Abstract
Purpose
Triple negative breast cancer (TNBC) represents a cancer stem cell enriched phenotype. Hypoxia-inducible factor-1 alpha (HIF-1α) induces the expression of proteins associated with stemness and is highly upregulated in TNBC. We questioned whether HIF-1α was immunogenic and whether vaccination targeting HIF-1α would impact the growth of basal-like mammary tumors in transgenic mice.
Experimental Design
We evaluated HIF-1α-specific IgG in sera from controls and patients with breast cancer. Class II epitopes derived from the HIF-1α protein sequence were validated by ELISPOT. To assess therapeutic efficacy, we immunized Tg-MMTVneu and C3(1)Tag mice with HIF-1α Th1-inducing peptides. Stem cells were isolated via magnetic bead separation. Levels of HIF-1α and stem cells in the tumor were quantitated by Western blotting and flow cytometry.
Results
The magnitude (p<0.001) and incidence (p<0.001) of HIF-1α-specific IgG was elevated in TNBC patients compared to controls. Both breast cancer patients and donors showed evidence of HIF-1α-specific T-helper (Th) 1 and Th2 immunity. Three HIF-1α-specific Th1 class II restricted epitopes that were highly homologous between species elicited Type I immunity in mice. After HIF-1α vaccination, mammary tumor growth was significantly inhibited in only C3(1)Tag (basal-like/stem cellhigh) (p<0.001) not TgMMTV-neu (luminal/neu/stem cell low) (p=0.859) murine models. Vaccination increased Type I T-cells in the tumor (p=0.001) and decreased cells expressing the stem cell marker, Sca-1, compared to controls (p=0.004).
Conclusions
A HIF-1α vaccine may be uniquely effective in limiting tumor growth in TNBC. Inhibiting outgrowth of breast cancer stem cells via active immunization in the adjuvant setting may impact disease recurrence.
Keywords: vaccine, HIF-1α, breast cancer, T-cells, stem cells, triple negative, HER2
INTRODUCTION
Triple negative breast cancers (TNBC) are more aggressive than other breast cancer subtypes and are associated with higher relapse rates, increased risk of distant recurrence, and a limited overall survival once the cancer has recurred (1). These unique disease characteristics have led to numerous investigations focused on defining biologic targets that may be therapeutically exploited in the adjuvant setting. Prevention of disease relapse is a major clinical goal in the treatment of TNBC.
TNBC is also the most immunogenic of the breast cancer subtypes. Recent analyses from randomized clinical trials have demonstrated that high levels of tumor infiltrating lymphocytes (TIL) were an independent predictor of favorable disease free and overall survival (2). Indeed, for every 10% increase in stromal TIL, there was a 19% reduction in the risk of death. Furthermore, tumor infiltrating Type I T-cells, specifically CD8+ T-cells, are the lymphocytic subtype most often associated with a survival benefit in TNBC (3, 4). High levels of tumor infiltrating CD8+ T-cells have also been shown to be associated with improved responses to chemotherapy in both the neoadjuvant and adjuvant setting (5, 6). Unfortunately, clinically relevant levels of TIL, especially Type I TIL, are present in only a minority of TNBC (7).
Clinical strategies aimed at inducing immunity in a majority of TNBC patients may have significant impact on the course of disease. Vaccines directed at immunogenic proteins expressed by these cancers could drive antigen-specific T-cells to the tumor. If Type I T-cells could be generated, the immune response could synergize with chemotherapy or immunotherapy to eliminate micrometastatic disease. Clinical evaluation of vaccines for TNBC has been limited by the lack of well-defined antigens present in the majority of such tumors.
Hypoxic pathways are operative in driving the malignant phenotype in TNBC and upregulation and activation of HIF-1α has been associated with aggressive disease, chemotherapy resistance, and maintenance of breast cancer stem cells (8). In a study of 383 TNBC patients, high expression of the protein in tumors was associated with a significantly shorter relapse free survival than those patients whose tumors expressed lower levels of HIF-1α (p=0.009) (9). As abnormal overexpression of a self-protein can enhance immunogenicity (10), we questioned whether HIF-1α was immunogenic in patients with TNBC and whether active immunization could impact tumor growth in a basal model of mammary cancer.
MATERIALS AND METHODS
Human subjects
Sample collection was approved by the University of Washington (UW) Human Subjects Division. Control sera from 92 female volunteer blood donors was collected at the Puget Sound Blood Center, Seattle, WA (median age: 51, range 33-73 years). Controls met all criteria for blood donation. Serum samples from 95 breast cancer patients (42 HER2+, 33 ER+, and 20 TNBC; median age 52, range 33-89 years) were obtained from individuals who consented to participate in the Fred Hutchinson Cancer Research Center/UW Breast Specimen Repository and Registry (IR file #5306). All patients were either stage I or II and blood was collected at the time of diagnosis, prior to definitive surgical resection. Peripheral blood mononuclear cells (PBMC) from 19 female volunteer controls (median age: 49, range 18-79 years) were collected and cryopreserved as previously described (11).
Analysis of antibody immunity
IgG specific for HIF-1α was assessed by indirect ELISA as previously described with the following modifications; recombinant HIF-1α (500 ng/ml) (Abnova) was used as the coating antigen and the sera was diluted 1:200 in 1% human serum albumin/PBS buffer (12). The ΔOD was calculated as the OD of the protein-coated wells minus the OD of the buffer-coated wells. The data are expressed as µg/ml HIF-1α-specific IgG. The mean and 2 standard deviations of the volunteer control population response, 0.49 µg/ml, defined the level above which a sample was considered positive.
