Abstract
Objectives
To evaluate the imaging performance of a handheld ultrasound system and the accuracy of an automated lumbar spine computer-aided detection (CAD) algorithm in the spines of human subjects.
Materials and Methods
This study was approved by the Institutional Review Board of the University of Virginia. The authors designed a handheld ultrasound system with enhanced bone image quality and fully automated computer-aided detection (CAD) of lumbar spine anatomy. The imaging performance was evaluated by imaging the lumbar spines of 68 volunteers with BMI between 18.5 and 48 kg/m2. The accuracy, sensitivity, and specificity of the lumbar spine CAD algorithm was assessed by comparing the algorithm’s results to ground-truth segmentations of neuraxial anatomy provided by radiologists.
Results
The lumbar spine CAD algorithm detected the epidural space with a sensitivity of 94.2% (95% CI: 85.1–98.1) and a specificity of 85.5% (95% CI: 81.7–88.6) and measured its depth with an error of approximately ± 0.5 cm compared to measurements obtained manually from the 2D ultrasound images. The spine midline was detected with a sensitivity of 93.9% (95% CI: 85.8–97.7) and specificity of 91.3% (95% CI: 83.6–96.9), and its lateral position within the ultrasound image was measured with an error of approximately ± 0.3 cm. The bone enhancement imaging mode produced images with 5.1 to 10-fold enhanced bone contrast when compared to a comparable handheld ultrasound imaging system.
Conclusions
The results of this study demonstrate the feasibility of CAD for assisting with real-time interpretation of ultrasound images of the lumbar spine at the bedside.
Keywords: lumbar puncture, ultrasound, computer-aided detection
Introduction
Palpation-based techniques are the most common approaches for facilitating lumbar spine bedside procedures such as lumbar puncture, sampling of cerebrospinal fluid, and spinal and/or epidural placement.1, 2 With these approaches, the needle insertion location is determined by palpating the iliac crest and spinous processes to identify the vertebral level and spine midline. Not surprisingly, recent studies have demonstrated that the efficacy of this technique decreases in patients whose surface landmarks are not identifiable and in patients with spinal deformities or degenerative effects of aging.3–6
Obesity, in particular, has been identified as a predictor of difficult needle placement, with multiple studies finding significantly decreased epidural and lumbar puncture (LP) success rates in patients with body mass index (BMI) greater than 35 kg/m2.4–7 Successful needle placement in these patients often requires additional needle insertions and passes,6 thereby increasing patient discomfort, prolonging the procedure, and increasing the risks of complications.8 In many cases, challenging patients are referred to interventional radiology (IR) for needle placement under fluoroscopic guidance, but this is not always an option, as is the case for obstetric patients or patients requiring immediate care.4
The use of pre-procedural ultrasound has been extensively studied as a means to improve epidural and lumbar puncture success rates in difficult patients by providing additional means to identify key neuraxial landmarks prior to needle insertion.6, 9–13 When used as a pre-procedural companion to palpation-based methods, ultrasound improves first-attempt epidural success rates, reduces the number of needle passes, and accurately estimates the depth to the epidural space to within approximately 1 cm.6, 14, 15 However, the evidence suggests that the acquisition and retention of neuraxial ultrasonography skills represents a major impediment to widespread use of ultrasound for neuraxial bedsides procedures.16, 17 In addition, ultrasound systems that are specifically designed to image soft tissue rather than bone structures may produce images that are difficult to interpret, thereby increasing the difficulty of the technique.18, 19
Attempts have been made to address these technical limitations by both enhancing ultrasound’s ability to image bone structures through image post-processing techniques20 and automating the detection of key neuraxial landmarks within ultrasound images.21, 22 Together, these technical solutions seek to reduce the learning curve by improving image quality and providing a secondary confirmation of the anatomical features contained within the ultrasound image. Given that ultrasound guidance is now the preferred approach for peripheral nerve blocks23 and central lines,24 it stands to reason that improving bone image quality may enable similarly improved efficacy for neuraxial needle guidance. To date, however, approaches to improve ultrasound spine imaging performance have been limited to proof-of-concept demonstrations in a limited number of human subjects or phantoms.20–22
In this work, we present the first imaging results of a handheld ultrasound system that was designed specifically for pre-procedural epidural and LP guidance. The system utilizes a piston ultrasound transducer rather than a linear or phased array so as to limit the deleterious effects of grating lobes and off-axis reflectors on bone image quality.19 In addition, the system employs bone-specific beamforming and image processing to enhance bone contrast and improve the conspicuity of key neuraxial landmarks, such as the spinous process, articular processes, transverse processes, and vertebral body. Finally, the system also comprises a real-time computer aided detection (CAD) algorithm that automatically identifies the location and depths of the spine midline, spinous processes, and epidural spaces to provide a secondary confirmation of the anatomy contained within the image. The primary objective of this imaging-only study was to evaluate the accuracy, sensitivity, and specificity of the CAD algorithm for the automated detection of neuraxial landmarks in human subjects. The long-term goal of this research is to enable physicians to use bedside neuraxial ultrasound and to ultimately improve the efficacy of neuraxial anesthesia and LP procedures.
Materials and Methods
This study was approved by the Institutional Review Board of the University of Virginia. Inclusion criteria were healthy volunteers, at least 18 years of age, and written informed consent. Exclusion criteria were the inability to give informed consent, known spinal deformities, or prior lumbar spine surgery. The ultrasound systems used in this study were provided on loan by Rivanna Medical to the University of Virginia. The study’s start date was July 11, 2014 and the end date was March 25, 2015.
Ultrasound imaging systems
Two handheld ultrasound systems were used in this study: the Accuro (Rivanna Medical, LLC, Charlottesville, VA, USA) and the GE V-Scan (GE Healthcare, Little Chalfont, United Kingdom). The Accuro weighed 0.31 kg with dimensions of 16.5 × 5.1 × 6.5 cm, and its screen was connected directly to the ultrasound transducer to permit one-handed scanning (Fig 1A). The V-Scan weighed 0.39 kg with display-unit dimensions of 13.5 × 7.3 × 2.8 cm and transducer dimensions of 12.0 × 3.3 × 2.6 cm, while its transducer was tethered to its screen by a cable (Fig 1B). Both devices were battery powered. The imaging frequency, maximum penetration depth, and scan angle were 2.5 – 5.0 MHz, 12.5 cm, and 60 ° for the Accuro, and 1.7 – 3.6 MHz, 24.0 cm, and 60 ° for the V-Scan.
