithwaite VA. The influence of habitat stability on landmark use during spatial learning in the threespined stickleback. Anim Behav. 2003;65:701–707. [Google Scholar]
- Ouyang JQ, Hau M, Bonier F. Within seasons and among years: when are corticosterone levels repeatable? Horm Behav. 2011;60:559–564. doi: 10.1016/j.yhbeh.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Pottinger TG, Carrick TR, Øverli E, Winberg S. Differences in behaviour between rainbow trout selected for high-and low-stress responsiveness. J Exp Biol. 2002;205:391–395. doi: 10.1242/jeb.205.3.391. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Winberg S, Pottinger TG. Behavioral and neuroendocrine correlates of selection for stress responsiveness in rainbow trout—a review. Integr Comp Biol. 2005;45:463–474. doi: 10.1093/icb/45.3.463. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Sørensen C, Pulman KG, Pottinger TG, Korzan W, Summers CH, Nilsson GE. Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev. 2007;31:396–412. doi: 10.1016/j.neubiorev.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA. Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Pintor L, McGhee KE, Roche DP, Bell AM. Individual variation in foraging behavior reveals a tradeoff between flexibility and performance in a top predator. Behav Ecol Sociobiol. 2014;68:1711–1722. doi: 10.1007/s00265-014-1779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R. The genetics of g in human and mouse. Nat Rev Neurosci. 2001;2:136–141. doi: 10.1038/35053584. [DOI] [PubMed] [Google Scholar]
- Pottinger TG, Carrick TR. Modification of the plasma cortisol response to stress in rainbow trout by selective breeding. Gen Comp Endocrinol. 1999;116:122–132. doi: 10.1006/gcen.1999.7355. [DOI] [PubMed] [Google Scholar]
- Pressley PH. Parental effort and the evolution of nest-guarding tactics in the threespine stickleback, Gasterosteus aculeatus L. Evolution. 1981;35:282–295. doi: 10.1111/j.1558-5646.1981.tb04887.x. [DOI] [PubMed] [Google Scholar]
- Raoult V, Brown C, Zuberi A, Williamson JE. Blood cortisol concentrations predict boldness in juvenile mulloway (Argyosomus japonicus) J Ethol. 2012;30:225–232. [Google Scholar]
- Roberts ML, Buchanan KL, Hasselquist D, Bennett AT, Evans MR. Physiological, morphological and behavioural effects of selecting zebra finches for divergent levels of corticosterone. J Exp Biol. 2007;210:4368–4378. doi: 10.1242/jeb.007104. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez ML, Huntingford FA, Øverli Ø, Thornqvist PO, Hoglund E. Response to environmental change in rainbow trout selected for divergent stress coping styles. Physiol Behav. 2011;102:317–322. doi: 10.1016/j.physbeh.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal dam- age associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short-and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr Zool. 2011;57:514–530. [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebire M, Katsiadaki I, Scott AP. Non-invasive measurement of 11-ketotestosterone, cortisol and androstenedione in male three-spined stickleback (Gasterosteus aculeatus) Gen Comp Endocrinol. 2007;152:30–38. doi: 10.1016/j.ygcen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Sebire M, Katsiadaki I, Scott AP. Further refinement of the non-invasive procedure for measuring steroid production in the male three-spined stickleback Gasterosteus aculeatus. J Fish Biol. 2009;75:2082–2094. doi: 10.1111/j.1095-8649.2009.02409.x. [DOI] [PubMed] [Google Scholar]
- Sih A, Del Giudice M. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc B. 2012;367:2762–2772. doi: 10.1098/rstb.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT. Fitness consequences of personality: a meta-analysis. Behav Ecol. 2008;19:448–455. [Google Scholar]
- Sneddon LU. The bold and the shy: individual differences in rainbow trout. J Fish Biol. 2003;62:971–975. [Google Scholar]
- Sorensen C, Johansen IB, Øverli Ø. Neural plasticity and stress coping in teleost fishes. Gen Comp Endocrinol. 2013;181:25–34. doi: 10.1016/j.ygcen.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Stamps JA. Individual differences in behavioural plasticities. Biol Rev. 2015;91:534–567. doi: 10.1111/brv.12186. [DOI] [PubMed] [Google Scholar]
- Steimer T, La Fleur S, Schulz PE. Neuroendocrine correlates of emotional reactivity and coping in male rats from the Roman high (RHA/Verh)-and low (RLA/Verh)-avoidance lines. Behav Genet. 1997;27:503–512. doi: 10.1023/a:1021448713665. [DOI] [PubMed] [Google Scholar]
- Stein LR, Bell AM. Consistent individual differences in fathering in threespined stickleback Gasterosteus aculeatus. Curr Zool. 2012;58:45–52. doi: 10.1093/czoolo/58.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milrgram NW. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Mem. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JS, Watts PC, Pottinger TG, Sneddon LU. Plasticity of boldness in rainbow trout: oncorhynchus mykiss: do hunger and predation influence risk-taking behaviour? Horm Behav. 2012;61:750–757. doi: 10.1016/j.yhbeh.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Thornton A, Lukas D. Individual variation in cognitive performance: developmental and evolutionary perspectives. Philos Trans R Soc B. 2012;367:2773–2783. doi: 10.1098/rstb.2012.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudorache C, Schaaf MJ, Slabbekoorn H. Covariation between behaviour and physiology indicators of coping style in zebrafish (Danio rerio) J Endocrinol. 2013;219:251–258. doi: 10.1530/JOE-13-0225. [DOI] [PubMed] [Google Scholar]
- Verbeek ME, Drent PJ, Wiepkema PR. Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav. 1994;48:1113–1121. [Google Scholar]
- Wolf M, Sander van Doorn G, Weissing FJ. Evolutionary emergence of responsive and unresponsive personalities. Proc Natl Acad Sci. 2008;105:15825–15830. doi: 10.1073/pnas.0805473105. [DOI] [PMC free article] [PubMed] [Google Scholar]