To validate the assay, a subset of ELISA positive and negative samples was confirmed by protein A/G agarose immunoprecipitation of rhHIF-1α protein (Abnova) with patient sera. The sera were first incubated with agarose control beads (Thermo Scientific) overnight at 4°C to decrease non-specific binding. After 24 hours, the columns were spun and flow-through was incubated with a 50 µL slurry of protein A/G agarose beads (Thermo Scientific) for 1h at 4°C. The columns were spun, flow-through removed, and the beads were washed three times with 500 µL 5% BSA/1X PBS blocking buffer. Prior to the final spin, the beads were left to incubate with the blocking buffer for 1h at 4°C. After removing the final wash, 10 ng of rHIF-1α (Abnova) was added to each column along with 200 µL 1X PBS and the mixture was incubated on a rotator at 4°C overnight. After 24 hours, the flow-through was removed, and the columns were washed three times with 500 µL 5% BSA/1X PBS. The captured protein was eluted using 20 µL 0.1 M glycine (pH 2.5), neutralized with 2 µL 0.1 M Tris (pH 9.5). The eluent was analyzed by immunoblot, probing with an anti-HIF-1α rabbit polyclonal primary antibody (2 µg/mL; Genetex), followed by a TrueBlot goat anti-rabbit HRP secondary (0.1 µg/mL; Rockland). Results demonstrated the HIF-1α-specific ELISA to have a sensitivity of 71% and specificity of 100%.
Analysis of peptide and protein-specific T-cell responses
Eleven HIF-1α peptides, (26% of the protein; Supplementary Table 1), predicted to promiscuously bind human MHCII, were selected using web based algorithms as previously described (13). The peptides were constructed and purified by high-performance liquid chromatography (>90% pure; JPT Peptide Technologies, GmbH). Human PBMC were evaluated by ELISPOT for antigen-specific IFN-gamma (γ) or IL-10 production as previously described (14) using 10 µg/ml HIF-1α peptides. Mouse splenic cells were evaluated by ELISPOT for antigen-specific IFN-γ secretion as published, except for the following modifications; splenic cells were incubated with antigens for 72hr and spots were developed with the AEC substrate kit (BD Biosciences) (13). Antigen-specific IL-4 was determined for murine T-cells using the ELISPOTPLUS for Mouse IL-4 kit (Mabtech) according to the manufacturer’s instructions after a 48hr incubation with antigen. Positive responses were defined by a statistically significant difference (p<0.05) between the mean number of spots from five replicates in the experimental wells and the mean number from no antigen control wells. T-cell lines were generated as previously described from volunteer controls demonstrating significant responses to the selected epitopes (12). Data are reported as the mean number of spots for each experimental antigen minus the mean number of spots detected in no antigen control wells ± SEM or SD (corrected spots per well: CSPW per 2×105 PBMC or 4.5×105 splenic cells).
Human antigen-specific T-cell lines were generated using published methods (15). ELISPOT for antigen-specific IFN-γ secretion was performed as described above using 10 µg/ml HIF-1α peptides or 1µg/ml human recombinant HIF-1α protein (Abnova) or low endotoxin human serum albumin (HSA) (Gemini Bio-products).
Animal models and syngeneic tumor cell lines
Animal care and use were in accordance with institutional guidelines. Female, FVB/N-TgN (MMTVneu)-202Mul mice (TgMMTV-neu) (7 weeks old; median weight: 18 g, range: 15.4-18.5 g; Jackson Laboratory) or FVB-Tg(C3-1-TAg)cJeg/Jeg (C3(1)-Tag) mice (6 weeks old; median weight: 18.5 g, range: 16.5-18.5 g; provided by Dr. Jeff Green, NCI) were used in this study. Tumors derived from the C3(1)Tag mouse exhibit a basal phenotype and those derived from the TgMMTV-neu mouse exhibit a luminal/neu phenotype (16). The mouse mammary tumor cell lines MMC and M6 were derived from spontaneous mammary tumors from TgMMTV-neu (17) and C3(1)-Tag (18) mice, respectively. Both cells lines were authenticated before use. The MMC cell line was verified to express rat neu by flow cytometry and the M6 cell line was verified to express the SV40 antigen by Western blot and the estrogen receptor by RT-PCR.
Vaccination, assessment of tumor growth and toxicity
Mice were immunized subcutaneously using a 26 ½ G needle. Each mouse was injected with 50 µl of a HIF-1α peptide pool (p38-53, p60-82, and p93-117; 50 μg each) as a mixture in complete Freund’s adjuvant/incomplete Freund’s adjuvant (Sigma). To generate an effective immune response to an overexpressed self-antigen, three immunizations were given two weeks apart (14). For tumor challenge, the corresponding syngeneic mouse mammary tumor cell line (0.5 × 106 cells) was implanted into the mammary fat pad two weeks after the last vaccine (n=10/group) (14). Tumors were measured as previously described (13). All tumor growth is presented as mean tumor volume (mm3 ± SEM). Data are representative of three independent experiments.
At study termination, brain, kidney, liver, lung and heart were collected in formalin. Sections were stained with hematoxylin and eosin and toxicities were determined by a certified veterinary pathologist. Heparinized and non-heparinized blood was analyzed for complete blood count and serum chemistries, respectively, by Phoenix Central Laboratories, Everett, WA.
in vivo T-cell depletion
Cell depletions were performed as previously described (19). Briefly, mice were vaccinated with HIF-1α peptides as described above. M6 cells were implanted two weeks after the last vaccine. Monoclonal antibodies were used for in vivo depletion (250 μg of anti-CD4; clone GK1.5 and 100 μg of anti-CD8; clone 2.43, UCSF Monoclonal Antibody Core) via intraperitoneal injection of the specific antibody three consecutive days before implant and twice per week until the experiment was terminated. Rat IgG2b was used as a control. Data are shown as mean ± SEM of 5 mice/group.
