Abstract
Background
Cellular and animal studies demonstrated relationships between sphingolipid metabolism and Alzheimer's disease (AD) pathology. High blood ceramide levels have been shown to predict cognitive impairment and AD, but these studies had small sample sizes and did not assess differences in risk by sex or APOE genotype.
Objective
To determine whether plasma ceramides and sphingomyelins were associated with risk of AD, and whether the association varied by sex and APOE genotype.
Methods
Participants included 626 men and 366 women, aged 55 years and older, enrolled in the Baltimore Longitudinal Study of Aging. Plasma ceramides and sphingomyelins were determined using quantitative analyses performed on a high-performance liquid chromatography coupled electrospray ionization tandem mass spectrometer. Cox proportional hazards models, stratified by sex, were used to examine the relationship of plasma ceramides and sphingomyelins with risk of AD over a mean (SD) follow-up of 15.0 (7.0) years for men and 13.1 (5.9) years for women.
Results
Among men, the highest tertile of most ceramides and sphingomyelins were associated with an increased risk of AD. Among women, there were no associations between any of the ceramides and risk of AD. In contrast, women in the highest tertile of most sphingomyelins had a reduced risk of AD, which was most pronounced among APOE ε4 carriers.
Conclusion
These results provide further evidence for the role of sphingolipid metabolism in AD and highlight the importance of considering sex and APOE genotype in assessing this relationship.
Keywords: Ceramides, lipids, Alzheimer disease, epidemiology, cohort study, longitudinal, sex differences
INTRODUCTION
Sphingolipids are ubiquitous lipids that have important structural roles in cell membranes and are essential components of lipid rafts. In addition to structural roles, sphingolipid metabolites function as second messengers for critical intra- and inter-cellular signaling that controls cellular growth, differentiation, proliferation, and apoptosis. In brain, the proper balance of sphingolipids is essential for normal neuronal function [1–3], as evidenced by a number of severe brain disorders that are the result of deficiencies in enzymes that control sphingolipid metabolism.
Multiple cellular and animal studies have demonstrated relationships between sphingolipid metabolism and Alzheimer's disease (AD) pathology. For example, both ceramides and sphingomyelins influence beta-secretase activity and amyloid-beta (Aβ) production [4–10]. Further, the ceramide transporter (CERT) binds to Aβ and modulates Aβ plaque formation [9, 11]. Lastly, ceramides can modulate Protein Phosphatase 2A activity, the major brain enzyme that regulates tau phosphorylation [12–16].
Few studies have attempted to translate these findings to understand whether sphingolipid metabolism is associated with risk of AD in humans. A blood-based biomarker or predictor of AD would have advantage over cerebrospinal fluid (CSF) or neuroimaging markers with regards to cost, feasibility, and invasiveness. Plasma ceramides have been found to be increased in AD patients compared to cognitively normal individuals [17]. However, AD has a long preclinical phase in which the biological changes of the disease are already affecting the brain. Therefore, identifying predictors of AD in the preclinical and early clinical stages is important for developing new treatment targets. One recent study reported significantly altered phospholipids and ceramides in Presenilin 1 mutation carriers compared to non-carriers [18], but additional research among individuals with late-onset AD is needed.
We previously reported that high blood ceramide levels were associated with risk of cognitive impairment and AD among cognitively normal women enrolled in the Women's Health and Aging Study (WHAS) II [19, 20]. However, these analyses were limited by a small sample size (n=100), only examined the relationship among women, and were not able to assess the effects of APOE genotype on the association.
The effects of sex differences and interactions with APOE genotype on the association between the sphingolipids and risk of AD are important to consider. We recently reported sex-specific trajectories of increasing plasma ceramides and sphingomyelins with aging [21, 22], and sex differences in the risk factors for AD are well known [23]. The APOE gene encodes the lipid/cholesterol carrier apolipoprotein E and the APOE ε4 allele may have a greater deleterious effect for women versus men with regards to hippocampal pathology, functional connectivity changes in the default mode network, cortical thickness, memory performance and risk of dementia in women compared with men [24–28]. In the proposed study, we examined whether plasma ceramides and sphingolipids were associated with risk of AD among 992 cognitively normal individuals enrolled in the Baltimore Longitudinal Study of Aging (BLSA). In addition, we investigated the effects of sex and the presence of an APOE ε4 allele on this association.
METHODS
Participants
Initiated in 1958, the Baltimore Longitudinal Study Aging (BLSA) is a longitudinal cohort study of community-dwelling individuals aimed at examining the physiological and psychological aspects of aging [29]. At each study visit, participants underwent an extensive medical examination, neuropsychological battery, blood draw, medical history and medication review. Historically, BLSA visits occurred every two years. In 2003, the sampling times were modified because historical data indicated non-linear changes at the oldest ages. To improve sampling of the epoch with accelerated physical and cognitive change, individuals aged 80 and older have been evaluated annually since 2003. The protocol was approved by the local Institutional Review Board and written informed consent was obtained at each study visit.
Over 3,100 BLSA participants have contributed data on the aging process. The present analyses included 992 individuals aged 55 years and older. The individuals included in the current analyses are representative of the larger BLSA cohort with regards to demographics and health characteristics.
Collection of plasma
Blood samples were drawn from the antecubital vein between 7–8 AM after an overnight fast [29]. Participants were not allowed to smoke, engage in physical activity, or take medications before the sample was collected. Plasma samples were immediately processed, catalogued, and stored at −80°C.
Diagnosis of Alzheimer's disease
All participants included in this analysis were cognitively normal at the time of the baseline blood draw. The diagnoses of dementia and Alzheimer's disease were determined by consensus case conference and were based on the Diagnostic and Statistical Manual (DSM)-III-R [30] and the National Institute of Neurological and Communication Disorders and Stroke — Alzheimer's Disease and Related Disorders Association criteria [31], respectively. Age at symptom onset was also determined through review of clinical and neuropsychological data at consensus conferences.
