Abstract
Background
Acute ischemic stroke is often due to thromboembolism forming over ruptured atherosclerotic plaque in the carotid artery (CA). The presence of intraluminal carotid artery thrombus is associated with a high risk of thromboembolic cerebral ischemic events. The cavitation induced by diagnostic ultrasound (DUS) high mechanical index (MI) impulses applied locally during a commercially available intravenous microbubble (IV MB) infusion has dissolved intravascular thrombi, especially when using longer pulse durations. The beneficial effects of this in acute carotid thromboembolism is not known.
Materials and Methods
An oversized balloon injury was created in the distal extracranial common carotid artery of 38 porcine carotid arteries. Following this, a 70-80% stenosis was created in the mid common carotid artery proximal to the injury site using partial balloon inflation. Acute thrombotic CA occlusions were created just distal to the balloon catheter by injecting fresh autologous arterial thrombi. Following angiographic documentation of occlusion, the common carotid thrombosis was treated with either diagnostic low MI imaging alone (0.2 MI; Philips S5-1) applied through a tissue mimicking phantom (TMP), or intermittent diagnostic high MI stable cavitation (SC) inducing impulses with a longer pulse duration (0.8 MI; 20 usec pulse duration) or inertial cavitation (IC) impulses (1.2 MI; 20 usec pulse duration). All treatment times were for 30 minutes. Intravenous ultrasound contrast (2% Definity; Lantheus Medical) was infused during the treatment period. Angiographic recanalization in four intra-and extracranial vessels downstream from the CA occlusion (auricular, ascending pharyngeal, buccinator, and maxillary) was assessed with both magnetic resonance three dimensional time of flight (TOF) and phase contrast (PCA) angiography. All magnetic resonance images were interpreted by an independent neuroradiologist using the TICI scoring system.
Results
By PCA, at least mild recanalization (TICI 2a or higher) was seen in 64% of downstream vessels treated with SC impulses compared to 33 % of IC treated and 29% of low MI alone treated downstream vessels (p=0.001), while moderate or complete recanalization (TICI 2b or higher) was seen in 39% of SC treated vessels compared to 10% IC treated and 21% of low MI alone treated vessels (p=0.001).
Conclusions
High MI 20 usec pulse duration impulses during a commercial microbubble infusion can be used to recanalize acutely thrombosed carotid arteries and restore downstream flow without anticoagulants. However, this effect is only seen with SC inducing impulses and not at higher mechanical indices, when a paradoxical reversal of the thrombolytic effect is observed. DUS induced SC can be a non-surgical method of dissolving carotid artery thrombi and preventing thromboembolization.
Keywords: Diagnostic Ultrasound, Thrombolysis, Microbubbles, Magnetic Resonance Angiography, Phase Contrast Angiography
Introduction
One of the treatable causes for ischemic stroke is carotid thromboembolism from ruptured carotid artery plaque (1-8). Emergent management in this setting involves either urgent endovascular or fibrinolytic therapy to prevent massive stroke (9,10). However all therapies must be administered within a certain time window to be effective and avoid the risk of intracranial hemorrhage. Furthermore, effective recanalization requires that both the treated vessel and the downstream branches be re-opened, in order to avoid microvascular damage. Transcranial ultrasound has been explored as a method of augmenting the effects of fibrinolytic therapy (11-16). Animal models have also demonstrated efficacy of both transcranial and transcutaneous ultrasound induced microbubble cavitation in dissolving intravascular thrombus without the need for any fibrinolytic agent (17-22).
More recently, diagnostic ultrasound (DUS) induced cavitation of systemically administered microbubbles has been shown to be an effective method of recanalizing both intra-arterial small vessels and microvessels without the need for fibrinolytic therapy (22-25). Since this has been effective in the absence of any antiplatelet or fibrinolytic therapy, it could be applied to patients with contraindications to these agents, and increase the number of eligible patients with acute ischemic stroke who have a viable treatment option.