Flow cytometry and immunohistochemistry
Stem cell antigen-1 (Sca-1) expression was documented in the dissociated tumor or tumor cell lines by incubating with anti-mouse Sca-1-FITC (clone D7; 0.1 µg/100µl; Miltenyi Biotec). Flow cytometry was performed on the FACSCanto (BD Biosciences) and data analyzed using FlowJo X software (BD Biosciences). Typically, 100,000 cells were collected per sample. Results are reported as a percentage of total cell number.
Immunohistochemistry was performed as previously described (19). Briefly, the fixed sections cut from frozen blocks were blocked with 10% goat serum (Vector Labs) 1h at room temperature then incubated overnight with anti-mouse CD4 (clone 1F6; 1:100; Abcam) or CD8 (clone KT15; 1:100; AbD Serotec). After extensive washing, the slides were incubated with Alexa Fluor 488 goat anti-rat (Abcam; 1:500) for 1h at room temperature. Cover slips were mounted with Prolong Gold antifade with DAPI (Life technologies). Positive cells and DAPI stained nuclei were counted in three random high powered fields per slide and expressed as a mean.
Protein and gene expression in M6 tumor cell subsets
Sca-1positive M6 cells were separated from Sca-1negtive cells using the Anti-Sca-1 MicroBead Kit (FITC) according to the manufacturer’s instructions (Miltenyi Biotec) with one exception; the Sca-1negtive cells were applied to a total of three consecutive columns to more effectively purify the population. The median percentage of Sca-1 FITC-staining cells was 78% (range 49-92%) in the positive population and 16% (range 2-22%) in the negative population. The cell lysates derived from each population were separated by SDS/PAGE (20) and the Sca-1positive population was confirmed to express HIF-1α, and other markers of CSC and epithelial-mesenchymal transition (EMT), including increased levels of the cell adhesion molecules P-cadherin, N-cadherin and Vimentin (21-23) and transcription factors SNAIL 1/2 and SIX-1 (24, 25) (p<0.05 for all; Supplemental Fig. 1). Antibodies used were rabbit anti-mouse HIF-1α (2 µg/mL; Genetex), rabbit anti-mouse P-Cadherin (1 µg/mL; Genetex), rabbit anti-mouse N-Cadherin (5 µg/mL; Genetex) goat anti-mouse Vimentin (1 µg/ml; Santa Cruz Biotech), rabbit anti-mouse Snail1/Snail2 (2 µg/mL; abcam), rabbit anti-mouse Six1 (0.5 µg/mL; Abnova), rabbit anti-mouse α/β Tubulin (diluted 1:1000; Cell Signaling Technology) and HRP-conjugated goat anti-rabbit and rabbit anti-goat (diluted 1:10,000; Invitrogen). Expression levels were quantitated by densitometry using NIH Image Processing and Analysis in Java (ImageJ) software. We verified the tumorigenicity of each population; when as few as 2×103 Sca-1-expressing cells were implanted in the mouse 100% of the implants were tumorigenic compared to only 25% of the implanted cells lacking Sca-1 expression (Supplementary Table 2).
Estrogen receptor-alpha (ER-α) RNA was isolated using the RNAqueous-4PCR (Life Technologies) kit according to manufacturer’s instructions. RNA quantity was determined with a NanoDrop Spectrophotometer. cDNA was synthesized from 100 pg of RNA using the SuperScript III RT (Life Technologies) kit according to the manufacturer’s instructions then quantified. ER expression was assessed via TaqMan (ABI 7900HT) Real time PCR using 50ng of cDNA and 1pg of ER TaqMan Gene Expression Array (Life Technologies).
Statistical analysis
The unpaired, two-tailed Student’s t-test was used to evaluate difference between two groups. Fisher’s exact test was used to evaluate differences between proportions. To compare more than three groups, a One-way ANOVA with Tukey’s Post-Hoc test was used when there was one variable and a Two-way ANOVA with Bonferoni’s post-test was used when there were two variables. p<0.05 was considered significant. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software).
RESULTS
HIF-1α-specific antibodies can be detected in the sera of breast cancer patients with the highest incidence and levels in those with the triple negative subtype
HIF-1α-specific IgG antibodies were detected in significantly more HER2+ (17 ± 2.3%; p=0.049), ER+ (30 ± 2.8%; p<0.001) and TNBC patient sera (55 ± 3.1%; p<0.001) than volunteer control sera (5 ± 1.3%) (Fig.1A). Furthermore, a higher incidence of HIF-1α-specific IgG was observed in TNBC patients compared to patients with the other breast cancer subtypes (p<0.001). The highest levels of HIF-1α-specific antibodies were also detected in sera from TNBC patients (mean, 1.5 µg/ml; range, 0-6.1 µg/ml) as compared to volunteer controls (mean, 0.049 µg/ml; range 0-1.35 µg/ml), HER2+ (mean, 0.40 µg/ml; range 0-3.86 µg/ml), or ER+ breast cancer sera (mean, 0.286 µg/ml; range 0-1.24 µg/ml; p<0.001 for all) (Fig. 1B).
Figure 1.