Lipid extraction and LC/ESI/MS/MS analysis
The lipid extractions and methods for measuring plasma ceramide and sphingomyelin levels in the BLSA have previously been described in detail, including the mass spectroscopy inter- and intra-day coefficients of variation [21, 22]. Briefly, a crude lipid extraction of plasma was conducted using a modified Bligh and Dyer procedure with ceramide or sphingomyelin C12:0 included as an internal standard (Avanti Polar Lipids, Alabaster, Alabama) [32, 33]. Plasma extracts were dried in a nitrogen evaporator (Organomation Associates Inc., Berlin, MA, USA), and re-suspended in pure methanol just prior to analysis. An autosampler (LEAP technologies Inc., Carrboro, NC) injected extracts into an HPLC (PerkinElmer, MA, USA) equipped with a reverse phase C18 column (Phenomenex, Torrance, CA). The eluted sample was then injected into an electrospray ion source coupled to a triple quadrupole mass spectrometer (API3000, AB Sciex Inc. Thornhill, Ontario, Canada) [32, 34, 35]. Analyses were conducted by multiple reaction monitoring (MRM). Eight point calibration curves (0.1 to 1,000 ng/ml) were constructed by plotting area under the curve (AUC), separately for ceramides and sphingomyelins, for each calibration standard d18:1/C16:0, d18:1/C18:0, d18:1/C20:0, d18:1/C22:0, d18:1/C24:0 (Avanti polar lipids, Alabaster, AL, USA) normalized to the internal standard. Correlation coefficients (R2) obtained were >0.999. Ceramide concentrations were determined by fitting the identified ceramide species to these standard curves based on acyl-chain length. Internal standards were run daily, and AUCs plotted weekly, to track instrument efficiency. Plasma extracts were reanalyzed if the internal standard deviated more than 25% of the median value. Instrument control and quantitation of spectral data was performed using Analyst 1.4.2 and MultiQuant software (AB Sciex Inc. Thornhill, Ontario, Canada). All sphingolipids are expressed in μg/ml. As previously reported, storage time was not associated with ceramide or sphingomyelin concentrations in the BLSA, indicating that that these classes of lipids were stable in long-term −80°C storage up to 30 years [21, 22].
Covariates
Covariates were chosen based on the literature and our previous studies in the BLSA describing variables associated with plasma ceramide and sphingomyelin levels and with factors associated with incident AD [21, 22]. Demographic variables included age, sex, race, and years of education. Height (in meters) and weight (in kilograms) were measured to calculate body mass index (BMI). Medical history information was self-reported and included hypertension, myocardial infarction, atrial fibrillation, angina, and chronic heart failure. The diagnoses of diabetes and pre-diabetes at each visit were established by combining information on medications, fasting glucose and glucose levels at 2-hours of a standard glucose tolerance test. In particular, participants who were taking anti-diabetes medication or had a fasting glucose >126 mg/dL and/or a 2-hour glucose >200 mg/dL were defined as diabetics. Among those who had no diabetes, participants with fasting glucose >100 mg/dL and/or a 2-hour glucose >140 mg/dL were defined as having pre-diabetes. Plasma total cholesterol and triglycerides were determined by an enzymatic method (Abbott Laboratories ABA-200 ATC Biochromatic Analyzer, Irving, Texas). Women who had used postmenopausal oral or transdermal estrogens were considered hormone therapy users. APOE ε4 genotype was determined using polymerase chain reaction amplification of leukocyte deoxyribonucleic acid followed by HhaI digestion and product characterization [36], or TaqMan relying on several single nucleotide polymorphisms around the APOE gene [37].
Statistical analyses
Differences in demographic and health-related characteristics at the visit with available sphingolipids, by sex and incident AD, were examined using Fisher's exact test for categorical variables and t-tests for continuous variables. All analyses were a priori determined to be examined separately for men and women because we have shown significant sex differences in both plasma ceramides and sphingomyelins in this cohort [21, 22].
We used time to event (or survival) analyses to evaluate whether plasma ceramides and sphingomyelins at the time of the first blood draw were associated with time to onset of AD. We used Cox proportional hazards regression models [38] to estimate the hazards ratio (HR), which is analogous to relative risk, and its 95% confidence interval (CI). Time at risk for these analyses was calculated to the symptom onset of AD, last follow-up visit, or death. We initially examined the lipids as continuous variables. However, because the relationship with risk of AD was not linear, all lipids were expressed and analyzed as tertiles. Multivariate models adjusted for for baseline age, race, education, APOE genotype, body mass index, diabetes, hypertension, triglycerides, cholesterol, and postmenopausal hormone therapy (women only). In additional analyses, we assessed an interaction between the ceramides or sphingomyelins and presence of an APOE ε4 allele in predicting risk of AD. All analyses were completed using Stata version 12.0 (Stata Corp, College Station, TX).
RESULTS
Participant characteristics by sex and incident AD
The 992 participants, all cognitively normal at baseline, were followed a maximum of 39 years (mean [SD]: 15.0 [7.0] for men and 13.1 [5.9] for women) and had 17,072.02 person-years at risk. Of the 626 men, 119 (19.0%) developed AD. Of the 366 women, 73 (19.9%) developed AD.
