Abstract
Objectives
Mesenchymal stem cells (MSCs) are frequently delivered via needle injection for treatment of musculoskeletal injuries. The purpose of this study was to evaluate the effect of needle diameter on the viability of MSCs.
Methods
Equine bone marrow-derived MSCs from 5 horses were suspended in PBS, and held at room temperature for 7 hours to mimic shipping conditions. Two replicate samples for each needle size (20, 22, 23, or 25-gauge (ga)) were aspirated into a 3ml syringe and re-injected into the holding vial 3 times, to reproduce the resuspension of cells prior to injection in clinical cases. Cells were stained with fluorescein diacetate and propidium iodide to measure viability. Flow cytometry (FC) was performed to compare cell debris and intact cells between groups.
Results
MSC viability was higher when cells were passed through a 20-ga rather than a 25-ga needle. Cell suspensions passed through a 20-ga needle contained a larger percentage of intact cells, compared to 25-ga samples. The percentage of debris present in cell suspensions tended to increase with decreasing needle diameter. Neither horse nor passage had a significant effect on viability.
Conclusions
Cell damage is more likely when MSCs are passed through 25-ga rather than 20-ga needles.
Clinical relevance
Use of needles larger than 25-ga is recommended to maintain the viability of MSCs injected in horses.
Keywords: Equine, mesenchymal stem cells, needle diameter, injection, regenerative medicine
Introduction
Mesenchymal stem cells (MSCs) are frequently used to treat musculoskeletal injuries in the horse.1–6 The most common clinical application of MSCs in the equine patient involves the treatment of tendon or ligament injuries via direct intralesional injection.2–13 MScs are also implanted in joints affected by osteoarthritis, cartilage damage, and meniscal damage.4–6,14,15 Regional limb perfusion of MSCs may be useful to treat inaccessible lesions such as those affecting tendons within the foot or to treat locally extensive disorders such as laminitis, diffuse tendon and ligament injuries, or multiple lesions within the same limb.6,16 All of these applications require that MSCs be injected through a needle or catheter; however there is very limited information on the effect of needle or catheter diameter on equine MSCs.
Research efforts in regenerative medicine have largely focused on proof-of-principle in vitro studies with few long-term clinical trials.1–13 Treatment protocols for each specific application have yet to be defined.2,4,6,12 Very few controlled studies have been published to optimize delivery methods1,6,11 and number of injected cells.2,4,6,12 Clinical application of equine MSCs typically rely on cells being shipped to practitioners from commercial or university laboratories. This practice prompted Bronzini et al1 to investigate the influence of temperature, time and media on MSCs shipped for clinical application. Typically 1 × 107 cells are shipped while cooled in Dulbecco’s modified Eagle’s medium (DMEM) or phosphate buffered saline (PBS) with or without antimicrobials and fetal bovine serum (FBS). Bronzini et al1 investigated the effects of combinations of these different media at physiologic (37 C), cooled (4 C) and room (20–22 C) temperature at times 3, 6, 9, 12, 24, 48, and 72 hours on the expression of typical MSC markers, resistance to apoptosis and beta-galactosidase activity. Results supported the possibility of shipping cells in PBS at room temperature, to be used within 9–12 hours.1 This information is important for optimizing cell viability during the shipping process.
Once received by the clinician, cells must be delivered to the patient without altering their viability.2,4,6 Small needle diameters are favored clinically to limit the development of hypoechoic tracks observed on ultrasonographic examinations.7,13. The needle tracks have been attributed to tissue damage, and should therefore correlate in size with needle diameter. However, too small of a needle diameter may damage cells due to shear stress during aspiration and injection of the cells.17,18 Tol et al17 showed that mononuclear cells from human bone marrow were not affected by needle diameter (16-, 18- and 22-ga) or infusion rates (1 mm/s vs. 0.5 mm/s). Walker et al18 questioned the extrapolation of this data to human MSCs as their diameter is larger (12–15 μm) than that of human mononuclear cells (5–6 μm). Walker et al18 therefore evaluated the effect of needle diameter (20-, 25-, and 30-ga needles as well as a 26-ga SL-10 microcatheter) and flow rate on rat and human MSC viability and cell surface markers. Whereas these variables had no effect on rat MSCs immediately or 24 hr post-injection, the viability of in human MSCs was decreased 24 hours after injection through 30-ga and microcatheters. In addition, apoptotic and dead cell fractions correlated inversely with needle sizes.18 Recently, Garvin et al19 reported that injecting equine MSCs through 23-, 21- and 19-ga needles did not influence their viability nor differentiation; however apoptotic cells were more abundant when cells were injected through 23- and 21-ga needles compared to 19-ga and non-injected controls.
