Abstract
Polo-like kinase 1 (Plk1) plays a role in numerous events in mitosis, but how the multiple functions of Plk1 are separated is poorly understood. We studied regulation of Plk1 through two putative phosphorylation residues, Ser-137 and Thr-210. Using phospho-specific antibodies, we found that Thr-210 phosphorylation precedes Ser-137 phosphorylation in vivo, the latter occurring specifically in late mitosis. We show that expression of two activating mutants of these residues, S137D and T210D, results in distinct mitotic phenotypes. Whereas expression of both phospho-mimicking mutants as well as of the double mutant leads to accelerated mitotic entry, further progression through mitosis is dramatically different: the T210D mutant causes a spindle assembly checkpoint-dependent delay, whereas the expression of the S137D mutant or the double mutant results in untimely activation of the anaphase-promoting complex/cyclosome (APC/C) and frequent mitotic catastrophe. Using nonphosphorylatable Plk1-S137A and Plk1-T210A mutants, we show that both sites contribute to proper mitotic progression. Based on these observations, we propose that Plk1 function is altered at different stages of mitosis through consecutive posttranslational events, e.g., at Ser-137 and Thr-210. Furthermore, our data show that uncontrolled Plk1 activation can uncouple APC/C activity from spindle assembly checkpoint control.
Since the identification of Polo in Drosophila over 15 years ago (54), the functions proposed for Polo-like kinases have been numerous. Most of the proposed actions of Plk are important for entry into, progression through, and exit from mitosis (16, 41). For instance, Polo-like kinase 1 (Plk1) has been suggested to promote mitotic entry by activating cyclin B1/Cdk1 in multiple ways: by phosphorylating cyclin B1 itself, by phosphorylating the Cdk1-activating phosphatase Cdc25C, and by phosphorylating the Cdk1-inhibiting kinases Myt1/Wee1 (1, 23, 30, 39, 45, 55, 58). Furthermore, Drosophila Polo and human Plk1 have been implicated in centrosome maturation and separation, with defects giving rise to monopolar spindles (31, 42, 45, 54). Proposed Plk targets regulating centrosome function are Hsp90, Asp, and Nlp (5, 9, 11). Additionally, budding yeast (Saccharomyces cerevisiae) Plk Cdc5 and Xenopus Plx1 phosphorylate a cohesin complex subunit, Scc1, thereby enhancing cleavage and regulating sister chromatid separation (2, 36, 53). Studies of budding yeast, Xenopus, murine, and human cells indicated a role for Plk1 in activating the anaphase-promoting complex/cyclosome (APC/C) (7, 10, 17, 29, 48). For one, Plk1 in combination with cyclin B1/Cdk1 can phosphorylate and activate the APC/C in vitro (17, 29). In addition, budding yeast Cdc5 has been proposed to contribute to the activation of the APC/C coactivator Cdh1, possibly by phosphorylating Cdc14 phosphatase (7, 47, 57). Cdc5 was shown to activate Cdc14 by releasing it from the nucleolus, which ultimately leads to mitotic exit and the onset of cytokinesis (21, 52). In fission and budding yeast, Plk1 homologues Plo1 and Cdc5 were implicated in the actual formation of the cytokinetic ring and septum (32, 42). Recently, MKlp2 and NudC were identified as possible direct Plk1 targets involved in cytokinesis in human cells (40, 59).
Looking at all of the above-listed functions of Plks, we wondered how these different actions are regulated. One level of regulation may be Plk's subcellular distribution. Plk1 is located mostly in the cytoplasm during interphase and translocates to the nucleus in early mitosis. Plk1 was shown to associate with centrosomes from G2 up to metaphase, to translocate to kinetochores at metaphase, and to locate at the midbody from anaphase to telophase (18, 33). The Polo box domain located in the C terminus is required for the localization of Plk1 to the centrosome and midbody (24, 51) and for the interaction with cell cycle regulators, e.g., Cdc25C in human cells and APC/C subunits in fission yeast (12, 46). The Polo box binds preferentially to phosphorylated serine/threonine motifs (12, 13), suggesting that the timely recruitment of Plk1 to a given substrate requires a priming phosphorylation event, e.g., by cyclin B1/Cdk1, that yields a high-affinity binding site for the Polo box. As such, sequential functions of Plk1 may require timed activation of different priming kinases.
Besides changes in subcellular localization, different phosphorylation events might also regulate the kinase Plk1 (19, 33, 38). Several kinases have been reported as upstream regulators of Polo-like kinases, e.g., Cdk1, mitogen-activated protein kinase, and protein kinase A (PKA). At different embryonic phases in starfish, distinct upstream kinases for Plk1 were identified, i.e., cyclin B/Cdk1 at meiosis I, mitogen-activated protein kinase as well as Cdk1 at meiosis II, and cyclin A/Cdk1 at embryonic M phase (43). In Xenopus, both xPlkk1 and PKA were shown to phosphorylate and activate Plx1 (28, 45). As with xPlkk1, related Ste20-like kinases were shown to target Plk1. For instance, human Ste20-like kinase phosphorylated mouse Plk1 in vitro (14) and mouse lymphocyte-oriented kinase was shown to phosphorylate Plk1 in vitro (25).
Possible target residues in Plk1 for upstream kinases are the highly conserved Ser-137 and Thr-210, latter being located in the so-called activation or T loop. When one or both residues are mutated into Asp to mimic phosphorylation, kinase activity is increased severalfold (32, 44). So far, no upstream kinase responsible for phosphorylating Ser-137 has been identified, and conflicting data exist about the kinase upstream of Thr-210, with xPlkk1 and PKA as candidates (25, 28, 44).
In this study, we examine the role of Ser-137 and Thr-210 in regulating Plk1 function in human cells. We show that phosphorylation of these residues in vivo occurs with different timing. Expression of activated Plk1 mutated at either residue affects mitotic progression differentially and demonstrate a role for Plk1 in APC/C activation. Our observations suggest that consecutive posttranslational modifications regulate separate Plk1 actions and that the order of events needs to be tightly controlled to prevent uncoupling of APC/C activation from spindle assembly checkpoint control.
MATERIALS AND METHODS
Construction of cell lines and plasmids.
UTA6 cells are a clonal population of human osteosarcoma U2OS cells stably transfected with the tetracycline-repressible transactivator tTA (provided by C. Englert, Karlsruhe, Germany) (15). UTA6 cells were grown in Dulbecco's modified Eagle's medium supplemented with 6 to 10% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 1 μg of tetracycline/ml. When washing and trypsinizing cells, 1 μg of tetracycline/ml was routinely added to the phosphate-buffered saline (PBS) and trypsin. pCMV-plasmids encoding wild-type (wt) Plk1, S137D, T210D, and S137D/T210D with an N-terminal Myc tag were a gift from E. Nigg (Martinsried, Germany). These (mutant) Myc-Plk1 constructs were subcloned into the tetracycline-repressible expression vector pUHD10-3 (provided by M. Gossen and H. Bujard, Berlin, Germany). To generate tetracycline-inducible (mutant) Plk1 cell lines, UTA6 cells were transfected with 10 μg of pUHD10-3 (mutant) Plk1 and 1 μg of pBabepuro (8) by the standard calcium phosphate transfection protocol. After 1 day, fresh medium containing 1 μg of puromycin/ml and 1 μg of tetracycline/ml was added. Two weeks later, individual colonies were picked and analyzed for Myc-Plk1 expression after induction by washing cells three times with PBS.
The murine nondegradable cyclin B1 plasmid pEF-B1DM-green fluorescent protein (GFP) was a gift of M. Brandeis (Jerusalem, Israel). Histone H2B-GFP (27), spectrin-GFP (26), the small interfering RNA (siRNA) vector pS (3), pS-Mad2 (34), and pS-Plk1 (56) have all been described previously, and pS-BubR1 was provided by G. Kops (La Jolla, Calif.). In short, the 19-mer targeting regions of pS-Plk1, pS-Mad2, and pS-BubR1 siRNA vectors were CGGCAGCGTGCAGATCAAC, GGAAGAGTCGGGACCACAG and AGATCCTGGCTAACTGTTC, respectively. A pRcCMV plasmid encoding Myc-wt Plk1 harboring silent mutations in the targeting region of pS-Plk1 (Plk1-sil) has been described elsewhere (56), rendering the protein insensitive to pS-Plk1-mediated degradation. These silent mutations were introduced in pRcCMV plasmids encoding Myc-Plk1-S137D and T210D (described above) by PCR-based mutagenesis (Stratagene) with the following primers: forward, 5′-AGCAACCGGCAGTGTTCAGATCAACTTC-3′ (silent mutations are indicated in boldface type); and reverse, 5′-GAAGTTGATCTGAACACTGCCGGTTGCT-3′. Plk1-S137A and T210A mutations were introduced into pRcCMV-Myc-Plk1-sil (56) with the following primers: S137A forward, 5′-TGCCGCCGGAGGGCCCTCCTGGAGC-3′; S137A reverse, 5′-GCTCCAGGAGGGCCCTCCGGCGGCA-3′; T210A forward, 5′-GGAGAGGAAGAAGGCCCTGTGTGGGAC-3′; and T210A reverse, 5′-GTCCCACACAGGGCCTTCTTCCTCTCC-3′.