Participant characteristics, stratified by incident AD and sex, at the time of the baseline blood draw to measure sphingolipids, are shown in Table 1. Compared to men, women were older (64.6 vs. 62.7 years, p = 0.001), had slightly less education (15.7 vs. 16.8 years, p < 0.001), were more frequently African American (18.3% vs. 9.3%, p < 0.001), were less likely to smoke (47.0% vs. 72.2%, p < 0.001), and less frequently had a history of a myocardial infarction (0.3% vs. 3.8%, p = 0.001) or pre-diabetes/diabetes (20.9% vs. 59.2%, p < 0.001). However, women did have higher total cholesterol levels at baseline compared to men (229.8 vs. 221.7, p = 0.003). Within each sex, plasma levels of ceramides and sphingomyelins did not differ for individuals with or without an APOE ε4 allele (data not shown).
Table 1.
Baseline participant characteristics by sex and incident Alzheimer's disease (AD)
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Variable | Developed AD (N=119) N(%)/mean (SD) | No AD (N=507) N(%)/mean (SD) | p-value | Developed AD (N=73) N(%)/mean (SD) | No AD (N=293) N(%)/mean (SD) | p-value |
| Age | 64.6 (8.8) | 62.2 (7.7) | 0.003 | 68.8 (8.9) | 63.6 (8.0) | <0.001 |
| African American | 8 (6.7%) | 50 (9.9%) | 0.379 | 4 (5.5%) | 63 (21.5%) | 0.001 |
| Education | 17.0 (2.9) | 16.7 (2.7) | 0.334 | 15.8 (2.3) | 15.7 (2.6) | 0.697 |
| Ever Smoking | 89 (74.8%) | 363 (71.6%) | 0.570 | 37 (50.7%) | 135 (46.1%) | 0.514 |
| Body Mass Index | 25.4 (2.4) | 26.4 (3.7) | 0.004 | 25.0 (4.6) | 25.9 (4.6) | 0.163 |
| Diagnosis of Hypertension | 28 (23.5%) | 150 (30.0%) | 0.213 | 16 (21.9%) | 69 (23.6%) | 0.451 |
| Myocardial Infarction | 22 (4.3%) | 2 (1.7%) | 0.285 | 0 | 1 (0.34%) | 1.000 |
| Diabetes | 0.934 | 0.464 | ||||
| None | 59 (49.6%) | 250 (49.3%) | 57 (78.1%) | 232 (79.2%) | ||
| Pre-diabetes | 49 (41.2%) | 203 (40.0%) | 15 (20.6%) | 49 (16.7%) | ||
| Diabetes | 11 (9.2%) | 54 (10.7%) | 1 (1.4%) | 12 (4.1%) | ||
| Apoe E4 allele | 35 (29.4%) | 119 (23.5%) | 0.193 | 30 (41.1%) | 80 (27.3%) | 0.032 |
| Total cholesterol | 230.5 (44.9) | 219.5 (39.3) | 0.008 | 237.7 (34.9) | 227.0 (42.0) | 0.046 |
| Triglycerides | 101.8 (45.8) | 121.0 (83.7) | 0.016 | 96.5 (42.9) | 111.8 (85.9) | 0.141 |
| Statin Use | 3 (2.5%) | 34 (6.7%) | 0.087 | 4 (5.5%) | 24 (8.2%) | 0.623 |
Plasma ceramides and risk of AD
The associations between tertiles of ceramides and sphingomyelins at the first visit and risk of AD, by sex, are shown in Table 2 and Figure 1. Among men, the highest tertiles of most ceramides were associated with an increased risk of AD in both univariate and multivariate analyses. The strongest association was with ceramide C16:0, which was associated with a greater than 2-fold increased risk (HR: 2.26, 95% CI: 1.20, 4.23) of developing AD for the top tertile compared to the lowest tertile. Among women, there were no associations between any of the ceramides and risk of AD. We found no significant interactions between ceramides and presence of an APOE ε4 allele in predicting risk of AD in either men or women.
Table 2.
Baseline tertiles of ceramides and sphingomyelins and risk of AD by sex
| Men | Women | ||||
|---|---|---|---|---|---|
| Lipid | univariate HR (95% CI) | multivariate HR (95% CI) | univariate HR (95% CI) | multivariate HR (95% CI) | |
| Ceramide | |||||
| C16:0 | 2 vs. 1 | 2.03 (1.08, 3.84) | 1.68 (0.88, 3.19) | 1.23 (0.67, 2.25) | 1.29 (0.66, 2.51) |
| 3 vs. 1 | 2.30 (1.25, 4.21) | 2.26 (1.20, 4.23) | 1.27 (0.70, 2.31) | 0.74 (0.37, 1.50) | |
