Abstract
Retinopathy of prematurity (ROP) is a major cause of childhood blindness in the world and is caused by oxygen-induced damage to the developing retinal vasculature, resulting in hyperoxia-induced vaso-obliteration and subsequent delayed retinal vascularization and hypoxia-induced pathological neovascularization driven by vascular endothelial growth factor (VEGF) signaling pathway in retina. Current anti-VEGF therapy has shown some effective in a clinical trial, but is associated with the unintended effects on delayed eye growth and retinal vasculature development of preterm infants. Notably, cellular responses to hypoxia are characterized by robust increases in extracellular adenosine production and the markedly induced adenosine receptors, which provide a novel target for preferential control of pathological angiogenesis without affecting normal vascular development. Here, we review the experimental evidence in support of adenosine receptor-based therapeutic strategy for ROP, including the aberrant adenosine signaling in oxygen-induced retinopathy and the role of three adenosine receptor subtypes (A1R, A2AR, A2BR) in development and treatment of ROP using oxygen-induced retinopathy models. The clinical and initial animal evidence that implicate the therapeutic effect of caffeine (a non-selective adenosine receptor antagonist) in treatment of ROP are highlighted. Lastly, we discussed the translational potential as well therapeutic advantage of adenosine receptor- and caffeine-based therapy for ROR and possibly other proliferative retinopathy.
Keywords: Adenosine; Adenosine (A1, A2A, A2B) receptors; Retinopathy of prematurity; Oxygen-induced retinopathy; Caffeine
1. Retinopathy of prematurity (ROP) is a leading cause of childhood blindness
Retinopathy of prematurity (ROP) is a disease of premature infants which disrupts normal retinal vascularization (Fleck and McIntosh, 2008). With increased survival of extremely premature infants due to advances in neonatology, ROP has become a major cause of childhood blindness (50,000–100,000 cases/year) in many parts of the world (Fleck and McIntosh, 2008; Gilbert, 2008). ROP is caused by oxygen-induced damage to the developing retinal vasculature (Gilbert, 2008; Chen et al., 2008; Dhaliwal et al., 2009) and is characterized by the hyperoxia-induced vaso-obliteration, subsequent delayed retinal vascularization, and hypoxia-induced pathological neovascularization (Fleck and McIntosh, 2008; Cavallaro et al., 2014) driven by hypoxia-induced factor-1α (HIF-1α) signaling pathway and increased vascular endothelial growth factor (VEGF) levels in retina (Cavallaro et al., 2014; Penn et al., 2008) (see Fig. 1A). Characteristic pathological changes include vaso-obliteration and proliferation of abnormal fibrovascular tissue at the border of the vascularised and non-vascularised retina (Fleck and McIntosh, 2008). Conventional therapies for ROP are limited to laser to ablate the avascular retina to prevent retinal detachment caused by ROP (Clark and Mandal, 2008), but the efficacy of ablative laser therapy are limited, and are associated with destruction to retina causing clinically significant loss of visual field. Anti-VEGF therapy (e.g. intra-vitreal injection of anti-VEGF-A antibody bevacizumab) was also proposed (Clark and Mandal, 2008) and has been recently shown to be effective in a randomized, controlled trial (Mintz-Hittner et al., 2011). However, the long term effect of intra-vitreal bevacizumab remains unclear with reported persistent avascular retina (Tokunaga et al., 2014) and recurrent intra-vitreal neovascularization (Hu et al., 2012). Importantly, VEGF acts as an angiogenic and a neurotrophic factor for normal retinal neural and vascular development (Tokunaga et al., 2014; Robinson et al., 2001; McCloskey et al., 2013). There are concerns on the unintended effects of anti-VEGF agents on delayed eye growth and retinal vasculature development of preterm infants who are still forming new blood vessels in many different organ systems (Nishijima et al., 2007; Saint-Geniez et al., 2008). Thus, there is a critical need to develop more effective and preferably non-invasive prophylactic and therapeutic strategies for ROP.
Fig. 1.
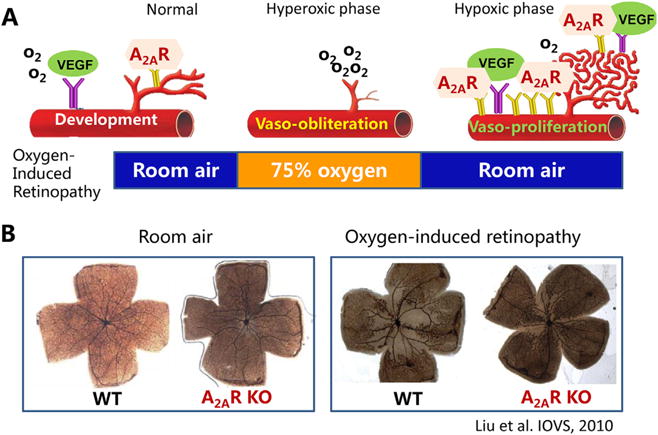
A2ARs preferentially modulate pathological angiogenesis without affecting normal retinal vascular development. (A) Adenosine receptors play a role in normal retinal vascular development (left panel) and pathological angiogenesis in oxygen-induced retinopathy model of ROP (middle and right panels). (B) In oxygen-induced retinopathy in mice, genetic inactivation of the A2AR selectively attenuates oxygen-induced retinopathy without affecting retinal vascular development under room air.