Figure 1.
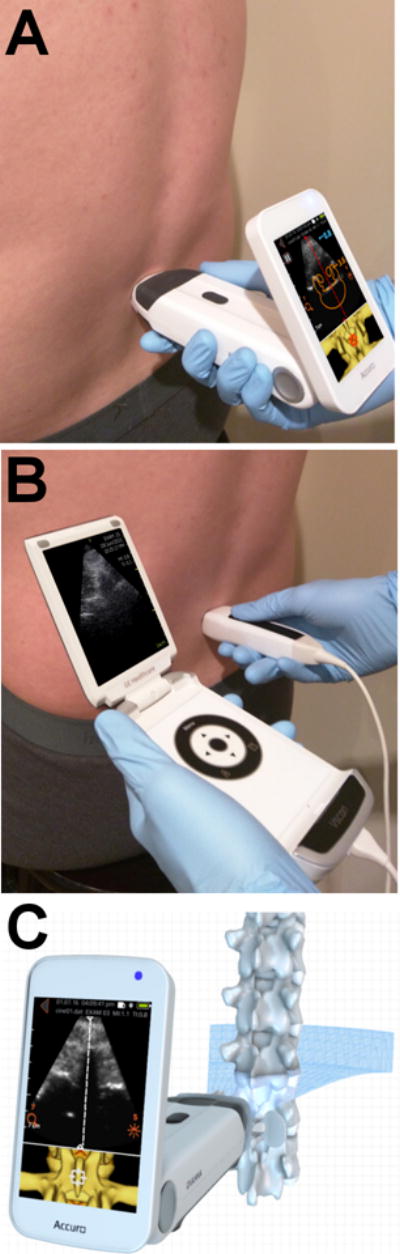
Demonstration of lumbar scanning technique with (A) Accuro and (B) V-Scan handheld ultrasound systems. (C) Schematic of the ultrasound beam intersecting the lumbar spine in the transverse plane.
Bone Enhancement
The Accuro possessed two imaging methods to facilitate imaging of the lumbar spine, a bone enhancement algorithm and a lumbar spine CAD algorithm.22 The bone enhancement algorithm provided 2D ultrasound images with accentuated bone surfaces and the CAD algorithm automatically detected spine anatomy within the 2D image and displayed anatomical overlays and indicators to the user.
Bone surfaces were enhanced by identifying the regions of dark signal dropout beneath the highly reflective bone surface, known as the acoustic shadow. Real-time detection of the acoustic shadow was performed by a shadow filter that quantified both the reflection amplitude and the degree of shadowing from the bone surface, as described by the following equation:
| Eqn (1) |
S(i,j) is the shadow intensity output, I(i,j) is the image frame data, M is the number of samples in each ultrasound A-line, α is the depth offset, τ is a small number used to avoid division by 0 (5×10−4 was used in this work, assuming image magnitude was between 0.0 and 1.0), and wk,i is a depth weighting. The depth weighting, wk,i, accounted for increased electronic noise through depth due to time gain compensation (TGC). In effect, the depth weighting was applied to ensure that electronic noise was not incorrectly treated as valid acoustic backscatter from tissue. The shadow intensity output, S(i,j), was used to map high intensity echoes immediately superficial to the shadow to bright pixel values using a conventional sigmoid intensity mapping function, as shown in Figure 4. To enhance the contrast and conspicuity of the bone in the final image, the background tissue signal was suppressed relative to the bone signal.
Figure 4.
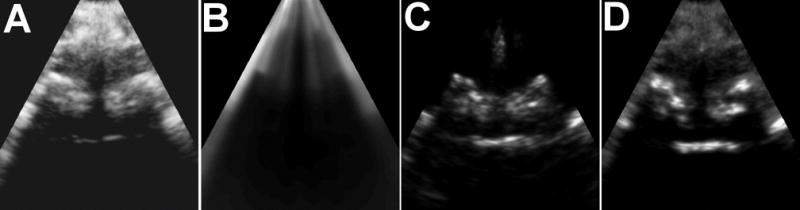
Intermediate processing results of the bone enhancement method. (A) Raw, unprocessed B-mode image. (B) The shadow image, S(i,j), computed as described in the methods section. (C) Intermediate image computed by dividing the raw image (A) by the shadow image, S(i,j), and scaling by a sigmoid to increase bone contrast. (D) The final image with enhanced bone signals and suppressed tissue signals.
Lumbar Spine CAD Algorithm
The high-contrast bone image served as the input to the lumbar spine CAD algorithm. The brightest pixels within the bone image corresponded to bone landmarks that were identified as either a spinous process or epidural space cross-section of the spine by a 3D model registration to the image data. The bright bone surfaces were mapped to the 3D model using a cost-function that maximized the similarity between the bone surfaces in the image and the 3D model.22, 25 The cost function took the form of the following equation:
| Eqn (2) |
where N is the number of points in the 3D model, M is the number of bright bone surfaces extracted from the bone image, dist is the Euclidean distance between a model point and a bone surface, and intensity is the brightness of the bone surface. This cost function can be viewed as minimizing the distance between the observed bone surfaces and the 3D model and preferentially weighting bright bone surfaces over dim bone surfaces. The 3D model points, model[i], undergo affine transformations in order to account for differences in scale, translation, and rotation between the underlying anatomy and the model.25 Example registration results between the 3D model and extracted bone points for spinous process and epidural space cross-sections are shown in Figures 2D and 3D, respectively.
Figure 2.
