Abstract
PTOV1 is a mitogenic protein that shuttles between the nucleus and the cytoplasm in a cell cycle-dependent manner. It consists of two homologous domains arranged in tandem that constitute a new class of protein modules. We show here that PTOV1 interacts with the lipid raft protein flotillin-1, with which it copurifies in detergent-insoluble floating fractions. Flotillin-1 colocalized with PTOV1 not only at the plasma membrane but, unexpectedly, also in the nucleus, as demonstrated by immunocytochemistry and subcellular fractionation of endogenous and exogenous flotillin-1. Flotillin-1 entered the nucleus concomitant with PTOV1, shortly before the initiation of the S phase. Protein levels of PTOV1 and flotillin-1 oscillated during the cell cycle, with a peak in S. Depletion of PTOV1 significantly inhibited nuclear localization of flotillin-1, whereas depletion of flotillin-1 did not affect nuclear localization of PTOV1. Depletion of either protein markedly inhibited cell proliferation under basal conditions. Overexpression of PTOV1 or flotillin-1 strongly induced proliferation, which required their localization to the nucleus, and was dependent on the reciprocal protein. These observations suggest that PTOV1 assists flotillin-1 in its translocation to the nucleus and that both proteins are required for cell proliferation.
PTOV1 was identified as a novel gene and protein during a differential display screening for gene expression in prostate cancer (4). PTOV1 is overexpressed in 71% of prostate carcinomas and in 80% of samples with prostate intraepithelial neoplasia, while it its barely detectable in normal prostate epithelium (34). In an independent study, PTOV1 was also found to be one of the genes most discriminant between normal and carcinomatous prostate (44). This gene codes for a protein that consists of two highly related sequence blocks arranged in tandem that are conserved in humans, rodents, and flies (4). The PTOV domain does not resemble any other protein motif described so far. A PTOV domain is also present in another protein, PTOV2 (4), later identified as ARC92, a component of transcriptional coregulator multisubunit complexes (28). Recently, PTOV2/ARC92, renamed as ACID-1, has been identified as a critical protein of Mediator complexes for the recruitment of activators to the basal transcriptional machinery (26). We have used yeast two-hybrid screenings to search for interaction partners of PTOV1, yielding a specific interaction with the lipid raft-associated protein flotillin-1 (5). Lipid rafts are specialized membrane microdomains enriched in glycosphingolipids, cholesterol, and glycosylphosphatidylinositol-anchored proteins (36). It is now believed that lipid rafts represent a central feature of cellular organization crucial for membrane trafficking events and for specific signaling cascades (36). Ubiquitous markers of lipid rafts are proteins of the Reggie/flotillin family (36, 38). Reggie-1 and Reggie-2, whose human orthologues are flotillin-2 and flotillin-1, respectively, were originally identified in regenerating neurons of goldfish optic nerves (35). Flotillin-1 is localized to noncaveolar membranes (20, 37) and has been shown to associate with lipid raft domains thanks to a palmitate modification on cysteine 34 (27). Reggie-1/flotillin-2 is also modified by myristoylation which contributes to anchoring the protein to the plasma membrane (29). Flotillin-1 interacts with the sorbin homology (SoHo) domain of the proteins c-cbl-associated protein (CAP), vinexin α, and ArgBP2, thus tethering them to lipid rafts (18). In addition to its postulated role in neuronal regeneration, flotillin-1 has been implicated in insulin signaling in adipocytes (3). Insulin stimulates the Cbl-CAP complex to bind flotillin-1 in lipid rafts, and this generates a signal crucial for the uptake of glucose in adipocytes (3). Lipid rafts enriched in flotillin-1 have also recently been identified on phagosomes (10).
In the present study, we show that PTOV1 interacts with flotillin-1 by coimmunolocalization and coimmunoprecipitation experiments in PC-3 and COS-7 cells. In addition, we show that both endogenous flotillin-1 and hemagglutinin (HA)-tagged flotillin-1 colocalize with PTOV1 in lipid rafts. Remarkably, we have found that flotillin-1 enters the nucleus concomitantly with the mitogen-induced entry of PTOV1. Modification of either end of the protein with a hemagglutinin epitope suggests that flotillin-1 requires a free carboxy terminus both for efficient interaction with PTOV1 and for its entry into the nucleus. Gene silencing experiments with RNA interference further support an ancillary role for PTOV1 in the nuclear entry of flotillin-1 and indicate that both proteins are required for normal cell proliferation. These observations reveal a new subcellular localization of flotillin-1 and thus suggest previously unsuspected functions for this protein.
MATERIALS AND METHODS
Cell culture, transfection, and antibodies.
Cells were grown at 37°C in an atmosphere of 5% CO2-95% air and 99% humidity. PC-3 cells (American Type Culture Collection, Rockville, Md.) were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 0.1 mM nonessential amino acids. COS-7 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium supplemented as described above. Culture media and reagents were from Gibco (Grand Island, N.Y.). COS-7 and PC-3 cells were transiently transfected for 24 or 48 h using Lipofectamine Plus reagent (Invitrogen, Carlsbad, Calif.). Antibodies were obtained from the following sources: mouse anti-flotillin-1 and anti-flotillin-2, Transduction Laboratories (Lexington, Ky.); rat anti-HA, Roche Diagnostics (Mannheim, Germany); rabbit anti-Ki67, Immunotek (Marseille, France); mouse anti-cyclin D1, Santa Cruz Biotechnology (Santa Cruz, Calif.) mouse anti-β-actin, Dako (Glostrup, Denmark); rabbit and mouse anti-FLAG, Sigma (Alcobendas, Spain). Rabbit antibody to human PTOV1 (4) was affinity purified by using Sulfolink columns from Pierce (Rockford, Ill.), and rabbit anti-urokinase-type plasminogen activator receptor (uPAR) was a kind gift from F. Blasi (Ospedale Universitario San Raffaele, Milan, Italy).
Plasmid constructs. (i) HA-tagged flotillin-1 constructs.
Full-length human flotillin-1 cDNA was amplified from the CM214-H04 IMAGE clone. A partial flotillin-1 cDNA lacking the first 23 amino-terminal residues was obtained by restriction enzyme digestion of pACT2. The products were cloned into EcoRI and XhoI in pCMV-HA (Clontech, Palo Alto, Calif.), in frame with the hemagglutinin tag, yielding pHA-Flot1 and pHA-ΔNFlot1. Primers containing EcoRI and EcoRV sites were used to remove the flotillin-1 stop codon to generate pFlot1-HA. pHA-Flot1C34A was obtained by mutating cysteine at position 34 to alanine by the Quick-Change site-directed mutagenesis procedure (Stratagene, La Jolla, Calif.). pHA-Flot1ΔC was obtained by introducing a stop codon at amino acid 382 by site-directed mutagenesis.
(ii) Flotillin-1-FLAG and green fluorescent protein (GFP)-flotillin-1 constructs.
Full-length flotillin-1 was amplified by PCR and cloned into the EcoRI and KpnI sites of pFLAG-CMV-5a (Sigma), and the flotillin-1 stop codon was mutated to Trp, yielding pFlot1-FLAG. To generate pGFP-Flot1, the same flotillin-1 PCR fragment was cloned into pEGFP-C1 (Clontech).
(iii) FLAG-tagged PTOV1 constructs.
Full-length PTOV1 cDNA from a BamHI-HindIII fragment (4) was used to generate a fusion protein with a FLAG epitope at its amino terminus in pFLAG (Sigma), yielding pFLAG-PTOV1. Improved expression of the FLAG-PTOV1 protein was obtained by mutating an internal Met in pFLAG-PTOV1 to a Leu. The pFLAG A domain (positions 14 to 188 of PTOV1) and pFLAG B domain (positions 184 to the end) were generated by cloning PCR products into the HindIII and EcoRI sites of pFLAG. pGFP-PTOV1 has been described previously (4).
Immunofluorescence.
Cells cultured on coverslips were fixed with 4% paraformaldehyde, incubated with 50 mM NH4Cl for 30 min, and blocked and permeabilized for 30 min with phosphate-buffered saline-0.1% saponin-1% bovine serum albumin. To detect HA-tagged proteins, cells were sequentially incubated with rat anti-HA, biotin-conjugated anti-rat immunoglobulin, and streptavidin-Alexa 488 (Molecular Probes, Eugene, Oreg.). For other antigens, cells were incubated for 2 h with primary antibody and for 1 h with the corresponding fluorescent secondary antibody. Coverslips were mounted in Immuno-Fluore mounting medium (ICN, Costa Mesa, Calif.). Fluorescence was visualized with a DM-IRBE inverted fluorescence microscope (Leica, Wetzlar, Germany) and captured by a TCS-NT argon/krypton confocal laser (Leica).