The purpose of this study was to evaluate the effect of needle diameter on the viability of equine bone marrow-derived MSCs when handled as recommended by Bronzini et al.1 Variables that may affect viability such as the horse, cell size, passage and needle shear damage creating cell debris were also evaluated. We hypothesized that cell viability would be affected as needle diameter decreases.
Materials and methods
Bone marrow aspiration
All procedures were approved by University Institutional Animal Care and Use Committee. Bone marrow was obtained from 5 adult Thoroughbred or Thoroughbred cross horses; 2 castrated males and 3 females, age 1–10. The horses were sedated, local anesthesia was instilled, and sternal bone marrow aspirates were obtained using an 11-ga Jamshidi bone marrow biopsy needle under sterile conditions. A total of 120 mL of bone marrow aspirate was collected from each horse into two 60-mL syringes each containing 25,000 units of heparin.
Culture of MSCs
Each none marrow aspirate was purified via Ficoll-Paque Plus (American Biosciences, Piscataway, NJ, USA) gradient centrifugation. Cells were plated (10,000 cells/cm2) and cultured at 37C at a 5% CO2, 95% air atmosphere, and 5% humidity. Media contained Dulbecco’s modified Eagle’s medium, 2 mM L-glutamine, penicillin (100 units/mL), streptomycin (100 units/mL), basic fibroblastic growth factor (bFGF, 1 ng/mL), and 10% fetal bovine serum. Cells were grown to confluency, lifted with trypsin, and resuspended in medium (10% fetal bovine serum,10% dimethyl sulfoxide, 80% media), prior to cryopreservation at 5 × 106 cells/vial until use.
Cells were then thawed, washed and plated in medium as described above. Cells were expanded to ≅ 6 × 107 passage 3 and ≅ 6 × 107 passage 4 for the first two horses. Cells collected from the third horse was expanded to ≅ 6 × 107 passage 3 and those from the last 2 horses were expanded to ≅ 6 × 107 passage 2.
Storage of MSCs
Cells were divided into 5 sample groups, with a duplicate per group, for a total of 10 samples (Fig 1). For each sample, MSCs were washed with medium and phosphate buffered saline (PBS), centrifuged, and resuspended to 1 × 107 cells in one mL in PBS. 0.5 mL of cell suspension was individually pipetted into a cryovial. To mimic shipping of cells for clinical use1, cryovials were kept at room temperature for approximately 7 hours. During this time, cells settle out of suspension and form a soft pellet at the bottom of the cryovial.
Fig 1.