Growth curves.
To assess proliferation in the (mutant) Plk1-inducible cell lines, 80,000 cells were plated in duplicate at day 0. Cells were either plated in the presence of tetracycline (noninduced) or were washed twice with PBS and plated in the absence of tetracycline (induced). Cells were harvested 1, 2, and 4 days after plating, after which the numbers of live, single cells with 12- to 30-μm diameters were determined by using a Casy 1 cell counter (RJM Sales, Scotch Plains, N.J.).
Immunofluorescence microscopy.
Cells grown on coverslips were fixed in 20 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.8), 0.2% Triton X-100, 1 mM MgCl2, 10 mM EGTA, and 4% formaldehyde for 10 min at room temperature. After blocking in 3% bovine serum albumin-PBS, cells were stained with mouse anti-Myc clone 4A6 (Upstate Biotechnology, Lake Placid, N.Y.), followed by chicken anti-mouse Alexa 488 and TO-PRO-3 (both from Molecular Probes, Eugene, Oreg.) for DNA staining.
Antibodies.
Rabbit anti-phospho-histone H3, rabbit anti-Plk1, and mouse anti-Myc clone 9E10 (used for immunoblotting and immunoprecipitation [IP]) and clone 4A6 (used for immunohistochemistry) were from Upstate Biotechnology; rabbit anti-Cdk4, rabbit anti-cyclin A, and mouse anti-cyclin B1 were from Santa Cruz Biotechnology (Santa Cruz, Calif.); phospho-Plk (Ser-137) antibody was from Cell Signaling Technology, Inc. (Beverly, Mass.). At our request, phospho-Plk1-Thr-210 antibody no. 98 was made by PhosphoSolutions (Aurora, Colo.). Donkey anti-rabbit antibody-Cy5 conjugate was from Jackson Immunoresearch Laboratories (Westgrove, Pa.). Peroxidase-conjugated goat anti-rabbit antiserum was from DAKO (Glostrup, Denmark). Propidium iodide (PI) was from Sigma (St. Louis, Mo.). Chicken anti-mouse antibody-Alexa 488 and TO-PRO-3 were from Molecular Probes.
Synchronization and spindle assembly checkpoint activation.
Plk1 (mutant) cell lines were synchronized at the G1/S transition by a double thymidine block. To this end, cells were treated with thymidine (2.5 mM; Sigma) for 24 h. Cells were released from the thymidine block by washing twice with PBS and then placed in the incubator for 15 min in fresh, warm medium before the medium was replaced again. Twelve hours after release, thymidine was added for another 24 h. To keep cells in a noninduced state, cells were always washed and cultured in the presence of 1 μg of tetracycline/ml. Next, cells were released from the second thymidine block as described above, either in the presence of tetracycline (noninduced) or in the absence of tetracycline (induced). When indicated, 250 ng of nocodazole/ml was added immediately after release or a 5 μM concentration of proteasome inhibitor MG132 (N-CBZ-Leu-Leu-Leu-Ala) (both from Sigma) was added for the last 3 h before harvesting. By using a cytocentrifuge (Shandon Elliot Cytospin; Frankfurt am Main, Germany) at 4,000 × g for 4 min, PI-stained cell suspension was spun on a microscope slide. The chromosome condensation state was assessed for >300 cells per condition.
For analysis with phospho-specific antibodies, cells were released from thymidine for 15 h in the presence of nocodazole. Mitotic cells were collected by shake-off and harvested or released from nocodazole by washing three times with PBS. Thirty minutes later, MG132 was added for 2.5 h to arrest cells in late mitosis. Only mitotic cells were harvested through shake-off. Plk1-depleted cells arrested in mitosis were harvested by shake-off 22 h after release from the thymidine block. For phosphatase treatment, cell lysates after nocodazole release (see above) were incubated at 30°C for 30 min in the presence of buffer and MnCl2 with or without λ-phosphatase (New England BioLabs, Inc., Beverly, Mass.). To test phospho-specificity of the Thr-210 antibody, we compared its recognition of peptides containing phospho- and dephospho-Thr-210 by densitometric analysis of dot blots with different amounts of both peptides.
Transfections.
Transient transfections in UTA6 cells were performed by using the standard calcium phosphate transfection protocol. Four micrograms of nondegradable cyclin B1 plasmid was transfected into Plk1-S137D cells. Directly after washing away the calcium phosphate precipitate, cells were arrested in thymidine for 24 h and used for time-lapse analysis after release.
For immunoblotting in reconstitution experiments, 10 μg of pS or pS-Plk1 was cotransfected into UTA6 cells with 1 μg of pBabepuro combined with the indicated amounts of wt Plk1-sil when indicated. Puromycin was added about 6 h after the calcium phosphate precipitate was washed away. After 24-h selection for transfected cells, cells were washed and puromycin-free medium was added. Another 24 h later, cells were harvested and lysed for immunoblotting.
For flow cytometry analysis in reconstitution experiments, 10 μg of pS or pS-Plk1 was cotransfected with 0.5 μg of spectrin-GFP combined with 0.5 μg of wt Plk1-sil, 0.5 μg of S137A, 10 μg of S137D, 0.5 μg of T210A, or 1 μg of T210D plasmid. Different amounts of reconstitution plasmids were used to equalize expression levels. Cells were synchronized in thymidine for 24 h and harvested 24 h after release. After 10 to 12 h of release, thymidine was re-added to collect cells at the G1/S transition.
For time-lapse analysis in reconstitution experiments, cells were cotransfected with 10 μg of pS-Plk1, 0.5 μg of H2B-GFP, and 0.5 μg of wt Plk1-sil or Plk1-S137A and monitored after synchronization with a single thymidine block.
Kinase assays, flow cytometry and immunoblotting.
Immunoblotting and in vitro kinase assays on cyclin B1 and Myc immunoprecipitates were performed as described previously (50) by using histone H1 (Roche, Basel, Switzerland) or dephosphorylated α-casein (Sigma) as a substrate. Tina 2.0 software (Raytest, Straubenhardt, Germany) was used to quantify kinase activity. Phospho-histone H3 and PI staining were performed as described previously (34).
Time-lapse analysis.
Plk1 (mutant) cell lines were plated on 35-mm-diameter glass-bottom culture dishes (WillCo-dish; WillCo Wells, Amsterdam, The Netherlands). When indicated, Plk1 (mutant) expression was induced the next day by washing three times with PBS. Cells were monitored with a Zeiss Axiovert 200 M microscope equipped with a 0.55 numerical aperture (NA) condenser and a ×20, 0.75 NA Plan-Apochromat objective in medium containing 33 μM HEPES (pH 7.4) and, when indicated, 250 ng of nocodazole (Sigma)/ml. Cells were heated to a temperature of 37°C with a microincubator ring (22). Images were taken by using Axio Vision 3.1 software (Zeiss, Oberkochen, Germany).
Plk1-depleted cells transfected with either wt Plk1 or Plk1-S137A in combination with H2B-GFP were monitored by time-lapse microscopy after release from thymidine block. Dishes were transferred to a heated culture chamber (37°C, 5% CO2) of a Zeiss Axiovert 200 M microscope equipped with a 0.55 NA condenser and a ×40, 1.3 NA Plan-Neo differential interference contrast (DIC) objective. Twelve-bit DIC and green fluorescence images were captured by using a Photometrics CoolSNAP HQ charged coupled device camera set at gain 1.0 (Scientific, Tucson, Ariz.) and a GFP filter cube (Chroma Technology Corp., Rockingham, Vt.) to select specific fluorescence. Images were processed by using MetaMorph software (Universal Imaging, Downingtown, Pa.).
RESULTS
Plk1 Ser-137 and Thr-210 are phosphorylated at different intervals in the cell cycle.
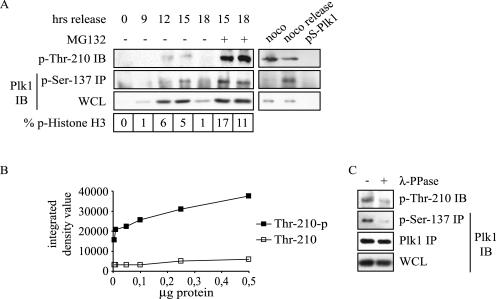
To study the regulation of Plk1 by phosphorylation, we examined the timing of in vivo Plk1 phosphorylation on the putative phosphorylation residues Ser-137 and Thr-210. Using phospho-specific antibodies, we analyzed phosphorylation at different points in the cell cycle in U2OS cell lysates. Expression of total Plk1 was most abundant at 12 and 15 h after thymidine release, at time points when the percentage of mitotic cells was at a maximum as measured by phospho-histone H3 positivity (Fig. 1A). Phosphorylation of Ser-137 was found mostly at 15 h after thymidine release, whereas Thr-210 phosphorylation was detected at both 12 and 15 h after thymidine release. This finding suggests that both residues are phosphorylated in vivo and that Thr-210 phosphorylation may precede phosphorylation of Ser-137. Adding the proteasome inhibitor MG132 for the last 3 h before harvesting resulted in increased percentages of mitotic cells and increased levels of Ser-137- and Thr-210-phosphorylated Plk1. We also examined cells arrested in mitosis by the microtubule-destabilizing agent nocodazole and cells released from nocodazole but blocked in late mitosis by the addition of MG132, thus comparing prometaphase cells with cells in late mitosis. In these two situations, Plk1 expression levels were equal (Fig. 1A, lower right panel). Ser-137 phosphorylation was not detected in nocodazole-blocked cells, but was found only in cells released from nocodazole. This finding indicates that Ser-137 phosphorylation occurs only in late mitosis. In contrast, phosphorylation of Thr-210 was found both in nocodazole and nocodazole release. These data indicate that Thr-210 phosphorylation precedes phosphorylation of Ser-137, which takes place only in late mitosis.