Diagnostic ultrasound (DUS) induced microbubble cavitation can be either a stable form (SC) which induces non-linear harmonic oscillations in microbubbles, or an inertial form (IC) which induces fluid jets that produce more shear stress along the thrombus and endothelium (26,27). Although IC may theoretically may be more beneficial for thrombus disruption, the fluid jets induced by this phenomenon have the potential to adversely alter platelet or endothelial function, which may lead to vascular spasm and injury (28-30). The effectiveness of these different forms of cavitation on recanalization of carotid arterial thrombi, and downstream restoration of flow in tributary vessels, has not been examined. The purpose of this study was to examine the effect of different forms of DUS induced cavitation in restoring branch flow following carotid artery thrombosis in a porcine model of common carotid stenosis and thromboembolism.
Methods
The study involved 21 pigs. Mean pig weight was 33.3 ± 4.5 kg. In four pigs only one carotid artery thrombotic artery occlusion was created, while in 17 pigs artery occlusions were created on separate days in the right and left carotid arteries so that each animal could be studied twice (resulting in a total of 38 carotid occlusions examined in 21 pigs). This was allowable because common carotid artery occlusion on one side did not induce stroke by MRI or any neurologic impairment in any pig due to their extensive rete collateral network (25). The Institutional Animal Care and Use Committee at the University of Nebraska Medical Center approved all procedures. In the 17 pigs that underwent two studies, the randomized treatment protocol for the contralateral side was the same as what they were randomized to receive on the first carotid artery treated. Whether the right or left common carotid was occluded first was determined by randomization.
On the day of the study, the pigs were sedated with a mixture of Telazol (4.4 mg/kg), ketamine (2.2 mg/kg), and xylazine (2.2 mg/kg) administered intramuscularly. Ketorolac (60 mg) and Solumedrol (40 mg) were administered intravenously to prevent the pulmonary hypertensive responses that occur in pigs given intravenous microbubbles (25). The animals were intubated and placed on a ventilator at a volume of 10 cc/kg of air and at a rate of 15 breaths per minute. Inhalation anesthesia was performed with isoflurane (induction at 4%, maintained at 1.2 to 2.0%). Subsequent to this, femoral arterial and venous lines (8F) were placed using Seldinger technique. A 7 F Multipurpose catheter (Merit Medical Systems, Inc., South Jordan, Utah) was guided into the mid common carotid artery (CA) using fluoroscopic guidance and intermittent small injections of radiopaque contrast (iohexol, GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The distal common CA was then balloon injured by using an overinflated 20 mm balloon catheter (Armada 35, Abbott Vascular, Santa Clara, CA) which stretched the vessel to 130% of its original measured diameter for 30 second inflations × 3. Then the deflated balloon was withdrawn until it was proximal to the balloon injury site, and re-inflated until it created a stenosis of 70-80% of the vessel diameter using digital calipers and catheter size (2.3 mm) as a reference diameter. Small 0.5-1.0 milliliters aliquots of thrombosing whole arterial blood less than one hour old from the same pig were then injected through the partially inflated balloon catheter until angiographic occlusion of the carotid artery and all four major branches (auricular, ascending pharyngeal, buccinator, and maxillary) was observed. If angiographic recanalization of any of the four downstream vessels was observed during this time period, repeat 0.5 ml injections of thrombi were performed until a persistent anterograde flow obstruction was observed for a total of 20 minutes. Total injected thrombus volume averaged 2.9 ± 1.2 ml, and was not different between groups. A requirement prior to randomization was complete absence of forward flow by angiography from the site of balloon injury and distal to this for 20 minutes (Figure 1). The duration of time between stenosis formation with the balloon catheter to persistent angiographic occlusion was 79 ± 26 minutes, and did not differ between groups.
Figure 1.