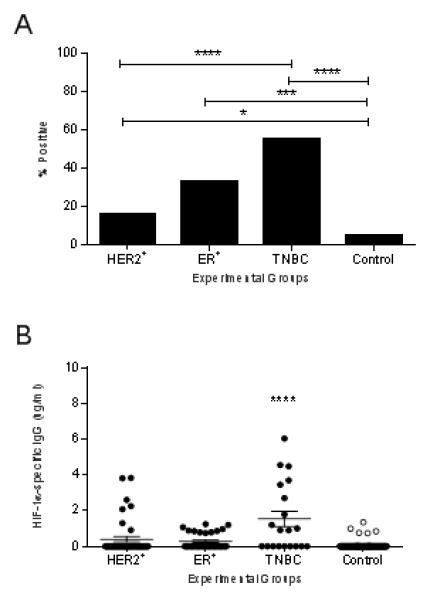
HIF-1α-specific antibodies can be detected in the sera of breast cancer patients with the highest incidence and levels in those with the triple negative subtype. (A) Incidence of positive HIF-1α-specific antibodies in each group. *p<0.05, **p<0.01, ***p<0.001 compared to the control group. (B) HIF-1α-specific IgG (μg/ml ± SEM) for the experimental groups. ***p<0.001 between groups. TNBC: triple negative breast cancer; ER+: estrogen receptor positive breast cancer; HER2+: human epidermal growth factor receptor 2 positive breast cancer.
Class II restricted Th1 epitopes derived from the HIF-1α protein sequence can be identified
Self-tumor antigens contain both Th1- and Th2-inducing epitopes and removal of the Th2-inducing portions of the antigen results in more effective anti-tumor immunity, dominated by Type I T-cells (14). We evaluated eleven putative class II epitopes, derived from the HIF-1α protein sequence, for both Th1 and Th2 immune responses. All peptides induced significant IFN-γ and IL-10 secretion in donor PBMC. Significantly more donors responded to the peptides by secreting IFN-γ (median 78%, range 39-83%) (Fig. 2A) than IL-10 (median, 33%; range 6-83%; p<0.001) (Fig. 2B), but there was no difference observed in the magnitude of the response between Th phenotype (p=0.886; Fig. 2 C, D). Epitopes were chosen for the vaccine based on a predominant Th1-inducing response as calculated by the following Th1/Th2 ratio: IFN-γ incidence X magnitude/IL-10 incidence X magnitude. Eighty-two percent of the peptides demonstrated a preference to induce secretion of IFN-γ, 9% demonstrated no preference and 9% induced IL-10 secretion (Supplementary Table 1). HIF-1α-p38,-p60, and -p93 were chosen for inclusion in the vaccine since these peptides demonstrated high ratios for IFN-γ preference, i.e. Th1 selective, and were positioned in the same region of the protein, mitigating potential differences associated from domain location.
Figure 2.
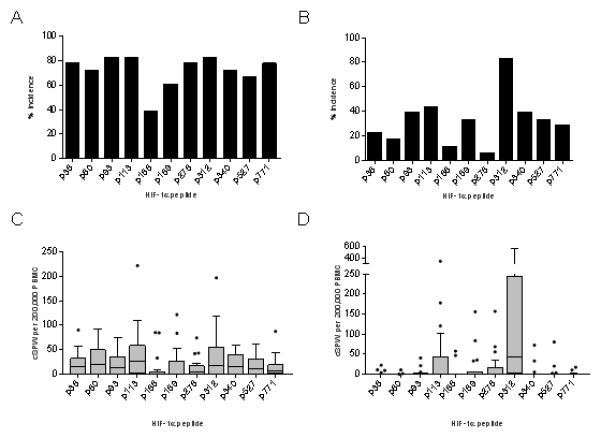
Th1-inducing epitopes derived from the HIF-1α protein sequence can be identified. Mean percent responding donors to HIF-1α epitopes with IFN-γ (A) or IL-10 (B). CSPW for the indicated epitope presented as interquartile box plots with Tukey whiskers for IFN-γ (C) and IL-10 (D). Median CSPW is indicated by the horizontal bar.
We confirmed whether responding sequences were native epitopes of HIF-1α by generating T-cell lines using the peptides described above. The T-cell lines generated (mean, 99.1%; range, 98-100% CD3+ cells) were predominantly CD8+ (mean, 70.5%; range, 50-99%), with fewer CD4+ (mean, 28.6%; range, 6-69%) cells. HIF-1α-p38 T-cells were both HIF-1α peptide- (mean, 15.6 SPW; range, 7-25 SPW; p=0.007 compared to HIVp52) and HIF-1α protein-specific (mean, 132 SPW; range, 91-158 SPW; p=0.032 compared HSA). The T-cells generated from HIF-1α-p60 responded to both peptide (mean, 129.3 SPW; range, 96-148 SPW; p<0.001) and recombinant protein (mean, 110 SPW range 92-140; p<0.001). HIF-1α-p93 T-cells were also both HIF-1α peptide- (mean, 172.6 SPW; range, 149-197; p<0.001) and HIF-1α protein-specific (mean, 61 SPW; range, 30-87 SPW; p=0.018; Supplementary Fig. 2).
HIF-1α poly-epitope vaccines are significantly more effective in inhibiting growth of basal than luminal mammary tumors
The HIF-1α epitopes chosen for the vaccine were highly homologous (91-100%) between human and mouse. The HIF-1α poly-epitope vaccine selectively elicits a Th1 rather than Th2 immune response. Indeed, we detected significantly more IFN-γ-secreting cells when the splenocytes were stimulated with the vaccinating peptides (mean, 117 CSPW; range, 64-152 CSPW; p<0.0001) as compared to the HIVp52 control (Fig. 3A). Conversely, while there was a significant difference observed in HIF-1α-specific IL-4-secreting Th2 compared to HIVp52 induced by ConA (mean 212 CSPW, range 161-238 CSPW; p<0.0001), there was no difference observed when cells were stimulated with the HIF-1α peptide pool (mean 17 CSPW; range 12-20 CSPW; p=0.921) (Fig. 3A).