FIG. 1.
Different timing of Plk1 Ser-137 and Thr-210 phosphorylation. (A) U2OS cells were released from a thymidine block for the indicated times, and MG132 was added during the last 3 h before harvesting where indicated (left panel). In fixed cells, PI staining was combined with phospho-histone H3 staining to detect mitotic cells by flow cytometry (bottom left panel). Cells were collected from nocodazole arrest (noco), after release from nocodazole for 3 h with MG132 present for the last 2.5 h to keep cells in mitosis (noco release), or after Plk1 depletion (pS-Plk1) (right panel). Only mitotic cells were harvested by mitotic shake-off. Whole-cell lysates (WCL) were used for IP and immunoblotting (IB) with the indicated antibodies. (B) Densitometric analysis of dot blots of the indicated amounts of peptides containing phospho-Thr-210 (Thr-210-p) or dephospho-Thr-210 (Thr-210). (C) Cell lysates of cells released from nocodazole (see legend to panel A) were treated with λ-phosphatase before IP and immunoblotting as indicated.
The Ser-137 phospho-specific antibody is a commercially available antibody and its phospho-specificity was demonstrated by the supplier by using an enzyme-linked immunosorbent assay (Cell Signaling Technology). At our request, a Thr-210 phospho-specific antibody was generated (PhosphoSolutions). This antibody was tested for phospho-specificity by densitometric analysis of dot blots with peptides containing phospho- or dephospho-Thr210 (Fig. 1B). We also tested the specificities of both phospho-specific antibodies by phosphatase treatment. Cells released from nocodazole but blocked in mitosis by MG132 were lysed and incubated with or without λ-phosphatase. Phosphorylation of both Ser-137 and Thr-210 was decreased after phosphatase treatment, demonstrating that the phospho-specific antibodies are indeed phosphorylation-specific (Fig. 1C).
Expression of mutant Plk1 induces proliferation defects.
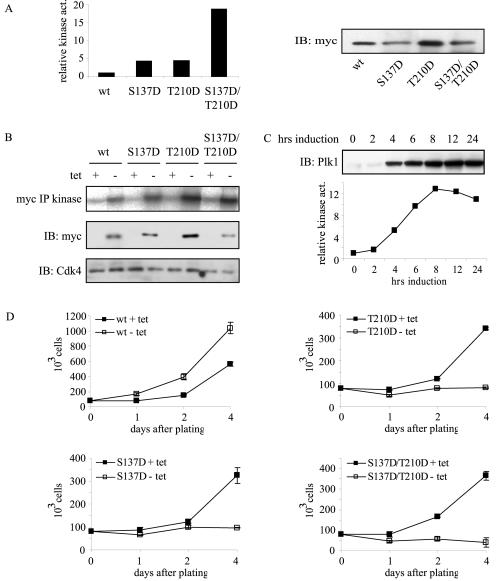
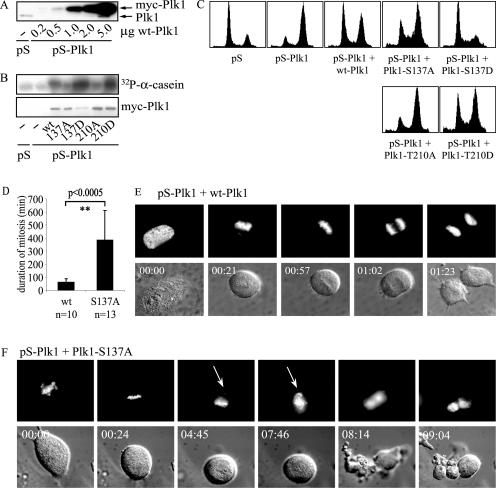
We wanted to study phosphorylation of Ser-137 and Thr-210 in more detail. Mutation of Ser-137 and Thr-210 to a phospho-mimicking residue has been described to result in elevated Plk1 kinase activity (25, 32, 44). When these mutants were expressed in U2OS cells and immunoprecipitated, we found that kinase activity of Plk1 in the respective mutants was indeed increased (Fig. 2A). We next generated inducible U2OS-derived cell lines that express (mutant) Plk1 under the control of a tetracycline-repressible promoter. Multiple clones expressing wt Plk1, Plk1-S137D, Plk1-T210D, or the double mutant Plk1-S137D/T210D, all N-terminally Myc-tagged, were generated. To compare expression and kinase activity in all cell lines, expression of Plk1 was induced by washing away the tetracycline-containing medium. After overnight induction, cells were harvested, lysed, and used for both immunoblotting and Myc-IP kinase assays using α-casein as a substrate. For our studies, we selected four cell lines with comparable Plk1 expression levels (Fig. 2B, middle panel). Kinase activity of the Plk1 mutants was elevated compared to wt Plk1 (Fig. 2B, upper panel). Noninduced control cells showed no or hardly any detectable expression and kinase activity (Fig. 2B). Induction of Plk1 expression in cells expressing Plk1-S137D was analyzed and was found to be very rapid (Fig. 2C, upper panel), with kinase activity reaching maximum levels around 8 h after induction (Fig. 2C, lower panel).
FIG. 2.
Expression of active Plk1 mutants results in proliferation defects. (A) wt Plk1, S137D, T210D, or S137D/T210D mutants were transfected in U2OS cells and subjected to Myc-IP kinase assays using α-casein as a substrate (left panel). Casein phosphorylation was quantified with wt Plk1 phosphorylation set as the reference. The amount of precipitated Myc-Plk1 was determined by anti-Myc immunoblotting (IB; right panel). (B) Expression of wt Plk1, S137D, T210D, or S137D/T210D mutants was induced in cell lines for 16 h by washing away tetracycline (− tet) or in samples left untreated (noninduced; + tet). Cell lysates were used for Myc-Plk1 IP kinase reactions using α-casein as a substrate (upper panel) or for immunoblotting and probed for Myc-Plk1 (middle panel) or Cdk4 as a loading control (lower panel). (C) Plk1-S137D expression was induced in asynchronous cells by washing away tetracycline. Cells were harvested and lysed at indicated time points. Lysates were used for immunoblotting and probed for Plk1 (upper panel) and used for Myc-Plk1 IP kinase assays with α-casein as a substrate (lower panel). Casein phosphorylation was quantified by phosphorylation with 0 h induction set as the reference. (D) Cell lines were induced (− tet) or noninduced (+ tet) and plated in duplicate on day 0. Cell numbers were counted 1, 2, and 4 days after plating.
To examine whether expression of (mutant) Plk1 affects proliferation, we obtained growth curves of all cell lines (Fig. 2D). Cells induced to express wt Plk1 proliferated more rapidly than noninduced cells. Remarkably, the expression of Plk1-S137D, Plk1-T210D, or Plk1-S137D/T210D resulted in severely impaired cell growth, indicating that the expression of constitutive active mutants of Plk1 is incompatible with normal cell proliferation.
Mutant Plk1 induces premature mitotic entry.
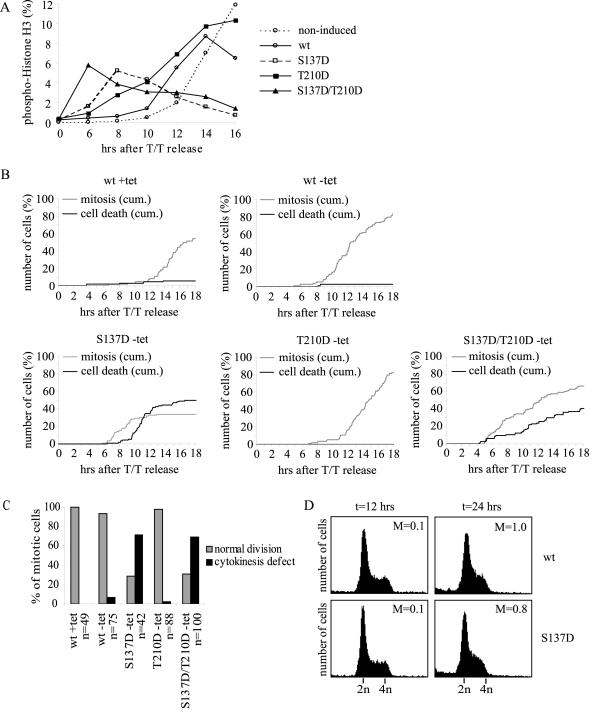
Plk1 has been implicated in promoting mitotic entry via activation of cyclin B1/Cdk1 complexes through activation of Cdc25C and inhibition of Myt1/Wee1. To determine if the timing of mitotic entry is affected in the cell lines expressing active mutants of Plk1, cells were released from a double thymidine block with Plk1 expression induced at the moment of release. At different time points after release, the cells were harvested and stained for phospho-histone H3 positivity to allow examination of mitotic cells by flow cytometry. Mitotic entry in noninduced control cells was observed around 12 to 14 h after release (Fig. 3A). Cells expressing wt Plk1 started to enter mitosis at 10 to 12 h after release. Plk1-T210D-expressing cells showed a more accelerated mitotic entry starting at 8 to 10 h after release, while cell expression of Plk1-S137D or S137D/T210D forced cells to enter mitosis even earlier, at 6 to 8 h after release (Fig. 3A). These data indicate that expression of active Plk1 can induce premature mitotic entry in human cells.