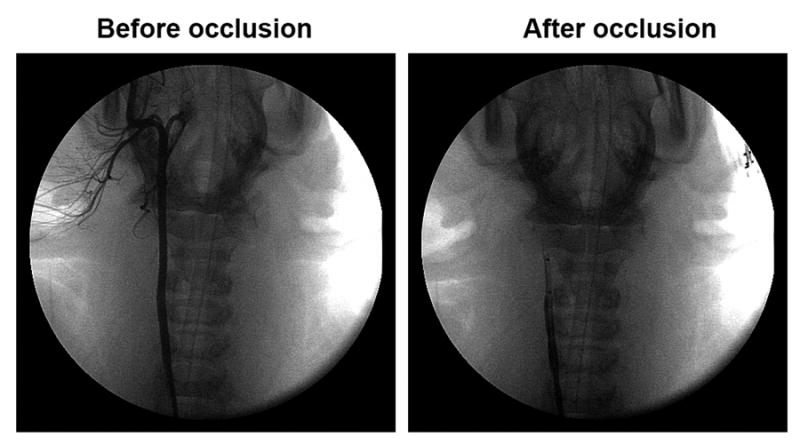
Angiographic demonstration of total thrombotic occlusion of the distal common carotid artery distal to 70-80% diameter occlusion produced by balloon inflation (right panel). The baseline angiogram is demonstrated in the left panel.
Intravenous Microbubbles
The intravenous microbubbles used for this study were the commercially available lipid encapsulated perfluorocarbon microbubble Definity (Lantheus Medical; North Billerica, MA). One vial (2.0 ml) was diluted in 100 milliliters of 0.9% normal saline (2% Definity) and infused over 30 minutes at an average rate of 3ml/min for all imaging and therapeutic applications.
Study Protocol
Following documented angiographic occlusion, pigs were randomized to one of three treatment regimens: low mechanical index (MI) imaging alone (0.2 MI), image guided stable cavitation (SC) inducing impulses, or image guided inertial cavitation (IC) inducing impulses. All ultrasound applications in the SC and IC randomized groups were performed through a tissue mimicking phantom (TMP) that contained either a three centimeter (n=8 for both SC and IC groups) or six centimeter (n=6 for SC; n=5 for IC) slab of material which had the same attenuation characteristics as human soft tissue (attenuation 1 dB/cm/MHz). The low MI imaging alone group was transmitted only through the three centimeter thick TMP. The SC and IC inducing high MI impulses were confirmed using a software-modified commercial ultrasound scanner iE33 with a sector imaging probe S5-1 (1.6 MHz; Philips Healthcare, Andover, MA) employed for microbubble cavitation imaging (25,31). The radiofrequency (RF) spectra created by the longer pulse duration (20 usec) impulses was measured by transmitting the impulses to excite microbubbles within the region of interest and backscattered RF beam-formed signals acquired with an internal acquisition board that was saved in the scanner's hard drive. The data were then transferred to an external personal computer and processed off-line for cavitation signatures within the region of interest. Cavitation imaging was based on the spectral analysis of acoustic signals radiated by the cavitating microbubbles: a dominant ultraharmonic waveform in response to the excitation frequency indicated SC (which occurred at an MI of 0.8), whereas dominant noise bands indicated IC (which occurred at an MI of 1.2). The degree of attenuation produced by the TMP was assumed to be equivalent to transthoracic cardiac imaging (24). The SC and IC impulses during treatments were applied only when microbubbles were visualized within the artery. Each high MI impulse was applied at a 20 Hertz frame rate as the transducer was moved over the TMP from the superior-most portion of the common carotid down to its inferior border over a five second period. Treatment times were for 30 minutes. Microbubble flow within the occluded graft was analyzed during low MI imaging at baseline, and at 15-20 and 25-30 minutes into treatment. This was graded as 0= no or minimal microbubble appearance within the arterial thrombus, 1+ = small 1-2 mm channels forming in the thrombus with slow microbubble flow, and 2+ = larger channels (>2 mm) with pulsatile flow.