2. Normal retinal vascular development and pathological angiogenesis in ROP
An ideal therapeutic strategy for ROP is to selectively control pathological neovascularization/angiogenesis without affecting normal retinal vasculature during postnatal development. The key to this strategy is to distinguish pathological angiogenesis process from normal retinal vascular development. Normal retinal vascular development starts with the de novo formation of blood vessels from endothelial precursor cells (vasculogenesis) (Lutty and McLeod, 2003; Gariano, 2003). This is followed by development of new blood vessels by budding from existing blood vessels (angiogenesis) (Gariano, 2003). A critical event in the pathogenesis of ROP is oxygen-induced damage to the developing retinal vasculature. ROP occurs in two distinct phases: first, the developing retina is exposed to a relatively hyperoxic environment, which damages developing retinal vessels, (Aiello et al., 1994; Alon et al., 1995). Consequently, retinal vascularization is delayed, resulting in vaso-obliteration. Second, as the avascular retina becomes critically hypoxic, increased VEGF production leads to physiological revascularization of the central retina and pathological angiogenesis with formation of preretinal vascular tufts (Fleck and McIntosh, 2008; Lutty and McLeod, 2003), ultimately resulting in traction retinal detachment and blindness. Oxygen-induced retinopathy (OIR) is an animal model of ROP that recapitulates some characteristic pathophysiological features of ROP, including vaso-obliteration, physiological revascularization and pathological angiogenesis (Fleck and McIntosh, 2008; Aiello et al., 1994; Alon et al., 1995). Normal vascular development and pathological angiogenesis share some common pathways: HIF-1α and angiogenic factors such as VEGF are involved in both processes (Lutty and McLeod, 2003; Gariano, 2003). Distinct molecular and morphological processes have been documented for those processes. While developmental and physiological vascularization is a highly organized process, producing distinct superficial and deep vasculature plexuses in retina (Gariano, 2003), pathological angiogenesis generates new vessels in the preretinal area that are unorganized and leaky, with a tortuous architecture (Gariano, 2003; Powers et al., 2008). Furthermore, distinct cellular mechanisms may also underlie these two processes. For instance, astrocytes play an important role in normal development of retinal vasculatures by forming a template that provides guidance for the developing vascular network (Stone et al., 1995, 1996). However, VEGF released from astrocytes reactive to hypoxia is critical for pathological angiogenesis in the retina following OIR but not essential to developmental angiogenesis (Weidemann et al., ; Dorrell et al.,). Furthermore, a recent study indicates that deletion of bone marrow derived cells by transplantation may preferentially affect developmental angiogenesis than pathological angiogenesis (Zou et al.,). These distinct characteristics provide a biological basis for selectively targeting pathological angiogenesis without affecting normal postnatal vascular development.
3. Aberrantly enhanced adenosine signaling in retina of oxygen-induced retinopathy
Current therapeutic development of ROP focuses on directly targeting VEGF and HIF-1α signaling pathway (Cavallaro et al., 2014; Penn et al., 2008; Mintz-Hittner et al., 2011; Hartnett and Penn, 2012). However, cellular responses to hypoxia are characterized by robust increases in extracellular adenosine production (up to 100 folds) and signaling events through the markedly induced adenosine receptors (up to 50 folds) locally (Chen et al., 2013). Adenosine is a naturally occurring nucleoside that is distributed ubiquitously throughout the body as a metabolic intermediary and neuromodulator in the brain. Extracellular adenosine acts through multiple G-protein-coupled receptors (i.e. A1, A2A, A2B and A3) (Fredholm et al., 2001) to exert control over blood vessel growth in various tissues, including retina, both under normal and pathological conditions (Adair et al., 2005; Patz, 1980). All four adenosine receptor subtypes have been detected in retina (Cui et al., 2010; Brito et al., 2012).
Hypoxia triggers the surge in extracellular adenosine level as a result of transcriptional induction of CD73 and equivalent nucleotide transporter 1 as well as suppression of adenosine kinase, thereby elevating the capacity of local tissues for extracellular adenosine production (Lutty and McLeod, 2003; Elsherbiny et al., 2013a). Indeed, pioneering studies by Lutty and colleagues showed that 5′ nucleotidase and adenosine were reduced during the hyperoxia phase but markedly increased in the hypoxic retina using a neonatal canine model of OIR, (Lutty and McLeod, 2003; Takagi et al., 1996; Taomoto et al., 2000; Lutty et al., 2000). Adenosine accumulating locally during hypoxia permits the local control of retinal vessel growth (Lutty and McLeod, 2003). Pathological conditions such as OIR are also accompanied by the increases of local inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor–α (TNF-α), which lead to a delayed (~24 h), marked and sustained increases in adenosine receptor (particularly the A2AR and the A2BR) expression in tissues and inflammatory cells (Frick et al., 2009; Schingnitz et al., 2010; Linden, 2011). In OIR models of ROP, the expression of A2AR was suppressed during the hyperoxic phase, but markedly increased in hypoxic retina, supporting the possible involvement of adenosine-A2AR signaling in retinal pathological angiogenesis (Lutty and McLeod, 2003; Takagi et al., 1996; Taomoto et al., 2000; Lutty et al., 2000) (see Fig. 1A).
Locally increased adenosine levels and adenosine receptor signaling might represent a local “find-me” signal and serve as a unique “purinergic chemotaxis” for a local resolution to pathological conditions (as revealed by genetic KO studies) (Chen et al., 2013). Thus, the surge of adenosine level and the induction of adenosine receptors in the hypoxic phase of OIR (Lutty and McLeod, 2003) may constitute a negative feedback and defense mechanism countering such pro-angiogenic statues triggered by hypoxia and HIF-1α-mediated expression of VEGF in retina. Increased adenosine-adenosine receptor signaling in hypoxic retina also offers an opportunity of targeting pathological angiogenesis of ROP with minimal effects on normal retinal vascular development. Consequently, we propose that A2AR activity in the retina has the potential to modulate normal retinal vascularization and/or pathological angiogenesis.
4. The role of adenosine receptors in development of ROP
Therapeutic potential of adenosine receptors-based therapy for ROP is supported by the ability of adenosine subtype receptors to modulate inflammation, neuroprotection and angiogenesis in retina. In particular, numerous studies support the role of adenosine receptors in modulating the angiogenic effects in various cell types and tissues (Adair, 2005), including cardiomyocyte (Deussen, 2000), skeletal muscle fiber (Lynge et al., 2001), skin (Feoktistov et al., 2009; Valls et al., 2009) and retina (Adair, 2005; Grant et al., 2001). The translational potential of adenosine receptor-based therapy for controlling proliferative retinopathy is substantiated by clinical evidence that clinical treatment of apnea of prematurity with caffeine (a non-selective adenosine receptor antagonist) reduced ROP related problems (Schmidt et al., 2007) (see below), and by genetic identification of the variants of the human A2AR gene that are associated with reduced risk of developing diabetic retinopathy (Charles et al., 2011).