FIG. 3.
Expression of active Plk1 mutants induces premature mitotic entry and different mitotic defects. (A) Expression of wt Plk1, S137D, T210D, or S137D/T210D was induced (or not induced) at the time of release from a double thymidine block. Cells were harvested at indicated time points after release. PI staining was combined with phospho-histone H3 staining to detect mitotic cells by flow cytometry. (B) Cells were plated on coverslips, released from the double thymidine (T/T) block, and replated in medium without (induced; − tet) or with (noninduced; + tet) tetracycline. After release, cells were analyzed for 18 h by using time-lapse microscopy. Mitotic and dead cells were counted and plotted as cumulative numbers. (C) Normal mitotic division and cytokinesis defects observed in time-lapse experiments were counted and plotted as percentages of the total mitotic population. (D) wt Plk1 and S137D cell lines were blocked in thymidine for 24 h, and expression was induced for 12 or 24 h in the presence of nocodazole, continuously arresting cells in thymidine. DNA profiles of fixed cells were analyzed together with phospho-histone H3 positivity by using flow cytometry. M, mitotic cells.
Furthermore, a very slow S phase progression was observed with the Plk1-S137D and Plk1-S137D/T210D cell lines when expression was induced 12 or 24 h before release from the second thymidine block instead of at the time of release (data not shown). This result indicates that inducing expression of constitutive active Plk1-S137D or Plk1-S137D/T210D at the G1/S transition, at a time when Plk1 is normally not expressed, inhibits DNA replication. Indeed, a function for Ser-137 in S phase was proposed earlier based on a reduction in the percentage of cells in S phase in HeLa cells overexpressing Plk1-S137D (25).
Different mitotic defects induced by S137D and T210D mutants.
To study cell cycle progression of the Plk1 cell lines in more detail, we monitored cells by time-lapse microscopy. After synchronization in G1/S by using a double thymidine block, cells expressing wt Plk1 entered mitosis slightly earlier than noninduced cells, with mitotic entry starting at 9 to 11 h and 12 to 14 h, respectively (Fig. 3B). The expression of wt Plk1 did not result in mitotic abnormalities (data not shown). Cells stably expressing Plk1-S137D or Plk1-S137D/T210D entered mitosis prematurely with kinetics similar to those we observed by flow cytometry (Fig. 3A and B). This finding confirms that the phospho-histone H3 positivity we used as a mitotic marker in flow cytometry indeed correlates with mitotic entry. Many of these cells underwent severe blebbing and subsequent cell death while in mitosis or shortly after exit from mitosis, a finding which suggests that these cells undergo mitotic catastrophe (Fig. 3B; Fig. S1 in the supplemental material). Indeed, approximately 50% of the cells expressing Plk1-S137D died during the course of the experiment. We observed 41% cell death with the Plk1-S137D/T210D-expressing cells. Of the mitotic cells expressing Plk1-S137D or Plk1-S137D/T210D that did not die during mitosis, 71 or 69%, respectively, failed at cytokinesis, ultimately giving rise to only one (multinucleated) daughter cell (Fig. 3C). Some mitotic cells expressing Plk1-S137D (7%, n = 42) started normal formation of a cleavage furrow, which subsequently regressed, while other mitotic cells (64%, n = 42) rounded only partially and flattened without undergoing a proper mitotic division. These partially rounded cells appeared not to degrade the nuclear envelope and in most cases died shortly after flattening (data not shown), which suggests that they underwent a very-early-onset mitotic catastrophe. Thus, expression of Plk1-S137D appears to result in mitotic catastrophe or aberrant cytokinesis in cells that do complete the early stages of mitosis.
In contrast to what we observed in cells expressing Plk1-S137D or the double mutant, cell lines expressing wt Plk1 or T210D showed only few dying cells (Fig. 3B) or cytokinesis defects (Fig. 3C). Interestingly, in Plk1-T210D cells, we found mitotic progression to be delayed. In these cells, the time from nuclear envelope breakdown until anaphase onset ranged from 50 min to approximately 10 h, with an average of 150 min (n = 91) compared to an average of 50 min in cells expressing wt Plk1 (n = 78). This finding suggests that Thr-210 in Plk1 may have to be dephosphorylated for proper mitotic progression. Plk1-T210D cells underwent successful cytokinesis, although it was often accompanied by excessive blebbing of the cell membrane coincident with cleavage furrow ingression (data not shown). Thus, cell lines expressing different active mutants of Plk1 give rise to distinct mitotic defects. The effect seen with the S137D/T210D mutant is very similar to that with the S137D mutant, while the T210D mutation appears to cause a very different mitotic defect, indicating that the S137D mutation has a dominant effect over the T210D mutation.
As described above, cells expressing Plk1-S137D or Plk1-S137D/T210D showed considerable cell death during mitosis or shortly after mitotic exit. Also, some cells seemed to die in late G2 or prophase, but before nuclear envelope breakdown. This finding suggests that the observed cell death occurs as a consequence of mitotic defects. Alternatively, the high expression of mutant Plk1 around this time may induce cell death independent of the cell cycle state of the cells. Thus, to test whether mere high expression of Plk1-S137D could lead to cell death, we induced expression in S phase-arrested cells. But contrary to the experimental setting described above, we now monitored the faith of these cells when they were kept arrested at the G1/S transition in the continued presence of thymidine. wt Plk1 or Plk1-S137D cell lines were arrested with thymidine for 24 h, after which expression was induced for 12 or 24 h in the continued presence of thymidine to keep cells in G1/S during the experiment (Fig. 3D). After 12 h induction, neither cell line showed less than 2N DNA content, indicative of cell death. After 24 h of induction, some cells were found to contain less than 2N DNA content, but percentages were similar in wt Plk1 and Plk1-S137D-expressing cells. Also, Plk1-S137D-expressing cells did not enter mitosis (Fig. 3D). Thus, mere high expression of Plk1-S137D could not account for the observed levels of cell death or mitotic entry, indicating that progression through the cell cycle is needed for these effects.
Expression of Plk1-S137D induces spindle assembly checkpoint failure.
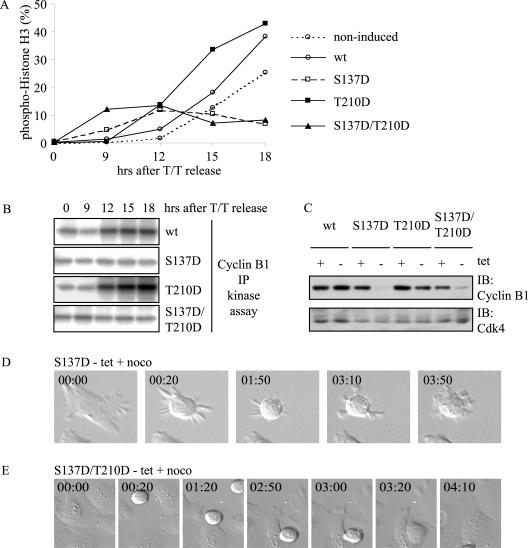
We wanted to examine other mitotic aspects in the mutant cell lines. The spindle assembly checkpoint monitors attachment and tension between chromosomes and the mitotic spindle to ensure that anaphase takes place only when all chromosomes are attached and proper tension is generated. We checked for proper spindle assembly checkpoint arrest by arresting cells in mitosis with nocodazole, which prevents spindle assembly checkpoint inactivation by depolymerizing microtubules. Again, we observed an early mitotic entry of all cell lines expressing mutant Plk1 compared to those expressing wt Plk1 (Fig. 4A). Surprisingly, however, cells expressing Plk1-S137D or Plk1-S137D/T210D could not be properly blocked in mitosis by nocodazole, in contrast to wt Plk1- and Plk1-T210D-expressing cells. A small but significant increase in the percentages of mitotic cells was consistently observed with the Plk1-S137D- and Plk1-S137D/T210D-expressing cultures at 9 to 12 h after release, but the percentages of mitotic cells markedly decreased at later time points. Concomitantly, cyclin B1/Cdk1 activity increased over time in the presence of nocodazole in cells expressing wt Plk1 or Plk1-T210D, but not in Plk1-S137D- or Plk1-S137D/T210D-expressing cells (Fig. 4B).
FIG. 4.