Magnetic Resonance Angiographic Treatment Outcomes
Following the treatment period, a repeat angiogram with radiopaque contrast was performed to examine for angiographic patency of the four major branches emanating from the common carotid artery (auricular, ascending pharyngeal, buccinator, and maxillary arteries). Following this, the pigs were transferred to a magnetic resonance scanner (Philips 1.5 T Achieva Platform) where measurements of angiographic patency in the four downstream vessels were analyzed using both three dimensional (3D) time of flight (TOF) angiography and phase contrast angiography (PCA) sequences and an eight-channel head coil. 3D TOF pixel sizes were .39mm × .39 mm; TR was 24msec, TE 6.9 msec, flip angle15 degrees, and reconstructed slice thickness 0.6mm. Pixel sizes for 3D PCA were .59mm × .59mm, and TR was 18.1 msec, TE 6.5 msec. Maximal velocity encoding in slice selection plane 20cm/sec. Echo-planar diffusion weighted imaging (DWI) was also performed with a b-value diffusion gradient of 0 and 1000.to examine for any evidence of stroke within the affected treatment side. A neuroradiologist who was completely blinded to treatment assignment assigned flow scores to each of the four downstream vessels supplied by the common carotid artery using the thrombolysis in cerebral infarction (TICI) scoring system (32). Scores of 2a, 2b, and 3 for each vessel using either PCA or TOF imaging following randomized treatments were grouped and compared to scores of 0-1.
Statistical Analysis of the Data
Comparisons in blood pressure, oxygen saturation, and heart rate before and after treatments were determined with analysis of variance (ANOVA). Contingency tables (3×2) were used to compared the proportions of downstream vessels in which there was return in flow signal (score of at least 2a, 2b, or 3) using either angiography, 3D TOF or 3D PCA analysis, as well as the number of pigs in which at least three downstream vessels had a TICI score of 2 or higher following treatment. A p value less than 0.05 was considered significant.
Results
There were no differences in mean arterial pressure, heart rate, or oxygen saturation before and after treatment in any of the treatment groups (Table 1). A total of nine common carotid occlusions were treated with low MI imaging only, 13 with image guided IC inducing impulses, and 14 with image guided SC inducing impulses. By conventional angiography, common carotid artery recanalization occurred in 3/9 vessels treated with low MI imaging only, 9/14 vessels treated with intermittent SC inducing impulses, and 0/14 vessels treated with IC inducing impulses (p<0.05). While minimal microbubble appearance was noted at time zero in all vessels with low MI imaging (score of zero), a microbubble flow score of 2+ was observed in 70% and 80% of SC treated vessels by 15-20 minutes and 25-30 minutes respectively, compared to 25% of IC treated vessels at these two time points. Low MI imaging alone groups had no 2+ flow scores at 15-20 minutes and 28% at 25-30 minutes (p=0.03 for SC treatment compared to other groups).
Table 1.
Mean arterial pressure, oxygen saturation, and heart rate before and after randomized treatments. Values were not statistically different between groups in any measurement.