4.1. A2AR and ROP
In the developing retina, immunoreactivity for adenosine and the A2A receptor (A2AR) are detected on endothelial cell precursors, angioblasts, and endothelial cells in formed blood vessels in the retina of newborn animals (Lutty and McLeod, 2003). In a neonatal canine model of OIR, the extracellular adenosine level is markedly increased in hypoxic retinal tissues supporting the possible involvement of A2ARs in the retinal vasoproliferation in OIR (Lutty and McLeod, 2003; Takagi et al., 1996). Thus, the A2AR activity in retina may contribute to modulation of normal retinal vascularization as well as pathological angiogenesis (Lutty and McLeod, 2003). Using A2AR knockout (KO) mice, we have demonstrated that genetic inactivation of the A2AR attenuates development of OIR pathology (Liu et al., 2010), as evidenced by: (a) reduced vaso-obliteration area in the center of the retina; (b) reduced pathological angiogenesis in retina; and (c) inhibition of hypoxia-induced VEGF gene expression. Notably, attenuation of pathological angiogenesis by the A2AR inactivation is selective for OIR since it does not affect the normal retinal vascularization during postnatal development (see Fig. 1B). This angiogenic role of the A2AR is also consistent with the ability of A2AR activation to increase angiogenesis in various cell types and tissues, including liver (Day et al., 2005), kidney (Okusa, 2002), skin (Montesinos et al., 1997) and retina (Taomoto et al., 2000), and with the ability of A2AR activation to modulate the expression of VEGF, a key regulator of tissue angiogenesis (Takagi et al., 1996; Grant et al., 1999). Collectively, these findings provide the direct evidence that the A2AR is critical for the development of OIR, and suggest a novel therapeutic approach of A2AR inactivation for ROP by targeting the pathological angiogenesis without affecting normal vascularization in the retina.
4.2. A2BR and ROP
Similar to A2ARs, the A2BRs are adenylyl-cyclase activating receptors, known to be expressed in the eye, and more specifically in the vasculature, microglia and macrophages (Saura et al., 2005; Brambilla et al., 2003; Boison et al., 1071; van Calker and Biber, 2005). Their expression is induced by oxidative stress (St Hilaire et al., 2008), and they control the expression of VEGF in various cells, including macrophages (Granata et al.,). In the cultured human retinal endothelial cells, pharmacological profiles are consistent with A2BR-mediated (but not with A1R- or A2AR-mediated) effects on growth factor expression and cell proliferation (Grant et al., 1999, 2001). Furthermore, studies with first generation of A2BR antagonists (Grant et al., 1999, 2001; Mino et al., 2001) and with ribozyme approach to inactivating A2BRs demonstrate that adenosine acting at the A2B receptors promotes pathological angiogenesis in retina through modulating VEGF level (Afzal et al., 2003). These results obtained with the A2BR antagonists are, however, intrinsically limited in their specificity. The exact role of the A2BR in normal retinal vascular development and the pathogenesis of OIR remains to be determined and no confirmatory OIR studies with genetic ablation of A2BRs are available.
4.3. A1R and ROP
The A1R mRNA and ligand binding are detected in developing retina (Brito et al., 2012) and is reduced in retina of OIR model (Zhang et al., 2015). Our characterization of the A1R KO and WT mice in normal and OIR model demonstrate that genetic deletion of the A1R does not affect normal retinal vascularization during postnatal development since ontogeny of the superficial, deep and intermedial layers of retinal vasculatures is largely indistinguishable between WT and A1R KO mice (Zhang et al., 2015). However, in OIR model, A1R activity distinctly controls hyperoxia-induced vaso-obliteration at postnatal day (P) 12 and hypoxia-induced revascularization at P17. Specifically, genetic deletion of the A1R reduces hyperoxia-induced retinal vaso-obliteration at P12. This effect of A1Rs on OIR is associated with A1R control of cellular apoptosis in the inner nuclear layer of retina at P12. At the vaso-proliferative phase (P17), A1R KO attenuates hypoxia-induced intra-retinal revascularization without affecting intra-vitreal neovascularization at P17. This effect of A1R KO on OIR is associated with the reduced number of endothelium tip cells, without modification of cellular proliferation and astrocytic activation (Zhang et al., 2015). Thus, A1R activity is not required for normal postnatal development of retinal vasculatures, but selectively controls hyperoxia-induced vaso-obliteration and hypoxia-driven revascularization by distinct cellular mechanisms.
5. Effective therapeutic window of AR actions on ROP
Retinal vasculature undergoes critical developmental changes postnatally: from P7 onward the superficial capillaries start sprouting vertically in retina to form first the deep then the intermediated vascular plexus in the retina of C57BL/6 mice (Smith et al., 1994; Stahl et al.,). Pathologic neovascularization formation might be particular sensitive to pharmacological manipulation at this stage. Furthermore, ROP is defined as a two-stage disease: the first stage is characterized by the vaso-obliteration while the second stage is characterized by hypoxia of the avascularized retina and resultant increase in neovascularization (Lutty and McLeod, 2003). Thus, it is critical to define the specific postnatal developmental stages and the specific disease course (hyperoxic vs hypoxic phases) that is sensitive to adenosine receptor modulation.