| C18:0 | 2 vs. 1 | 1.44 (0.85, 2.46) | 1.27 (0.74, 2.18) | 1.41 (0.78, 2.55) | 1.04 (0.53, 2.04) |
| 3 vs. 1 | 1.93 (1.15, 3.24) | 1.83 (1.07, 3.11) | 1.59 (0.92, 2.77) | 0.90 (0.48, 1.68) | |
| C20:0 | 2 vs. 1 | 1.22 (0.78, 1.92) | 1.29 (0.81, 2.04) | 1.08 (0.62, 1.89) | 1.06 (0.56, 2.00) |
| 3 vs. 1 | 1.32 (0.83, 2.09) | 1.50 (0.93, 2.42) | 1.17 (0.66, 2.06) | 0.67 (0.35, 1.27) | |
| C22:0 | 2 vs. 1 | 1.25 (0.78, 2.01) | 1.11 (0.68, 1.81) | 0.95 (0.52, 1.73) | 1.02 (0.50, 2.08) |
| 3 vs. 1 | 1.42 (0.91, 2.23) | 1.66 (1.04, 2.66) | 1.36 (0.78, 2.37) | 1.16 (0.59, 2.27) | |
| C24:0 | 2 vs. 1 | 1.33 (0.82, 2.16) | 1.20 (0.73, 1.96) | 1.01 (0.57, 1.80) | 1.28 (0.66, 2.47) |
| 3 vs. 1 | 1.49 (0.94, 2.35) | 1.67 (1.04, 2.69) | 1.14 (0.65, 2.02) | 1.13 (0.57, 2.25) | |
| C26:0 | 2 vs. 1 | 1.21 (0.76, 1.93) | 1.14 (0.70, 1.84) | 1.02 (0.55, 1.88) | 0.88 (0.44, 1.75) |
| 3 vs. 1 | 1.35 (0.86, 2.13) | 1.27 (0.80, 2.02) | 1.20 (0.67, 2.14) | 0.95 (0.48, 1.89) | |
| C22:1 | 2 vs. 1 | 0.88 (0.56, 1.39) | 0.72 (0.44, 1.15) | 1.20 (0.65, 2.21) | 0.87 (0.43, 1.74) |
| 3 vs. 1 | 1.04 (0.68, 1.59) | 1.02 (0.66, 1.57) | 1.21 (0.67, 2.17) | 0.81 (0.42, 1.55) | |
| C24:1 | 2 vs. 1 | 1.31 (0.84, 2.06) | 1.31 (0.82, 2.09) | 1.30 (0.74, 2.27) | 1.13 (0.61, 2.12) |
| 3 vs. 1 | 1.56 (1.01, 2.42) | 1.51 (0.97, 2.36) | 1.01 (0.55, 1.87) | 0.70 (0.36, 1.34) | |
| Sphingomyelins | |||||
| C16:0 | 2 vs. 1 | 1.01 (0.64, 1.59) | 0.92 (0.57, 1.47) | 0.90 (0.51, 1.60) | 0.65 (0.34, 1.21) |
| 3 vs. 1 | 1.24 (0.79, 1.93) | 0.96 (0.59, 1.57) | 0.96 (0.55, 1.69) | 0.50 (0.26, 0.97) | |
| C18:0 | 2 vs. 1 | 1.95 (1.25, 3.05) | 1.84 (1.15, 2.93) | 1.44 (0.77, 2.69) | 1.27 (0.63, 2.56) |
| 3 vs. 1 | 1.54 (0.97, 2.44) | 1.60 (0.97, 2.63) | 1.39 (0.75, 2.58) | 0.89 (0.44, 1.83) | |
| C20:0 | 2 vs. 1 | 0.81 (0.51, 1.28) | 0.74 (0.46, 1.20) | 0.94 (0.53, 1.65) | 1.01 (0.54, 1.89) |
| 3 vs. 1 | 1.46 (0.96, 2.23) | 1.20 (0.76, 1.90) | 0.91 (0.51, 1.63) | 0.74 (0.39, 1.41) | |
| C22:0 | 2 vs. 1 | 0.99 (0.62, 1.57) | 0.87 (0.54, 1.39) | 1.13 (0.65, 1.95) | 1.21 (0.66, 2.23) |
| 3 vs. 1 | 1.38 (0.89, 2.12) | 1.03 (0.64, 1.65) | 0.93 (0.50, 1.70) | 0.64 (0.33, 1.23) | |
| C24:0 | 2 vs. 1 | 1.06 (0.66, 1.71) | 0.69 (0.42, 1.13) | 1.21 (0.69, 2.14) | 1.15 (0.61, 2.19) |
| 3 vs. 1 | 1.61 (1.05, 2.46) | 1.37 (0.87, 2.15) | 1.03 (0.57, 1.85) | 0.86 (0.44, 1.67) | |
| C16:1 | 2 vs. 1 | 1.10 (0.72, 1.69) | 0.85 (0.55, 1.31) | 1.33 (0.74, 2.40) | 1.11 (0.59, 2.10) |
| 3 vs. 1 | 1.28 (0.81, 2.01) | 0.91 (0.57, 1.48) | 0.97 (0.54, 1.75) | 0.73 (0.39, 1.39) | |
| C18:1 | 2 vs. 1 | 1.55 (0.99, 2.43) | 1.51 (0.95, 2.40) | 1.52 (0.84, 2.78) | 1.12 (0.58, 2.17) |
| 3 vs. 1 | 2.00 (1.25, 3.18) | 1.86 (1.14, 3.03) | 0.98 (0.53 1.81) | 0.66 (0.32, 1.38) | |
| C20:1 | 2 vs. 1 | 1.17 (0.76, 1.81) | 1.03 (0.66, 1.62) | 1.93 (1.05, 3.53) | 1.53 (0.77, 3.02) |
| 3 vs. 1 | 1.88 (1.21, 2.93) | 1.58 (1.00, 2.52) | 1.08 (0.56, 2.07) | 0.71 (0.35, 1.45) | |
| C22:1 | 2 vs. 1 | 1.34 (0.86, 2.10) | 1.17 (0.73, 1.86) | 1.13 (0.63, 2.04) | 1.04 (0.54, 1.98) |
| 3 vs. 1 | 1.88 (1.19, 2.97) | 1.69 (1.04, 2.76) | 0.79 (0.44, 1.43) | 0.52 (0.27, 0.99) | |
| C24:1 | 2 vs. 1 | 1.22 (0.78, 1.89) | 0.89 (0.57, 1.41) | 1.30 (0.71, 2.38) | 1.03 (0.52, 2.06) |
| 3 vs. 1 | 1.52 (0.98, 2.37) | 1.18 (0.73, 1.89) | 1.35 (0.74, 2.46) | 1.00 (0.52, 1.91) | |
Multivariate models adjust for baseline age, race, education, APOE genotype, body mass index, diabetes hypertension, triglycerides, cholesterol, and postmenopausal hormone therapy (women only).
Figure 1.