Plk1-S137D and S137D/T210D mutants undergo a defective spindle assembly checkpoint arrest. (A) Expression of wt Plk1, S137D, T210D, or S137D/T210D was induced upon release from a double thymidine (T/T) block in the presence of nocodazole or was left noninduced. Cells were analyzed for phospho-histone H3 positivity combined with DNA profiles by flow cytometry. (B) See legend for panel A. In parallel, cells were lysed and cyclin B1-IP kinase assays were performed with histone H1 as a substrate. (C) Plk1 expression in cell lines was induced for 20 h by washing away tetracycline (− tet) or left noninduced (+ tet) in the presence of nocodazole. Immunoblots of cell lysates were probed for cyclin B1 and Cdk4. (D) Plk1-S137D cells synchronized with thymidine were monitored by time-lapse microscopy. At release, expression was induced and nocodazole was added. Elapsed time (h:min) is indicated in the upper left corner of each panel. noco, nocodazole. (E) Conditions were as for panel D, but Plk1-S137D/T210D-expressing cells were used.
We determined cyclin B1 expression levels in cells released from a single thymidine block for 20 h in the presence of nocodazole. We observed decreased expression of cyclin B1 when Plk1-S137D or Plk1-S137D/T210D mutants were expressed (Fig. 4C, upper panel). This result suggests that these cells have failed to establish spindle assembly control of the APC/C, leading to premature cyclin B1 degradation.
To confirm mitotic progression past an active spindle assembly checkpoint in Plk-S137D- or Plk1-S137D/T210D-expressing cells, we filmed these cells in the presence of nocodazole after synchronization by a double thymidine block. Even though nocodazole was present, cells could exit mitosis (Fig. 4D and E). Cells showed defective cytokinesis, similar to the cytokinesis defects observed without nocodazole. These results confirm that the expression of Plk1-S137D or Plk1-S137D/T210D results in a defective spindle assembly checkpoint-mediated arrest.
Plk1-S137D expression leads to untimely activation of the APC/C.
Spindle assembly checkpoint inactivation allows activation of the APC/C, which targets its substrates for degradation by the 26S proteasome. We investigated APC/C activation by examining proteasomal degradation of its target proteins. Therefore, we determined the expression level of several APC/C substrates with or without the addition of a proteasome inhibitor (MG132). Because adding MG132 for longer than 3 h was toxic to U2OS cells, we added the proteasome inhibitor only the last 3 h before harvesting.
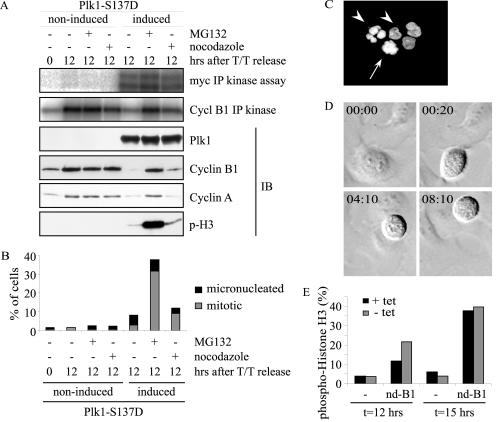
After induction of Plk1-S137D expression and release for 12 h, both expression of cyclin A and B1 and cyclin B1/Cdk1 kinase activity were dramatically reduced (Fig. 5A). The decreased expression and kinase activity was restored by adding MG132 (even though it was added only for 3 h), indicating that cyclin A and B1 had been actively degraded by the proteasome (Fig. 5A). We examined histone H3 phosphorylation to check whether Plk1-S137D-expressing cells were indeed blocked in mitosis after the addition of MG132. We observed a significantly higher phospho-histone H3 positivity after the addition of MG132, indicative of a mitotic arrest (Fig. 5A). Again, the addition of nocodazole at the time of release only marginally restored the expression of APC/C target proteins and histone H3 phosphorylation (Fig. 5A), similar to paclitaxel (Taxol) (data not shown), another spindle poison that blocks progression through mitosis. We quantified the number of mitotic cells and observed that 32% of cells were mitotic after 3 h of MG132 addition. In sharp contrast, only 9% of cells were mitotic after nocodazole treatment for 12 h (Fig. 5B). In comparison, around 40% of the cells expressing wt Plk1 were mitotic at 18 h after release into nocodazole (Fig. 4A). In Plk1-S137D-expressing cells, we also observed micronucleated cells (6%), indicative of a defective spindle checkpoint, defective cytokinesis, or cell death (Fig. 5B and C). These data confirm a spindle assembly checkpoint defect and suggest untimely APC/C activation in cells expressing Plk1-S137D or Plk1-S137D/T210D.
FIG. 5.
The APC/C is untimely activated by Plk1-S137D expression. (A) Plk1-S137D cells were released for 12 h from a double thymidine (T/T) block in medium with (noninduced) or without (induced) tetracycline. When indicated, nocodazole was added at release. Proteasome inhibitor MG132 was added only 3 h before harvesting. Cell lysates were used for the indicated immunoblots (IB) and Myc-IP or cyclin B1-IP kinase assays with α-casein or histone H1 as a substrate, respectively. (B) The experimental setup was as for panel A. More than 300 cells were counted by cytospinning in an examination of DNA condensation to identify mitotic and micronucleated cells. (C) See legend to panel B. Examples of a mitotic cell (arrow) and micronucleated cells (arrowheads) are shown. (D) The Plk1-S137D cell line was transfected with a nondegradable cyclin B1 plasmid combined with H2B-GFP. After synchronization, Plk1-S137D expression was induced and cells were monitored by time-lapse microscopy. Numbers in the upper left corners are the elapsed time (h:min). (E) The Plk1-S137D cell line was transfected with spectrin-GFP alone (−) or in combination with nondegradable cyclin B1 (nd-B1). At release from thymidine block, Plk1-S137D expression was induced (− tet) or not induced (+ tet) and cells were harvested for flow cytometric analysis of mitotic cells at the indicated time points.
Thus far, our data suggest that cells expressing Plk-S137D mutants enter mitosis prematurely, but fail to maintain the mitotic state in the presence of spindle poisons. Concomitantly, we observe untimely degradation of APC/C substrates, such as cyclin B1, that are normally degraded only upon mitotic exit. Therefore, we wanted to test if we could prevent mitotic exit by the expression of a nondegradable cyclin B1 mutant shown to arrest cells in late mitosis (60). To this end, we transfected a plasmid encoding nondegradable cyclin B1 and released cells from a thymidine block while inducing Plk1-S137D expression at release. Time-lapse microscopy demonstrated that Plk1-S137D-expressing cells could be arrested in mitosis for an extensive period of time by the expression of nondegradable cyclin B1 (Fig. 5D). This result was confirmed by flow cytometry analysis (Fig. 5E). So, Plk1-S137D-expressing cells can indeed be arrested in mitosis, as long as the degradation of cyclin B1 is prevented.
Plk1-T210D causes a spindle assembly checkpoint-dependent delay.
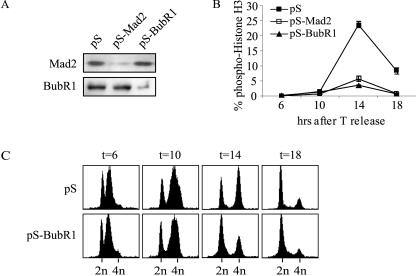
We next investigated whether the mitotic delay in cells expressing Plk1-T210D was possibly due to a spindle assembly checkpoint-dependent arrest. To this end, we silenced the expression of the essential checkpoint components Mad2 or BubR1 to see if we could rescue the mitotic delay. Knocking down expression of either Mad2 or BubR1 was reported to be sufficient to decrease the duration of mitosis by approximately half and to lead to mitotic exit before full congression (34). We transfected cells with a control vector (pS) or a vector encoding small interfering RNA (siRNA) for Mad2 or BubR1 (pS-Mad2 or pS-BubR1) (34) and released cells from a single thymidine block, leading to down-regulation of expression (Fig. 6A). Mitotic cells were visualized with anti-phospho-histone H3 antibody and measured by flow cytometry. About 25% of the control-transfected Plk1-T210D cells were phospho-histone H3 positive 14 h after release (Fig. 6B). In contrast, only 3 to 5% of Plk1-T210D cells were in mitosis after inhibiting spindle assembly checkpoint function by knocking down Mad2 or BubR1 expression (Fig. 6B). These cells did not arrest in G2 or undergo apoptosis, but progressed to G1 as shown by DNA profiles (Fig. 6C). From this finding, we conclude that the spindle assembly checkpoint is functional in Plk1-T210D cells, in contrast to Plk1-S137D mutants that show a checkpoint override. Thus, mimicking phosphorylation at Ser-137 and Thr-210 in Plk1 has distinct and partly opposing effects on the spindle assembly checkpoint and consequent mitotic progression.
FIG. 6.
Plk1-T210D induces a spindle assembly checkpoint-dependent delay. Plk1-T210D-expressing cells were transfected with control siRNA vector pS, pS-Mad2 or pS-BubR1. (A) After release from thymidine (T) block for 20 h, the nonselected total cell population was lysed and immunoblotted with the indicated antibodies. (B) After release from thymidine block for the indicated time, cells were harvested and fixed. PI staining was combined with staining for phospho-histone H3 to identify mitotic cells by flow cytometry. Transfected cells were identified through spectrin-GFP cotransfection. (C) See legend to panel B. PI profiles of Plk1-T210D cells transfected with control siRNA vector pS or pS-BubR1 were harvested at the indicated times after thymidine release.