Adenosine-based actions are also presumably to be most evident at the hypoxic phase with the surge of adenosine level. Indeed, our preliminary study indicates the protection against pathological angiogenesis at the hypoxic phase (P17) by A2AR KO and by A2AR antagonists (KW6002) and by caffeine (non-selective adenosine receptor antagonists). However, detailed analysis indicated that A1R KO reduced avascular areas at during the hyperoxic phase (P12) (Zhang et al., 2015). Similarly, A2AR KO and caffeine was effective in protecting OIR not only at P17, but also at P12 (unpublished data). Our findings highlight the important function of adenosine signaling in modulating retinal vascular function even under hyperoxic environments. This may indicate that retinal vasculature development at P7–12 might be particular sensitive to interference since angiogenic sprouting from retinal superficial capillaries into the vitreous take places at P7. However, this finding is somewhat surprising since despite clearly vaso-obliteration at the retina center at the hyperoxic phase, there is no “hypoxia” in retina as shown by in vivo detection with nitroimidazole EF5 (Scott and Fruttiger, 2010) and the adenosine concentration and the expression of ecto-5′ nucleotidase (CD73) are low during the hyperoxic phase. The exact reason for this is not clear. If the hypothesis that the hyperoxia phase is the critical to AR-mediated protection against OIR is validated by future investigations, this finding suggests that the hyperoxic damage to developing retinal vasculatures is the primary and critical effect during ROP pathogenesis despite the fact that pathological angiogenesis is most evident at the hypoxic phase of ROP (Zhang et al., 2015).
6. Cellular (type) mechanism of adenosine receptor actions on ROP
ROP pathology is characterized by abnormal/pathological angiogenesis and the endothelial cell is a final common pathway of abnormal endothelial proliferation (Xu et al., 2012). In retina, endothelial tip cells are mainly located at the leading edge of vascular plexus and the fusion sites of the remodeling area. Numerous studies support the role of adenosine signaling in endothelial cell proliferation and migration in vitro and vascular growth in vivo (Adair, 2005). In retinal endothelial cells, A2AR activation increases production of VEGF and GLUT-1 (Takagi et al., 1996, 1998), indicating a pro-angiogenic effect of A2AR. Consistent with this, genetic inactivation of the A2AR reduces endothelial cell proliferation in OIR model (Liu et al., 2010). Thus, increased endothelial sprouting and proliferation likely play a major role in control of pathological angiogenesis in ROP (Xu et al., 2012; Horowitz and Simons, 2008; Carmeliet and Tessier-Lavigne, 2005). However, the exact role of the endothelial A2ARs remains to be determined by study using endothelial A2AR KO mice. Future studies of caffeine and KW6002 control of gene expression in the tip cell fraction of sprouting vessels in OIR would shed light on the transcriptional mechanism underlying AR control of tip cells in promoting retinal vascularization.
Control of retinal vascularization during development and OIR likely involves close interactions among endothelial cells, neurons and glial cells (microglial and astrocytes) (see Fig. 2). In particular, the interaction between endothelial tip cells and astrocytes plays a critical role in developmental blood vessel formation and physiological revascularization (Wood and Martin, 2002). Astrocytes play a significant role in angiogenesis in response to hypoxia through their high expression of VEGF (Dorrell and Friedlander, 2006). Studies in OIR models showed that the density of astrocytes in the retina decreases during hyperoxia and then increases following hypoxia (Chan-Ling et al., 1992; Downie et al., 2008), and that restoring retinal astrocytes reduces vascular pathology associated with OIR (Weidemann et al., ; Dorrell et al.,). Neuronal mechanisms in retina may also contribute to retinal vascularization of ROP, particularly during the hyperoxic phase. In the vaso-obliteration phase, hyperoxia induces apoptosis of ganglia cells and developing endothelial cells and inhibits endothelial cell proliferation and migration, resulting in vaso-obliteration (Aiello et al., 1994; Alon et al., 1995). Activation of retinal A1Rs has been shown to inhibit Ca++ channels in retinal ganglion cells of mini-slices (Sun et al., 2002; Santos et al., 2000), protect NMDA-induced cell death in cultured retinal neurons (Oku et al., 2004), and mediate the interleukin-6 effect on the survival of cultured retinal ganglion cells (Perigolo-Vicente et al., 2013). Consistent with the A1R-mediated neuroprotective effect, early studies indicated that cytotoxicity and cell death were generally more pronounced in neurons and astrocytes derived from A1R KO mice (Bjorklund et al., 2008; Dunwiddie and Masino, 2001; Johansson et al., 2001). In addition, in parallel with the avascular area, A2AR KO attenuates TUNEL-positive cells in the inner nuclear layer of retina (unpublished data), suggesting that A1R and A2AR KO probably protect against hyperoxia-induced damage to developing retinal vessels by modulating neuronal apoptosis.
Fig. 2.
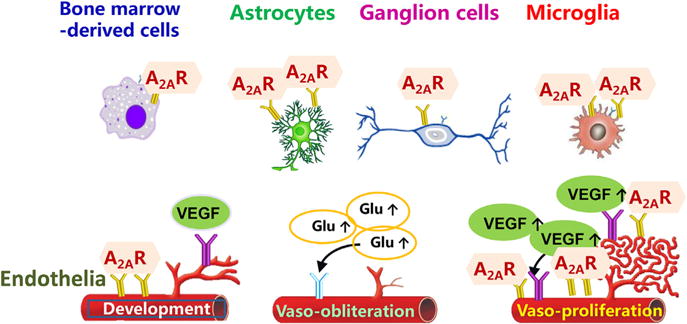
A2ARs in distinct cell types play differential roles in development of retinopathy of prematurity. A2ARs are expressed in endothelial cells as well as astrocytes, microglial cells, neuronal cells and bone marrow-derived cells. We postulate that the A2AR in certain cells (such as endothelial cells and bone marrow-derived cells) may play an important role in normal development of retinal vascularization while A2ARs in microglial cells may be upregulated in OIR model and contribute to pathological angiogenesis.