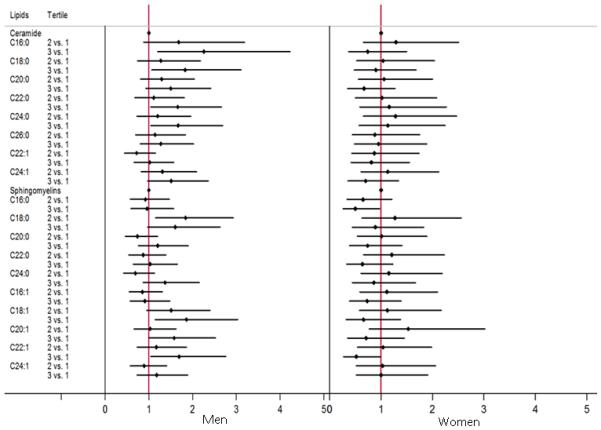
Forest plot for baseline tertiles of ceramides and sphingomyelins and risk of AD by sex.
Plasma sphingomyelins and risk of AD
Among men, the middle tertile of sphingomyelin C18:0 (HR: 1.84, 95% CI: 1.15, 2.93) and the highest tertile of sphingomyelins C18:1 (HR: 1.86, 95% CI: 1.14, 3.03), C20:1 (HR: 1.58, 95% CI: 1.00, 2.52), and C22:1 (HR: 1.69, 95% CI: 1.04, 2.76) were associated with an increased risk of AD compared to the lowest tertile (Table 2 and Figure 1). We did not find an interaction between sphingomyelin and the presence of an APOE ε4 allele on AD risk among men.
In contrast, women in the highest tertile of all sphingomyelins (with the exception of C24:1) had a reduced risk of AD. While the confidence intervals of most sphingomyelins did cross 1, the highest, versus lowest, tertiles of sphingomyelins C16:0 (HR 0.50, 95% CI: 0.26, 0.97) and C22:1 (HR: 0.52, 95% CI: 0.27, 0.99) were associated with a significantly reduced risk of AD among women. Most other sphingomyelins trended in the same direction (Table 2 and Figure 1).
Among women, there were also significant interactions between most sphingomyelins and APOE genotype. Across all sphingomyelins except C20:1, women who were APOE ε4 carriers and had sphingomyelin levels in either the middle or highest tertile, compared to the lowest, had reduced risks of AD (Table 3). For example, women who were APOE ε4 carriers and had levels of sphingomyelin C24:0 in the middle (HR: 0.24, 95% CI: 0.07, 0.90) or highest (HR: 0.26, 95% CI: 0.07, 0.93) tertile, compared to the lowest tertile, had an approximate 75% reduced risk of AD.
Table 3.
Interaction between baseline ceramide tertiles in APOE E4 genotype for predicting AD among women
| Lipid | Lipid*APOE E4 | ||
|---|---|---|---|
|
| |||
| Sphingomyelin | HR (95% CI) | HR (95% CI) | |
| C16:0 | 2 vs. 1 | 0.89 (0.39, 2.01) | 0.42 (0.12, 1.53) |
| 3 vs. 1 | 0.84 (0.37, 1.91) | 0.25 (0.07, 0.86) | |
| C18:0 | 2 vs. 1 | 2.28 (0.82, 6.32) | 0.28 (0.07, 1.17) |
|
| |||
| 3 vs. 1 | 1.70 (0.60, 4.81) | 0.25 (0.06, 1.00) | |
| C20:0 | 2 vs. 1 | 1.20 (0.51, 2.82) | 0.69 (0.20, 2.37) |
| 3 vs. 1 | 0.95 (0.41, 2.24) | 0.56 (0.15, 2.01) | |
| C22:0 | 2 vs. 1 | 2.20 (0.94, 5.12) | 0.24 (0.07, 0.88) |
|
| |||
| 3 vs. 1 | 1.20 (0.48, 2.98) | 0.23 (0.06, 0.87) | |
| C24:0 | 2 vs. 1 | 2.09 (0.87, 5.00) | 0.24 (0.07, 0.90) |
| 3 vs. 1 | 1.57 (0.63, 3.92) | 0.26 (0.07, 0.93) | |
| C16:1 | 2 vs. 1 | 1.28 (0.55, 3.01) | 0.72 (0.20, 2.58) |
|
| |||
| 3 vs. 1 | 0.83 (0.36, 1.95) | 0.75 (0.21, 2.64) | |
| C18:1 | 2 vs. 1 | 2.22 (0.86, 5.74) | 0.20 (0.05, 0.81) |
| 3 vs. 1 | 1.00 (0.37, 2.73) | 0.41 (0.10, 1.65) | |
| C20:1 | 2 vs. 1 | 1.37 (0.56, 3.36) | 1.25 (0.34, 4.62) |
|
| |||
| 3 vs. 1 | 0.63 (0.24, 1.63) | 1.29 (0.32, 5.17) | |
| C22:1 | 2 vs. 1 | 1.60 (0.67, 3.87) | 0.34 (0.09, 1.30) |
| 3 vs. 1 | 0.81 (0.34, 1.96) | 0.32 (0.09, 1.15) | |
| C24:1 | 2 vs. 1 | 2.34 (0.91, 5.99) | 0.14 (0.04, 0.55) |
|
| |||
| 3 vs. 1 | 1.75 (0.71, 4.32) | 0.26 (0.07, 0.94) | |
Models adjust for baseline age, race, education, body mass index, diabetes, hypertension, triglycerides, cholesterol, and postmenopausal hormone therapy
In additional analyses, we examined the ratio of sphingomyelins to ceramides, but did not find an association with risk of AD in any of the models. We also examined the interaction between the sphingomyelin to ceramide ratio and APOE ε4 carrier status and did not find an association. Lastly, adjusting for other potential confounders, including statins, myocardial infarction, and depressive symptoms, did not change the results.