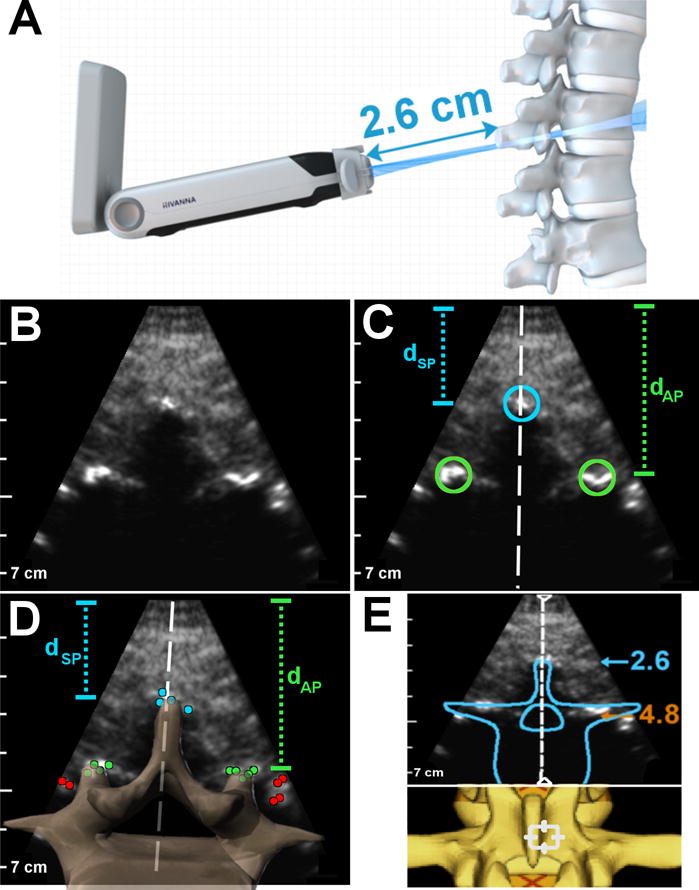
Summary of image analysis performed on spinous process cross-sections of the lumbar spine. (A) Schematic of ultrasound beam intersecting a spinous process cross-section. (B) Image acquired by Accuro of a spinous process cross-section (with bone enhancement). (C) Radiologist measurements on the spinous process image: the blue circle denotes location of the spinous process tip, the green circles denote location of the articular processes, the white line is the spine midline. (D) Schematic of CAD results on the same image: the blue, green, and red dots are bright bone points identified by the algorithm, a cross-section of the 3D spine model is superimposed on the image to show the registration result, and the dashed white line is the spine midline. In both (C) and (D), dSP is depth to spinous process tip and dAP is depth to articular processes. (E) Graphics rendered to the Accuro screen by the CAD algorithm to indicate that the image contains a spinous process. Also shown are depth and midline indicators, along with a birds eye view of the 3D spine model. The blue number is the depth to the spinous process tip and the orange number is the depth to the epidural space (in cm).
Figure 3.
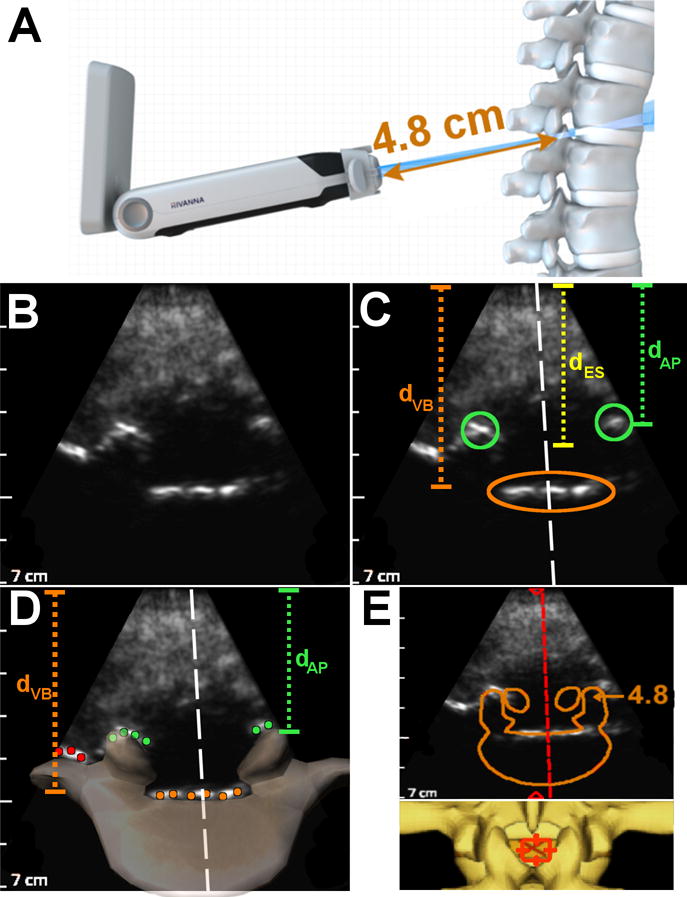
Summary of image analysis performed on epidural space cross-sections of the lumbar spine. (A) Schematic of ultrasound beam intersecting an epidural space cross-section. (B) Image acquired by Accuro of an epidural space cross-section (with bone enhancement). (C) Radiologist measurements on the epidural space image: the green circles denote location of the articular processes, the orange ellipse denotes the location of the posterior surface of the vertebral body, and the white line is the spine midline. (D) Schematic of CAD results on the same image: the orange, green, and red dots are bright bone points identified by the algorithm, a cross-section of the 3D spine model is superimposed on the image to show the registration result, and the dashed white line is the spine midline. dVB is depth to spinous process tip, dAP is depth to articular processes, and dES is the depth to the epidural space. (E) Graphics rendered to the Accuro screen by the CAD algorithm to indicate that the image contains an epidural space. Also shown are depth and midline indicators, along with a birds eye view of the 3D spine model. The orange number is the depth to the epidural space (in cm).
The CAD algorithm operates on images of the lumbar spine acquired in the transverse plane and is tolerant to rotation and translation of the cross-sectional spine anatomy within the transverse plane. The algorithm can also accommodate angulation of the scan plane in the elevational dimension, provided the scan plane cleanly intersects a spinous process or an epidural space cross-section. If elevational angulation is too severe, then the scan plane may intersect multiple vertebral units, in which case the CAD algorithm will not discriminate between spinous process or epidural space cross-sections.