Proliferation assays.
For Ki67 proliferation assays, PC-3 cells were plated onto coverslips, transfected, and, after 48 h, fixed and processed for immunocytochemistry as described above. Transfection was monitored by immunocytochemistry with antibodies to HA or FLAG or by GFP fluorescence, and a double immunofluorescence assay was performed to assess Ki67 reactivity. Cells positive for nuclear staining of Ki67 were scored as proliferating. At least 200 transfected cells were counted for each condition in each experiment, with at least two independent experiments per condition. For [3H]thymidine incorporation, cells (5 × 104 cells/well) seeded in 24-well plates in quintuplicate experiments per condition were transfected with pEGFP, pGFP-PTOV1, or pGFP-Flot1. After 48 h, 1 μCi of [methyl-3H]thymidine (ICN, Aurora, Ohio) per well was added for 3 h, and incorporated radioactivity was determined by precipitation in 5% trichloroacetic acid and scintillation counting. Results were corrected for transfection efficiency by counting the GFP-positive cells obtained for each plasmid in parallel wells.
Subcellular fractionation, sucrose density gradients, immunoprecipitation, and Western blotting.
For subcellular fractionation, 106 cells were scraped into 0.3 ml of cold hypotonic buffer (25 mM MES [morpholineethanesulfonic acid], pH 6.5) containing protease inhibitors and homogenized by 10 strokes of a Teflon Dounce homogenizer. The lysates were centrifuged at 800 × g at 4°C, yielding nuclear pellets and nonnuclear fractions. Pelleted nuclei were washed four times in hypotonic buffer to eliminate cytosolic contamination. For Western blotting or immunoprecipitation, nuclear pellets were resuspended in extraction buffer (25 mM MES [pH 6.5], 1% Triton X-100, 0.5 mM EDTA, 150 mM NaCl), incubated for 45 min at 4°C, and centrifuged for 10 min at 10,000 × g. The resulting supernatants were defined as crude nuclear extracts. Nonnuclear fractions were adjusted to the final concentration of extraction buffer containing 60 mM octyl-glucoside (Sigma), incubated for 45 min at 4°C, and centrifuged at 10,000 × g for 10 min, and the resulting supernatants were used for immunoprecipitations or Western blotting. For coimmunoprecipitation, either total or fractionated cell lysates in extraction buffer were precleared with protein G- or protein A-agarose (Roche) and incubated overnight at 4°C with 5 μg of anti-PTOV1, anti-flotillin-1, anti-HA, or M2 anti-FLAG, and immune complexes were recovered by incubation with protein G- or protein A-agarose. Immunoprecipitation experiments with unrelated antibodies of the same isotype were also carried out as the control for the specificity of each primary antibody. After five washes with extraction buffer, samples were boiled in Laemmli buffer and analyzed by Western blotting.
To obtain membrane domains enriched in detergent-insoluble lipid rafts, cell lysates were fractionated according to established methods with some modifications (5, 23). Briefly, nonnuclear fractions from 3 × 106 cells were obtained as described above, adjusted to a final sucrose concentration of 40% (wt/vol) in cold extraction buffer without octyl-glucoside and without EDTA, overlayed with a 5 to 30% discontinuous sucrose gradient, and centrifuged at 100,000 × g for 16 to 18 h at 4°C. Fractions were collected and concentrated by precipitation in 10% ice-cold trichloroacetic acid, washed, and boiled in Laemmli buffer.
Metabolic labeling.
Cells (106) were transfected with pHA-Flot1 and, after 48 h, incubated overnight with [35S]methionine (50 μCi/ml) (TRANS35S-LABEL; ICN) in methionine-free medium. After washing with cold phosphate-buffered saline, cells were lysed with ice-cold extraction buffer, and whole-cell lysates were processed for immunoprecipitation as described above. The immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and gels were fluorographed, dried, and exposed to film at −80°C.
RNA interference.
Small interfering RNA (siRNA) duplexes were synthesized by in vitro transcription by using a Silencer RNA construction kit from Ambion (Austin, Tex.). The target sequences were 5′-AACAACAAGTTTCTGGCATGG-3′ (PTOV1 siRNA), 5′-AAGGACATTCACGATGACCAG-3′ (Flot-1 siRNA 1), and 5′-AACAGATCCAGAGGATCTCTC-3′ (Flot-1 siRNA 2). Control (scrambled) siRNAs were synthesized for sequences that did not match any human transcripts or genes by BLAST searches in the GenBank database. For transfection of duplexed siRNAs, PC-3 cells plated at 2 × 105 cells/well in 6-well plates or 7 × 104 cells in coverslips were cotransfected with siRNA duplexes and pEGFP vector (0.1 μg/well) (Clontech) to monitor transfected cells using Lipofectamine Plus reagent (Invitrogen). Forty-eight hours after transfection, cells were lysed for Western blotting analysis or fixed with 4% paraformaldehyde for immunocytochemistry.
Statistics.
Results are expressed as means ± standard errors of the means, and the Student's t test was used for statistical analysis. A P value of <0.05 was taken as the level of significance.
RESULTS
Interaction of PTOV1 with flotillin-1.
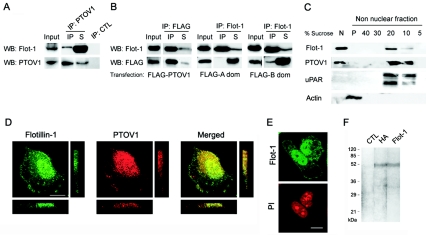
In order to identify proteins that can interact with PTOV1 and possibly mediate its function, a yeast two-hybrid assay was performed to screen a human prostate cDNA library using full-length PTOV1 cDNA as bait. Nine distinct clones were identified in a screen of 3.5 × 106 clones, of which two were identified as flotillin-1. The flotillin-1 inserts retrieved in this screening lacked the sequences corresponding to the first 23 amino acids from the N terminus of the protein. The interaction of PTOV1 with flotillin-1 was also shown to occur in mammalian cells by coimmunoprecipitation experiments of endogenous proteins in PC-3 (Fig. 1A) or of FLAG-tagged PTOV1-transfected cells (Fig. 1B). These experiments show that both endogenous and exogenous PTOV1 pull down a fraction of endogenous flotillin-1. Similar experiments were performed with each of the two duplicated domains of PTOV1, domains A and B. Specifically, the B domain, but not the A domain, of PTOV1 was able to partially coimmunoprecipitate with endogenous flotillin-1 (Fig. 1B). Therefore, although domains A and B are very similar in sequence to each other (4), they are not functionally interchangeable.
FIG. 1.
PTOV1 interacts with flotillin-1 in mammalian cells. (A) Coimmunoprecipitation of endogenous PTOV1 and flotillin-1 (Flot-1) from PC-3 cells. Total cell lysates were immunoprecipitated with anti-PTOV1 antibody or control rabbit immunoglobulin (IP: CTL). Ten percent of the volume of total cell extracts (Input), the IP, and 10% of the volume of the nonprecipitated fractions (S) were analyzed by Western blotting (WB) with anti-flotillin-1 or anti-PTOV1 antibodies. (B) Flotillin-1 interacts with the second PTOV domain of PTOV1. Total cell lysates from pFLAG-PTOV1, pFLAG A domain (dom), or pFLAG B domain transfections were immunoprecipitated with anti-FLAG or anti-flotillin-1 antibodies. Total cell extracts (10% of the volume [Input]) and the IP and nonimmunoprecipitated fractions (10% of the volume [S]) were analyzed by Western blotting with anti-flotillin-1 or anti-FLAG antibodies. Immunoprecipitation analyses with control rabbit (as shown in panel A) or isotype-matched mouse antibodies were consistently negative and are not shown. (C) PTOV1 and flotillin-1 are distributed in detergent-insoluble floating fractions and also in nuclear fractions. Cells were lysed, and crude nuclear (N) and nonnuclear fractions were extracted. This last fraction was used for sucrose density gradient centrifugation. The fractions (5 to 40% of sucrose) were precipitated by trichloroacetic acid and resuspended in Laemmli buffer, and the entire fractions were analyzed together with the entire crude nuclear extract (N) and the pellet of the gradient (P) from each experiment by Western blotting with antibodies to flotillin-1, PTOV1, uPAR, and actin. (D) Immunocytochemical colocalization of PTOV1 and flotillin-1. Cells were fixed and double labeled for PTOV1 (red) and flotillin-1 (green) endogenous proteins and analyzed by confocal microscopy. Confocal images of the samples were also analyzed along the z axis with sections of 0.6 μm. (E) Nuclei were counterstained with propidium iodide (PI). (F) Immunoprecipitation of flotillin-1 from metabolically labeled PC-3 cells. Cells transfected with pHA-Flot1 were labeled with [35-S]methionine, lysed, and immunoprecipitated with anti-HA, anti-flotillin-1, or a control antibody. Immunoprecipitated proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography. Scale bars, 10 μm.