DISCUSSION
The aim of this study was to determine whether plasma ceramide and sphingomyelin levels were associated with risk of AD, and whether this association differed by sex and APOE genotype. We found that higher ceramide levels were associated with an increased risk of AD among men, but not women. Further, higher sphingomyelin levels were associated with an increased risk of AD among men but a reduced risk of AD among women. Notably, the association between sphingomyelins and the lower risk of AD in women was strongest among APOE ε4 carriers. These results provide further evidence for the role of sphingolipid metabolism in AD and highlight the importance of considering sex and APOE genotype in assessing this relationship.
Studies examining CSF sphingolipids have reported higher levels of ceramides or sphingomyelins among AD patients compared to cognitively normal individuals [39, 40], and cross-sectional associations between either CSF ceramides or sphingomyelins and CSF levels of amyloid or tau [41–43]. However, few studies have examined plasma ceramides and sphingomyelins in relation to risk of AD. One cross-sectional study (mean age of 75 years) reported higher levels of plasma ceramides and lower levels of plasma sphingomyelins in 26 AD patients compared to 26 cognitively normal individuals [17]. Although sex was not considered in the analyses, differences by APOE ε4 genotype were examined. Overall, plasma lipids were similar across APOE genotypes, similar to our findings. However, individuals with an APOE ε4 allele and AD, but not among those who were cognitively normal, had significantly higher levels of sphingomyelin, but not ceramides, compared to subjects without an ε4 allele. Interestingly, an autopsy study also reported disturbed sphingolipid metabolism among AD patients who were APOE ε4 carriers [44]. These demonstrated effects of APOE on the sphingolipid-AD association in humans led us to further explore the modifying effects of APOE in the present study.
Longitudinal studies are needed to determine whether plasma sphingolipids are associated with an increased risk of AD. The first two longitudinal studies examined serum ceramides and sphingomyelins and risk of cognitive impairment and dementia among 100 women (aged 70–79 years, mean age of 74, at baseline) enrolled in the Women's Health and Aging Study (WHAS) II in Baltimore, Maryland [19, 20]. While ceramides were associated with greater risk for AD, there were no associations with sphingomyelins. Limitations of this study included the small sample size, examination of the sphingolipid-dementia relationship only among women, and the lack of information on APOE genotype.
In the proposed study, we sought to further extend this work by examining whether plasma ceramides and sphingolipids were associated with risk of AD among a larger sample of 992 cognitively normal individuals enrolled in the Baltimore Longitudinal Study Aging (BLSA), and to determine the effect of sex and the presence of an APOE ε4 allele on this association. Interestingly, among men we did find that elevated ceramides were associated with a higher risk of AD and that APOE was not an effect modifier. However, among women, we did not find an association with ceramide, but instead with sphingomyelins. Further, the relationship between low sphingomyelins and greater risk of AD was strongest among APOE ε4 carriers
The present findings are not fully consistent with the previous WHAS II results. Interestingly, the findings among men in the present study do replicate the WHAS II results, which was a women only sample. However, the results for women in the BLSA do not replicate the results observed in WHAS II. Reasons for the discrepant ceramide results for women in WHAS II versus the current BLSA study are not readily apparent. One hypothesis for the difference is that there are some important sample characteristics that differentiate women in WHAS II and BLSA. Women enrolled in the BLSA were, on average, a decade younger at the time of the sphingolipid measurement and had fewer medical comorbidities than women in WHAS II. Indeed, most BLSA participants have good access to medical care and have remained relatively healthy over the follow-up interval. There are few BLSA participants with uncontrolled hypertension, hypercholesterolemia, or diabetes. It has been increasingly recognized, from the cellular to human level, that perturbations in ceramide metabolism are associated with longevity [45–48] and the development and progression of many age-related diseases including cancer [49], atherosclerosis [50], insulin resistance and diabetes [51–53], and chronic kidney disease [54]. Thus, ceramides have been both directly associated with AD pathology and indirectly through increased risk of the above ageing-related diseases that also increase the risk of AD [3]. The healthier BLSA sample could have therefore impeded our ability to observe an association among women. While the men enrolled in the BLSA are also relatively healthy compared to the population, they were more frequently smokers, obese, and more likely to have a history of myocardial infarction and diabetes compared to women. Additional epidemiological studies examining the sex differences between plasma ceramides and risk of AD are needed in order to better understand these results.
In the present study, we also found large sex differences in the associations between plasma sphingomyelins and risk of AD. High sphingomyelin levels among men were associated with an increased risk of AD. In contrast, high levels among women were associated with a decreased risk, especially among those with an APOE ε4 allele. Multiple studies, including the BLSA, have reported that women have higher sphingomyelin levels than men (e.g., [22, 55, 56]). A study of longevity markers did find that elevated sphingomyelins were associated with longevity among women, but not men, which does support our results [46]. However, the majority of studies examining sphingomyelins and disease onset and progression have simply adjusted for sex. Additional studies examining the effects of sphingomyelins separately in men and women are needed to better understand the sex differences.
Limitations of the study warrant consideration. The BLSA is a community-dwelling volunteer cohort that is predominantly white, of upper-middle socioeconomic status, and with an above average educational level. Thus, the results may not be generalizable to populations with other characteristics. Further, ceramides are hydrophobic so they are carried on lipoproteins in the blood, with the greatest concentrations in VLDL and LDL [57] and the ceramide transporter (CERT) [58]. Sphingomyelins are carried by all major lipoproteins but the percent carried differs among the lipoprotein subclasses and by APOE genotype [59]. The composition and quantification of the specific acyl chain lengths of ceramides and sphingomyelins on lipoproteins may differ by age and with disease onset. However, we were unable to quantitate all of the lipids by specific lipoproteins and CERT.