7. Caffeine and ROP
The translational potential of adenosine receptor-based therapy for controlling proliferative retinopathy is substantiated by its clinical potentials of caffeine treatment in reducing ROP related problems in premature infants. In a recent large prospective clinical phase III trial with caffeine treatment for apnea in premature infants, caffeine treatment apparently reduces the severity of ROP as compared to that of the control in a two-year follow-up observation (Schmidt et al., 2007). The therapeutic potential of caffeine for ROP is further supported by the ability of caffeine to control angiogenic factors HIF-1α and VEGF (Merighi et al., 2007; Ryzhov et al., 2007), angiogenesis (Ryzhov et al., 2007; Hsu et al., 2015) and apoptosis of endothelium cells (Li et al., 2013) and other vascular actions (Echeverri et al., 2010). This raises an exciting possibility that caffeine, the ubiquitous trimethylxanthine that is widely used in premature infants with apnea of prematurity (Abdel-Hady et al., 2015) may protect against pathological neovascularization in ROP. The protection against ROP by caffeine is in general agreement with the finding that caffeine treatment reduced the vulnerability of the immature brain to hypoxic ischemia (Bona et al., 1995), reduced the effects of NMDA on e.g. seizure susceptibility (Georgiev et al., 1993) in neonates. Moreover, our recent study demonstrate that caffeine treatment at the concentration of 0.1 g/L −1.0 g/L from P0–P17 reduced nonvascular areas by 31.28–53.78%, respectively, and also reduced neovascular nuclei counting (Zhou et al., 2015). Furthermore, we also found that repeated treatment of the A2AR antagonist KW6002 at P7–P14 reduced avascular areas as well as neo-vascularization at P17 as revealed by isolectin B4-immunostaining, consistent with notion that adenosine receptors are the main pharmacological targets of caffeine’s actions (Zhou et al., 2015). Lastly, chronic treatment with caffeine or KW6002 did not affect normal retinal neovascularization during postnatal development (Zhou et al., 2015). These findings provide the biological basis for the clinical finding that the use of caffeine in treatment of apnea in premature infants is associated with reduced ROP. Collectively, these findings support the novel caffeine- and adenosine receptor-based pharmacologic treatment for ROP. Further studies to identify the molecular targets, the cellular mechanism and effective therapeutic window underlying the protective effects of caffeine are needed to optimize caffeine treatment regime to target specific molecular pathways to achieve maximize prophylactic benefits for ROP while maintaining their impressive safety profiles.
8. Therapeutic advantages and clinical implications of caffeine treatment in ROP
The demonstration of the role of the adenosine receptor in development of ROP and protection by caffeine suggests two potential therapeutic strategies with high translational potential: a) modification of caffeine treatment paradigm for apnea of prematurity to the specific prophylaxis and treatment for ROP; and b) development of A2AR antagonists -based treatment for ROP. A2AR antagonists such as KW6002 and preledenet are safe according to the safety profiles in clinical phase III trials and have been approved for clinical use in Japan. Importantly, our analyses demonstrate that genetic inactivation of A2ARs (Liu et al., 2010) or A1Rs (Zhang et al., 2015) or treatment with A2AR antagonists (caffeine and KW602) (Zhou et al., 2015) selectively controls pathological OIR without affecting normal retinal development. The mechanism underlying this selectivity may be related to local increase of adenosine-adenosine receptor signaling in response to stress, hypoxia and inflammation (Chen et al., 2013). Thus, the preferential effect of caffeine and KW6002 on OIR confers a critical advantage over anti-VEGF antibody strategy which is limited by delayed eye growth and abnormal vasculature and neural development of preterm retina since VEGF activity is necessary not only for pathological angiogenesis, but also for normal retinal vascularization during development. Since the publication of the Caffeine for Apnea of Prematurity randomized clinical trial in 2006, the use of caffeine for prophylactic purposes has been suggested (i.e, administration is commenced very soon after birth or before a diagnosis of apnea of prematurity is made). Thus, caffeine may also represent a novel prophylactiv strategy for ROP.
Additionally, dissection of the adenosine receptor and VEGF signaling pathways leading to distinct physiological development and pathological angiogenesis are needed to fulfill the potential of caffeine and A2AR antagonists to achieve maximal therapeutic effects with minimal unwanted side effects. In particular, it should be noted that despite of lack of the general developmental effect on postnatal brain (specifically retinal vasculature) functions, there is lingering concerns on the possible specific effect of caffeine on embryonic development (Ma et al., 2014; Back et al., 2006) and possible postnatal development and maturation of cortical GABAergic neurons at the microstructural level by perinatal exposure to caffeine (Ardais et al., 2014; Silva et al., 2013).
9. Adenosine receptors and other proliferative retinopathies
Lastly, it would be important to explore whether the protection of OIR by A2AR inactivation can be extended to other pathological proliferative retinopathy including diabetic retinopathy in adults and MD in aging population. In diabetic retina, A1R and A2AR levels were elevated (Vindeirinho et al., 2013). Interestingly, the variants of the human A2AR gene are associated with reduced risk of developing diabetic retinopathy in a prospective study (Charles et al., 2011), suggesting the involvement of the A2AR in diabetic retinopathy. In diabetic retinopathy model, genetic inactivation of the A2AR increased apoptotic cells, TNF-α release, and intercellular adhesion molecule-1 expression compared with wild-type mice (Liou et al., 2011; Ibrahim et al., 2011; Elsherbiny et al., 2013b). Thus, it is expected that caffeine and KW6002 may exacerbate retinal damage in diabetic retinopathy, in contrast with the caffeine-mediated protection against OIR-induced retina (Liu et al., 2010; Zhou et al., 2015). This suggesting the distinct molecular/cellular mechanisms (including neuronal, inflammatory and vascular mechanisms) may underlie distinct effects of A2AR-mediated modulation of retinal vascularization. Future studies to clarify the possible distinct role of A2AR in development of DR and MD may stimulate required clinical studies to translate this novel adenosine receptor-based pharmacologic therapies for the treatment of ROP and other proliferative retinopathies.