Subcellular colocalization of PTOV1 and flotillin-1.
Flotillin-1 is a major protein component of lipid rafts (5, 36). These membrane domains are resistant to solubilization with nonionic detergents at low temperatures (5, 7) and have low buoyant density on sucrose density gradients (5). To analyze the presence of flotillin-1 and PTOV1 in lipid rafts, PC-3 cells extracts were separated into crude nuclear and nonnuclear fractions, and the latter extracts were used to isolate lipid rafts by sucrose density gradient centrifugation. Flotillin-1, as expected, was clearly enriched in the 20 to 30% sucrose fractions, and PTOV1 cofractionated with flotillin-1 in the same floating fractions (Fig. 1C). These results show that PTOV1, originally described as a cytoplasmic and nuclear protein (34), also associates with lipid rafts. Unexpectedly, not only PTOV1 but also flotillin-1 was clearly present in the nuclear fraction (Fig. 1C). The urokinase receptor (uPAR), a glycophosphatidylinositol-anchored protein that is associated with membrane lipid rafts and abundantly expressed in PC-3 cells (19), was present exclusively in the floating, nonnuclear fractions (Fig. 1C), indicating the absence of significant cell membrane contamination of the nuclear fractions. In addition, the finding of actin in the pellet of the sucrose density gradient, where cytoskeletal proteins are expected to fractionate, but not in the floating fractions also supports the lack of contamination among the fractions.
The subcellular localizations of flotillin-1 and PTOV1 were also analyzed by confocal immunofluorescence in PC-3 cells. As expected from the previous cofractionation experiments, PTOV1 and flotillin-1 partially colocalized at the plasma membrane (Fig. 1D). Also, in agreement with the fractionation experiments described above, both proteins also colocalized in the nucleus (Fig. 1D). For unequivocal localization, nuclei were counterstained with propidium iodide (Fig. 1E). In parallel experiments, flotillin-2, a paralogue of flotillin-1 (16), was seen exclusively localized to the cell membrane and was never seen associated with the nucleus (data not shown). Immunoelectron microscopy for flotillin-1 showed localizations at the cell membrane, in the cytoplasm, and in the nucleus without association of flotillin-1 with any identifiable structures, including the nucleolus or the nuclear envelope (data not shown). As an additional characterization to confirm the specificity of the commercial anti-flotillin-1 antibody employed in these studies (41), immunoprecipitation experiments were performed on samples metabolically labeled with [35S]methionine. Immunoprecipitation of PC-3 cells transfected with pHA-Flot1 with either anti-flotillin-1 or anti-HA antibodies yielded a band that comigrated at 48 kDa, the size expected for flotillin-1; no other detectable proteins were pulled down in these experiments (Fig. 1F).
Subcellular localization of HA-tagged forms of flotillin-1.
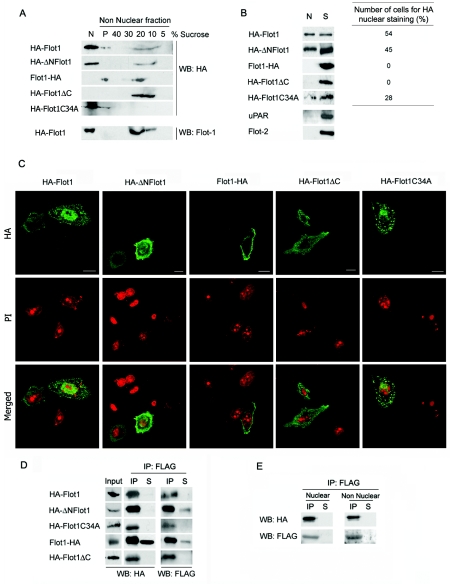
To further confirm that flotillin-1 is indeed capable of nuclear localization, HA-tagged versions of flotillin-1 (Fig. 2A) were transfected into PC-3 cells and analyzed for their subcellular localization. Full-length flotillin-1 was tagged with a hemagglutinin epitope at either its amino terminus or its carboxy terminus (pHA-Flot1 and pFlot1-HA, respectively). An HA tag was also placed at the amino end of the truncated flotillin-1 form that lacked the first 23 amino acids at its amino terminus, corresponding to the clone that interacted with PTOV1 in the original yeast two-hybrid screening (pHA-ΔNFlot1) (Fig. 2A). Two other constructs were used, one lacking the carboxy-terminal 38 residues of the protein (pHA-Flot1ΔC), and one in which the cysteine at position 34 was substituted for alanine (pHA-Flot1C34A). Cysteine 34 has been shown by others (27) to be a site for palmitoylation of flotillin-1 required for optimal localization of the protein to the plasma membrane and to lipid rafts. As shown in Fig. 2A, both amino-terminally tagged forms of flotillin-1, HA-flotillin-1 (HA-Flot1) and HA-ΔNFlot1, presented a sedimentation behavior on sucrose density gradients similar to that of endogenous flotillin-1, with fractionation both to the lipid raft-enriched fractions and to the nuclear fraction, although the chimeric proteins appeared to have a less prominent raft distribution than endogenous flotillin-1 (Fig. 2A). These localizations were confirmed by Western blotting of purified nuclear and cytosolic fractions (Fig. 2B) and by immunocytochemistry (Fig. 2C). Approximately 50% of the cells transfected with HA-Flot1 or HA-ΔNFlot1 showed nuclear reactivity when stained with anti-HA (Fig. 2B, right panel). In contrast, the carboxy-terminally tagged HA-Flot1 and HA-Flot1ΔC proteins, although clearly detected in lipid rafts, were not present in the nuclear fraction, and no cells with nuclear staining could be identified (Fig. 2A to C). This result suggests that modifications of full-length flotillin-1 at its carboxy terminus, but not at its amino terminus, compromise its nuclear localization. Failure of nuclear localization of flotillin-1 tagged at its carboxy terminus is independent of the specific epitope used for tagging, since a Flot1-FLAG fusion protein is also excluded from the nucleus (data not shown). Deletion of the carboxy-terminal 38 residues of flotillin-1 also prevented the nuclear localization of the protein (Fig. 2A to C), thus reinforcing the notion that the carboxy terminus of flotillin-1 contains a function that is essential for its nuclear localization which is sensitive to relatively small modifications, such as the addition of short epitopes. The nonpalmitoylable mutant Flot1C34A failed to associate with lipid rafts, as expected (27), but it was still capable of nuclear localization, albeit with a reduced efficiency (50% reduction) compared to that of HA-Flot1 (Fig. 2A to C). This result suggests that prior association of flotillin-1 with lipid rafts may facilitate, but is not an absolute requirement for, its nuclear localization. As in the previous fractionation experiments, uPAR was found only in the nonnuclear fractions. In addition, the absence of flotillin-2 signal in the nuclear fractions further confirmed that these were not contaminated with cytoplasmic or membrane-associated proteins (Fig. 2B).
FIG. 2.
