Abstract
Purpose
To assess the radio-sensitizing effect of the biguanide drug Metformin used alone or in combination with reactive oxygen species (ROS) modifying agents N-acetyl-L-cysteine (NAC) or emodin, and contrasted to the mitochondrial complex 1 inhibitor rotenone in altering the radiation responses of the p53 wild type SA-NH and p53 mutant FSa mouse tumor lines grown either in vitro or in vivo.
Materials and Methods
Tumor cells were grown to confluence in vitro and exposed to a single 4 Gy dose in the presence or absence of Metformin (5 mM) and ROS modifiers or to 10 Gy with or without Metformin as tumors in the flanks of C3H mice using a tumor growth delay assay.
Results
Both Metformin and rotenone protected SA-NH (P<0.001) while sensitizing FSa (P<0.001) to 4 Gy. Neither NAC nor emodin altered Metformin’s action. Metformin was also directly toxic to FSa cells (P=0.002). In contrast, in vivo Metformin (250 mg/kg) sensitized both SA-NH (9 day growth delay, P<0.05) and FSa (4 day growth delay, P<0.05) tumors if administered 1 h before irradiation.
Conclusion
Metformin effects on tumor cells measured under in vitro conditions may differ from those determined in vivo due to p53 and heterogeneous environmental factors.
Keywords: Metformin, p53, N-acetyl-L-cysteine, Emodin, Rotenone
INTRODUCTION
Metformin, 1,1-dimethylbiguanide hydrochloride, is the first line drug used in the treatment of type II diabetes (Khouri et al. 2004). It is currently invoking considerable interest for repurposing as a potential anti-cancer agent for use in radiation therapy (Song et al. 2012, Zhang T et al. 2014, Zhang Y et al. 2014, Cheng et al. 2015, Koritzinsky 2015). Metformin has been reported to moderately protect mouse embryo fibroblasts from cytotoxicity induced by ionizing radiation while being directly cytotoxic to human cancer cells (Muaddi et al. 2013). It has been reported to induce a direct cytotoxic response in malignant cells and impair tumor growth (Song et al. 2012, Muaddi et al. 2013, Buzzai et al. 2007, Storozhuk et al. 2013, Zannella et al. 2013, Skinner et al. 2013). These properties offer the excellent potential for the exploitation of metformin to enhance the therapeutic ratio when combined with radiation therapy (Song et al. 2012, Koritzinsky 2015).
Numerous studies conducted to characterize metformin’s properties as a potential anti-cancer agent have, however, produced at times contradictory results. While metformin was observed to radio-sensitize various tumor cell lines to ionizing radiation (Song et al. 2012, Zhang T et al. 2014, Zhang Y et al. 2014, Cheng et al. 2015, Koritzinsky 2015) it has also been reported to protect human HCT116 colorectal cancer (Muaddi et al. 2013, Skinner et al. 2013) and A549 lung cancer cells (Skinner et al. 2013) along with SA-NH murine sarcoma cells (Miller et al. 2014). Its actions modifying radiation response have been linked to its ability to modify mitochondrial metabolism and associated reactive oxygen species (ROS) production (Wheaton et al. 2014, Andrzejewski et al. 2014, Weinberg et al. 2015). In particular, metformin can inhibit mitochondrial complex I activity which in turn affects intracellular ROS production. In non-malignant cells it is proposed that this leads to an inhibition of the entry of electrons into the electron transport chain resulting in reduced ROS production, a protective mechanism (Algire et al. 2012). Inhibition of mitochondrial complex I in cancer cells, in contrast, appears to result in elevated ROS production presumably leading to sensitization and cell death. This has been observed following exposure of cancer cells to the mitochondrial complex I inhibitors BAY 87-2243 and rotenone (Wheaton et al. 2014, Andrzejewski et al. 2014, Weinberg et al. 2015, Algire et al. 2012, Schockel et al. 2015, Cheng et al. 2016). The role of metformin in affecting ROS production in cells is further complicated by observations in some cell systems that metformin can act as an anti-oxidant by inhibiting or repressing ROS production by the NADPH enzymes NOX 2 and 4 (Cheng et al. 2015, Bost et al. 2012), while elevating ROS production in other cell lines through the elevation of NOX 2 and 5 (Zhang Y et al. 2014, Picone et al. 2015). Caution is therefore required in interpreting metformin’s effects on modifying the intracellular redox environment of individual normal and malignant cell systems as it relates to expected changes in its use for affecting therapeutic efficacy.