Figure 3.
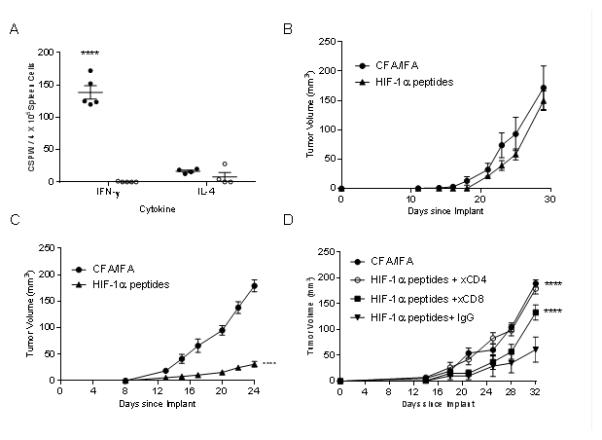
HIF-1α poly-epitope vaccines are significantly more effective in inhibiting growth of basal than luminal mammary tumors. (A) IFN-γ and IL-4 ELISPOT in splenocytes after completion of three immunizations. Antigens include a pool of the HIF-1α vaccinating peptides (●), HIV p52 (○) as a negative control and ConA (▲) as a positive control. The data are presented as corrected spots per well (CSPW). The horizontal bar indicates the mean CSPW ± SEM. n=5 mice/group; ***p<0.001 compared to HIV. Mean tumor volume (mm3 ± SEM) from mice injected with adjuvant alone (●) or HIF-1α poly-epitope vaccine (▲) in TgMMTV-neu mice (B) or C3(1)Tag mice (C), n=5 mice/group; ****p<0.0001. (D) Mean tumor volume (mm3 ± SEM) from C3(1)Tag mice injected with adjuvant alone (●), or HIF-1α poly-epitope vaccine treated with mouse IgG (●), anti-CD4 (○) or anti-CD8 (■) n=5 mice/group; ****p<0.0001 compared to HIF-1α poly-epitope vaccine+IgG.
HIF-1α vaccination was more effective in the C3(1)Tag than the TgMMTV-neu mice in limiting tumor growth. The mean tumor volume of HIF-1α-vaccinated TgMMTV-neu mice (155.8 ± 37 mm3) was equivalent to the control tumor (149.4 ± 17.9 mm3; p=0.875) (Fig. 3B). Tumor growth was inhibited by 83% in HIF-1α-vaccinated C3(1)Tag mice. The mean tumor volume of HIF-1α-vaccinated C3(1)Tag mice (31.2 ± 5.1 mm3) was significantly less than that observed in the adjuvant only control (178.9 ± 11.3 mm3; p<0.001; Fig. 3C).
Tumor inhibition was mediated by both CD4+ and CD8+ T-cells (Fig. 3D). Depletion of either CD4+ or CD8+ T-cells resulted in a significant loss of the tumor inhibitory effect of the HIF-1α poly-epitope vaccine (mean volume, 179±10.5 mm3 or 132±14.7 mm3, respectively; p<0.0001 for both) compared to vaccine with control IgG (mean volume, 61±24.2 mm3).
Basal-like breast cancer contains greater levels of cancer stem cells than luminal/neu breast cancer
Given that vaccination could inhibit tumor growth only in the C3(1)Tag model, we compared HIF-1α expression between both tumor lines. M6 cells express significantly more HIF-1α than MMC (p=0.032; Fig 4A). HIF-1α has been shown to modulate cancer stem cells (26). Sca-1 is a marker for murine cancer stem cells (27). Sca-1 is present on the majority of M6 cells (61.8±5.6%) as compared to MMC (1.1±0.45%, p<0.001; Fig. 4B). We also observed a greater expression of HIF-1α in the Sca-1positive cells compared to the Sca-1negative cells (p=0.003; Fig. 4C). Since it was recently shown that both early recurrence and more aggressive disease is associated with greater numbers of cancer stem cells in ER+/HER2− breast cancer (28), we examined the ER expression in the M6 subsets. There is a 79% increase in ER gene expression in the Sca-1positive cells compared to the Sca-1negative cells (p=0.009; Supplementary Fig. 3).
Figure 4.
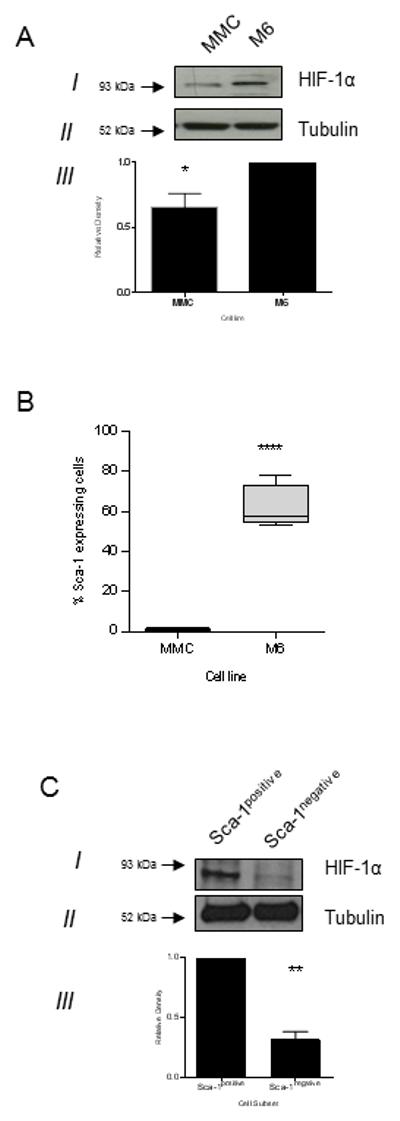
Basal-like breast cancer contains greater levels of cancer stem cells than luminal/neu breast cancer. (A) Representative Western blot of cell lysate for HIF-1α (I) and Tubulin (II). Relative pixel density ± SD as measured by densitometry from 3 independent experiments (III), **p<0.05. (B) Percent of Sca-1 expressing cells in the indicated cell line. ****p<0.0001; n=4 independent experiments. (C) Representative Western blot of Sca-1positive and Sca-1negative cell lysate for HIF-1α (I) and Tubulin (II). Relative pixel density ± SD as measured by densitometry from 3 independent experiments (III), **p<0.01.