Subcellular localization and interaction with PTOV1 of HA-tagged forms of flotillin-1. (A) Association of epitope-tagged flotillin-1 variants with lipid rafts. Nuclear and nonnuclear fractions were obtained from PC-3 cells transfected with the indicated variants. Nonnuclear fractions were further fractionated by sucrose density gradient (Fig. 1). The fractions (5 to 40% of sucrose), the pellet of the gradient (P), and the total crude nuclear extract (N) were analyzed by Western blotting (WB) with anti-HA. The same membranes were reanalyzed for endogenous flotillin-1 (Flot1) partitioning and gave results similar to those of Fig. 1C (endogenous flotillin-1 is shown only for the HA-Flot1 transfectant). (B) Nuclear localization of epitope-tagged variants of flotillin-1. (Left panel) Cells were transfected with the indicated plasmids, and 10% of the crude nuclear extract (N) and of nonnuclear fractions (S) were analyzed by Western blotting with anti-HA antibody. The same membranes were reanalyzed with antibodies to uPAR and to flotillin-2 and found to be negative for plasma membrane protein contamination of nuclear extracts(shown are the HA-Flot1 transfectants). (Right panel) In parallel transfections, the percentage of cells with nuclear HA was assessed for each construction, as recorded by immunocytochemistry. At least 200 cells were counted in each case in two independent experiments. (C) Representative confocal immunofluorescence images of transfected flotillin-1 variants stained with anti-HA (top) and with propidium iodide (PI) (bottom). Control reactions eliminating the primary antibody gave negative staining. Scale bars, 10 μm. (D) Coimmunoprecipitation of FLAG-PTOV1 and flotillin-1 variants. Cells were cotransfected with pFLAG-PTOV1 and HA-tagged flotillin-1 variants, lysates were immunoprecipitated with anti-FLAG antibody, and total cell extracts (10% [Input]), IP, nonprecipitated material (10% [S]), or precipitated proteins from a control antibody (data not shown) were detected by Western blotting with anti-HA and anti-FLAG antibodies. (E) Coimmunoprecipitation of FLAG-PTOV1 and HA-Flot1 in nuclear or nonnuclear fractions. Lysates from cells cotransfected with pFLAG-PTOV1 and pHA-Flot1 were separated into nuclear and nonnuclear fractions and immunoprecipitated with anti-FLAG, and coimmunoprecipitates were detected by Western blotting with anti-HA and anti-FLAG antibodies.
We next determined the capacity of PTOV1 to interact with the HA-tagged forms of flotillin-1. FLAG-PTOV1 very efficiently pulled down the amino-terminally tagged full-length flotillin-1, the HA-ΔNFlot1 variant, and the nonpalmitoylable mutant HA-FlotC34A (Fig. 2D). The efficiency of coimmunoprecipitation was greatly compromised when an HA tag was present at the carboxy terminus of flotillin-1 (pFlot1-HA), suggesting that flotillin-1 requires a free carboxy terminus for an efficient interaction with PTOV1. However, the carboxy-terminal deletion variant HA-Flot1ΔC fully retained its capacity to interact with PTOV1 (Fig. 2D). PTOV1 interacted indistinguishably with both nuclear and nonnuclear flotillin-1 (Fig. 2E), indicating that this interaction is not a function of a particular subcellular localization of flotillin-1. Immunoprecipitation with an isotype-matched mouse immunoglobulin was negative in all cases (data not shown). These results suggest that the carboxy terminus of flotillin-1 does not interact directly with PTOV1, but its modification by the addition of an HA tag partially interferes with this interaction. Deletion or epitope tagging of the carboxy terminus of flotillin-1 completely inhibits its nuclear localization.
Cell cycle-dependent nuclear localization of PTOV1 and flotillin-1.
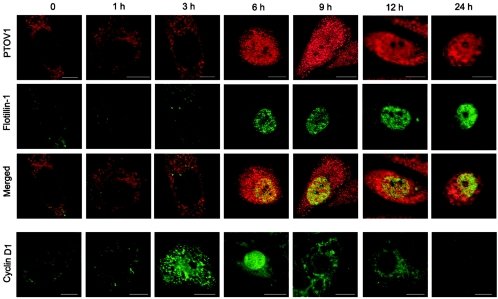
To determine if the nuclear localization of flotillin-1 was associated with the mitogen-dependent nuclear translocation of PTOV1 (34), time course serum stimulation experiments were performed on quiescent, serum-deprived PC-3 cells, and localization of both proteins was assessed by double immunofluorescence (Fig. 3). The proliferative status of the cells was monitored by the expression of Ki67 (40). In quiescent cells, PTOV1 was localized mostly in the cytoplasm, while flotillin-1 was barely detectable. At 6 h after serum stimulation, coincident with the initiation of the S phase of the cell cycle, marked by nuclear exit of cyclin D1 (2), PTOV1 underwent nuclear translocation. Nuclear localization of flotillin-1 was observed exclusively in those cells in which PTOV1 was also present in the nucleus (Fig. 3). The two proteins remained in the nucleus throughout the S phase, after which the number of cells with nuclear staining for both proteins progressively declined (Fig. 4B). The proportion of cells with nuclear PTOV1 was coincidental with that of cells with nuclear flotillin-1 throughout the serum stimulation experiment (Fig. 4B). In these experiments, confocal images were captured at 2 to 5 μm above the most basal plane of the cells to select for optimal images of nuclear localization of the proteins. This resulted in suboptimal images for the simultaneous localization of flotillin-1 in cell membranes. Serum depletion and readdition to PC-3 cells resulted in synchronous populations of cells, as assessed by the homogeneous oscillation of the proportion of cells with nuclear cyclin D1 (Fig. 4B). Under these conditions, flotillin-2 was localized exclusively to the cell membrane at all times, without detectable association with the nucleus (data not shown).
FIG. 3.
Cell cycle-dependent nuclear translocation of endogenous PTOV1 and flotillin-1. Quiescent serum-starved PC-3 cells were stimulated with 10% FBS for the indicated times and stained by double fluorescence for PTOV1 (red) and flotillin-1 (green). In parallel coverslips, cells were also stained for cyclin D1 (green). Scale bars, 10 μm.
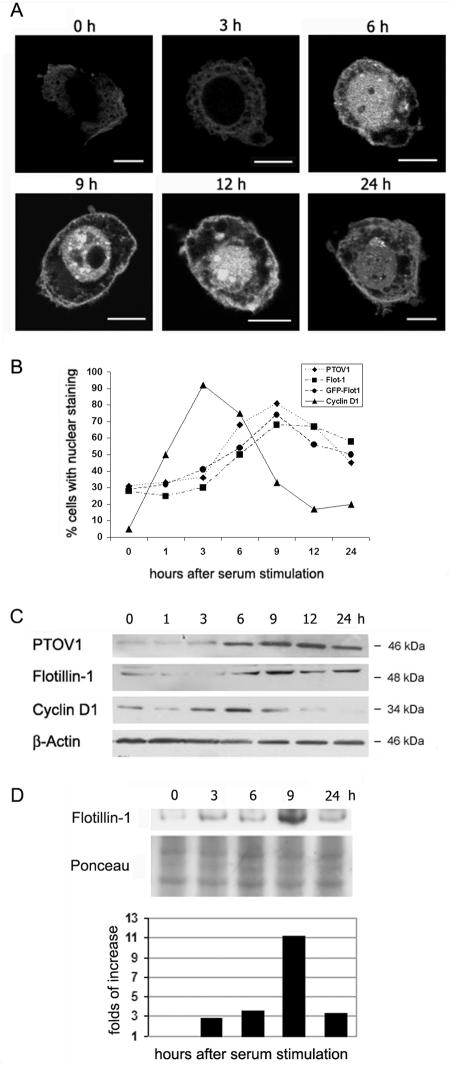
FIG. 4.
Cell cycle-dependent protein turnover of endogenous PTOV1 and flotillin-1 and of GFP-flotillin-1. (A) Cell cycle-dependent nuclear translocation of GFP-flotillin-1. PC-3 cells transiently transfected with pGFP-Flot1 were serum starved and restimulated with 10% FBS and fixed at the indicated times, and GFP fluorescence was captured by confocal microscopy. Scale bars, 10 μm. (B) Association of the nuclear localization of endogenous PTOV1 and flotillin-1 (Flot-1) and of transfected GFP-flotillin-1 with cell cycle progression. PC-3 cells were treated as described in the legend of Fig. 3, and the cells with nuclear PTOV1, nuclear flotillin-1, and nuclear cyclin D1 fluorescent staining were scored at each time point. To determine GFP-flotillin-1 localization, transfected PC-3 cells were treated as described above (A), and nuclear GFP fluorescence was scored. At least 200 cells were counted for each condition. (C) Cell cycle-associated variation in protein levels of PTOV1 and flotillin-1. In experiments performed inparallel to those described above (A), whole-cell lysates were analyzed by Western blotting with specific antibodies. Antibody to β-actin was used as a control for protein loadings. (D) Cell cycle-associated changes in the levels of nuclear endogenous flotillin-1. PC-3 cells were subjected to serum depletion and stimulation as described in the legend of Fig. 3, nuclei were isolated (see Materials and Methods) at the indicated time points, and proteins were extracted and analyzed by Western blotting with anti-flotillin-1. Loading normalizations were done relative to total protein content as assessed by Ponceau red staining of the transferred membranes. The bar graph corresponds to the normalized values of the densitometric scans of the bands.