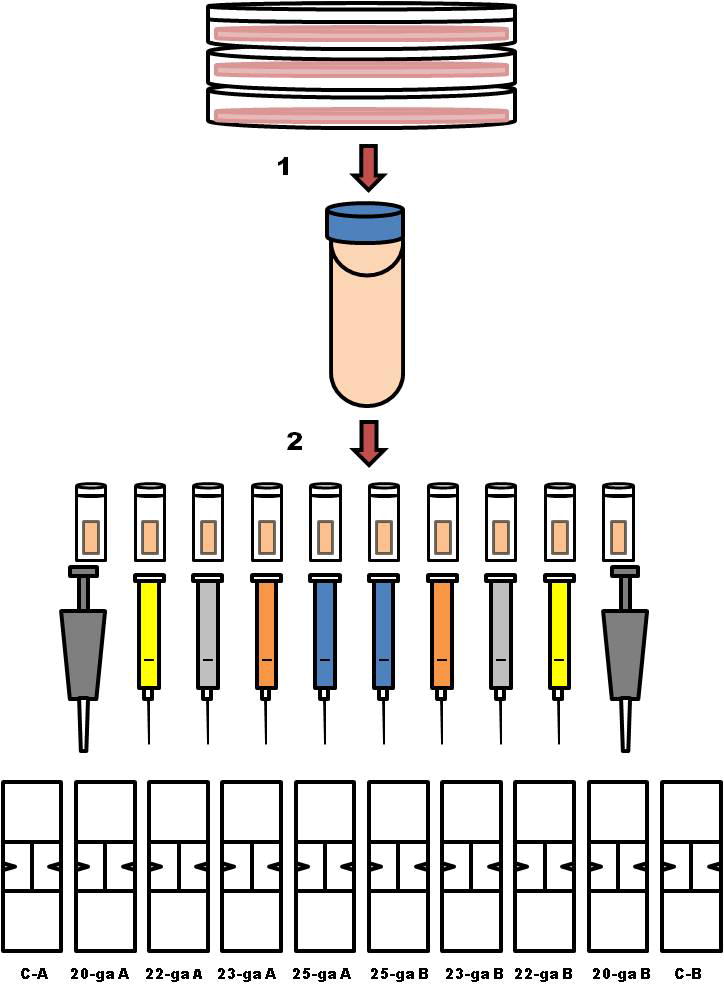
Methods flow diagram. Cells were culture expanded to ≅ 6 × 107 for each horse. (1) Cells were lifted with trypsin, washed with media and phosphate buffered saline (PBS), centrifuged, and resuspended to 1 × 107 cells/mL in PBS. (2) Of that cell suspension, 0.5 mL was individually pipetted into 10 cryovials. To mimic shipping of the cells for clinical use were allowed to sit at room temperature for approximately 7 hours. To account for variability in time to cell processing, cell aspiration, staining and counting were performed in the following order (A and B groups): control A (C-A), 20-ga A, 22-ga A, 23-ga A, 25-ga A; 25-ga B, 23-ga B, 22-ga B, 20-ga B, control B (C-B).
Needle aspiration and injection of MSCs
Two cryovials were used as controls and two were assigned to each hypodermic needle size: 20-ga 1 inch, 22-ga 1 inch, 23-ga 1 inch, and 25-ga 5/8 inch, respectively (Fig 2). The pellet of cells was resuspended by gently inverting the cryovial several times. For each needle size group, the suspended cells were aspirated through the needle into a 3 ml syringe and re-injected into the cryovial a total of 3 times immediately prior to counting. This step was designed to simulate the practice commonly followed by practitioners to resuspend cells prior to injection into the patient. Cells in the control group were aspirated with a micropipette (1000 μL).
Fig 2.
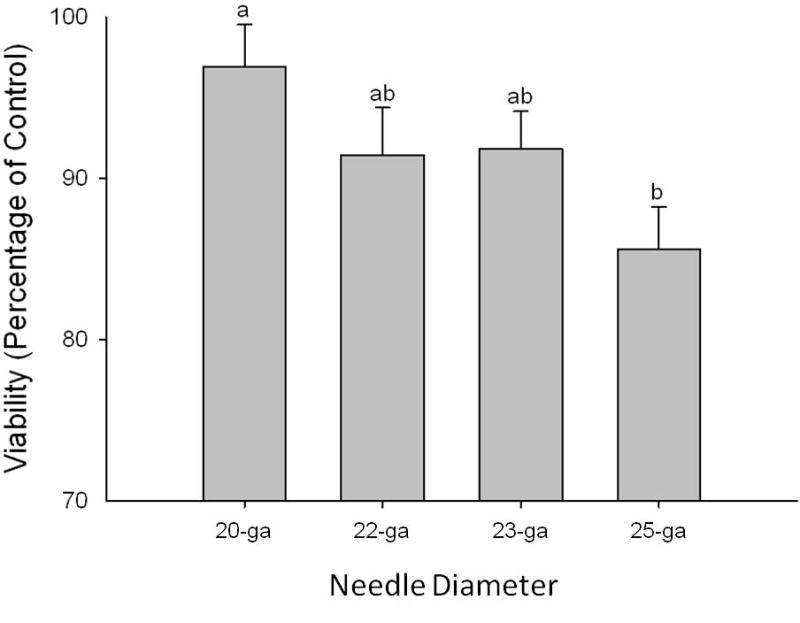
Effect of needle diameter on MSC viability measured as a percentage of control cell viability. Bars represent mean +/− S.D. Superscript letters indicate significant differences in MSC viability between needle diameter groups (20-ga, 22-ga, 23-ga, 25-ga). ANCOVA, with horse and passage number as covariates, followed by LSD test for multiple comparisons was performed, significance was set at p < 0.05.