| Parameter | O2 sat Before | O2 sat After | MAP Before | MAP After | HR Before | HR After |
|---|---|---|---|---|---|---|
| Low MI Alone | 98 ± 3.0 | 97 ± 3.7 | 70 ± 9.6 | 70 ± 5.8 | 78 ± 9.2 | 77 ± 9.2 |
| SC impulses | 100 ± 0.6 | 98 ± 2.8 | 70 ± 10.3 | 72 ± 8.9 | 86 ± 11.5 | 86 ± 9.8 |
| IC impulses | 99 ± 2.9 | 98 ± 2.3 | 71 ± 12.0 | 73 ± 10.7 | 84 ± 8.4 | 85 ± 6.8 |
Time of Flight and Phase Contrast Angiography
TOF analyses were able to be analyzed in eight vessels treated with low MI imaging only, 13 treated with intermittent IC-inducing impulses, and 14 vessels treated with intermittent SC-inducing impulses. PCA data was analyzable in eight vessels treated with low MI imaging only, 12 vessels treated with intermittent IC-inducing impulses, and 14 vessels treated with intermittent SC-inducing impulses. TOF and PCA were in agreement in grading flow in the four downstream vessels in over 95% of scored vessels (Table 2 and Table 3). Restoration of TICI 2 or 3 flow in at least three of the four downstream vessels supplied by the common carotid was seen in 9 of 14 CA of pigs treated with SC inducing impulses, 2/13 CA of pigs treated with IC inducing impulses, and 1/8 vessels treated with low MI imaging only (p=0.028). A TICI score of 2a, 2b, or 3 was seen in 64% (38/59) of SC treated vessels compared to 33% (17/52) of IC treated and 29 % (10/34) of low MI alone treated CA (p<0.001). Although SC treated vessels had higher TICI 2b-3 flow in all downstream vessels, the proportion with highest return of TICI 2 b-3 flow was in the larger maxillary artery (7/15 in the SC treated group versus 0/13 in the IC treated group and 0/8 in the low MI imaging group; p=0.002) and to a lesser degree in the ascending pharyngeal artery (6/15 in the SC treated group versus 3/13 in the IC and 3/8 in the low MI only groups, P=NS). Figure 2 demonstrates a common CA treated with diagnostic ultrasound guided SC impulses through a TMP, compared to another pig with common carotid artery occlusion treated with IC impulses. No pig exhibited evidence of stroke on diffusion weighted imaging following any treatment.
Table 2.
Number of vessels with angiographic recanalization (Grade 2a or higher) following treatment with SC versus IC inducing impulses at the 20 usec pulse duration. *p<0.005 compared to US alone and IC inducing impulses.
| Low MI Alone | IC impulses | SC impulses | |
|---|---|---|---|
| MRI PCA | 10 (29%) | 17(35%) | 34 (61%)* |
| MRI TOF | 10 (29%) | 17 (33%) | 38 (64%)* |
Abbreviations: IC=Inertial Cavitation, MRI PCA=magnetic resonance angiography phase contrast angiography, MRI TOF=magnetic resonance angiography time of flight imaging; MI=mechanical index; SC=stable cavitation
Table 3.
Number of vessels with angiographic recanalization (Grade 2b or higher) following treatment with SC versus IC inducing impulses at the 20 usec pulse duration. *p<0.005 compared to US alone and IC inducing impulses.
| Low MI Alone | IC impulses | SC impulses | |
|---|---|---|---|
| MRI PCA | 5 (15%) | 7 (14%) | 23 (41%)* |
| MRI TOF | 7 (21%) | 5 (10%) | 23 (39%)* |
Abbreviations: Same as Table 2.
Figure 2.
Angiographic (left three panels) and phase contrast angiogram (PCA; right panel) of a pig acute carotid artery thrombotic occlusion treated with stable cavitation inducing impulses at a 20 usec pulse duration (top panels) compared to a pig common carotid occlusion treated with inertial cavitation (IC) inducing impulses (bottom panels). The downstream distal vessel branches (1=maxillary artery, 2=buccinators, 3=auricular, 4=ascending pharyngeal) are recanalized following treatment with SC inducing impulses, but not following IC inducing impulses.
Discussion
Acute carotid artery thromboses typically occurs in the setting of either an underlying carotid stenosis or as an embolus from a left ventricular cavity thrombus, and is associated with a high risk of downstream ischemic events (33). When detected by computed tomography, it is often treated as an emergency requiring angiography and endovascular intervention (9,10,33,34). In this study, we demonstrated that in treating acute CA thromboses that develop in the region of a stenosis, diagnostic ultrasound high MI impulses which induce SC can effectively recanalize the CA thrombus, and restore downstream flow in the major branches emanating from the common carotid artery. This includes the ascending pharyngeal artery which in pigs supplies the internal carotid and circle of Willis. The recanalization occurred without the need for antiplatelet agents, anticoagulants, or fibrinolytic agents. Secondly, we demonstrated that higher ultrasound MI's which elicit a more violent form of cavitation (IC), are not effective. The reasons for this reversal of effect at a higher ultrasound peak negative pressures is unclear, but demonstrates than an ultrasound dose response curve exists for in vivo applications of sonothrombolysis. This dose response curve is potentially related to both the MI and the pulse duration, and must be taken into account for subsequent clinical trials.