HIF-1α immunization results in a significant reduction of cancer stem cells in mammary tumors
Immunization against HIF-1α significantly induced the influx of CD8+ T-cells into the tumor by 20-fold compared to control (p=0.001), whereas there was no difference observed in CD4+T-cell levels (Fig 5A). Moreover, targeting HIF-1α by vaccination reduced both HIF-1α protein expression and Sca-1positive cells in the tumor. We detected a 34% decrease in tumor HIF-1α protein (p=0.018; Fig 5B) as well as a 34% reduction in Sca-1 expression following HIF-1α-specific vaccination in C3(1)-Tag mice as compared to controls (p=0.044; Fig. 5C).
Figure 5.
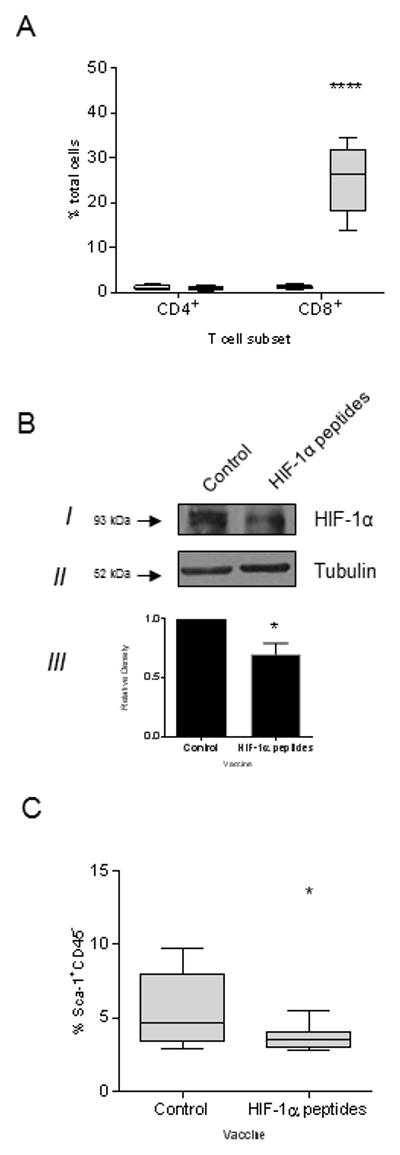
HIF-1α immunization results in a significant reduction of cancer stem cells in mammary tumors. (A) Percent CD4+ or CD8+ T-cells in the tumor from mice immunized with adjuvant alone (white bar) or HIF-1α peptides (gray bar) presented as box and whisker plot with Tukey outliers, n=5 mice/group; ****p<0.0001. (B) Representative Western blot of tumor cell lysate from mice immunized with adjuvant alone (control) or HIF-1α peptides for HIF-1α (I) and Tubulin (II). Relative pixel density ± SD as measured by densitometry from 3 independent experiments (III), *p<0.05. (C) Percent of Sca-1 expressing cells in tumors from mice immunized with adjuvant alone (control) or HIF-1α peptides. *p<0.05; n=9 mice/group.
There was no evidence of vaccine induced toxicity. All serum chemistry and complete blood count values between groups were not significantly different (p>0.05 for all values; Supplementary Tables 3 and 4). There were no treatment related lesions, including evidence of autoimmunity, consistent with a toxic response that distinguished one group from another (Supplementary Fig. 4).
DISCUSSION
HIF-1α protein is overexpressed in more than 80% of triple negative breast cancers thus has the potential to be a near universal immunologic target in this breast cancer subtype (9). Marked overexpression of self-tumor antigens has been shown to be a dominant mechanism by which immunity is enhanced (10). Data presented here demonstrates that HIF-1α is immunogenic in patients with breast cancer, especially TNBC. Immunization with a HIF-1α epitope-based vaccine, designed to elicit a Th1 response, demonstrates greater anti-tumor activity against basal-like than luminal-like mammary tumors. Finally, HIF-1α vaccination can result in the elimination of mammary stem cells.
The HIF-1α protein has been shown to be overexpressed in a majority of invasive breast cancers as well as poorly differentiated ductal carcinoma in situ (29). Increased levels of HIF-1α are significantly associated with high rates of proliferation in breast cancers. The protein has not been shown to be upregulated in normal breast tissue (29). Overexpression of oncogenic self-tumor associated proteins enhances their immunogenicity. In a study of over 100 breast cancer patients, the highest level of expression of the HER-2/neu protein (3+ vs. 1, 2+) was significantly associated with the presence of both antibody (p<0.001) and T-cell immunity (p=0.001) directed to the protein (10). Data presented here demonstrates that autoantibodies directed against HIF-1α can be detected in all subtypes of breast cancer with nearly 60% of patients with TNBC demonstrating some level of humoral immunity to the protein. Indeed, the highest levels of HIF-1α-specific IgG were found in the sera of patients with TNBC. Whether these data indicate the inherent immunogenicity of TNBC as compared to other breast cancer subtypes or reflect an increased incidence of HIF-1α overexpression in TNBC as compared to other breast cancers is unknown. However, the high incidence and levels of tumor associated autoantibodies in TNBC suggest this subtype manifests an active tumor specific Type II immune response (30).