Viability of MSCs
Cell viability was assessed by staining cells with the supra vital dyes fluorescein diacetate (green fluorescent) and propidium iodide (red fluorescent), prior to examination under fluorescent microscopy.20 Total and viable cell numbers were counted in replicate for each sample, including duplicates. Percent viability was calculated for each sample. Duplicates and replicate samples were averaged prior to statistical analysis.
To account for variability in time to cell processing, cell aspiration, staining and counting were performed in the following order (groups A and B): control A, 20-ga A, 22-ga A, 23-ga A, 25-ga A; 25-ga B, 23-ga B, 22-ga B, 20-ga B, control B (Fig 2).
Determination of equine MSC diameter
The range and average diameter of 100 MSCs was only measured from control samples. Cell diameter was measured from microscopy images and calculated using quantitative image analysis in Adobe® Photoshop® CS4 Extended (Adobe Systems Inc., San Jose, CA, USA). The measurements were calibrated by measuring the length of one side of a small square in a hemocytometer in a microscopic image and setting it to the known length of one side of the small squares (0.25 mm). The distribution of cell sizes was subjectively observed during cell viability assessment with fluorescent microscopy.
Flow Cytometry
Unstained MSC samples were evaluated for the presence of cellular debris based on forward scatter (FSC) using a FACS Calibur flow cytometer (BD Biosciences, Mississauga, ON, Canada). Fifty thousand events were counted for all controls and needle samples. Flow cytometry data was analyzed using FlowJo software (TreeStar, Inc., Ashland, OR, USA) to compare events with FSC consistent with debris versus intact cells. Since a single sheared cell could give rise to multiple debris events, this data was analyzed in terms of trends rather than absolute numbers or percentages.
Statistical analysis
For each sample set, percent viability compared to control was determined. Data were analyzed with analysis of covariance (ANCOVA), with horse and passage number as covariates, followed by the Least Significant Difference (LSD) test for multiple comparisons. Analyses were performed using Statistix 9 software (Analytical Software, Tallahassee, FL, USA) and significance was set at p < 0.05.
Results
Viability of MSCs aspirated through by various diameter needles
MSC viability was greater in the 20-ga needle group compared to the 25-ga group (Fig 2; p = 0.03). No difference in MSC viability was found between cells injected through 20, 22, and 23-ga needles. Similarly, no difference was found between 22, 23, and 25-ga needle groups. Horse and MSC passage number did not influence MSC viability (p = 0.25 and 0.24, respectively).
Size distribution of equine MSCs
Cell diameter ranged from 9.1–37.3 μm, with an average of 21.6 μm. Size distribution of viable cells seemed subjectively more heterogenous after mixing cells in the control and larger diameter needle groups, including more cells at the larger end of the size range. As needle diameter decreased, viable cells appeared more homogeneous in size, with viable cells appearing smaller (Fig 3). Cell clumping was not observed.
Fig 3.