In conclusion, our results support previous animal and human studies suggesting that alterations in sphingolipid metabolism are associated with risk of AD or AD-related pathology. However, our results indicate that the relationship between sphingolipids and AD in humans are influenced by multiple factors, including sex and APOE genotype. Thus, future research examining sphingolipids and risk of AD in large cohorts should consider these factors – not just by simply adjusting for them, but by examining them as effect modifiers. Additional research also is needed to determine whether the timing of the sphingolipid measure (mid-life versus late-life) poses a different risk for AD and whether plasma sphingolipids will be useful prognostic biomarkers.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health/National Institute on Aging (U01 AG37526 and R01 AG49704) and by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Dr Mielke served as a consultant to AbbVie and Lysosomal Therapeutics, Inc, and receives research support from the National Institute on Aging, National Institutes of Health, the Michael J Fox Foundation, and Biogen.
Footnotes
Conflict of Interest Disclosures:All other authors have no conflicts of interest to declare.
REFERENCES
- [1].Mielke MM, Haughey NJ. Could plasma sphingolipids be diagnostic or prognostic biomarkers for Alzheimer's disease? Clin Lipidol. 2012;7:525–536. doi: 10.2217/clp.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mencarelli C, Martinez-Martinez P. Ceramide function in the brain: When a slight tilt is enough. Cell Mol Life Sci. 2013;70:181–203. doi: 10.1007/s00018-012-1038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mielke MM, Lyketsos CG. Alterations of the sphingolipid pathway in Alzheimer's disease: New biomarkers and treatment targets? Neuromolecular Med. 2010;12:331–340. doi: 10.1007/s12017-010-8121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- [6].Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- [8].Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- [9].Mencarelli C, Bode GH, Losen M, Kulharia M, Molenaar PC, Veerhuis R, Steinbusch HW, De Baets MH, Nicolaes GA, Martinez-Martinez P. Goodpasture antigen-binding protein/ceramide transporter binds to human serum amyloid P-component and is present in brain amyloid plaques. J Biol Chem. 2012;287:14897–14911. doi: 10.1074/jbc.M111.299545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hur JY, Welander H, Behbahani H, Aoki M, Franberg J, Winblad B, Frykman S, Tjernberg LO. Active gamma-secretase is localized to detergent-resistant membranes in human brain. FEBS J. 2008;275:1174–1187. doi: 10.1111/j.1742-4658.2008.06278.x. [DOI] [PubMed] [Google Scholar]
- [11].Bode GH, Losen M, Buurman WA, Veerhuis R, Molenaar PC, Steinbusch HW, De Baets MH, Daha MR, Martinez-Martinez P. Complement activation by ceramide transporter proteins. J Immunol. 2014;192:1154–1161. doi: 10.4049/jimmunol.1301673. [DOI] [PubMed] [Google Scholar]
- [12].Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- [13].Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- [14].Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, Ogretmen B. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23:751–763. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem. 1995;65:2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- [16].Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer's disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience. 1994;61:765–772. doi: 10.1016/0306-4522(94)90400-6. [DOI] [PubMed] [Google Scholar]
- [17].Han X, Rozen S, Boyle SH, Hellegers C, Cheng H, Burke JR, Welsh-Bohmer KA, Doraiswamy PM, Kaddurah-Daouk R. Metabolomics in early Alzheimer's disease: Identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS One. 2011;6:e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chatterjee P, Lim WL, Shui G, Gupta VB, James I, Fagan AM, Xiong C, Sohrabi HR, Taddei K, Brown BM, Benzinger T, Masters C, Snowden SG, Wenk MR, Bateman RJ, Morris JC, Martins RN. Plasma phospholipid and sphingolipid alterations in presenilin1 mutation carriers: A pilot study. J Alzheimers Dis. 2015;50:887–894. doi: 10.3233/JAD-150948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31:17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mielke MM, Bandaru VVR, Xia J, Haughey NJ, Fried LP, Yasar S, Albert M, Varma V, Harris G, Schneider EG, Rabins PV, Bandeen-Roche K, Lyketsos CG, Carlson MC. Serum ceramides increase the risk of Alzheimer disease: The Women's Health and Aging Study II. Neurology. 2012;79:633–641. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell. 2015;14:1014–1023. doi: 10.1111/acel.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mielke MM, Bandaru VV, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ. Factors affecting longitudinal trajectories of plasma sphingomyelins: The Baltimore Longitudinal Study of Aging. Aging Cell. 2015;14:112–121. doi: 10.1111/acel.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fleisher A, Grundman M, Jack CR, Jr., Petersen RC, Taylor C, Kim HT, Schiller DH, Bagwell V, Sencakova D, Weiner MF, DeCarli C, DeKosky ST, van Dyck CH, Thal LJ. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- [25].Liu Y, Paajanen T, Westman E, Wahlund LO, Simmons A, Tunnard C, Sobow T, Proitsi P, Powell J, Mecocci P, Tsolaki M, Vellas B, Muehlboeck S, Evans A, Spenger C, Lovestone S, Soininen H. Effect of APOE epsilon4 allele on cortical thicknesses and volumes: The AddNeuroMed study. J Alzheimers Dis. 2010;21:947–966. doi: 10.3233/JAD-2010-100201. [DOI] [PubMed] [Google Scholar]
- [26].Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD. Gender modulates the APOE epsilon4 effect in healthy older adults: Convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: Modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24–28. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- [28].Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O'Brien R, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33:720–731.e724. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Jr, Lakatta EG, Tobin JD. Normal Human Aging: The Baltimore Longitudinal Study of Aging. U.S. Government Printing Office; Washington, DC: 1984. [Google Scholar]