10. Concluding remarks
ROP is a major cause of childhood blindness in the world. Current pharmacological therapy focus on anti-VEGF strategy, but this strategy is associated with the unintended effects on delayed eye growth and retinal vasculature development of preterm infants. Preclinical studies using OIR demonstrate that elevated A2AR and A2BR signaling promotes pathological angiogenesis while A1R signaling apparently confers protection OIR. We recently identified a pathway that affects pathologic, but not developmental, angiogenesis of the eye, and involves the A2AR: genetic inactivation of the A2AR attenuated OIR without affecting normal postnatal retinal vascularization (Liu et al., 2010). This raises the exciting possibility that A2AR activity in the retina may be selectively targeted for treatment of ROP. This notion is further substantiated by clinical evidence that caffeine treatment of apnea of prematurity is associated with reduced ROP (Schmidt et al., 2007). Further understanding of the A2AR, A2BR and A1R signaling interacting with other molecular and cellular pathways leading to distinct physiological development and pathological angiogenesis may lead to new strategy to achieve maximal therapeutic effects of adenosine receptor-based treatment with minimal unwanted side effects. Identification of the effective therapeutic window and cellular (endothelium and neuronal and glial) mechanisms of adenosine receptor strategies for the prevention and treatment of pathological retinal neovascularization will provide the required preclinical evidence to translate adenosine receptor-based treatment for ROP. The caffeine- and A2AR-based therapeutic strategies have high translational potential since caffeine is widely used in neonate care and KW6002 shows noted safety profile in phase III clinical trials.
Acknowledgments
The work by author’s lab as described in this review was supported by the Start-up Fund from Wenzhou Medical University (No. 89211010; No. 89212012), National Natural Science Foundation of China (No. 81630040, No. 81600753, No. 81100672) and Zhejiang Provincial Natural Science Foundation Grant (LY12H12007) and by Boston University School of Medicine special research fund DTD 4-30-14.
Footnotes
Jiang-Fan Chen obtained MD from Wenzhou Medical College, China and PhD from Medical College of Pennsylvania, USA. Dr. Chen is currently a professor at Wenzhou Medical University (China) and Boston University School of Medicine (USA). Dr. Chen’s work has advanced the prospective of A2AR antagonists as a novel treatment strategy for neurological disorders such as Parkinson’s disease. Dr. Chen’s recent investigation into the role of A2AR in retinopathy led to the discovery of the abnormal A2AR signaling in retina as a therapeutic target for retinopathy of prematurity. Dr. Chen has published >150 papers on adenosine receptor neurobiology.
References
- Abdel-Hady H, Nasef N, Shabaan AE, Nour I. Caffeine therapy in preterm infants. World J Clin Pediatr. 2015;4(4):81–93. doi: 10.5409/wjcp.v4.i4.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol. 2005;289(2):R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- Adair TH, Cotten R, Gu JW, Pryor JS, Bennett KR, McMullan MR, McDonnell P, Montani JP. Adenosine infusion increases plasma levels of VEGF in humans. BMC Physiol. 2005;5(10) doi: 10.1186/1472-6793-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal A, Shaw LC, Caballero S, Spoerri PE, Lewin AS, Zeng D, Belardinelli L, Grant MB. Reduction in preretinal neovascularization by ribozymes that cleave the A2B adenosine receptor mRNA. Circ Res. 2003;93(6):500–506. doi: 10.1161/01.RES.0000091260.78959.BC. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Ardais AP, Borges MF, Rocha AS, Sallaberry C, Cunha RA, Porciuncula LO. Caffeine triggers behavioral and neurochemical alterations in adolescent rats. Neuroscience. 2014;270:27–39. doi: 10.1016/j.neuroscience.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, Rivkees SA. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60(6):696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- Bjorklund O, Shang M, Tonazzini I, Dare E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol. 2008;596(1–3):6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 17(7):1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona E, Aden U, Fredholm BB, Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995;38(3):312–318. doi: 10.1203/00006450-199509000-00007. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43(2):190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- Brito R, Pereira MR, Paes-de-Carvalho R, Calaza Kda C. Expression of A1 adenosine receptors in the developing avian retina: in vivo modulation by A(2A) receptors and endogenous adenosine. J Neurochem. 2012;123(2):239–249. doi: 10.1111/j.1471-4159.2012.07909.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Cavallaro G, Filippi L, Bagnoli P, La Marca G, Cristofori G, Raffaeli G, Padrini L, Araimo G, Fumagalli M, Groppo M, et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 2014;92(1):2–20. doi: 10.1111/aos.12049. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Tout S, Hollander H, Stone J. Vascular changes and their mechanisms in the feline model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1992;33(7):2128–2147. [PubMed] [Google Scholar]
- Charles BA, Conley YP, Chen G, Miller RG, Dorman JS, Gorin MB, Ferrell RE, Sereika SM, Rotimi CN, Orchard TJ. Variants of the adenosine A(2A) receptor gene are protective against proliferative diabetic retinopathy in patients with type 1 diabetes. Ophthalmic Res. 2011;46(1):1–8. doi: 10.1159/000317057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li XX, Yin H, Gilbert C, Liang JH, Jiang YR, Zhao MW. Risk factors for retinopathy of prematurity in six neonatal intensive care units in Beijing, China. Br J Ophthalmol. 2008;92(3):326–330. doi: 10.1136/bjo.2007.131813. [DOI] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets–what are the challenges? Nat Rev Drug Discov. 2013;12(4):265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Mandal K. Treatment of retinopathy of prematurity. Early Hum Dev. 2008;84(2):95–99. doi: 10.1016/j.earlhumdev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Cui D, Trier K, Zeng J, Wu K, Yu M, Ge J. Adenosine receptor protein changes in Guinea pigs with form deprivation myopia. Acta Ophthalmol. 2010;88(7):759–765. doi: 10.1111/j.1755-3768.2009.01559.x. [DOI] [PubMed] [Google Scholar]
- Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174(8):5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- Deussen A. Metabolic flux rates of adenosine in the heart. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362(4–5):351–363. doi: 10.1007/s002100000318. [DOI] [PubMed] [Google Scholar]
- Dhaliwal C, Wright E, Graham C, McIntosh N, Fleck BW. Wide-field digital retinal imaging versus binocular indirect ophthalmoscopy for retinopathy of prematurity screening: a two-observer prospective, randomised comparison. Br J Ophthalmol. 2009;93(3):355–359. doi: 10.1136/bjo.2008.148908. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 58(1):43–54. doi: 10.1002/glia.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25(3):277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. AT1 receptor inhibition prevents astrocyte degeneration and restores vascular growth in oxygen-induced retinopathy. Glia. 2008;56(10):1076–1090. doi: 10.1002/glia.20680. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Echeverri D, Montes FR, Cabrera M, Galan A, Prieto A. Caffeine’s vascular mechanisms of action. Int J Vasc Med. 2010;2010:834060. doi: 10.1155/2010/834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsherbiny NM, Naime M, Ahmad S, Elsherbini AM, Mohammad S, Fulzele S, El-Remessy AB, Al-Gayyar MM, Eissa LA, El-Shishtawy MM, et al. Potential roles of adenosine deaminase-2 in diabetic retinopathy. Biochem Biophys Res Commun. 2013;436(3):355–361. doi: 10.1016/j.bbrc.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Elsherbiny NM, Ahmad S, Naime M, Elsherbini AM, Fulzele S, Al-Gayyar MM, Eissa LA, El-Shishtawy MM, Liou GI. ABT-702, an adenosine kinase inhibitor, attenuates inflammation in diabetic retinopathy. Life Sci. 2013;93(2–3):78–88. doi: 10.1016/j.lfs.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I, Cronstein BN. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb Exp Pharmacol. 2009;193:383–397. doi: 10.1007/978-3-540-89615-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008;84(2):83–88. doi: 10.1016/j.earlhumdev.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182(8):4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF. Cellular mechanisms in retinal vascular development. Prog Retin Eye Res. 2003;22(3):295–306. doi: 10.1016/s1350-9462(02)00062-9. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Johansson B, Fredholm BB. Long-term caffeine treatment leads to a decreased susceptibility to NMDA-induced clonic seizures in mice without changes in adenosine A1 receptor number. Brain Res. 1993;612(1–2):271–277. doi: 10.1016/0006-8993(93)91672-f. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, Oslund R, Gelb MH, Lambeau G, Marone G, et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol. 184(9):5232–5241. doi: 10.4049/jimmunol.0902501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res. 1999;85(8):699–706. doi: 10.1161/01.res.85.8.699. [DOI] [PubMed] [Google Scholar]
- Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Invest Ophthalmol Vis Sci. 2001;42(9):2068–2073. [PubMed] [Google Scholar]
- Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367(26):2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Simons M. Branching morphogenesis. Circ Res. 2008;103(8):784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- Hsu SJ, Lee FY, Wang SS, Hsin IF, Lin TY, Huang HC, Chang CC, Chuang CL, Ho HL, Lin HC, et al. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology. 2015;61(5):1672–1684. doi: 10.1002/hep.27679. [DOI] [PubMed] [Google Scholar]
- Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130(8):1000–1006. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, El-Shishtawy MM, Zhang W, Caldwell RB, Liou GI. A((2)A) adenosine receptor (A((2)A)AR) as a therapeutic target in diabetic retinopathy. Am J Pathol. 2011;178(5):2136–2145. doi: 10.1016/j.ajpath.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98(16):9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jin SY, Son HJ, Seo JH, Jeong GB. Caffeine-induced endothelial cell death and the inhibition of angiogenesis. Anat Cell Biol. 2013;46(1):57–67. doi: 10.5115/acb.2013.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Regulation of leukocyte function by adenosine receptors. Adv Pharmacol. 2011;61:95–114. doi: 10.1016/B978-0-12-385526-8.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GI, Ahmad S, Naime M, Fatteh N, Ibrahim AS. Role of adenosine in diabetic retinopathy. J Ocular Biol Dis Inf. 2011;4(1–2):19–24. doi: 10.1007/s12177-011-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Zhou R, Pan QQ, Jia XL, Gao WN, Wu J, Lin J, Chen JF. Genetic inactivation of the adenosine A2A receptor attenuates pathologic but not developmental angiogenesis in the mouse retina. Invest Ophthalmol Vis Sci. 2010;51(12):6625–6632. doi: 10.1167/iovs.09-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, McLeod DS. Retinal vascular development and oxygen-induced retinopathy: a role for adenosine. Prog Retin Eye Res. 2003;22(1):95–111. doi: 10.1016/s1350-9462(02)00058-7. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Merges C, McLeod DS. 5′ nucleotidase and adenosine during retinal vasculogenesis and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2000;41(1):218–229. [PubMed] [Google Scholar]
- Lynge J, Juel C, Hellsten Y. Extracellular formation and uptake of adenosine during skeletal muscle contraction in the rat: role of adenosine transporters. J Physiol. 2001;537(Pt 2):597–605. doi: 10.1111/j.1469-7793.2001.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZL, Wang G, Cheng X, Chuai M, Kurihara H, Lee KK, Yang X. Excess caffeine exposure impairs eye development during chick embryogenesis. J Cell Mol Med. 2014;18(6):1134–1143. doi: 10.1111/jcmm.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME. Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Investig Ophthalmol Vis Sci. 2013;54(3):2020–2026. doi: 10.1167/iovs.13-11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol. 2007;72(2):395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- Mino RP, Spoerri PE, Caballero S, Player D, Belardinelli L, Biaggioni I, Grant MB. Adenosine receptor antagonists and retinal neovascularization in vivo. Investig Ophthalmol Vis Sci. 2001;42(13):3320–3324. [PubMed] [Google Scholar]
- Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186(9):1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171(1):53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku H, Goto W, Kobayashi T, Okuno T, Hirao M, Sugiyama T, Yoneda S, Hara H, Ikeda T. Adenosine protects cultured retinal neurons against NMDA-induced cell death through A1 receptors. Curr Eye Res. 2004;29(6):449–455. doi: 10.1080/02713680490522443. [DOI] [PubMed] [Google Scholar]