Type I T-cells, both CD4 and CD8, and an IFN-γ rich tumor microenvironment is needed for successful elimination of cancer by the immune system. Studies have shown that high activation of Type I immune pathways and low activation of Type II cytokines is associated with improved clinical outcomes in breast cancer (31). Investigation of breast cancers and surrounding normal tissue have revealed that antigen presenting cells present in the tumor drive the development of Type II T-cells which secrete cytokines that prevent the generation of CD8 cytotoxic T-cells needed for direct tumor killing (32). Successful immunomodulation of any breast cancer would require a shift in the immune response to elicit Type I immune cells. We have recently demonstrated non-mutated self-tumor antigens contain epitopes within the protein sequence that preferentially elicit antigen-specific IFN-γ-secreting Th1 or IL-10-secreting Th2 cells (14). Type II cytokines, such as IL-10, inhibit the development of effective cytotoxic T-cells and suppress Th1 responses (33). Experiments in a mouse mammary model, evaluating the breast cancer antigen IGFBP-2, demonstrated that unless the Th2 inducing sequences were identified and removed from a subunit vaccine, that vaccine would not elicit an anti-tumor response (14). Here we show that the HIF-1α protein sequence also includes epitopes that elicit Th2; some induce IL-10 secretion in the majority of individuals studied. Our immunization approach focused on including only epitopes that elicited a dominant Type I response (Supplementary Table 1). The high degree of homology of HIF-1α between species allowed evaluation of a multi-epitope vaccine in mice where immunization demonstrated the generation of HIF-1α-specific Th1. Immunization with a HIF-1α Th1 selective vaccine could inhibit tumor growth in a basal-like transgenic mammary model. The anti-tumor response was mediated by both CD4 and CD8 T-cells, underscoring the role of IFN-γ-secreting Th1 in propagating the generation and expansion of activated CD8 cytotoxic T-cells in the tumor microenvironment (34).
HIF-1α-specific Th1 vaccine selectively inhibited tumor growth in the C3(1)-Tag model. We noted that the MMC tumor cell line, derived from TgMMTV-neu mouse mammary tumor, and the M6 tumor cell line, derived from the C3(1)-Tag, differed significantly in the level of expression of HIF-1α as well as Sca-1, a marker for murine mammary stem cells (27). HIF-1α is a regulator of cancer stem cells. HIF-1α is upregulated during hypoxia and intratumoral hypoxia induces recruitment of mesenchymal stem cells (35). Expression of HIF-1α activates signaling pathways that control stem cell renewal and multipotency (26). We demonstrated that immunization directed against HIF-1α significantly reduced the Sca-1high population in the basal mammary model as compared to controls. In addition, although tumors were not completely eliminated, the rate of tumor growth was significantly reduced by 92% in the animals receiving the HIF-1α vaccine as compared to controls. These data provide evidence that active immunization is capable of biologically remodeling the tumor in the case where the targeted antigen is an oncogenic driver.
Several studies have validated that cancer stem cells (CSCs) are strongly resistant to chemotherapy and their enrichment in residual breast cancer tumors following treatment may be responsible for driving relapse. One study demonstrated a three-fold increase in the percentage of CSC in recurring platinum-refractory basal-cell like tumors compared to platinum-responsive tumors. Additionally, in the presence of platinum, these CSC have a three-fold increase in the ability to form colonies (36). Another study demonstrated that HIF-1 activity was essential for the enrichment of CSC in TNBC, but not in HER2+ disease, following treatment with paclitaxel or gemcitabine. Furthermore, it was shown that an increase in a HIF-1 genetic signature in TNBC patients after receiving chemotherapy was associated with decreased overall survival (37). Immunization against HIF-1α in the adjuvant setting may eliminate the cells that are responsible for mediating drug and radiation resistance and drive Type I T-cells to the tumor which may enhance the anti-tumor effects of chemotherapy in patients with TNBC. If safety of vaccination is determined in Phase I clinical trials, the addition of Th1 selective vaccination against HIF-1α in the adjuvant setting could be assessed to determine if immunization could prevent disease relapse.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Triple negative breast cancer (TNBC) is typically associated with earlier recurrence, increased metastasis and worse prognosis than other breast cancer subtypes. Efforts to prevent disease recurrence have been confounded by the lack of specific therapeutic targets. HIF-1α is associated with a stem cell phenotype. Vaccination against HIF-1α can inhibit the growth of basal breast cancers and reduce the number of breast cancer stem cells in immunized mice. HIF-1α vaccines represent a targeted therapy that may be suitable for adjuvant treatment in patients with TNBC.
Acknowledgments
Financial Support: This work was supported by the DOD Breast Cancer Program; W81XWH-11-1-0760. Human samples were collected through the Clinical Research Center Facility at the University of Washington (NIH grant UL1TR000423).
Footnotes
Conflict of Interest: MLD is a stockholder in Epithany and VentiRx and receives grant support from Celgene, EMD Serono, VentiRx, Jansen, and Seattle Genetics.