- [30].American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. 1987. [Google Scholar]
- [31].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [32].Bandaru VV, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S, Chang L, Wojna V, Pardo C, Calabresi P, Munsaka S, Haughey NJ. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81:1492–1499. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- [34].Bandaru VV, Patel N, Ewaleifoh O, Haughey NJ. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV-gp120. J Neuroimmune Pharmacol. 2011;6:640–649. doi: 10.1007/s11481-011-9289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, Haughey NJ. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- [37].Koch W, Ehrenhaft A, Griesser K, Pfeufer A, Muller J, Schomig A, Kastrati A. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123–1131. doi: 10.1515/CCLM.2002.197. [DOI] [PubMed] [Google Scholar]
- [38].Cox DR. Regression models and life-tables (with discussion) J Royal Stat Soc (B) 1972;34:187–202. [Google Scholar]
- [39].Kosicek M, Kirsch S, Bene R, Trkanjec Z, Titlic M, Bindila L, Peter-Katalinic J, Hecimovic S. Nano-HPLC-MS analysis of phospholipids in cerebrospinal fluid of Alzheimer's disease patients--a pilot study. Anal Bioanal Chem. 2010;398:2929–2937. doi: 10.1007/s00216-010-4273-8. [DOI] [PubMed] [Google Scholar]
- [40].Kosicek M, Zetterberg H, Andreasen N, Peter-Katalinic J, Hecimovic S. Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer's disease. Neurosci Lett. 2012;516:302–305. doi: 10.1016/j.neulet.2012.04.019. [DOI] [PubMed] [Google Scholar]
- [41].Mielke MM, Haughey NJ, Bandaru VV, Zetterberg H, Blennow K, Andreasson U, Johnson SC, Gleason CE, Blazel HM, Puglielli L, Sager MA, Asthana S, Carlsson CM. Cerebrospinal fluid sphingolipids, beta-amyloid, and tau in adults at risk for Alzheimer's disease. Neurobiol Aging. 2014;35:2486–2494. doi: 10.1016/j.neurobiolaging.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Koal T, Klavins K, Seppi D, Kemmler G, Humpel C. Sphingomyelin SM(d18:1/18:0) is significantly enhanced in cerebrospinal fluid samples dichotomized by pathological amyloid-beta42, tau, and phospho-tau-181 levels. J Alzheimers Dis. 2015;44:1193–1201. doi: 10.3233/JAD-142319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fonteh AN, Ormseth C, Chiang J, Cipolla M, Arakaki X, Harrington MG. Sphingolipid metabolism correlates with cerebrospinal fluid Beta amyloid levels in Alzheimer's disease. PloS One. 2015;10:e0125597. doi: 10.1371/journal.pone.0125597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bandaru VV, Troncoso J, Wheeler D, Pletnikova O, Wang J, Conant K, Haughey NJ. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer's but not normal brain. Neurobiol Aging. 2009;30:591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yu Z, Zhai G, Singmann P, He Y, Xu T, Prehn C, Romisch-Margl W, Lattka E, Gieger C, Soranzo N, Heinrich J, Standl M, Thiering E, Mittelstrass K, Wichmann HE, Peters A, Suhre K, Li Y, Adamski J, Spector TD, Illig T, Wang-Sattler R. Human serum metabolic profiles are age dependent. Aging Cell. 2012;11:960–967. doi: 10.1111/j.1474-9726.2012.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gonzalez-Covarrubias V, Beekman M, Uh HW, Dane A, Troost J, Paliukhovich I, van der Kloet FM, Houwing-Duistermaat J, Vreeken RJ, Hankemeier T, Slagboom EP. Lipidomics of familial longevity. Aging Cell. 2013;12:426–434. doi: 10.1111/acel.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Huang X, Withers BR, Dickson RC. Sphingolipids and lifespan regulation. Biochim Biophys Acta. 2014;1841:657–664. doi: 10.1016/j.bbalip.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cutler RG, Thompson KW, Camandola S, Mack KT, Mattson MP. Sphingolipid metabolism regulates development and lifespan in Caenorhabditis elegans. Mech Ageing Dev. 2014;143–144:9–18. doi: 10.1016/j.mad.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Alberg AJ, Armeson K, Pierce JS, Bielawski J, Bielawska A, Visvanathan K, Hill EG, Ogretmen B. Plasma sphingolipids and lung cancer: a population-based, nested case-control study. Cancer Epidemiol Biomarkers Prev. 2013;22:1374–1382. doi: 10.1158/1055-9965.EPI-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, Maruyama T, Miwa Y, Harada-Shiba M, Tsushima M, Kojo S. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- [51].Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65:S39–46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- [52].Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: New insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62:401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mitsnefes M, Scherer PE, Friedman LA, Gordillo R, Furth S, Warady BA. Ceramides and cardiac function in children with chronic kidney disease. Pediatr Nephrol. 2014;29:415–422. doi: 10.1007/s00467-013-2642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, Illig T. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ishikawa M, Maekawa K, Saito K, Senoo Y, Urata M, Murayama M, Tajima Y, Kumagai Y, Saito Y. Plasma and serum lipidomics of healthy white adults shows characteristic profiles by subjects' gender and age. PLoS One. 2014;9:e91806. doi: 10.1371/journal.pone.0091806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J, Bielawska A. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. J Lipid Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mencarelli C, Losen M, Hammels C, De Vry J, Hesselink MK, Steinbusch HW, De Baets MH, Martinez-Martinez P. The ceramide transporter and the Goodpasture antigen binding protein: One protein--one function? J Neurochem. 2010;113:1369–1386. doi: 10.1111/j.1471-4159.2010.06673.x. [DOI] [PubMed] [Google Scholar]
- [59].Jeong T, Schissel SL, Tabas I, Pownall HJ, Tall AR, Jiang X. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J Clin Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]