To further confirm that flotillin-1 can be localized in the nucleus in cycling cells, we studied the behavior of GFP-flotillin-1 fusion protein in transiently transfected PC-3 cells in time course serum stimulation experiments performed as described above for the endogenous protein (Fig. 4A). In quiescent serum-deprived cells, GFP-flotillin-1 is visible only outside the nucleus, being localized in the cytoplasm and the plasma membrane. At 6 h after serum stimulation, GFP-flotillin-1 translocated to the nucleus with kinetics that were identical to those of endogenous flotillin-1 (Fig. 4A). The distribution of the fusion protein, analyzed by scoring the proportion of cells with nuclear GFP-flotillin-1 at each time point, follows very closely that of the endogenous protein at all time points after serum stimulation (Fig. 4B). The variations in the strength of the GFP signal throughout the serum starvation and stimulation experiment are a reflection of the serum-dependent regulation of the cytomegalovirus promoter-enhancer (6) and consequently of the levels of GFP-flotillin-1 protein in PC-3 cells (data not shown).
In parallel experiments, Western blotting of extracts from synchronized cells showed that protein levels of PTOV1 and flotillin-1 follow a cyclic behavior, with low levels in quiescence followed by a gradual increase after serum stimulation that reached its maximum levels approximately 9 h after serum stimulation, with the majority of cells in the S phase of the cell cycle (Fig. 4C). In addition, serum depletion and stimulation experiments in which nuclei were fractionated and analyzed by Western blotting also indicated that flotillin-1 accumulates in the nucleus with a peak at 9 h after serum stimulation (Fig. 4D). Therefore, nuclear localization of flotillin-1 followed cell cycle-dependent kinetics equivalent to that of PTOV1, and both proteins showed similar cyclic variations in their levels, with a maximum at S, concomitant with the translocation of both proteins to the nucleus.
PTOV1 is required for nuclear translocation of flotillin-1.
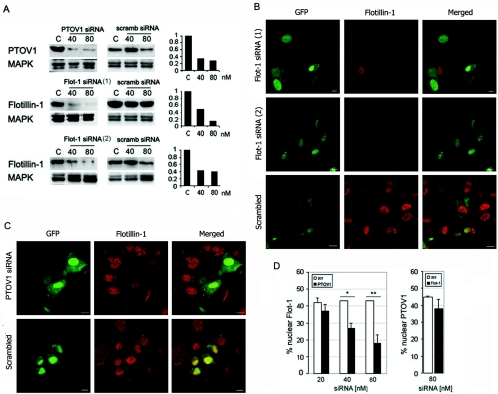
Thus far, our observations suggested that PTOV1 plays an important role in the nuclear localization of flotillin-1. PTOV1, but not flotillin-1, contains putative nuclear localization signals (NLS) that might account for its nuclear localization (4). We reasoned that PTOV1 could provide flotillin-1 with the NLS necessary for its transport into the nucleus and that, in the absence of PTOV1, flotillin-1 would not localize to the nucleus. To test this hypothesis, we studied the nuclear localization of flotillin-1 in PC-3 cells depleted of PTOV1 by RNA interference and assessed the number of cells with nuclear flotillin-1. Conversely, nuclear localization of PTOV1 was determined in cells depleted of flotillin-1. Forty-eight hours after transfection of siRNA for PTOV1, protein levels were specifically decreased by 67 and 73% with 40 and 80 nM siRNA, respectively, compared to cells transfected with a control siRNA (Fig. 5A). Depletion of PTOV1 was accompanied by a statistically significant reduction in the number cells with flotillin-1 in the nucleus compared to cells treated with equivalent concentrations of control siRNA (Fig. 5C and D). The reduction in nuclear localization of flotillin-1 was directly proportional to the degree of depletion of PTOV1 (Fig. 5D, left panel) and suggested that nuclear translocation of flotillin-1 is largely dependent on the presence of PTOV1.
FIG. 5.
Depletion of PTOV1 and flotillin-1 by RNA interference suggests a dependence on PTOV1 for the nuclear localization of flotillin-1. (A) Specific depletion of PTOV1 and flotillin-1 proteins attained by transfecting PC-3 cells with the indicated double-stranded siRNAs. Forty-eight hours posttransfection, PTOV1 or flotillin-1 levels were analyzed by Western blotting and quantitated by densitometric scanning of the signal (right graphs). Normalization was done against signals for mitogen-activated protein kinase (MAPK). As controls, nonspecific (scrambled [scramb]) siRNAs were transfected in parallel at the same concentrations. (B) Confocal immunofluorescent images of cells transfected with either of two distinct siRNA duplexes targeting flotillin-1, and control scrambled siRNA. RNA duplexes were cotransfected with pEGFP to identify transfected cells. The majority of cells transfected with duplexes targeting flotillin-1, but not those transfected with the control RNA duplexes, have a markedly decreased staining for endogenous flotillin-1. (C) Effect of the depletion of PTOV1 on the nuclear localization of flotillin-1. Confocal immunofluorescent images of cells transfected with siRNA targeting PTOV1 (80 nM) and control scrambled siRNA (80 nM) are shown. RNA duplexes were cotransfected with pEGFP to identify transfected cells. Cells transfected with duplexes targeting PTOV1 have a markedly decreased staining for endogenous nuclear flotillin-1, analyzed by fluorescent staining with anti-flotillin-1. (D) Effect of the depletion of PTOV1 or flotillin-1 on the nuclear localization of the reciprocal proteins. Cells transfected with pEGFP plus different concentrations of PTOV1 siRNA (left panel) or 80 nM Flot-1 siRNA 1 (right panel) or scrambled (scr) siRNA as controls were analyzed by fluorescent staining with anti-flotillin-1 (left) or anti-PTOV1 (right). Nuclear localization of flotillin-1 or PTOV1 was scored in GFP-positive cells (*, P = 0.008; **, P = 0.0001). At least 200 cells were scored for each condition. In panels B and C, images in the red fluorescence range were captured at equal voltages; scale bars, 10 μm.
Transfection of siRNA 1 for flotillin-1 caused a specific and marked decrease (55 and 90% reduction for 40 and 80 nM siRNA, respectively) in its protein levels after 48 h (Fig. 5A and B). A second, unrelated siRNA, Flot-1 siRNA 2, also markedly and specifically depleted flotillin-1 (Fig. 5A and B). Both siRNA 1 and siRNA 2 induced a clear decrease in membrane and nuclear flotillin-1 staining in the majority of cells (Fig. 5B). However, depletion of flotillin-1 by siRNA 1 had no significant effect on the nuclear localization of endogenous PTOV1 (Fig. 5D, right panel). We conclude that nuclear localization of flotillin-1 requires PTOV1, while nuclear translocation of PTOV1 occurs independently of flotillin-1.
PTOV1 and flotillin-1 are mitogenic and are required for normal cell proliferation.
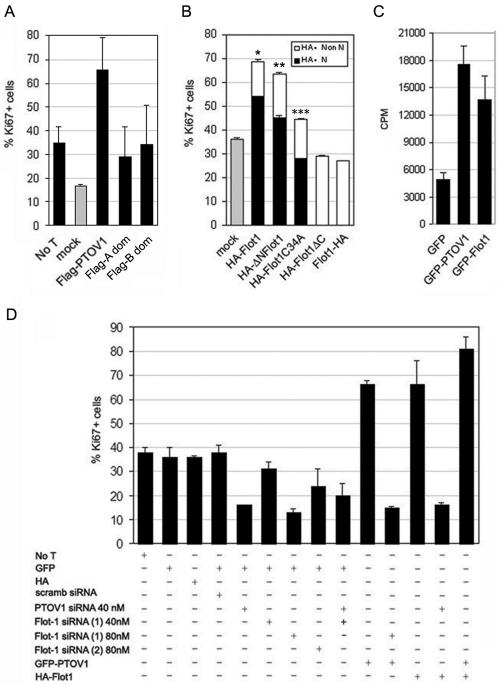
We have previously shown that overexpression of GFP-PTOV1 is mitogenic in different cell types (35). As shown in Fig. 6A, the FLAG-PTOV1 fusion protein was also strongly mitogenic. Each of the two PTOV modules of PTOV1 bears a putative NLS and, when transfected separately (FLAG A domain and FLAG B domain), were capable of entering the nucleus. Despite this capacity, transfection of either of the two individual PTOV domains had significantly decreased mitogenic capacity compared to that of the full-length protein (Fig. 6A), indicating that both domains are needed to reproduce the full proliferative effect of PTOV1.