When an appropriate fit between the 3D model and the underlying image data was found, graphics were displayed on the screen to indicate the presence of the spine midline, a spinous process, or an epidural space. In addition, depths to the spinous process tip and epidural space were also displayed on the screen. Representative graphics produced by the CAD algorithm for spinous process and epidural space cross-sections are shown in Figures 2E and 3E, respectively. Both the bone enhancement and CAD algorithms operate in real-time on the Accuro imaging system.
Imaging Study Protocol
Volunteer demographics (age, height, weight and BMI) were collected following consent. Volunteers were instructed to sit upright, relax their shoulders, and arch their lumbar spine to simulate patient positioning during a routine epidural or LP procedure. An anesthesiologist skilled in the use of neuraxial ultrasound acquired multiple cine video datasets of each subject’s lumbar vertebrae using both the GE V-Scan and the Accuro imaging systems. All imaging was performed in the transverse plane (Fig 1C). Three sonographers independently reviewed the cine datasets to screen for data of insufficient quality for further analysis (e.g. images not containing relevant anatomy were excluded from further study). The GE V-Scan is a comparable handheld ultrasound unit that served as an image quality comparison.
Neuraxial Landmark Detection
Three radiology fellows independently identified image frames from the cine datasets that contained clearly visible neuraxial landmarks. First, each radiologist independently classified each image frame as either containing a spinous process or an epidural space. Second, each radiologist identified the following spine landmarks in each image (if present): the spinous process tip, the lamina, the articular processes, the epidural space, the posterior surface of the vertebral body, and the spine midline. Distances from the surface (skin) to the landmarks were also measured. Representative measurements made by the radiologist observers for spinous process and epidural space cross-sections are shown in Figures 2C and 3C, respectively. The radiologists were blinded to lumbar spine CAD results when interpreting and segmenting spinal anatomy in the ultrasound images. In addition, prior to making official measurements for the study, each radiologist was trained to identify spine landmarks on 10 practice datasets (BMI between 20 – 40 kg/m2) in order to standardize image measurement techniques.
The accuracy of the lumbar spine CAD algorithm was assessed by comparing its automated measurements to the average value of the measurements provided by the three radiologists. First, the CAD algorithm used the 3D model-fit to the underlying image data to determine if a spinous process or epidural space cross-section was present in the image. Second, the CAD algorithm determined the location of the spine midline and depths to the spinous process and/or epidural space from the model fit, as shown in Figures 2D and 3D. In this study, the accuracy of the measurement and detection of the following anatomical features were assessed: (i) presence of a spinous process cross-section, (ii) presence of an epidural space cross-section, (iii) location of the spine midline, (iv) depth to the epidural space, and (v) depth to the spinous process tip.
Bone Image Quality
A quantitative comparison of the bone contrast in images acquired by the V-Scan and the Accuro was made based on the expert segmentations of neuraxial anatomy. The bone-to-tissue contrast ratio for the spinous process, lamina/articular processes, and posterior surface of the vertebral body was computed using the following equation:
| Eqn (3) |
where Ibone is the bone intensity and Itissue is the soft tissue intensity.
Statistical Analysis
In all cases, positional errors between the lumbar spine CAD measurements and radiologist measurements are expressed as mean ± standard deviation. Sensitivity and specificity for the classification of spinous processes and epidural spaces was performed using the measurements provided by the radiologists as a benchmark. Confidence intervals for all metrics were computed by assuming independent, random sampling from a Gaussian-distributed random variable. Statistical significance and p-values were computed using two-sided t-tests and the threshold for significance was considered to be α = 0.05. All statistical analysis was performed in MATLAB (Mathworks, Natick, MA, USA).
Results
A total of eighty volunteers with BMI between 18.5 and 48 kg/m2 were recruited for this study. Data from the first 12 volunteers could not be analyzed due to inconsistencies in image acquisition techniques, thereby limiting the final analysis to sixty-eight. Demographic and baseline characteristics are presented in Table 1. Volunteers were grouped into not-obese (BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2) categories according to BMI as defined by the World Health Organization (WHO). The only parameters that differed significantly between the two groups were subject BMI and weight.
Table 1.
Subject demographic and baseline characteristics
| BMI* Range | p-value | ||
|---|---|---|---|
| Characteristic | 18.5 – 29.9 | 30 – 49.9 | |
| N | 32 | 36 | |
| Age (yr.) | 37.8 ± 12.9 | 40.5 ± 11.4 | 0.363 |
| BMI | 24.2 ± 2.9 | 38.9 ± 5.0 | <0.001 |
| Height (cm) | 170.5 ± 9.5 | 168.5 ± 12.1 | 0.534 |
| Weight (kg) | 70.9 ± 12.3 | 110.2 ± 18.9 | <0.001 |
| Male Sex (%) | 34.3 | 30.5 | 0.739 |
BMI: body mass index
Bone Image Quality
The V-Scan was used as the control for assessing bone image quality. Representative images acquired by the V-Scan and the Accuro are displayed in Figure 5 (subject BMI of 44 kg/m2). Spine anatomical landmarks are annotated in each image. Bone-to-tissue contrast was evaluated for neuraxial bone landmarks in both the V-Scan and Accuro images. The results of this analysis are given in Table 2. In subjects with BMI < 30 kg/m2, the contrast of the spinous process tip was significantly lower than that of the other bone landmarks in images acquired by both systems (p < 0.05 for both systems). The average bone-to-tissue contrast in Accuro images of subjects with BMI ≥ 30 kg/m2 was 12% lower for vertebral body landmarks relative to images acquired of subjects with BMI < 30 kg/m2. The average contrast of all bone landmarks was 5.1 to 10-fold greater when imaged using the bone enhancement technique on the Accuro compared to conventional B-mode on the V-Scan (p < 0.05 for all landmarks).
Figure 5.