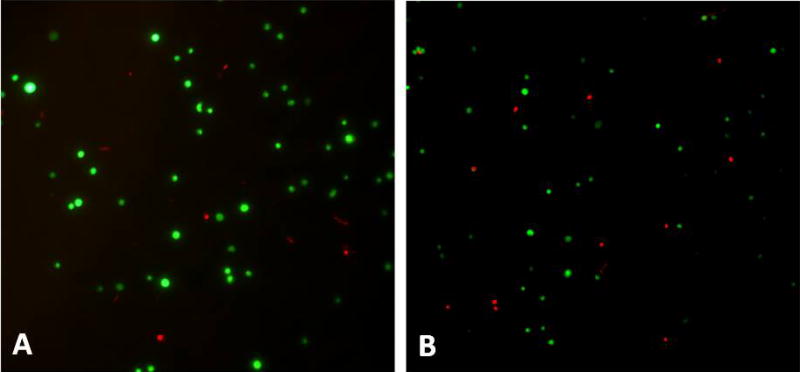
Fluorescent microscopic images. Fluorescein diacetate (green fluorescent= viable cells) and propidium iodide (red fluorescent= non-viable cells). (A) Cells injected through a 20-ga needle, and (B) cells injected through a 25-ga needle. Note the more heterogeneous population of cells, including some large viable cells in A, versus the more homogeneous smaller cells in B.
MSC debris after aspiration through needles
The flow cytometry data were consistent with viability staining. Based on the evaluation of FSC, an indication of relative cell size, a larger percentage of events with size value range consistent with intact cells were present in 20-ga samples compared to 25-ga samples. The percentage of events in the debris gate tended to increase relative to those in the viable cell gate with decreasing needle gauge size. Figure 4 illustrates an example of flow cytometry data output, with a larger percentage of events in the intact cell gate in the control and 20-ga needle sample, compared to the 25-ga needle sample.
Fig 4.
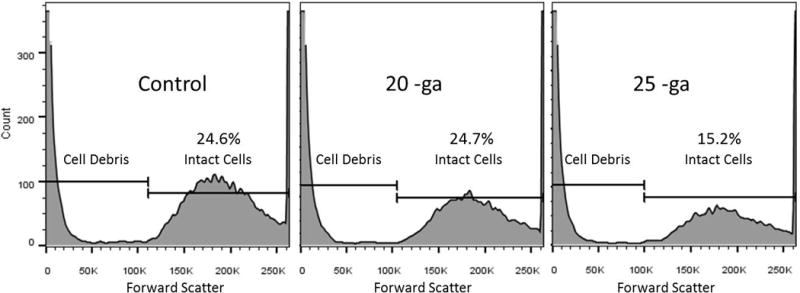
Flow cytometry data output. Event cell size distribution shows decreasing percentage of events in intact cell gate versus the debris gate with decreasing needle diameter. Fifty thousand events were analyzed on flow cytometry from each sample and then gated to differentiate events with forward scatter (FSC) values (values increase with cell size) consistent with debris versus intact cells. The above an example flow cytometry data output, with a larger percentage of events in the intact cell gate in the control and 20-ga needle sample compared to the 25-ga needle sample, which had more debris.
Discussion
The primary objective of this study was to determine the effect of injecting equine MSCs through different size needle diameters on cell viability. Larger MSCs are more likely to be sheared and killed when passed through smaller diameter needles, as indicated by the decrease in MSC viability injected through 25-ga compared to 20-ga needles. MSC viability did not vary significantly with horse or passage. Cell debris tended to increase as needle diameter decreased.
The decrease cell viability observed in 25-ga needle in our study may result from the mechanical shear created by small-diameter needle as proposed by Tol et al.17 and Walker et al.18 Cell suspensions seemed to include fewer larger cells as the diameter of the needle decreased, and contained a greater number of non-viable cells. Viability of human MSCs was negatively affected by smaller needle diameters in a study, but this finding was not confirmed when human bone marrow mononuclear cells, whose diameter is smaller, were tested in another study.17,18 The discrepancy between these results justifies a relative comparison of cell and needle diameters. The inner diameter of 20, 22, 23, and 25-ga hypodermic needles measure 23.75, 16.25, 13.25, and 10.25 μm respectively. The equine MSCs tested in our study were larger than human MSCs, as their diameter averaged 21.6 μm. Based on this data, one should expect cells to be exposed to shear as needle diameter decreases, thereby generating cell debris. In that context, the lack of difference in MSC viability between the 20-ga, 22 and 23-ga needles is intriguing. Comparing cell to needle diameters, one could reason that the 20-ga needle would leave a larger safety margin against cell damage compared to the smaller diameter needles evaluated. Viability tended to improve and cell debris tended be fewer on flow cytometry when comparing the 20-ga and to both the 22- and 23-ga needles in our study. This data converge with the increase in apoptotic cells reported by Gavin et al19, when comparing cell suspensions injected through 23- and 21-ga needles compared to 19-ga needles and non-injected controls. Combined, these results support the use of a 20-ga needle to administer stem cells suspensions. The proposed benefits, in terms of cell viability, should however by weighed against the size of associated needle tracks, the clinical relevance of these warranting further research.