Ser-137 and Thr-210 phosphorylation both contribute to proper mitotic progression.
To study the function of Ser-137 in more detail, we compared a nonphosphorylatable S137A mutation to the phospho-mimetic S137D mutation in a background devoid of endogenous Plk1. To this end, we depleted endogenous Plk1 in U2OS cells by vector-driven siRNA (pS-Plk1) (56) and reconstituted these cells with the respective Plk1 mutants. Two silent mutations in the siRNA-target region of the Plk1 constructs were introduced to allow expression of the exogenous Plk1, without possible interference of endogenous Plk1. We could indeed demonstrate that this approach results in the replacement of endogenous Plk1 with the exogenous Plk1 (Fig. 7A). Routinely, we obtain a knockdown efficiency of more than 95% (56). To avoid possible overexpression artifacts, we used 0.5 μg of nontargetable wt Plk1, since this approach resulted in wt Plk1 expression comparable to or slightly lower than endogenous Plk1 expression (Fig. 7A). Next, cells were reconstituted with the different Ser-137 and Thr-210 mutants and harvested 48 h after transfection. Compared to wt Plk1, mutating Ser-137 or Thr-210 into Asp increased kinase activity approximately fivefold, whereas kinase activity was lowered after mutation into Ala (Fig. 7B). These kinase activities are comparable to those of the same Plk1 mutants as those described in other experiments with Xenopus and U2OS cells (25, 32, 44).
FIG. 7.
Phosphorylation of Plk1 Ser-137 and Thr-210 both contribute to proper mitotic progression. (A) Cells were transfected with control siRNA vector pS or pS-Plk1 together with the indicated amounts of nontargetable wt Plk1 and pBabepuro to allow selection of transfected cells. Cells were lysed at 48 h after transfection to analyze Plk1 expression by immunoblotting. (B) Cells were transfected with pS or pS-Plk1 together with the indicated nontargetable Plk1 mutants. Cells were lysed 48 h after transfection and subjected to Myc-IP kinase assays using α-casein as a substrate (upper panel). The amount of Plk1 that was immunoprecipitated was assayed by Myc-Plk1 immunoblotting (lower panel). (C) Cells were transfected with pS or pS-Plk1 in combination with spectrin-GFP and reconstituted with the indicated nontargetable (mutant) Plk1, released from thymidine block, and collected at the next G1/S transition after 24 h by the readdition of thymidine. After fixation, DNA profiles were analyzed by flow cytometry. (D) Average duration of mitosis from prophase until anaphase onset or cell death in wt Plk1- versus Plk1-S137A-reconstituted cells. The P value comparing both populations is indicated. (E) Cells were transfected with pS-Plk1, H2B-GFP, and nontargetable wt Plk1 to monitor by time-lapse imaging after synchronization with a single thymidine block. The arrows point out chromosomes detaching from the metaphase plate. Numbers in the upper left corners of the panels refer to the elapsed time (h:min). (F) See legend to panel E. Cells were reconstituted with nontargetable Plk1-S137A instead of wt Plk1.
Next, we examined whether the Plk1-Ser-137 mutants could reconstitute Plk1-depleted cells, which arrest in G2/M (Fig. 7C) (56). Using 0.5 μg of wt Plk1, we almost fully restored normal cell cycle progression in Plk1-depleted cells as analyzed by flow cytometry (Fig. 7C). Interestingly, we found that the expression of Plk1-S137A could not induce a rescue of cell cycle progression to the level we observed with wt Plk1. The cultures reconstituted with Plk1-S137A had a higher content of 4N cells (Fig. 7C), indicating that they are delayed at some point in G2/M, suggesting that Ser-137 phosphorylation does contribute to normal Plk1 function. In contrast, reconstitution with Plk1-S137D seemed to result in a better rescue than with wt Plk1, as judged by an increased G1 content and decreased mitotic population (Fig. 7C). However, it should be noted that about one-third of the Plk1-S137D-expressing cells did not progress through the cell cycle properly after release from a thymidine block and remained arrested at the G1/S transition (data not shown). This finding is consistent with the result described above, that expression of the active Plk1-S137D during a thymidine block can inhibit DNA replication.
By time-lapse analysis, we found that Plk1-S137D-reconstituted cells that did enter mitosis underwent either a normal division (79%, n = 19) or one with defective cytokinesis (11%, n = 19), and approximately 20% of the cells died. Percentages of cells showing these phenotypes are lower than in the Plk1-S137D-inducible cell line (Fig. 3B and C), possibly due to the observed G1/S arrest or to a difference in expression levels of the mutant protein.
In addition, we tested Plk1 Thr-210 mutants in the reconstitution assay. Plk1-T210A could not substitute for wt Plk1 in Plk1-depleted cells, and those cells arrested in G2/M (Fig. 7C). Plk1-T210D had somewhat delayed cell cycle progression compared to wt Plk1, possibly due to slower mitotic progression, as described above. These data indicate that Thr-210 is indeed a crucial in vivo phosphorylation site necessary for proper Plk1 function and mitotic progression. Concomitantly, we examined Myc-Plk1 localization in cells reconstituted with Plk1-S137A, Plk1-S137D, Plk1-T210A, or Plk1-T210D by immunohistochemistry. Like wt Plk1, all mutants were able to localize to centrosomes and kinetochores (see Fig. S2 in the supplemental material). For the mutants for which we could find cells at late stages of mitosis, we also observed localization at the midbody. The different mutant Plk1 effects can thus not be attributed to obvious differences in subcellular localization.
The above data suggested that Plk1-S137A could reconstitute Plk1-depleted cells but to a lesser extent than wt Plk1. Since the above in vivo data with a phospho-specific antibody suggested that Ser-137 is phosphorylated in late mitosis, we hypothesized that a late mitotic function of Plk1 might be defective in Plk1-S137A-expressing cells and therefore that they might show problems during late mitotic stages. We examined Plk1-S137A-reconstituted cells by cotransfecting GFP-tagged histone H2B and analyzed mitotic progression in these cells by time-lapse microscopy. We measured the duration of mitosis starting from prophase until either anaphase or cell death. In wt Plk1-reconstituted cells, the average duration of mitosis was 1.0 h (n = 10). In Plk1-S137A-reconstituted cells, the average duration of mitosis was at least 6.4 h (Fig. 7D). This estimate is low because 5 of 13 Plk1-S137A-reconstituted cells were still in mitosis at the end of the experiment. Cells reconstituted with wt Plk1 underwent a normal mitosis (Fig. 7E), although it was sometimes accompanied by severe blebbing during anaphase and telophase (data not shown). Plk1-S137A-reconstituted cells reached mostly metaphase alignment and consequently arrested (8 of 13 cells), although sometimes one or more chromosomes did not congress (4 of 13). Rotation of the metaphase plate was frequently observed (data not shown). During metaphase arrest, single chromosomes detached shortly from the metaphase plate and realigned, or multiple chromosomes detached and then moved towards one or both spindle poles (see Fig. 7F and Video S3 in the supplemental material). Eventually, Plk1-S137A-reconstituted cells divided (5 of 13), died while in mitosis (3 of 13), or stayed in arrest for the duration of imaging (5 of 13). Thus, the expression of Plk1-S137A gives rise to errors in the late stages of mitosis, suggesting that Ser-137 phosphorylation may be needed at this time. These data confirm a late mitotic function as suggested by our in vivo data with a phospho-specific antibody (Fig. 1A).
DISCUSSION
Here, we have studied the role of the conserved Ser-137 and Thr-210 residues in Plk1. Our data indicate that phosphorylation of Ser-137 and Thr-210 on Plk1 occurs with distinct timing. Cells expressing phospho-mimicking mutations on these residues displayed different mitotic phenotypes, suggesting that timed activation of distinct Plk1 functions may be regulated by consecutive posttranslational modifications. Furthermore, our results suggest that tight control of Plk1 activity is important to maintain APC/C activation under control of the spindle assembly checkpoint.
By using a phospho-specific antibody for Ser-137, we could immunoprecipitate Plk1 only after release from the nocodazole block, but not during the arrest, providing the first evidence that Ser-137 is actually phosphorylated in vivo. So far, a putative kinase for Ser-137 has not been found (25, 28, 44). Since we show that phosphorylation of this residue may occur during only a very limited period during mitosis, it may as such have gone undetected. With a Thr-210 phospho-specific antibody, we found an up-regulation of Thr-210 phosphorylation at the same time as the increase in total Plk1 expression. This finding suggests that in contrast to phosphorylation of Ser-137, phosphorylation of Thr-210 in the activation loop of Plk1 may be needed for an earlier, more general Plk1 activation.