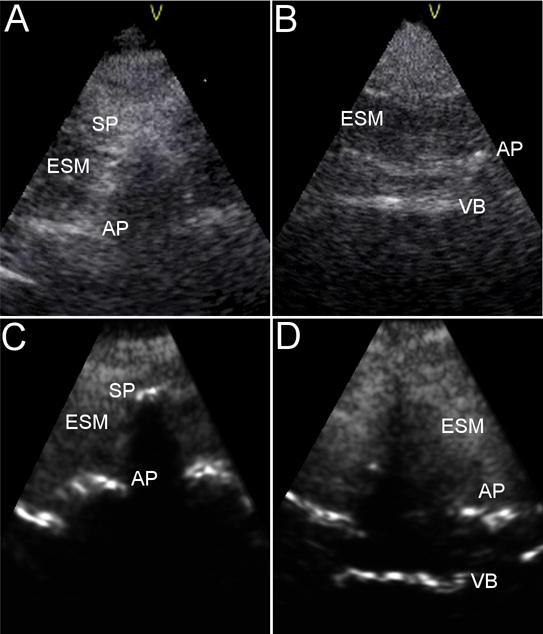
Representative images of a spinous process cross section (left) and an epidural space cross section (right) as acquired by the V-Scan (A,B) and Accuro (C,D) in a subject with BMI of 44 kg/m2. Labelled landmarks are spinous process tip (SP), articular processes (AP), vertebral body (VB), and erector spinae muscle (ESM).
Table 2.
Bone to Tissue Contrast Ratios of Spine Landmarks for V-Scan and Accuro
| BMI* Range | ||||||
|---|---|---|---|---|---|---|
| 18.5 – 29.9 | 30 – 49.9 | |||||
| VB§ | SP | L | VB | SP | L | |
| V-Scan | 0.8 ± 0.2 [0.4, 1.3]† |
0.3 ± 0.1 [0.1, 0.6] |
1.0 ± 0.2 [0.4, 1.7] |
0.7 ± 0.3 [0.2, 1.1] |
0.6 ± 0.2 [0.2, 1.0] |
0.6 ± 0.2 [0.1, 0.9] |
| Accuro | 4.1 ± 0.9 [2.3, 5.9] |
3.0 ± 0.6 [1.8, 4.7] |
5.2 ± 0.8 [2.7, 7.3] |
3.6 ± 0.6 [1.9, 4.8] |
4.6 ± 0.7 [2.6, 5.9] |
4.0 ± 0.4 [2.7, 5.4] |
BMI: body mass index
Abbreviations: vertebral body (VB), spinous process (SP), lamina (L)
Numbers in brackets are the minimum and maximum observed values, [min, max]
Neuraxial Landmark Detection
A positive correlation (Pearson’s r = 0.68, 95% CI: 0.54 – 0.79) was found between the radiologist’s measurements of epidural depth and subject BMI (Fig 6). The average depth to the epidural spaces imaged was 4.8 ± 0.8 cm (range 3.3 – 5.8 cm) in subjects with BMI < 30 kg/m2 and 5.7 ± 0.9 cm (range 4.4 – 6.9 cm) in subjects with BMI ≥ 30 kg/m2 (p < 0.005). The average depth to the spinous process tips was 1.9 ± 0.7 cm (range 1.4 – 4.1 cm) in subjects with BMI < 30 kg/m2 and 3.8 ± 0.8 cm (range 2.3 – 5.2 cm) in subjects with BMI ≥ 30 kg/m2 (p < 0.001). These depths are averages of measurements made at the L2-L3, L3-L4, and L4-L5 vertebral levels.
Figure 6.
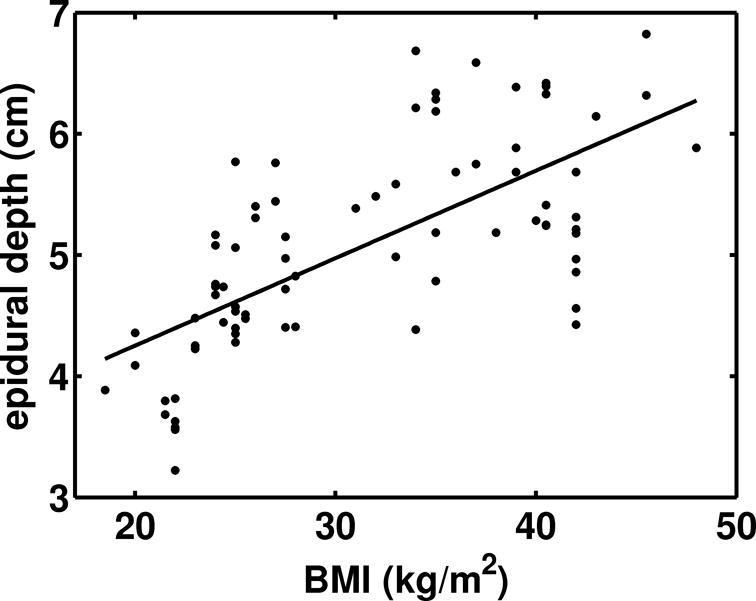
Relationship between epidural depths measured in the ultrasound images by radiologists and subject BMI. The equation for the line of best fit is: depth (cm) = 2.82 + 0.072×BMI. The correlation between epidural depth and BMI is: Pearson’s r = 0.68, 95% CI: 0.54 – 0.79).
Estimates of epidural depth generated by the lumbar spine CAD algorithm were compared to the radiologist’s measurements (Fig 7). The two measures were highly correlated across the entire range of epidural depths observed in this study (Fig 7A, Pearson’s r = 0.96, 95% CI: 0.93 – 0.98). A Bland-Altman analysis is shown in Figure 7B. The upper 95% confidence limit was 0.5 cm and the lower 95% confidence limit was −0.3 cm. A bias of 0.1 cm was observed, indicating that, on average, the lumbar spine CAD estimates were 0.1 cm shallower than the radiologist’s measurements.
Figure 7.
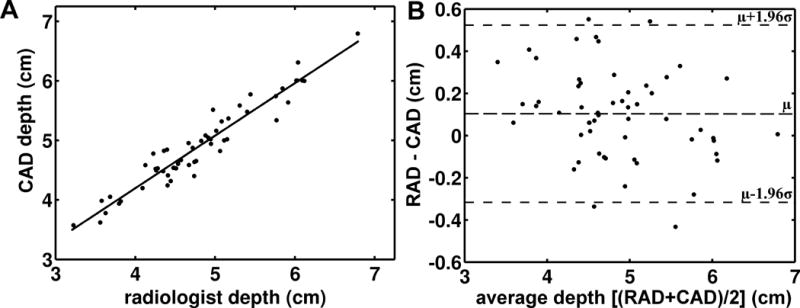
(A) Depth to the epidural space measured by the lumbar spine CAD algorithm versus depth to the epidural space measured by radiologists. (B) Bland-Altman analysis of epidural depths as measured by the lumbar spine CAD algorithm and radiologists. The difference between the depths measured by radiologists (RAD) and the depths measured by the CAD algorithm (CAD) are plotted against the average of the two measures (RAD+CAD)/2. The horizontal dashed lines represent the average difference (0.1 cm) and the 95% CI. μ: mean, σ: standard deviation.