FIG. 6.
PTOV1 and flotillin-1 are required for normal cell proliferation. The effect of overexpression of exogenous PTOV1 or flotillin-1 or the depletion of the corresponding endogenous proteins on the proliferative state of PC-3 cells was assessed by double fluorescence for GFP, anti-FLAG or anti-HA, and anti-Ki67. At least 200 cells were scored for each condition. The results shown are the means from three independent experiments for each condition. (A) Mitogenic effects of full-length PTOV1 and the two separate PTOV domains of PTOV1. FLAG constructs were transfected, and cells were analyzed 48 h after transfection by double fluorescence staining with anti-Ki67 and anti-FLAG antibodies. For mock transfections with empty FLAG vector and untransfected cells (No T), only the Ki67 staining was scored. (B) Mitogenic activity of flotillin-1 variants. Forty eight hours after transfection of the indicated plasmids, cells were analyzed by double fluorescence for Ki67 and HA reactivities to determine their proliferative capacities. Nuclear (N) or nonnuclear (Non N) staining of the HA reactivity was also scored to determine the capacity for each variant to enter the nucleus. Significance for Ki67 values relative to mock controls is as follows: *, P = 2.97 × 10−6; **, P = 0.0001; ***, P = 0.19. (C) Overexpression of GFP-flotillin-1 induces cell proliferation. PC-3 cells were transfected with pEGFP, pGFP-PTOV1, or pGFP-Flot1 and analyzed 48 h after transfection for [3H]thymidine incorporation. Induction of proliferation for each plasmid, corrected for transfection efficiencies, is expressed as counts per minute (CPM). (D) Effects of the depletion of PTOV1 or flotillin-1 on the proliferative status of PC-3 cells. Cells were transfected with pEGFP or pCMV-HA as controls, pGFP-PTOV1, or pHA-Flot1 and the indicated siRNAs and assessed by double fluorescence detection for Ki67 and GFP or HA. For cells transfected with pGFP-PTOV1 and pHA-Flot1, fluorescent staining for Ki67 was determined for GFP-positive cells.
Transfection of full-length HA-flotillin-1 also produced a marked mitogenic effect (Fig. 6B) which was completely inhibited by depletion of PTOV1 (Fig. 6D). Transfection of other flotillin-1 variants that retained the capacity to enter the nucleus (pHA-ΔN-Flot1 and pHA-Flot1C34A) also resulted in significantly augmented proliferative indexes (Fig. 6B). The nonpalmitoylable variant HA-Flot1C34A, which has an attenuated capacity for nuclear localization (Fig. 2B), showed an attenuated capacity to augment the proliferative index that was not statistically significant compared to that of the mock-transfected control (Fig. 6B). In all cases, nuclear localization of the transfected forms of flotillin-1 was strictly associated with Ki67 positivity such that all of the cells in which the transfected flotillin-1 variants localized to the nucleus stained for Ki67. In contrast to the flotillin-1 variants that can enter the nucleus, transfection of the flotillin-1 variants that fail to enter the nucleus (HA-Flot1 and HA-Flot1ΔC) did not augment the numbers of Ki67-positive cells (Fig. 6B) and were therefore not mitogenic. The mitogenic effect of flotillin-1 overexpression was also confirmed by [3H]thymidine incorporation assay (Fig. 6C). In these experiments, pGFP-Flot1 induced a very significant (2.8-fold) increase in proliferation compared to controls in which control pEGFP was transfected (Fig. 6C). The GFP-flotillin 1 protein entered the nucleus with efficiencies equivalent to those of HA-Flot1 (data not shown).
Next, the proliferative status of PC-3 cells depleted of either PTOV1 or flotillin-1 by RNA interference was assessed by determining the expression of Ki67 in cells cotransfected with siRNA and constructs for the expression of GFP or HA-modified proteins in double-fluorescence assays. Cells transfected with pEGFP or with pEGFP plus control siRNAs yielded very similar frequencies of double-GFP- and Ki67-positive cells (Fig. 6D). Specific depletion of either PTOV1 or flotillin-1 by treatment with the corresponding siRNAs markedly reduced the number of double-GFP- and Ki67-positive cells after 48 h of RNA interference (Fig. 6D). Depletion of PTOV1 with 40 nM siRNA was used in these experiments to avoid nonspecific effects observed with higher concentrations of the control scrambled siRNA (Fig. 5A). Simultaneous depletion of both proteins by cotransfection of Flot-1 siRNA 1 and PTOV1 siRNA did not further reduce the number of proliferating cells. Therefore, both PTOV1 and flotillin-1 are necessary for normal cell proliferation.
Transfection of pGFP-PTOV1 had a strong mitogenic effect (Fig. 6C), as had been previously shown by fluorescence-activated cell sorter and Ki67 analyses (35). Depletion of flotillin-1 completely abolished the proliferative effect induced by GFP-PTOV1 (Fig. 6D). Conversely, transfection of pHA-flotillin-1 was similarly mitogenic, and depletion of PTOV1 with PTOV1 siRNA completely abolished this proliferative effect (Fig. 6D). Simultaneous overexpression of GFP-PTOV1 and HA-flotillin-1 resulted in an increase in the fraction of proliferating PC-3 cells (82%) that was slightly larger than that induced by overexpression of either protein alone (66 and 65%) (Fig. 6D). These observations lend further support to the notion that the two proteins depend on each other for their proliferative effects. In addition, the increase in the number of proliferating cells when both PTOV1 and flotillin-1 are overexpressed suggests a direct relationship between the amount of complexes formed between both proteins and the induction of proliferation.
In conclusion, both PTOV1 and flotillin-1 are strong inducers of cell proliferation, each of these two proteins is required for normal cell proliferation, and they show mutual interdependence in order to attain their mitogenic effects. In addition, the mitogenic effect of flotillin-1 is strictly associated with its capacity to enter the nucleus.
DISCUSSION
We have previously reported that overexpression of PTOV1 is associated with neoplastic prostate epithelium (4) and that it induces proliferation in different cell types (34). Here, we show that PTOV1 interacts with the lipid raft protein flotillin-1 in yeast and mammalian cells. Remarkably, the two proteins not only copurify in lipid rafts and colocalize at membrane sites, they also colocalize in the nucleus. Both PTOV1 and flotillin-1 enter the nucleus several hours after a mitogenic stimulus, shortly before the beginning of the S phase. The nuclear localization of flotillin-1 had not been previously reported, and the supporting experiments performed in the present study include confocal immunofluorescence, immunoelectron microscopy, and subcellular fractionation analyses for endogenous and transfected flotillin-1. Work reported previously by others (14, 27) has also provided images consistent with nuclear localization of flotillin-1 in different cell types and with different antibodies, but this was not further analyzed or discussed in those reports. Our study also strongly suggests that the nuclear entry of flotillin-1 requires an intact carboxy terminus, that nuclear entry requires PTOV1, and that once in the nucleus flotillin-1 functions to stimulate cell proliferation. The most relevant observations that lead to these conclusions are as follows: (i) truncation or modification of the carboxy terminus, but not the amino terminus, of flotillin-1 prevents its translocation to the nucleus; (ii) mitogen-stimulated nuclear entry of flotillin-1 is simultaneous with nuclear entry of PTOV1; (iii) depletion of PTOV1 by RNA interference prevents the nuclear localization of flotillin-1; and (iv) PTOV1 and flotillin-1 have a mutually dependent mitogenic effect.