Induced expression of Plk1 mutants, containing phospho-mimicking mutations on Ser-137 and/or Thr-210 (S137D, T210D, and S137D/T210D) all caused severe proliferation defects in U2OS-derived cells. In contrast, cell growth was enhanced in cells expressing wt Plk1 compared to noninduced control cells, demonstrating an important role for Plk1 in cellular proliferation. In this respect, it is interesting that Plk1 is overexpressed in many cancer types and that Plk1 overexpression can oncogenically transform NIH 3T3 cells (20, 49). Expression of the active mutants resulted in premature mitotic entry that was most prominent in cells expressing Plk1-S137D or Plk1-S137D/T210D. Expression of Plk1-T210D also resulted in acceleration of mitotic entry, similar to what was recently described by Jackman et al. (23). However, the effect of a T210D mutation on mitotic entry appeared to be subtle compared to the effect of the Plk1-S137D or double mutant, indicating that the S137D mutation leads to a greater gain of function for this particular aspect of Plk1 functioning.
The observed premature mitotic entry could be caused by early activation of Cdc25C and cyclin B1/Cdk1 since Plk1 is known to directly regulate these proteins. Indeed, injection of the corresponding Plx1 double mutant S128D/T201D mRNA into Xenopus oocytes induces premature activation of both Cdc25C and cyclin B1/Cdk1 (44). Injection of the less active Plx1-S128D or Plx1-T210D single mutants or wt Plx1 mRNA did not cause premature entry into mitosis. In contrast, we did observe early mitotic entry using single mutants. This difference may reflect different regulation of Plk1 function in human cells versus Xenopus oocytes. Possibly, Xenopus oocytes require higher Plk1 activity to allow mitotic entry, given that the kinase activity of the Plx1 double mutant is about 10 times higher than that of either single mutant (44).
Besides regulating mitotic entry, Plks are suggested to regulate mitotic exit and cytokinesis. At least in budding yeast, the initiation of mitotic exit is known to require Plk activity, whereas Plk needs to be degraded for the completion of cytokinesis (41). Here, we observed major cytokinesis defects in U2OS cells induced to express Plk1-S137D or S137D/T210D, while cells expressing wt Plk1 or Plk1-T210D underwent proper cytokinesis. This result suggests that dephosphorylation of Ser-137 may be required for proper execution of cytokinesis, or that the S137D mutation interferes with normal Plk1 degradation. Alternatively, the observed cytokinesis defects may be an indirect consequence of improper chromosome segregation. Our results with induced expression of wt Plk1 are inconsistent with experiments performed with HeLa cells transiently expressing wt Plk1. These cells were temporarily delayed in mitosis (25, 38) and become multinucleated (38). These more dramatic effects could be due to higher levels of Plk1 expression or could reflect a difference in p53 status, since HeLa cells are p53 deficient and may therefore more easily accumulate multinucleated cells.
In contrast to what we find with S137D mutants, T210D-expressing cells were transiently delayed in mitosis. Consistent with this finding, others have shown an accumulation of mitotic HeLa cells 2 days after transfection with Plk1-T210D (25). Concomitantly, a delayed mitosis before anaphase and a small but significant delay in mitotic exit was shown with Plk1-T210D-expressing HeLa cells, with mitotic exit measured as the time from sister chromatid separation until the completion of cleavage (35). Our data suggest that phosphorylation of Thr-210 delays inactivation of the spindle assembly checkpoint through a yet-unidentified mechanism. Recently, a human kinase of the Plk1 family, Plk2/Snk, has also been suggested to function in the mitotic spindle assembly checkpoint. Depletion of Plk2 in the presence of nocodazole or paclitaxel gives a significant increase in apoptosis in mitosis, which suggests that Plk2 may prevent mitotic catastrophe following spindle damage (4).
Using time-lapse microscopy, we found that expression of Plk1-S137D or Plk1-S137D/T210D resulted in a substantial induction of cell death. Cells died either after a defective cytokinesis or in mitosis. Cell death was not apparent when the Plk1-S137D or Plk1-S137D/T210D mutants were expressed in cells continuously arrested in G1/S by thymidine, indicating that the observed cell death requires cell cycle progression and cannot be attributed simply to excess kinase activity. Instead, we propose that the observed cell death occurs only after mitotic entry. The exact mechanism of this mitotic catastrophe remains to be elucidated, but in this respect it is interesting that premature cyclin B1/Cdk1 activation before completion of S or G2 can induce premature chromatin condensation and apoptosis (6). Since cells expressing Plk1-S137D and Plk1-S137D/T210D enter mitosis ∼6 h after thymidine release, S or G2 phase may not have been completed, and this may result in mitotic catastrophe. Alternatively, premature inactivation of the spindle assembly checkpoint has also been shown to result in mitotic catastrophe (37). Indeed, we found that Plk1-S137D or Plk1-S137D/T210D-expressing cells could not be arrested in mitosis through actions of the spindle assembly checkpoint. Upon entry into mitosis, Plk1-S137D-expressing cells activated the APC/C even in the presence of spindle poisons, such as nocodazole or paclitaxel. The addition of a proteasome inhibitor was able to arrest these cells in mitosis, indicating that the override of the spindle assembly checkpoint requires proteasome-dependent protein degradation. Taken together, these data demonstrate that the expression of S137D mutants can uncouple APC/C activation from spindle assembly checkpoint control. This uncoupling could be the result of direct inactivation of the spindle assembly checkpoint by Plk1-S137D, or Plk1-S137D could somehow render APC/C activation nonresponsive to the inhibitory signals generated by the spindle assembly checkpoint. Alternatively, failure of the spindle assembly checkpoint could arise as a secondary consequence of the premature mitotic entry, as certain checkpoint components may not have accumulated to sufficiently high levels to enforce a normal checkpoint arrest. We also studied the importance of Ser-137 phosphorylation by reconstituting Plk1-depleted cells with a Plk1-S137A mutant, and we observed a marked delay in metaphase. Together with the in vivo data with the phospho-specific antibody, these results indicate that Ser-137 is phosphorylated in late mitosis after the spindle assembly checkpoint has been silenced, possibly contributing to functions of Plk1 at the later stages of mitosis.
Taken together, our data show that different activating mutations in Plk1 at putative phosphorylation sites have distinct and partly opposite effects on spindle assembly checkpoint activity. Our data indicate that consecutive phosphorylation events on Plk1 are important to maintain timed execution of distinct mitotic events and to prevent mitotic catastrophe. As also suggested by our results with phospho-specific antibodies in vivo, phosphorylation of Thr-210 may regulate early mitotic roles of Plk1, whereas later mitotic roles may be brought about by Ser-137 phosphorylation. Our data with a Plk1-S137D/T210D mutant indicate that phosphorylation of Ser-137 has a dominant effect over Thr-210 phosphorylation, suggesting that consequent Ser-137 phosphorylation may somehow alter the effect of Thr-210 phosphorylation. Ser-137 phosphorylation may then promote mitotic progression past a satisfied spindle assembly checkpoint. Further investigation on other residues may shed more light on how other Plk1 functions are separated.
Supplementary Material
Acknowledgments
We thank members of the Medema lab and J. Pines for helpful discussions, L. Oomen and L. Brocks for support with time-lapse microscopy, M. Browning and K. Nixon for help in generating and testing the phospho-Thr-210 antibody, M. Brandeis for the nondegradable cyclin B1 plasmid, and H. and J. van de Weerdt for logistic support.