Efficacy of Stable Cavitation versus Inertial Cavitation Inducing Impulses
The porcine model utilized in this study created a common carotid thrombotic occlusion that resulted in obstruction in blood flow to four major downstream vessels within the pig extracranial and intracranial circulation. Low MI diagnostic ultrasound during a commercially available microbubble infusion was not effective in restoring blood flow, but when intermittent SC inducing impulses were applied, we observed moderate to complete recanalization in not only the common carotid artery but also the downstream vessels in over half of the pigs treated. Not only was low MI imaging alone not effective at restoring flow in downstream vessels, high MI impulses that induced IC were also not effective. Although our study does not provide a mechanism as to why the thrombolytic effect of DUS at the longer pulse duration was reversed at a higher MI, there is data from other studies to suggest that adverse bioeffects may have been responsible. SC inducing impulses would be expected to cause a form of non-linear oscillatory behavior that would be capable of dissolving thrombi (28), but would not create the fluid jets and cell membrane damage that often accompany IC inducing impulses (19). These fluid jets may have produced endothelial and platelet injury, resulting in thromboxane or serotonin release, inducing vasoconstriction that inhibited any return of flow from thrombus dissolution. Vasoconstriction in response to the high MI longer pulse durations used in this study have been observed following transthoracic applications in patients with acute ST segment elevation myocardial infarction (29). The potential role for platelet induced vasoconstriction is supported by studies in animal models of coronary injury and arterial thrombus formation. In this setting, diagnostic transthoracic high MI impulses (>1.0) at 20 usec pulse duration produced vasodilation of balloon injured coronary artery segments, but this vasodilatory effect was reversed when intra-arterial thrombus was also present (29). The peak negative pressure that produced penetrating tunnel-forming clots in vitro in the study by Acconcia et.al. is similar to the SC inducing high MI impulse used in our study (albeit at a longer pulse duration). This tunnel like effect would have dissolved the carotid thrombus and prevented the thromboembolic phenomena. While the IC inducing impulses also should have produced this bubble penetration effect, the fluid jet effect of IC may induce hemolysis of red blood cells cascading off the clot surface (35).
Other potential explanations for the reversal of the beneficial effect at higher MI's may be that IC produced an equivalent degree of thrombus dissolution than SC impulses, but the residual fragments were bigger and more likely to cause downstream embolization and obstruction. This is less likely to be a cause since the common carotid artery remained occluded by angiography in 13 of the vessels treated with IC inducing impulses. Furthermore, in vitro studies have demonstrated that thrombolysis achieved with IC generating impulses for the most part produce fragments that are in the micron-size range (36).
Study Limitations
The effect of pulse duration was not examined in the current study, but previous investigations in both animals and humans have shown that high MI (>1.0) short pulse duration (<5 usec) impulses intermittently applied to the coronary microcirculation have improved early epicardial recanalization rates and microcirculatory blood flow in acute myocardial infarction (15). In the current study, these same >1.0 MI impulses were not effective when applied for a longer pulse duration. This would suggest that a shorter IC inducing impulse may be as effective as a longer SC inducing impulse. In vitro studies have confirmed that the 20 usec pulse duration is more effective than a 5 usec pulse duration at a lower MI, and therefore it is critical that clinical trials in treating both ischemic stroke and acute myocardial infarction take into account both the MI and the pulse duration. Since most commercially available diagnostic systems use short <5 usec pulse durations, a higher MI that induces transient IC may be most effective, as has been observed with transtemporal ultrasound (25). A single element transducer with long pulse durations of up to 5000 microseconds at 1 MHz has been shown to be more effective than shorter pulse durations (37). However, such long pulse duration and lower frequency (1 MHz) are currently unavailable from a diagnostic imaging scanner. The treatment efficacy is likely to be increased by the use of long pulse, but other effects (such as the reverberation of very long pulses within biologic systems) may introduce complications.