Lipid raft-associated flotillin-1 tethers signaling complexes to these specialized membrane domains through interaction with a SoHo motif present in proteins like CAP and vinexin (18). PTOV1 does not have a recognizable SoHo motif, and thus, its interaction with flotillin-1 may be mediated by a domain on the latter protein different from that involved in interactions with SoHo motifs. In contrast to flotillin-1, we show that its paralogue, flotillin-2/Reggie-1, which is also associated with lipid rafts, associates exclusively with membranes. These two proteins are very similar in sequence, except at their divergent amino termini and for the presence of a glycine-rich stretch near the carboxy terminus of flotillin-1 that is absent from flotillin-2 (5, 16, 35). Removal of the amino-terminal 28 residues from flotillin-1 does not affect its localization to the nucleus, whereas deletion of the carboxy-terminal 38 residues or the addition of an HA or FLAG epitope at the carboxy terminus of the protein completely inhibited its nuclear localization. The carboxy-terminally deleted form of flotillin-1 was still able to efficiently interact with PTOV1, indicating that interaction with PTOV1 is not sufficient for nuclear localization of flotillin-1. Interestingly, the addition of an HA epitope at the carboxy terminus of flotillin-1 partially compromised its interaction with PTOV1. This finding might suggest that the carboxy terminus of flotillin-1 is normally folded such that it is placed near the surface of interaction with PTOV1 and that the addition of an HA tag hinders this interaction. Taken together, these observations indicate that the carboxy terminus of flotillin-1 is necessary for its nuclear localization and that this nuclear localization function is sensitive to modifications such as the addition of a hemagglutinin peptide.
Our RNA interference experiments show that nuclear entry of PTOV1 is autonomous in that it does not require flotillin-1, whereas nuclear entry of flotillin-1 depends on the presence of PTOV1. PTOV1 contains two putative consensus bipartite nuclear localization signals (4), while flotillin-1 lacks any putative NLS. The entry into the nucleus of many proteins that lack a functional NLS proceeds through interactions with other proteins that provide such signals in trans (15). PTOV1 consists of a duplicated module (4), each with a functional NLS, and our experiments show that only the second, most carboxy-terminal PTOV module binds flotillin-1 and could thus provide the signal for nuclear import of flotillin-1. Therefore, PTOV1 might provide a second signal, together with the carboxy-terminal region of flotillin-1, for the nuclear localization of this protein.
The insertion of flotillin-1 in membranes is mediated by a palmitoyl moiety (27, 29) and also possibly by the addition of myristoyl moieties, which modify flotillin-2 (33). Palmitoylation is a reversible modification of proteins that can regulate the localization of proteins in membranes (25, 43). Proteins with palmitoylable cysteine residues can cycle between palmitoylated and depalmitoylated states in response to a number of signals, including agonist-induced receptor activation and oxidative stress (11, 22, 32, 33, 43). It is not known if flotillin-1 can undergo such cycles of acylation-deacylation under physiological conditions, with associated cycles in its subcellular localization. Mutation of the cysteine residue at position 34 in flotillin-1, which renders it nonpamitoylable, inhibited its localization to membrane lipid rafts (27, 29) and attenuated, but did not inhibit, its capacity to enter the nucleus compared to that of HA-Flot1, causing the mutated protein to localize mostly in the cytoplasm. This result suggests that release of flotillin-1 from the membranes into the cytoplasm may not be sufficient for efficient translocation into the nucleus. The nonpalmitoylable form of flotillin-1 is able to interact very efficiently with PTOV1, and therefore, the latter protein, although necessary, is not sufficient for the nuclear translocation of flotillin-1. In consequence, in addition to its interaction with PTOV1 and an intact carboxy terminus, flotillin-1 may be required to reside in lipid rafts before it can translocate efficiently to the nucleus. This requirement has been shown for other proteins that reversibly associate with membranes through palmitoyl moieties, like phospholipid scramblase 1, which undergoes nuclear translocation when depalmitoylated or after stimulation of cells with cytokines (45). Other integral membrane proteins also translocate to the nucleus under a number of stimuli. Examples are Notch (1, 12), amyloid precursor protein (8, 9, 13, 17), ErbB4 (21, 30), E-cadherin (24), CD44 (31), and DCC (39). All these proteins are processed by γ-secretase-presenilin type mechanisms in response to specific stimuli, resulting in the proteolytic cleavage of their cytoplasmic domains which can translocate into the nucleus (12). In most cases, these proteins have two functions, one in signal transduction at the plasma membrane and a second function in transcriptional regulation in the nucleus (12).
The present observations raise the question of the possible functions of nuclear PTOV1 and flotillin-1 and, more specifically, by what mechanisms they stimulate cell proliferation. The architecture of PTOV1 of two consecutive, almost-identical modules arranged in tandem suggests that it could play the role of an adaptor molecule, bridging two proteins through protein-protein interactions. A second protein, conserved in humans, rodents, and flies, bears a PTOV module within a polypeptide otherwise unrelated to PTOV1. This protein has been variously termed PTOV2 (4), ARC92 (46), p78 (42), and ACID-1 (26) and was recently shown to be a critical component of multiprotein Mediator/ARC complexes that recruit activators to the basal transcriptional machinery (26, 46). In PTOV2/ARC92/ACID-1, the PTOV (or ACID) domain makes direct contacts with the acidic transcriptional activator VP16 (26, 46). A duplicated PTOV module, as found in PTOV1, could in principle act either to tether proteins with similar sequence motifs recognized by the PTOV module into functional complexes or, on the contrary, to titrate out the recruitment of proteins that function by interacting with PTOV2/ARC92/ACID-1 through the PTOV module of the latter protein. Although PTOV1 does not interact directly with VP16 (46), it will be interesting to examine whether it can modulate other functions of PTOV2/ARC92/ACID-1. Whether flotillin-1 participates in such functions also warrants further study.
Acknowledgments
We thank I. Prieto and J. L. Barbero for their generous support and help with immunocytochemistry and M. Scaltriti, M. Soler, and M. Valeri for valuable help and suggestions.
This study was supported by grants SAF2001-1969 from the Ministry of Science and Technology (to T.M.T.) and FIS 02/0764 from the Ministry of Health (to R.P.) and by the Fundación para la Investigación en Urología (to J.M.). A.S. is a recipient of a BEFI fellowship from the Instituto Nacional de la Salud Carlos III.