- Okusa MD. A(2A) adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Ren Physiol. 2002;282(1):F10–F18. doi: 10.1152/ajprenal.2002.282.1.F10. [DOI] [PubMed] [Google Scholar]
- Patz A. Studies on retinal neovascularization. Friedenwald Lecture Invest Ophthalmol Vis Sci. 1980;19(10):1133–1138. [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27(4):331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perigolo-Vicente R, Ritt K, Pereira MR, Torres PM, Paes-de-Carvalho R, Giestal-de-Araujo E. IL-6 treatment increases the survival of retinal ganglion cells in vitro: the role of adenosine A1 receptor. Biochem Biophys Res Commun. 2013;430(2):512–518. doi: 10.1016/j.bbrc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Powers MR. In: Eye, Retina, and Visual System of the Mouse. Chalupa L, Williams R, editors. The MIT Press; Cambridge, Mass achustts London, England: 2008. pp. 285–297. [Google Scholar]
- Robinson GS, Ju M, Shih SC, Xu X, McMahon G, Caldwell RB, Smith LE. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15(7):1215–1217. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, McCaleb JL, Goldstein AE, Biaggioni I, Feoktistov I. Role of adenosine receptors in the regulation of angiogenic factors and neovascularization in hypoxia. J Pharmacol Exp Ther. 2007;320(2):565–572. doi: 10.1124/jpet.106.114850. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D’Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PloS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos PF, Caramelo OL, Carvalho AP, Duarte CB. Adenosine A1 receptors inhibit Ca2þ channels coupled to the release of ACh, but not of GABA, in cultured retina cells. Brain Res. 2000;852(1):10–15. doi: 10.1016/s0006-8993(99)02155-1. [DOI] [PubMed] [Google Scholar]
- Saura J, Angulo E, Ejarque A, Casado V, Tusell JM, Moratalla R, Chen JF, Schwarzschild MA, Lluis C, Franco R, et al. Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J Neurochem. 2005;95(4):919–929. doi: 10.1111/j.1471-4159.2005.03395.x. [DOI] [PubMed] [Google Scholar]
- Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184(9):5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- Scott A, Fruttiger M. Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye Lond Engl. 2010;24(3):416–421. doi: 10.1038/eye.2009.306. [DOI] [PubMed] [Google Scholar]
- Silva CG, Metin C, Fazeli W, Machado NJ, Darmopil S, Launay PS, Ghestem A, Nesa MP, Bassot E, Szabo E, et al. Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci Transl Med. 2013;5(197):197ra04. doi: 10.1126/scitranslmed.3006258. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- St Hilaire C, Koupenova M, Carroll SH, Smith BD, Ravid K. TNF-alpha upregulates the A2B adenosine receptor gene: the role of NAD(P)H oxidase 4. Biochem Biophys Res Commun. 2008;375(3):292–296. doi: 10.1016/j.bbrc.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, et al. The mouse retina as an angiogenesis model. Investig Ophthalmol Vis Sci. 51(6):2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7 Pt 1):4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Chan-Ling T, Pe’er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37(2):290–299. [PubMed] [Google Scholar]
- Sun X, Barnes S, Baldridge WH. Adenosine inhibits calcium channel currents via A1 receptors on salamander retinal ganglion cells in a mini-slice preparation. J Neurochem. 2002;81(3):550–556. doi: 10.1046/j.1471-4159.2002.00832.x. [DOI] [PubMed] [Google Scholar]
- Takagi H, King GL, Robinson GS, Ferrara N, Aiello LP. Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest Ophthalmol Vis Sci. 1996;37(11):2165–2176. [PubMed] [Google Scholar]
- Takagi H, King GL, Aiello LP. Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes. 1998;47(9):1480–1488. doi: 10.2337/diabetes.47.9.1480. [DOI] [PubMed] [Google Scholar]
- Taomoto M, McLeod DS, Merges C, Lutty GA. Localization of adenosine A2a receptor in retinal development and oxygen-induced retinopathy. Investig Ophthalmol Vis Sci. 2000;41(1):230–243. [PubMed] [Google Scholar]
- Tokunaga CC, Mitton KP, Dailey W, Massoll C, Roumayah K, Guzman E, Tarabishy N, Cheng M, Drenser KA. Effects of anti-VEGF treatment on the recovery of the developing retina following oxygen-induced retinopathy. Investig Ophthalmol Vis Sci. 2014;55(3):1884–1892. doi: 10.1167/iovs.13-13397. [DOI] [PubMed] [Google Scholar]
- Valls MD, Cronstein BN, Montesinos MC. Adenosine receptor agonists for promotion of dermal wound healing. Biochem Pharmacol. 2009;77(7):1117–1124. doi: 10.1016/j.bcp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Calker D, Biber K. The role of glial adenosine receptors in neural resilience and the neurobiology of mood disorders. Neurochem Res. 2005;30(10):1205–1217. doi: 10.1007/s11064-005-8792-1. [DOI] [PubMed] [Google Scholar]
- Vindeirinho J, Costa GN, Correia MB, Cavadas C, Santos PF. Effect of diabetes/hyperglycemia on the rat retinal adenosinergic system. PloS One. 2013;8(6):e67499. doi: 10.1371/journal.pone.0067499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 58(10):1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Martin P. Structures in focusefilopodia. Int J Biochem Cell Biol. 2002;34(7):726–730. doi: 10.1016/s1357-2725(01)00172-8. [DOI] [PubMed] [Google Scholar]
- Xu Z, Gong J, Maiti D, Vong L, Wu L, Schwarz JJ, Duh EJ. MEF2C ablation in endothelial cells reduces retinal vessel loss and suppresses pathologic retinal neovascularization in oxygen-induced retinopathy. Am J Pathol. 2012;180(6):2548–2560. doi: 10.1016/j.ajpath.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li H, Li B, Zhong D, Gu X, Tang L, Wang Y, Wang C, Zhou R, Li Y, et al. Adenosine A1 receptors selectively modulate oxygen-induced retinopathy at the hyperoxic and hypoxic phases by distinct cellular mechanisms. Investig Ophthalmol Vis Sci. 2015;56(13):8108–8119. doi: 10.1167/iovs.15-17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Wang C, Lin B, Wang Y, Chen J, Liu X. Assoc Res Vis Ophthalmol. 2015:214. [Google Scholar]
- Zou H, Otani A, Oishi A, Yodoi Y, Kameda T, Kojima H, Yoshimura N. Bone marrow-derived cells are differentially involved in pathological and physiological retinal angiogenesis in mice. Biochem Biophys Res Commun. 391(2):1268–1273. doi: 10.1016/j.bbrc.2009.12.057. [DOI] [PubMed] [Google Scholar]