Shear stress is not only influenced by cross sectional area, but also varies with fluid flow, velocity and the length of the channel.19 Although no flow rate were not measured in our study, the technique used to draw and inject the cell solution through the needle and syringe was kept as consistent as possible between samples, and was designed to simulate the handling of cell suspensions in the clinical setting. Standardizing flow between groups would inherently increase the velocity of cells passing through the smaller diameter needles, possibly increasing cell damage.17,18 Needle sizes commonly used for injection of MSCs in horses include 1 inch long, 22- and 23-ga needles. We widened our range of investigation by including needles one size larger and smaller than those typically selected in clinics. Garvin et al19 did not detect any difference in equine MSC viability or differentiation when passed through 23-, 21- and 19-ga needles. However, they did not evaluate smaller needle sizes, nor did they test the influence of serial passes through the needle, as often done in practice. These factors were included in our study. Comparing the results of our study with Gavin’s, needle diameter seems more important for inciting cell death than the method used to mix cells prior to injection. A 5/8 inch 25-ga needle was used in our study as this type of needle is routinely used in our hospital for superficial injections. However, the length of this needle is 3/8 inch shorter than the other needles included in our study. Although not evaluated, it is likely that cell viability would have been more severely affected if the 25-ga needle had been 1 inch long, as turbulent flow causes shear stress and is directly proportional to the length of the tube.19 Injection pressure is another factor that could influence cell viability. No methods or devices are available to measure injection pressure in the clinical setting. Without such information, laboratory findings relating injection pressure to cell death would have no clinical significance. Injection pressure was therefore neither measured, nor controlled in our study. Rather, the study was designed to evaluate the influence of methods, including cell injection, currently used in clinical practice by the authors.
Clinical applications of stem cell therapy, in particular those involving tendons and ligaments, required that healthy normal tissue be punctured with a needle or catheter to deliver the MSCs. Horses are often more reactive to the pain associated with insertion of larger needles so naturally, smaller diameter needles would be desirable to minimize pain of treatment administration. Needle tracks can sometimes be seen in tendons for months following the injection7,13 and presumably, larger diameter needles would cause more tissue damage due to needle track development. Although no evidence has linked the development of needle tracks with increased pain or loss of function, these tracks could serve as an escape route for MSCs away from the site of the lesion. These tracks could therefore jeopardize the retention of cells at the injection site, and attenuate their therapeutic effect. These potential disadvantages, combined with owners’ concerns over needle tracks, support the use of the smallest needle diameter that will not damage the cells.
Based on the results of our study, careful re-suspension of the cells, through needles larger than 25-ga is recommended. This study provides additional information toward establishing injection protocols for clinical applications of equine MSCs. While our study addresses one aspect of such protocols, ideal cell number and concentration, timing and frequency of administrations will warrant future research to optimize stem cell therapy.2,4,6,12
Acknowledgments
Funding for the proposal was provided by the Grayson Jockey Club Research Foundation (XXX). Dr. XXX is supported by National Institutes of Health grant #1K08AR060875 (XXX). The authors thank MsXXX and Dr. XXX for their technical support.
Footnotes
Disclosure Statement (conflicts of interest)
No authors have a conflict of interest.