Similarly, lumbar spine CAD estimates of spine midline location within each image were compared to the radiologist’s measurements (Fig 8). The two measures were positively correlated (Fig 8A, Pearson’s r = 0.85, 95% CI: 0.82 – 0.86). A Bland-Altman analysis is shown in Figure 8B. The upper 95% confidence limit was 0.2 cm and the lower 95% confidence limit was −0.4 cm.
Figure 8.
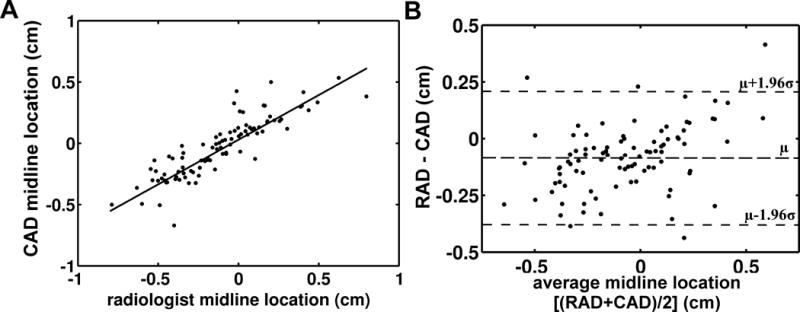
(A) Midline location measured by the lumbar spine CAD algorithm versus midline location measured by radiologists. (B) Bland-Altman analysis of midline locations as measured by the lumbar spine CAD algorithm and radiologists. The difference between the midline locations measured by radiologists (RAD) and the midline locations measured by the CAD algorithm (CAD) are plotted against the average of the two measures (RAD+CAD)/2. The horizontal dashed lines represent the mean difference (−0.1 cm) and the 95% CI. μ: mean, σ: standard deviation.
In addition to measuring the depth to the epidural space and the position of the midline, the lumbar spine CAD algorithm also automatically identified the spine midline and distinguished between epidural spaces and spinous processes. A summary of the detection sensitivity and specificity of epidural spaces, spinous processes, and the spine midline within the ultrasound images is given in Table 3. The lumbar spine CAD algorithm exhibited an overall epidural space detection sensitivity of 94.2% (95% CI: 85.1 – 98.1) and a specificity of 85.5% (95% CI: 81.7 – 88.6). Overall midline detection sensitivity and specificity were 93.9% (95% CI: 85.8 – 97.7) and 90.5% (95% CI: 81.6 – 95.5), respectively. Representative lumbar spine CAD graphics that are displayed on the Accuro screen to assist image interpretation are shown in Figure 2E, Figure 3E, and in Supplementary Videos (see Supplemental Digital Content 1 and 2).
Table 3.
Spine Landmark Identification in Accuro Images
| BMI§ Range | |||
|---|---|---|---|
| 18.5 – 29.9 | 30 – 49.9 | All Subjects | |
| CAD Positional Error Relative to Radiologist Segmentations | |||
| Epidural Depth [mm] | 2.0 ± 1.5 | 2.3 ± 2.4 | 2.1 ± 1.8 |
| Midline Location [mm] | 0.7 ± 0.7 | 0.9 ± 1.3 | 0.8 ± 1.0 |
| Spinous Process Depth [mm] | 1.9 ± 3.0 | 2.7 ± 2.9 | 2.3 ± 3.0 |
| CAD Anatomy Classification Sensitivity and Specificity | |||
| Epidural Space Sensitivity (%) | 94.1 [82.8 – 98.4]† |
94.4 [80.6 – 99.7] |
94.2 [85.1 – 98.1] |
| Epidural Space Specificity (%) | 84.4 [78.7 – 88.9] |
86.5 [81.0 – 90.6] |
85.5 [81.7 – 88.6] |
| Midline Sensitivity (%) | 95.1 [82.1 – 99.1] |
94.8 [83.4 – 98.7] |
93.9 [85.8 – 97.7] |
| Midline Specificity (%) | 91.2 [84.9 – 96.8] |
91.4 [82.9 – 97.3] |
91.3 [83.6 – 96.9] |
| Spinous Process Sensitivity (%) | 84.7 [79.3 – 89.0] |
85.8 [80.3 – 90.1] |
85.3 [81.6 – 88.4] |
| Spinous Process Specificity (%) | 91.9 [80.0 – 97.9] |
93.3 [76.4 – 99.6] |
92.3 [80.6 – 97.5] |
All measurements are mean ± standard deviation [mm].
BMI: body mass index
Numbers in brackets are 95% confidence intervals
Discussion
Accurate identification of the spine midline and interlaminar space prior to bedside procedures of the lumbar spine helps to ensure the technical success of epidural and LP procedures. The surface landmark-guided technique is effective in patients with palpable surface landmarks, but its efficacy is diminished in patients with anatomical landmarks that are difficult to palpate.3–6 In these patients, ultrasound can aid in the identification of key anatomical structures and can also estimate the depth to the epidural space to within approximately ± 1 cm accuracy.26, 27 Multiple randomized controlled trials (RCTs) have demonstrated that pre-procedural ultrasonography both increases the efficacy of neuraxial procedures (e.g. lower rates of incomplete analgesia, 2 vs 8%, p < 0.0328) and decreases the technical difficulty of the procedure (e.g. increased first-attempt success rate, 62 vs 32%, p < 0.001, and reduced number of needle passes, 6 vs 13, p < 0.0036).