The current study also did not explore what effect ultrasound induced cavitation may have in potentiating the effect of fibrinolytics. Although our goal was to assess the sole effects of cavitation on thrombus dissolution, others have shown that transcranial ultrasound induced cavitation of microbubbles can potentiate the effects of fibrinolytic agents over five-fold while also reducing intracranial hemorrhage rates (38).
Conclusions
In an animal model of acute carotid artery thromboembolism with significant underlying carotid stenosis, stable cavitation inducing impulses from a modified diagnostic ultrasound scanner were able to restore common carotid blood flow and downstream blood flow without the need for fibrinolytic, anti-platelet, or anticoagulant therapy. This effect may be reversed if a more prolonged pulse duration at a higher MI is used to create cavitation. Nonetheless, the addition of diagnostic ultrasound induced stable cavitation may be a rapid non-invasive method of treating this form of ischemic stroke even when anticoagulation is contraindicated.
Acknowledgments
Conflicts of Interest and Source of Funding: Dr. Porter receives grant support from Lantheus Medical Imaging and educational support from Bracco Research. This study was supported by National Institute of Health (R21EB015163) and by the Theodore F. Hubbard Foundation.
References
- 1.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–31. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 3.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 5.Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–75. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 6.Sacco RL, Shi T, Zamanillo MC, et al. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: the Northern Manhattan Stroke Study. Neurology. 1994;44:626–34. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 7.Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet. 2008;37:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 8.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Demchuk AM, Menon M, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 10.Mokin M, Kass-Hout T, Kass-Hout O, et al. Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion. Stroke. 2012;43:2362–68. doi: 10.1161/STROKEAHA.112.655621. [DOI] [PubMed] [Google Scholar]
- 11.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound enhanced systemic thrombolysis for acute ischemic stroke. New Engl J Med. 2004;351:2170–78. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 12.Eggers J, Koch B, Meyer K, Konig I, et al. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797–800. doi: 10.1002/ana.10590. [DOI] [PubMed] [Google Scholar]
- 13.Tsivgoulis G, Eggers J, Ribo M, et al. Safety and efficacy of ultrasound-enhanced thrombolysis. A comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke. 2010;41:280–87. doi: 10.1161/STROKEAHA.109.563304. [DOI] [PubMed] [Google Scholar]
- 14.Alexandrov AV, MIkulik R, Ribo M, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39:1464–69. doi: 10.1161/STROKEAHA.107.505727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina CA, Ribo M, Rubiera M, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissues plasminogen activator. Stroke. 2006;37:425–29. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 16.Molina CA, Barreto AD, Tsivgoulis, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009;66:28–38. doi: 10.1002/ana.21723. [DOI] [PubMed] [Google Scholar]
- 17.Flores R, Hennings LJ, Lowery JD, et al. Microbubble-augmented ultrasound sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute Ischemic stroke. Stroke. 2011;42:2280–5. doi: 10.1161/STROKEAHA.110.607150. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culp WC, Porter TR, McCowan TC, et al. Microbubble-augmented ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs. J Vasc Interv Radiol. 2003;14:343–47. doi: 10.1097/01.rvi.0000058409.01661.b4. [DOI] [PubMed] [Google Scholar]
- 19.Culp WC, Erdem E, Roberson PK, et al. Microbubble potentiated ultrasound as a method of stroke therapy in a pig model: preliminary findings. J Vasc Interv Radiol. 2003;14:1433–36. doi: 10.1097/01.rvi.0000096767.47047.fa. [DOI] [PubMed] [Google Scholar]