REFERENCES
- 1.Artavanis-Tsakonas, S., M. D. Rand, and R. J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science 284:770-776. [DOI] [PubMed] [Google Scholar]
- 2.Baldin, V., J. Lukas, M. J. Marcote, M. Pagano, and G. Draetta. 1993. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 7:812-821. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, C. A., V. Ribon, M. Kanzaki, D. C. Thurmond, S. Mora, S. Shigematsu, P. E. Bickel, J. E. Pessin, and A. R. Saltiel. 2000. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407:202-207. [DOI] [PubMed] [Google Scholar]
- 4.Benedit, P., R. Paciucci, T. M. Thomson, M. Valeri, M. Nadal, C. Caceres, I. de Torres, X. Estivill, J. J. Lozano, J. Morote, and J. Reventos. 2001. PTOV1, a novel protein overexpressed in prostate cancer containing a new class of protein homology blocks. Oncogene 22:1455-1464. [DOI] [PubMed] [Google Scholar]
- 5.Bickel, P. E., P. E. Scherer, J. E. Schnitzer, P. Oh, M. P. Lisanti, and H. F. Lodish. 1997. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272:13793-13802. [DOI] [PubMed] [Google Scholar]
- 6.Brightwell, G., V. Poirier, E. Cole, S. Ivins, and K. W. Brown. 1997. Serum-dependent and cell cycle-dependent expression from a cytomegalovirus-based mammalian expression vector. Gene 194:115-123. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. A., and E. London. 1997. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 240:1-7. [DOI] [PubMed] [Google Scholar]
- 8.Cao, X., and T. C. Sudhof. 2001. A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293:115-120. [DOI] [PubMed] [Google Scholar]
- 9.Cupers, P., I. Orlans, K. Craessaerts, W. Annaert, and B. De Strooper. 2001. The amyloid precursor protein (APP)-cytoplasmic fragment generated by γ-secretase is rapidly degraded but distributes partially in a nuclear fraction of neurones in culture. J. Neurochem. 78:1168-1178. [DOI] [PubMed] [Google Scholar]
- 10.Dermine, J.-F., S. Duclos, J. Garin, F. St-Louis, S. Rea, R. G. Parton, and M. Desjardins. 2001. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 276:18507-18512. [DOI] [PubMed] [Google Scholar]
- 11.El-Husseini, A.-D., E. Schnell, S. Dakoji, N. Sweeney, Q. Zhou, O. Prange, C. Gauthier-Campbell, A. Aguilera-Moreno, R. A. Nicoll, and D. S. Bredt. 2002. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108:849-863. [DOI] [PubMed] [Google Scholar]
- 12.Fortini, M. E. 2002. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat. Rev. Mol. Cell Biol. 3:673-684. [DOI] [PubMed] [Google Scholar]
- 13.Gao, Y., and S. W. Pimplikar. 2001. The γ-secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc. Natl. Acad. Sci. USA 98:14979-14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gkantiragas, I., B. Brugger, E. Stuven, D. Kaloyanova, X. Y. Li, K. Lohr, F. Lottspeich, F. T. Wieland, and J. B. Helms. 2001. Sphingomyelin-enriched microdomains at the Golgi complex. Mol. Biol. Cell 12:1819-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 16.Hazarika, P., N. Dham, P. Patel, M. Cho, D. Weidner, L. Goldsmith, and M. Duvic. 1999. Flotillin 2 is distinct from epidermal surface antigen (ESA) and is associated with filopodia formation. J. Cell. Biochem. 75:147-159. [PubMed] [Google Scholar]
- 17.Kimberly, W. T., J. B. Zheng, S. Y. Guenette, and D. J. Selkoe. 2001. The intracellular domain of the β-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a Notch-like manner. J. Biol. Chem. 276:40288-40292. [DOI] [PubMed] [Google Scholar]
- 18.Kimura, A., C. A. Baumann, S.-H. Chiang, and A. R. Saltiel. 2001. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc. Natl. Acad. Sci. USA 98:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiling, J. L., J. C. Byrd, R. J. Deisz, I. F. Mizukami, R. F. Todd III, and R. G. MacDonald. 2003. Binding of urokinase-type plasminogen activator receptor (uPAR) to the mannose 6-phosphate/insulin-like growth factor II receptor: contrasting interactions of full-length and soluble forms of uPAR. J. Biol. Chem. 278:20628-20637. [DOI] [PubMed] [Google Scholar]
- 20.Lang, D. M., S. Loomed, M. Jung, R. Ankerhold, B. Petrausch, U. Laessing, M. F. Wiechers, H. Plattner, and C. A. O. Stuerme. 1998. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J. Neurobiol. 37:502-523. [DOI] [PubMed] [Google Scholar]
- 21.Lee, H.-J., K.-M. Jung, Y. Z. Huang, L. B. Bennett, J. S. Lee, L. Mei, and T.-W. Kim. 2002. Presenilin-dependent γ-secretase-like intramembrane cleavage of ErbB4. J. Biol. Chem. 277:6318-6323. [DOI] [PubMed] [Google Scholar]
- 22.Linder, M. E., and R. J. Deschenes. 2003. New insights into the mechanisms of protein palmitoylation. Biochemistry 42:4311-4320. [DOI] [PubMed] [Google Scholar]
- 23.Lisanti, M. P., Z. Tang, P. E. Scherer, and M. Sargiacomo. 1995. Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol. 250:655-668. [DOI] [PubMed] [Google Scholar]
- 24.Marambaud, P., J. Shioi, G. Serban, A. Georgakopoulos, S. Sarner, V. Nagy, L. Baki, P. Wen, S. Efthimiopoulos, Z. Shao, T. Wisniewski, and N. K. Robakis. 2002. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 21:1948-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milligan, G., M. Parenti, and A. I. Magee. 1995. The dynamic role of palmitoylation in signal transduction. Trends Biochem. Sci. 20:181-187. [DOI] [PubMed] [Google Scholar]
- 26.Mittler, G., T. Stühler, L. Santolin, T. Uhlmann, E. Kremmer, F. Lottspeich, L. Berti, and M. Meisterernst. 2003. A novel docking site on Mediator is critical for activation by VP16 in mammalian cells. EMBO J. 22:6494-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow, I. C., S. Rea, S. Martin, I. A. Prior, R. Prohaska, J. F. Hancock, D. E. James, and R. G. Parton. 2002. Flotillin-1/Reggie-2 traffics to surface raft domains via a novel Golgi-independent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. J. Biol. Chem. 277:48834-48841. [DOI] [PubMed] [Google Scholar]
- 28.Näär, A. M., P. A. Beaurang, S. Zhou, S. Abraham, W. Solomon, and R. Tjian. 1999. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature 398:828-832. [DOI] [PubMed] [Google Scholar]
- 29.Neumann-Giesen, C., B. Falkenbach, P. Beicht, S. Claasen, G. Luers, C. A. Stuermer, V. Herzog, and R. Tikkanen. 2004. Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J. 378:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni, C.-Y., M. P. Murphy, T. E. Golde, and G. Carpenter. 2001. Gamma-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294:2179-2181. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto, I., Y. Kawano, D. Murakami, T. Sasayama, N. Araki, T. Miki, A. J. Wong, and H. Saya. 2001. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J. Cell Biol. 155:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parat, M. O., R. Z. Stachowicz, and P. L. Fox. 2002. Oxidative stress inhibits caveolin-1 palmitoylation and trafficking in endothelial cells. Biochem. J. 361:681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson, L. J., L. Busconi, and T. Michel. 1995. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J. Biol. Chem. 270:995-998. [DOI] [PubMed] [Google Scholar]
- 34.Santamaria, A., P. L. Fernandez, X. Farre, P. Benedit, J. Reventos, J. Morote, R. Paciucci, and T. M. Thomson. 2003. PTOV-1, a novel protein overexpressed in prostate cancer, shuttles between the cytoplasm and the nucleus and promotes entry into the S phase of the cell division cycle. Am. J. Pathol. 162:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulte, T., K. Paschke, U. Laessing, F. Lottspeich, and C. Stuermer. 1997. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development 124:577-587. [DOI] [PubMed] [Google Scholar]
- 36.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 37.Souto, R. P., G. Vallega, J. Wharton, J. Vinten, J. Tranum-Jensen, and P. F. Pilch. 2003. Immunopurification and characterization of rat adipocyte caveolae suggest their dissociation from insulin signaling. J. Biol. Chem. 278:18321-18329. [DOI] [PubMed] [Google Scholar]
- 38.Stuermer, C. A. O., D. M. Lang, F. Kirsch, M. Wiechers, S.-O. Deininger, and H. Plattner. 2001. Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol. Biol. Cell 12:3031-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi, Y., S.-H. Kim, and S. S. Sisodia. 2003. Presenilin-dependent “γ-secretase” processing of deleted in colorectal cancer (DCC). J. Biol. Chem. 278:30425-30428. [DOI] [PubMed] [Google Scholar]
- 40.Verheijen, R., H. J. Kuijpers, R. van Driel, J. L. Beck, J. H. van Dierendonck, G. J. Brakenhoff, and F. C. Ramaekers. 1984. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. Localization in mitotic cells and association with chromosomes. J. Cell Sci. 92:531-540. [DOI] [PubMed] [Google Scholar]
- 41.Volonte, D., F. Galbiati, S. Li, K. Nishiyama, T. Okamoto, and M. P. Lisanti. 1999. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J. Biol. Chem. 274:12702-12709. [DOI] [PubMed] [Google Scholar]
- 42.Wang, C., I. M. McCarty, L. Balazs, Y. Li, and M. S. Steiner. 2002. A prostate-derived cDNA that is mapped to human chromosome 19 encodes a novel protein. Biochem. Biophys. Res. Commun. 296:281-287. [DOI] [PubMed] [Google Scholar]
- 43.Wedegaertner, P. B., and H. R. Bourne. 1994. Activation and depalmitoylation of Gs alpha. Cell 77:1063-1070. [DOI] [PubMed] [Google Scholar]
- 44.Welsh, J. B., L. M. Sapinoso, A. I. Su, S. G. Kern, J. Wang-Rodriguez, C. A. Moskaluk, H. F. Frierson, Jr., and G. M. Hampton. 2001. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 61:5974-5978. [PubMed] [Google Scholar]
- 45.Wiedmer, T., J. Zhao, M. Nanjundan, and P. J. Sims. 2003. Palmitoylation of phospholipid scramblase 1 controls its distribution between nucleus and plasma membrane. Biochemistry 42:1227-1233. [DOI] [PubMed] [Google Scholar]
- 46.Yang, F., R. DeBeaumont, S. Zhou, and A. M. Naar. 2004. The activator-recruited cofactor/Mediator coactivator subunit ARC92 is a functionally important target of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. USA 101:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]