The primary impediment to widespread adoption of pre-procedural ultrasonography for bedside LP and epidural procedures appears to be its steep learning curve. Margarido et al and Deacon et al determined that fewer than 40% of trainees were able to identify an appropriate needle insertion location on a subject with normal BMI after comprehensive training by a team of experts.16, 17 Image interpretation is even more difficult in obese and elderly patients due to degraded image quality, and key landmarks are only detectable by experts in 70 – 80% of obese patients.5, 26 These results imply that improved training methods and innovations in ultrasound technology may be required for the positive results of the RCTs to be realized outside of select clinical centers with ultrasonography experts.29
This study evaluated the imaging performance of a handheld ultrasound device that was developed to provide real-time feedback about the presence and location of anatomical landmarks within ultrasound images of the lumbar spine. As this was the first study with a device of this kind, we limited its scope to be an imaging-only study and sought to establish the agreement between automated anatomical landmark detection by the lumbar spine CAD algorithm and manual landmark identification by experienced radiologists. As shown in Table 3, if an epidural space, a spinous process, or the spine midline was manually identified within the ultrasound image by the radiologists, then it was automatically detected by the lumbar spine CAD algorithm with sensitivity and specificity between 85 – 95%. Importantly, the accuracy of automated neuraxial landmark identification was preserved in patients with obesity (up to BMI of 48 kg/m2), a group for which landmarks are difficult to identify via surface palpation.5, 6, 26 These results are comparable to the results of previously reported efforts to automatically detect lumbar anatomy in ultrasound images using computer vision techniques, which have demonstrated sensitivities and specificities in a limited number of subjects between 80 – 95 %.21, 22
In addition, a strong correlation was observed between the lumbar spine CAD algorithm and radiologist measurements for the epidural depth and location of the spine midline. The Bland-Altman analysis suggests that the location of these anatomical features within the ultrasound images was automatically identified to within approximately ± 0.5 cm. This degree of accuracy is expected to be sufficient for determining where to place the needle along the spine midline and for providing an estimate of the depth to the epidural space. Vallejo et al demonstrated that knowledge of these two parameters prior to needle placement reduced the incidence of failed epidurals and reduced the number of insertion attempts required for epidural placement.27
The intent of enhancing the bone surfaces is to improve the image quality of spine ultrasound images so as to reduce the learning curve for interpreting neuraxial ultrasound images. As evidenced by the results of Figure 5 and Table 2, the contrast and conspicuity of key bone landmarks was significantly higher in the enhanced images than in conventional B-mode images acquired by the V-Scan. The V-Scan was selected for an image quality comparison because it is a comparable handheld, highly portable system intended for use at the bedside. Accessibility of ultrasound systems has been identified as an impediment to adoption of neuraxial ultrasound in obstetric and ICU settings,18 and a highly portable, low-cost system is best suited to address this need. In addition, the lumbar spine CAD algorithm seeks to provide a secondary confirmation during bedside imaging evaluation of the lumbar spine to help inform the physician’s interpretation of features within the ultrasound images. The long-term goal is to enable physicians to use bedside neuraxial ultrasound and to ultimately improve the efficacy of neuraxial anesthesia and LP procedures. As stated above, this imaging-only study was the first step towards this goal, and clinically relevant outcomes must be evaluated in future RCTs.
This study is not without limitations. Although the Accuro ultrasound system can acquire ultrasound images in any orientation, the lumbar spine CAD algorithm only assists with anatomical landmark recognition when imaging in the transverse plane. There is evidence that transverse plane imaging is sufficient for identifying the midline and depth to the epidural space,9, 26 but imaging in longitudinal and sagittal orientations has also been shown to improve efficacy.12, 27 In addition, the deepest epidural space observed was only 6.9 cm deep, and while this was deeper than average values frequently reported in the literature, it is not representative of the most challenging patients, whose lumbar epidural spaces can often exceed 8 cm in depth.26 Given that this was an imaging-only study in volunteers, the reported depths measured from the ultrasound images could not be validated by true depths confirmed by needle placement. It is likely that the depths reported in this study underestimate the depths at which loss of resistance or CSF would be detected due to tissue compression by the ultrasound probe during imaging.9, 26 Finally, the impact of the lumbar spine CAD technology on clinically relevant endpoints must be evaluated in one or more RCTs before definitive conclusions may be reached.
In conclusion, we have demonstrated that a handheld ultrasound system with 3D spine navigation technology can automatically and accurately identify key neuraxial landmarks within ultrasound images of the lumbar spine acquired in the transverse plane.
Supplementary Material
Supplemental Digital Content 1: Representative lumbar spine CAD graphics and bone imaging in a subject with BMI of 25 kg/m2. The dashed vertical line indicates the position of the spine midline within the image, the blue number is the estimated depth to the spinous process tip, and the orange number is the estimated depth to the epidural space. An orange cross-section of vertebral anatomy is overlaid on the ultrasound image when an interlaminar space is identified, and a blue cross-section of the spinous process is overlaid on the ultrasound image when a spinous process is identified. This information is also related in the form of a 3D model of the lumbar spine at the bottom of the screen.
Supplemental Digital Content 2: Representative lumbar spine CAD graphics and bone imaging in a subject with BMI of 42 kg/m2. A description of the real-time lumbar spine CAD graphics is provided in the caption to Supplemental Digital Content 1.
Acknowledgments
Source of Funding
The authors acknowledge funding from NIH NIBIB SBIR grant R44 EB015232 to Rivanna Medical. MT made final decisions regarding inclusion of information in this submitted manuscript and has no financial relationship with Rivanna Medical.
Footnotes
Conflicts of Interest
M.T. is a shareholder of ADial Pharmaceuticals. A.J.D. is an employee of Rivanna Medical. F.W.M. is a shareholder of Rivanna Medical and has pending patents PCT/US2011/022984, PCT/US2012/034945, PCT/US2013/045576, US/61/662,481, PCT/US14/18732, PCT/US14/18732, and PCT/US13/77917 that are of relevance to this work. D.S. disclosed no relevant relationships. A.K. disclosed no relevant relationships.