References
- 1.Bronzini I, Patruno M, Iacopetti I, et al. Influence of temperature, time and different media on mesenchymal stomal cells shipped for clinical application. Vet J. 2012;194:121–123. doi: 10.1016/j.tvjl.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Fortier LA. Stem cells: classifications, controversies, and clinical applications. Vet Surg. 2005;34:415–423. doi: 10.1111/j.1532-950X.2005.00063.x. [DOI] [PubMed] [Google Scholar]
- 3.Fortier LA, Smith RK. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet Clin North Am Equine Pract. 2008;24:191–201. doi: 10.1016/j.cveq.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Fortier LA, Travis AJ. Stem cells in veterinary medicine. Stem Cell Res Ther. 2011;2:9. doi: 10.1186/scrt50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisbie DD, Smith RK. Clinical update on the use of mesenchymal stem cells in equine orthopedics. Equine Vet J. 2010;42:86–89. doi: 10.2746/042516409X477263. [DOI] [PubMed] [Google Scholar]
- 6.Schnabel LV, Fortier LA, McIlwraith CW, et al. Therapeutic use of stem cells in horses: which type, how, and when? Vet J. 2013;3:570–577. doi: 10.1016/j.tvjl.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Alves AG, Stewart AA, Dudhia J, et al. Cell-based therapies for tendon and ligament injuries. Vet Clin North Am Equine Pract. 2011;27:315–333. doi: 10.1016/j.cveq.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Caniglia CJ, Schramme MC, Smith RK. The effect of intralesional injection of bone marrow derived mesenchymal stem cells and bone marrow supernatant on collagen fibril size in a surgical model of equine superficial digital flexor tendonitis. Equine Vet J. 2012;44:587–593. doi: 10.1111/j.2042-3306.2011.00514.x. [DOI] [PubMed] [Google Scholar]
- 9.Crovace A, Lacitignola L, Rossi G, et al. Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendonitis of equine superficial digital flexor tendon. Vet Med Int. 2010;2010:250978. doi: 10.4061/2010/250978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godwin EE, Young NJ, Dudhia J, et al. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44:25–32. doi: 10.1111/j.2042-3306.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- 11.Pacini S, Spinabella S, Trombi L, et al. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007;13:2949–2955. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- 12.Schnabel LV, Lynch ME, van der Meulen MC, et al. Mesenchymal stem cells and insulin-like growth factor-1 gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 13.Smith RK. Mesenchymal stem cell therapy for equine tendinopathy. Disabil Rehabil. 2008;30:1752–1758. doi: 10.1080/09638280701788241. [DOI] [PubMed] [Google Scholar]
- 14.Ferris DJ, Frisbie DD, Kisiday JD, et al. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. 2014;43:255–265. doi: 10.1111/j.1532-950X.2014.12100.x. [DOI] [PubMed] [Google Scholar]
- 15.Frisbie DD, Kisiday JD, Kawcak CE, et al. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27:1675–1680. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- 16.Sole A, Spriet M, Galuppo LD, et al. Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using (99m) Tc-HMPAO. Equine Vet J. 2012;44:594–599. doi: 10.1111/j.2042-3306.2011.00530.x. [DOI] [PubMed] [Google Scholar]
- 17.Tol M, Akar AR, Durdu S, et al. Comparison of different needle diameters and flow rates on bone marrow mononuclear stem cell viability: an ex vivo experimental study. Cytotherapy. 2008;10:98–99. doi: 10.1080/14653240701762356. [DOI] [PubMed] [Google Scholar]
- 18.Walker PA, Jimenez F, Gerber MH, et al. Effect of needle diameter and flow rate on rat and human mesenchymal stromal cell characterization and viability. Tissue Eng Part C Methods. 2010;16:989–997. doi: 10.1089/ten.tec.2009.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garvican ER, Cree S, Bull L, et al. Viability of equine mesenchymal stem cells during transport and implantation. Stem Cell Res Ther. 2014;5:94. doi: 10.1186/scrt483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortier LA, Nixon AJ, Lust G. Phenotypic expression of equine articular chondrocytes grown in three-dimensional cultures supplemented with supraphysiologic concentrations of insulin-like growth factor-1. Am J Vet Res. 2002;63:301–305. doi: 10.2460/ajvr.2002.63.301. [DOI] [PubMed] [Google Scholar]