References
- 1.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 2.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Chen X, Zhou E, Chen G, Qian K, Wu X, et al. Intratumoral CD8(+) cytotoxic lymphocyte is a favorable prognostic marker in node-negative breast cancer. PloS One. 2014;9:e95475. doi: 10.1371/journal.pone.0095475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyashita M, Sasano H, Tamaki K, Chan M, Hirakawa H, Suzuki A, et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2014;148:525–34. doi: 10.1007/s10549-014-3197-y. [DOI] [PubMed] [Google Scholar]
- 6.West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disis ML, Stanton SE. Triple-negative breast cancer: immune modulation as the new treatment paradigm. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2015;35:e25–30. doi: 10.14694/EdBook_AM.2015.35.e25. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi R, Gianni L. Hallmarks of triple negative breast cancer emerging at last? Cell Res. 2014;24:904–5. doi: 10.1038/cr.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–7. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodell V, Waisman J, Salazar LG, de la Rosa C, Link J, Coveler AL, et al. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449–54. doi: 10.1158/1535-7163.MCT-07-0386. [DOI] [PubMed] [Google Scholar]
- 11.Disis ML, dela Rosa C, Goodell V, Kuan LY, Chang JC, Kuus-Reichel K, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–8. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Cecil DL, Park KH, Gad E, Childs JS, Higgins DM, Plymate SR, et al. T-helper I immunity, specific for the breast cancer antigen insulin-like growth factor-I receptor (IGF-IR), is associated with increased adiposity. Breast Cancer Res Treat. 2013;139:657–65. doi: 10.1007/s10549-013-2577-z. [DOI] [PubMed] [Google Scholar]
- 13.Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, et al. Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res. 2008;68:8400–9. doi: 10.1158/0008-5472.CAN-07-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecil DL, Holt GE, Park KH, Gad E, Rastetter L, Childs J, et al. Elimination of IL-10-inducing T-helper epitopes from an IGFBP-2 vaccine ensures potent antitumor activity. Cancer Res. 2014;74:2710–8. doi: 10.1158/0008-5472.CAN-13-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang Y, Knutson KL, Goodell V, dela Rosa C, Salazar LG, Higgins D, et al. Tumor antigen-specific T-cell expansion is greatly facilitated by in vivo priming. Clin Cancer Res. 2007;13:1883–91. doi: 10.1158/1078-0432.CCR-06-2083. [DOI] [PubMed] [Google Scholar]
- 16.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–33. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 18.Holzer RG, MacDougall C, Cortright G, Atwood K, Green JE, Jorcyk CL. Development and characterization of a progressive series of mammary adenocarcinoma cell lines derived from the C3(1)/SV40 Large T-antigen transgenic mouse model. Breast Cancer Res Treat. 2003;77:65–76. doi: 10.1023/a:1021175931177. [DOI] [PubMed] [Google Scholar]
- 19.Disis ML, Gad E, Herendeen DR, Lai VP, Park KH, Cecil DL, et al. A multi-antigen vaccine targeting neu, IGFBP-2 and IGF-IR prevents tumor progression in mice with pre-invasive breast disease. Cancer Prev Res (Phila) 2013 doi: 10.1158/1940-6207.CAPR-13-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005;52:144–54. doi: 10.1002/art.20748. [DOI] [PubMed] [Google Scholar]
- 21.Guler G, Balci S, Costinean S, Ussakli CH, Irkkan C, Suren D, et al. Stem cell-related markers in primary breast cancers and associated metastatic lesions. Mod Pathol. 2012;25:949–55. doi: 10.1038/modpathol.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian X, Anzovino A, Kim S, Suyama K, Yao J, Hulit J, et al. N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene. 2014;33:3411–21. doi: 10.1038/onc.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro AS, Paredes J. P-Cadherin Linking Breast Cancer Stem Cells and Invasion: A Promising Marker to Identify an "Intermediate/Metastable" EMT State. Front Oncol. 2014;4:371. doi: 10.3389/fonc.2014.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkatout I, Wiedermann M, Bauer M, Wenners A, Jonat W, Klapper W. Transcription factors associated with epithelial-mesenchymal transition and cancer stem cells in the tumor centre and margin of invasive breast cancer. Exp Mol Pathol. 2013;94:168–73. doi: 10.1016/j.yexmp.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Baron AE, Harrell JC, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. The J Clin Investig. 2009;119:2678–90. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grange C, Lanzardo S, Cavallo F, Camussi G, Bussolati B. Sca-1 identifies the tumor-initiating cells in mammary tumors of BALB-neuT transgenic mice. Neoplasia. 2008;10:1433–43. doi: 10.1593/neo.08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyoshi Y, Shien T, Ogiya A, Ishida N, Yamazaki K, Horii R, et al. Differences in expression of the cancer stem cell marker aldehyde dehydrogenase 1 among estrogen receptor-positive/human epidermal growth factor receptor type 2-negative breast cancer cases with early, late, and no recurrence. Breast Cancer Res. 2016;18:73. doi: 10.1186/s13058-016-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309–14. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- 30.Katayama H, Boldt C, Ladd JJ, Johnson MM, Chao T, Capello M, et al. An Autoimmune Response Signature Associated with the Development of Triple-Negative Breast Cancer Reflects Disease Pathogenesis. Cancer Res. 2015;75:3246–54. doi: 10.1158/0008-5472.CAN-15-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teschendorff AE, Gomez S, Arenas A, El-Ashry D, Schmidt M, Gehrmann M, et al. Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer. 2010;10:604. doi: 10.1186/1471-2407-10-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. The J Exp Med. 2011;208:479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19:3755–63. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Z, Cuss SM, Singh V, Gurusamy D, Shoe JL, Leighty R, et al. CD4+ T Cell Help Selectively Enhances High-Avidity Tumor Antigen-Specific CD8+ T Cells. J Immunol. 2015;195:3482–9. doi: 10.4049/jimmunol.1401571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci U S A. 2014;111:E2120–9. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–50. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:E5429–38. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