References
- 1.Hawkins JL. Epidural Analgesia for Labor and Delivery. New England Journal of Medicine. 2010;362(16):1503–10. doi: 10.1056/NEJMct0909254. [DOI] [PubMed] [Google Scholar]
- 2.Wright BL, Lai JT, Sinclair AJ. Cerebrospinal fluid and lumbar puncture: a practical review. J Neurol. 2012;259(8):1530–45. doi: 10.1007/s00415-012-6413-x. [DOI] [PubMed] [Google Scholar]
- 3.Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth. 2012;109(2):144–54. doi: 10.1093/bja/aes214. [DOI] [PubMed] [Google Scholar]
- 4.Edwards C, Leira EC, Gonzalez-Alegre P. Residency training: a failed lumbar puncture is more about obesity than lack of ability. Neurology. 2015;84(10):e69–72. doi: 10.1212/WNL.0000000000001335. [DOI] [PubMed] [Google Scholar]
- 5.Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766–71. doi: 10.1034/j.1399-6576.2001.045006766.x. [DOI] [PubMed] [Google Scholar]
- 6.Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94–101. doi: 10.1097/ALN.0b013e31821a8ad4. [DOI] [PubMed] [Google Scholar]
- 7.Ellinas EH, Eastwood DC, Patel SN, et al. The effect of obesity on neuraxial technique difficulty in pregnant patients: a prospective, observational study. Anesth Analg. 2009;109(4):1225–31. doi: 10.1213/ANE.0b013e3181b5a1d2. [DOI] [PubMed] [Google Scholar]
- 8.Horlocker TT, McGregor DG, Matsushige DK, et al. A retrospective review of 4767 consecutive spinal anesthetics: central nervous system complications. Perioperative Outcomes Group. Anesth Analg. 1997;84(3):578–84. doi: 10.1097/00000539-199703000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188–92. doi: 10.1213/01.ane.0000250912.66057.41. [DOI] [PubMed] [Google Scholar]
- 10.Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262–8. doi: 10.1097/EJA.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 11.Grau T, Leipold RW, Fatehi S, et al. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25–31. doi: 10.1017/s026502150400105x. [DOI] [PubMed] [Google Scholar]
- 12.Karmakar MK, Li X, Ho AM, et al. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845–54. doi: 10.1093/bja/aep079. [DOI] [PubMed] [Google Scholar]
- 13.Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358–68. doi: 10.1212/CPJ.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlas A, Chaparro LE, Chin KJ. Lumbar Neuraxial Ultrasound for Spinal and Epidural Anesthesia: A Systematic Review and Meta-Analysis. Reg Anesth Pain Med. 2016;41(2):251–60. doi: 10.1097/AAP.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 15.Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. Bmj. 2013;346:f1720. doi: 10.1136/bmj.f1720. [DOI] [PubMed] [Google Scholar]
- 16.Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480–6. doi: 10.1177/0310057X1404200409. [DOI] [PubMed] [Google Scholar]
- 17.Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120–6. doi: 10.1007/s12630-009-9219-2. [DOI] [PubMed] [Google Scholar]
- 18.Gambling DR. Lumbar ultrasound: useful gadget or time-consuming gimmick? Int J Obstet Anesth. 2011;20(4):318–20. doi: 10.1016/j.ijoa.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Mauldin FW, Owen K, Tiouririne M, Hossack JA. The effects of transducer geometry on artifacts common to diagnostic bone imaging with conventional medical ultrasound. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2012;59(6):1101–14. doi: 10.1109/tuffc.2012.2301. [DOI] [PubMed] [Google Scholar]
- 20.Hacihaliloglu I, Abugharbieh R, Hodgson AJ, et al. Automatic bone localization and fracture detection from volumetric ultrasound images using 3-D local phase features. Ultrasound Med Biol. 2012;38(1):128–44. doi: 10.1016/j.ultrasmedbio.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Tran D, Rohling RN. Automatic detection of lumbar anatomy in ultrasound images of human subjects. IEEE Transactions on Biomedical Engineering. 2010;57(9):2248–56. doi: 10.1109/TBME.2010.2048709. [DOI] [PubMed] [Google Scholar]
- 22.Tiouririne M, Nguyen S, Hossack JA, et al. Handheld Real-Time Volumetric Imaging of The Spine: Technology Development. Journal of medical engineering & technology. 2014;38(2):100–3. doi: 10.3109/03091902.2013.877989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis SR, Price A, Walker KJ, et al. Ultrasound guidance for upper and lower limb blocks. Cochrane Database Syst Rev. 2015;(9):Cd006459. doi: 10.1002/14651858.CD006459.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brass P, Hellmich M, Kolodziej L, et al. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:Cd006962. doi: 10.1002/14651858.CD006962.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohr K. Landmark-Based Image Analysis - Using Geometric and Intensity Models Dordrecht. Boston, London: Kluwer Academic Publishers; 2001. [Google Scholar]
- 26.Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876–81. doi: 10.1213/ane.0b013e3181a323f6. [DOI] [PubMed] [Google Scholar]
- 27.Vallejo MC, Phelps AL, Singh S, et al. Ultrasound decreases the failed labor epidural rate in resident trainees. International Journal of Obstetric Anesthesia. 2010;19(4):373–8. doi: 10.1016/j.ijoa.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Grau T, Leipold RW, Conradi R, et al. Efficacy of ultrasound imaging in obstetric epidural anesthesia. Journal of Clinical Anesthesia. 2002;14(3):169–75. doi: 10.1016/s0952-8180(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 29.Chin KJ, Karmakar MK, Peng P. Ultrasonography of the adult thoracic and lumbar spine for central neuraxial blockade. Anesthesiology. 2011;114(6):1459–85. doi: 10.1097/ALN.0b013e318210f9f8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: Representative lumbar spine CAD graphics and bone imaging in a subject with BMI of 25 kg/m2. The dashed vertical line indicates the position of the spine midline within the image, the blue number is the estimated depth to the spinous process tip, and the orange number is the estimated depth to the epidural space. An orange cross-section of vertebral anatomy is overlaid on the ultrasound image when an interlaminar space is identified, and a blue cross-section of the spinous process is overlaid on the ultrasound image when a spinous process is identified. This information is also related in the form of a 3D model of the lumbar spine at the bottom of the screen.
Supplemental Digital Content 2: Representative lumbar spine CAD graphics and bone imaging in a subject with BMI of 42 kg/m2. A description of the real-time lumbar spine CAD graphics is provided in the caption to Supplemental Digital Content 1.