- 20.Culp WC, Porter TR, Lowery J, et al. Intracranial clot lysis with intravenous microbubbles and transcranial ultrasound in swine. Stroke. 2004;35:2407–11. doi: 10.1161/01.STR.0000140890.86779.79. [DOI] [PubMed] [Google Scholar]
- 21.Culp WC, Flores R, Brown AT, et al. Successful microbubble sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute ischemic stroke. Stroke. 2011;42:2280–5. doi: 10.1161/STROKEAHA.110.607150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie F, Tsutsui JM, Lof J, et al. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;31:979–85. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Mathias W, Jr, Tsutsui JM, Tavares BG, et al. Diagnostic ultrasound impulses improve microvascular flow in patients with STEMI receiving intravenous microbubbles. J Am Coll Cardiol. 2016;67:2506–2515. doi: 10.1016/j.jacc.2016.03.542. [DOI] [PubMed] [Google Scholar]
- 24.Xie F, Lof J, Everbach C, et al. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. JACC Cardiovasc Imaging. 2009;2:511–518. doi: 10.1016/j.jcmg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Gao S, Zhang Y, Wu J, et al. Improvements in Cerebral Blood Flow and Recanalization Rates with Transcranial Diagnostic Ultrasound and Intravenous Microbubbles Following Acute Cerebral Cerebral Emboli. Invest Radiol. 2014;40:593–600. doi: 10.1097/RLI.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 26.Porter TR, Radio S, Lof J, et al. Diagnostic ultrasound high mechanical index impulses restore microvascular flow in peripheral arterial thromboembolism. Ultrasound Med Biol. 2016;42:1531–40. doi: 10.1016/j.ultrasmedbio.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodama T, Tatsuno M, Sugimoto S, et al. Liquid jets, accelerated thrombolysis: a study for revascularization of cerebral embolism. Ultrasound Med Biol. 1999;25:977–983. doi: 10.1016/s0301-5629(99)00050-2. [DOI] [PubMed] [Google Scholar]
- 28.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–69. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- 29.Roos ST, Juffermans L, van Royen N, et al. Unexpected high incidence of coronary vasoconstriction in the reduction of microvascular injury using sonolysis (ROMUS) trial. Ultrasound Med Biol. 2016;42:1919–28. doi: 10.1016/j.ultrasmedbio.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Miller DL, Driscoll EM, Dou C, et al. Microvascular permeabilization and cardiomyocyte injury provoked by myocardial contrast echocardiography in a canine model. J Am Coll Cardiol. 2006;47:1464–8. doi: 10.1016/j.jacc.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 31.Vignon F, Shi WT, Powers JE, et al. Microbubble cavitation imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:661–70. doi: 10.1109/TUFFC.2013.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Technology assessment committee of the American Society of Interventional and Thereapeutic Neruobiology: Technology assessment committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 33.Vellimana AK, Kadkhodayan Y, Rich KM, et al. Symptomatic patients with intraluminal carotid artery thrombus: outcome with a strategy of initial anticoagulation. J Neurosurg. 2013;118:34–41. doi: 10.3171/2012.9.JNS12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthet JP, Marty-Ane CH, Picard E, et al. Acute carotid artery thrombosis: description of 12 surgically treated cases. Ann Vasc. Surg. 2005;19:11–18. doi: 10.1007/s10016-004-0074-x. [DOI] [PubMed] [Google Scholar]
- 35.Acconia CN, Leung BYC, Goertz DE. The microscale evolution of the erosion front of blood clots exposed to ultrasound stimulated microbubbles. J Acoust Soc Am. 2016;139:EL135–41. doi: 10.1121/1.4946045. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell AD, Cain CA, Duryea AP, et al. Non-invasive thrombolysis using pulsed ultrasound cavitation therapy-histotripsy. Ultrasound Med Biol. 2009;35:1982–94. doi: 10.1016/j.ultrasmedbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leeman JE, Kim JS, Yu FT, et al. Effect of acoustic conditions on microbubble-mediated microvascular sonothrombolysis. Ultrasound Med Biol. 2012;38:1589–98. doi: 10.1016/j.ultrasmedbio.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Brown AT, Flores R, Hamilton E, et al. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol. 2011;46:202–7. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]