This work was supported by grant 901-28-145 from The Netherlands Organization for Scientific Research (NWO) and grant NKI 2000-2191 from the Dutch Cancer Society (KWF).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abrieu, A., T. Brassac, S. Galas, D. Fisher, J. C. Labbe, and M. Doree. 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 111:1751-1757. [DOI] [PubMed] [Google Scholar]
- 2.Alexandru, G., F. Uhlmann, K. Mechtler, M. A. Poupart, and K. Nasmyth. 2001. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105:459-472. [DOI] [PubMed] [Google Scholar]
- 3.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 4.Burns, T. F., P. Fei, K. A. Scata, D. T. Dicker, and W. S. El-Deiry. 2003. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (Taxol)-exposed cells. Mol. Cell. Biol. 23:5556-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casenghi, M., P. Meraldi, U. Weinhart, P. I. Duncan, R. Korner, and E. A. Nigg. 2003. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell. 5:113-125. [DOI] [PubMed] [Google Scholar]
- 6.Castedo, M., J. L. Perfettini, T. Roumier, K. Andreau, R. Medema, and G. Kroemer. 2004. Cell death by mitotic catastrophe: a molecular definition. Oncogene 23:2825-2837. [DOI] [PubMed] [Google Scholar]
- 7.Charles, J. F., S. L. Jaspersen, R. L. Tinker-Kulberg, L. Hwang, A. Szidon, and D. O. Morgan. 1998. The polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr. Biol. 8:497-507. [DOI] [PubMed] [Google Scholar]
- 8.Danos, O., and R. C. Mulligan. 1988. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc. Natl. Acad. Sci. USA 85:6460-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Carcer, G., M. do Carmo Avides, M. J. Lallena, D. M. Glover, and C. Gonzalez. 2001. Requirement of Hsp90 for centrosomal function reflects its regulation of Polo kinase stability. EMBO J. 20:2878-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descombes, P., and E. A. Nigg. 1998. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 17:1328-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.do Carmo Avides, M., A. Tavares, and D. M. Glover. 2001. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nat. Cell Biol. 3:421-424. [DOI] [PubMed] [Google Scholar]
- 12.Elia, A. E., L. C. Cantley, and M. B. Yaffe. 2003. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299:1228-1231. [DOI] [PubMed] [Google Scholar]
- 13.Elia, A. E., P. Rellos, L. F. Haire, J. W. Chao, F. J. Ivins, K. Hoepker, D. Mohammad, L. C. Cantley, S. J. Smerdon, and M. B. Yaffe. 2003. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo box domain. Cell 115:83-95. [DOI] [PubMed] [Google Scholar]
- 14.Ellinger-Ziegelbauer, H., H. Karasuyama, E. Yamada, K. Tsujikawa, K. Todokoro, and E. Nishida. 2000. Ste20-like kinase (SLK), a regulatory kinase for polo-like kinase (Plk) during the G2/M transition in somatic cells. Genes Cells 5:491-498. [DOI] [PubMed] [Google Scholar]
- 15.Englert, C., X. Hou, S. Maheswaran, P. Bennett, C. Ngwu, G. G. Re, A. J. Garvin, M. R. Rosner, and D. A. Haber. 1995. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 14:4662-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glover, D. M., I. M. Hagan, and A. A. Tavares. 1998. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 12:3777-3787. [DOI] [PubMed] [Google Scholar]
- 17.Golan, A., Y. Yudkovsky, and A. Hershko. 2002. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J. Biol. Chem. 277:15552-15557. [DOI] [PubMed] [Google Scholar]
- 18.Golsteyn, R. M., K. E. Mundt, A. M. Fry, and E. A. Nigg. 1995. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 129:1617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamanaka, R., M. R. Smith, P. M. O'Conner, S. Maloid, K. Mihalic, J. L. Spivak, D. L. Longo, and D. K. Ferris. 1995. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J. Biol. Chem. 270:21086-21091. [DOI] [PubMed] [Google Scholar]
- 20.Holtrich, U., G. Wolf, A. Brauninger, T. Karn, B. Bohme, H. Rubsamen-Waigmann, and K. Strebhardt. 1994. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc. Natl. Acad. Sci. USA 91:1736-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, F., Y. Wang, D. Liu, Y. Li, J. Qin, and S. J. Elledge. 2001. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 107:655-665. [DOI] [PubMed] [Google Scholar]
- 22.Ince, C., D. L. Ypey, M. M. Diesselhoff-Den Dulk, J. A. Visser, A. De Vos, and R. Van Furth. 1983. Micro-CO2-incubator for use on a microscope. J. Immunol. Methods 60:269-275. [DOI] [PubMed] [Google Scholar]
- 23.Jackman, M., C. Lindon, E. A. Nigg, and J. Pines. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5:143-148. [DOI] [PubMed] [Google Scholar]
- 24.Jang, Y. J., C. Y. Lin, S. Ma, and R. L. Erikson. 2002. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc. Natl. Acad. Sci. USA 99:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang, Y. J., S. Ma, Y. Terada, and R. L. Erikson. 2002. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J. Biol. Chem. 277:44115-44120. [DOI] [PubMed] [Google Scholar]
- 26.Kalejta, R. F., T. Shenk, and A. J. Beavis. 1997. Use of a membrane-localized green fluorescent protein allows simultaneous identification of transfected cells and cell cycle analysis by flow cytometry. Cytometry 29:286-291. [DOI] [PubMed] [Google Scholar]
- 27.Kanda, T., K. F. Sullivan, and G. M. Wahl. 1998. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8:377-385. [DOI] [PubMed] [Google Scholar]
- 28.Kelm, O., M. Wind, W. D. Lehmann, and E. A. Nigg. 2002. Cell cycle-regulated phosphorylation of the Xenopus polo-like kinase Plx1. J. Biol. Chem. 277:25247-25256. [DOI] [PubMed] [Google Scholar]
- 29.Kotani, S., S. Tugendreich, M. Fujii, P. M. Jorgenson, M. Watanabe, C. Hoog, P. Hieter, and K. Todokoro. 1998. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell 1:371-380. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai, A., and W. G. Dunphy. 1996. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 273:1377-1380. [DOI] [PubMed] [Google Scholar]
- 31.Lane, H. A., and E. A. Nigg. 1996. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135:1701-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, K., and R. Erikson. 1997. Plk is a functional homolog of Saccharomyces cerevisiae Cdc5, and elevated Plk activity induces multiple septation structures. Mol. Cell. Biol. 17:3408-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, K. S., Y. L. Yuan, R. Kuriyama, and R. L. Erikson. 1995. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol. Cell. Biol. 15:7143-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lens, S. M., R. M. Wolthuis, R. Klompmaker, J. Kauw, R. Agami, T. Brummelkamp, G. Kops, and R. H. Medema. 2003. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J. 22:2934-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindon, C., and J. Pines. 2004. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol. 164:233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losada, A., M. Hirano, and T. Hirano. 2002. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 16:3004-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michel, L., E. Diaz-Rodriguez, G. Narayan, E. Hernando, V. V. Murty, and R. Benezra. 2004. Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc. Natl. Acad. Sci. USA 101:4459-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mundt, K. E., R. M. Golsteyn, H. A. Lane, and E. A. Nigg. 1997. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem. Biophys. Res. Commun. 239:377-385. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima, H., F. Toyoshima-Morimoto, E. Taniguchi, and E. Nishida. 2003. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 278:25277-25280. [DOI] [PubMed] [Google Scholar]
- 40.Neef, R., C. Preisinger, J. Sutcliffe, R. Kopajtich, E. A. Nigg, T. U. Mayer, and F. A. Barr. 2003. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162:863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nigg, E. A. 1998. Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol. 10:776-783. [DOI] [PubMed] [Google Scholar]
- 42.Ohkura, H., I. M. Hagan, and D. M. Glover. 1995. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9:1059-1073. [DOI] [PubMed] [Google Scholar]
- 43.Okano-Uchida, T., E. Okumura, M. Iwashita, H. Yoshida, K. Tachibana, and T. Kishimoto. 2003. Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles. EMBO J. 22:5633-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian, Y., E. Erikson, and J. L. Maller. 1999. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol. Cell. Biol. 19:8625-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian, Y. W., E. Erikson, C. Li, and J. L. Maller. 1998. Activated Polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus leavis. Mol. Cell. Biol. 18:4262-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds, N., and H. Ohkura. 2003. Polo boxes form a single functional domain that mediates interactions with multiple proteins in fission yeast polo kinase. J. Cell Sci. 116:1377-1387. [DOI] [PubMed] [Google Scholar]
- 47.Schwab, M., M. Neutzner, D. Mocker, and W. Seufert. 2001. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20:5165-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shirayama, M., W. Zachariae, R. Ciosk, and K. Nasmyth. 1998. The polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 17:1336-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, M. R., M. L. Wilson, R. Hamanaka, D. Chase, H. Kung, D. L. Longo, and D. K. Ferris. 1997. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem. Biophys. Res. Commun. 234:397-405. [DOI] [PubMed] [Google Scholar]
- 50.Smits, V. A., M. A. van Peer, M. A. Essers, R. Klompmaker, G. Rijksen, and R. H. Medema. 2000. Negative growth regulation of SK-N-MC cells by bFGF defines a growth factor-sensitive point in G2. J. Biol. Chem. 275:19375-19381. [DOI] [PubMed] [Google Scholar]
- 51.Song, S., T. Z. Grenfell, S. Garfield, R. L. Erikson, and K. S. Lee. 2000. Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol. Cell. Biol. 20:286-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stegmeier, F., R. Visintin, and A. Amon. 2002. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108:207-220. [DOI] [PubMed] [Google Scholar]
- 53.Sumara, I., E. Vorlaufer, P. T. Stukenberg, O. Kelm, N. Redemann, E. A. Nigg, and J. M. Peters. 2002. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell 9:515-525. [DOI] [PubMed] [Google Scholar]
- 54.Sunkel, C. E., and D. M. Glover. 1988. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci. 89:25-38. [DOI] [PubMed] [Google Scholar]
- 55.Toyoshima-Morimoto, F., E. Taniguchi, N. Shinya, A. Iwamatsu, and E. Nishida. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215-220. [DOI] [PubMed] [Google Scholar]
- 56.Van Vugt, M. A. T. M., B. C. M. Van De Weerdt, G. Vader, H. Janssen, J. Calafat, R. Klompmaker, R. M. F. Wolthuis, and R. H. Medema. 2004. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J. Biol. Chem. 279:36841-36854. [DOI] [PubMed] [Google Scholar]
- 57.Visintin, R., F. Stegmeier, and A. Amon. 2003. The role of the polo kinase Cdc5 in controlling Cdc14 localization. Mol. Biol. Cell 14:4486-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe, N., H. Arai, Y. Nishihara, M. Taniguchi, T. Hunter, and H. Osada. 2004. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc. Natl. Acad. Sci. USA 101:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, T., J. P. Aumais, X. Liu, L. Y. Yu-Lee, and R. L. Erikson. 2003. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev. Cell. 5:127-138. [DOI] [PubMed] [Google Scholar]
- 60.Zur, A., and M. Brandeis. 2002. Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes. EMBO J. 21:4500-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.