Another important factor in assessing metformin’s ability to modify intracellular ROS is the p53 mutational status of cells (Buzzai et al. 2007, Skinner et al. 2013). While the effects of metformin on cancer cell viability have been identified as being mainly related to modulation of AMPK activity and autophagy, p53 is known to be a downstream effector of AMPK (Sui et al. 2015). Since it is one of the more frequently mutated genes in cancer, we chose to exam the effects of metformin on both a p53 wild type (WT) and a mutant (Mut) murine tumor model, e.g. SA-NH WT and FSa Mut sarcoma mouse tumors. The transcription factor p53 is known to play an important role in regulating mitochondrial respiration by modulating the balance between the utilization of aerobic respiratory and glycolytic pathways (Matoba et al. 2006, Sinthupibulyakit et al. 2010). Oxidative stress in p53 wild type cells can be protected against through the up-regulation of oxidative phosphorylation along with modulation of anti-oxidants in contrast to p53 mutant cells which are solely dependent upon glycolysis (Sinthupibulyakit et al. 2010). In addition to effects on mitochondrial complex I, production of ROS via NOX 4 and overall intracellular oxidative stress are recognized as being differentially affected by the p53 mutational status of cells, with p53 mutant as compared to functional p53 wild type cells exhibiting an elevated level of oxidative stress (Boudreau et al. 2014, Pervin et al. 2013). Metformin’s ability to affect intracellular ROS production in cells and modify their responses to exposure to ionizing radiation should therefore also be affected by the p53 mutational status of cells.
In our previous report we demonstrated the radio-protective properties of metformin on SA-NH sarcoma cells, human microvascular endothelial cells, and mouse embryo fibroblasts grown and treated in vitro, and mouse bone marrow progenitor cells exposed under in vivo conditions (Miller et al. 2014). Each of these cell systems are characterized as having functional wild type p53, and each was observed to be significantly protected by metformin against radiation-induced cell killing. In the present study we expand our investigation of metformin as a radiation response modifier by including two murine tumor model systems differing in p53 mutational status. Metformin’s effect on radiation response was investigated under in vitro conditions utilizing a colony forming assay and compared to in vivo responses using a standard tumor growth delay method. The mitochondrial complex I inhibitor rotenone was also investigated as a radiation response modifier for comparison with metformin (Goncalves et al. 2011, Green et al. 2011). The free radical scavenger N-acetyl-L-cysteine (NAC) and free radical generating drug emodin were previously successfully used to modify the very low radiation dose adaptive response in both SA-NH and FSa sarcoma tumor models (Miller et al. 2016). Utilizing these same drug concentrations and exposure times found to be previously effective we expanded our investigation to determine their effects, if any, on metformin’s ability to modify radiation response in these tumor model systems (Srinivas et al. 2007, Miller et al. 2016).
MATERIALS AND METHODS
Cells and Culture Conditions
SA-NH (p53 wild-type) cells derived from a spontaneously arisen SA-NH murine sarcoma tumor and FSa (p53 mutant) cells from a methylcholanthrene-induced C3H female mouse fibrosarcoma and adapted for in vitro growth were supplied by Dr. Luka Milas, the University of Texas MD Anderson Cancer Institute. SA-NH cells stably transfected with a pcDNA3 plasmid containing a mutant IκBα gene under the control of a CMV promoter in which serine’s 32 and 36 are mutated and a neomycin resistance gene to allow the selection of stable transfectants using G-418 and designated SA-NH+mIκBα1 were developed as described in detail elsewhere (Murley et al. 2004) and were used to assess the role of NFκB activation in the ability of metformin to protect p53 WT cells. Growth conditions for SA-NH+mIκBα1 cells were identical to those of SA-NH cells. All three cell lines were routinely checked for mycoplasma contamination and were used at passages 5–10. SA-NH and SA-NH+mIκBα1 cells were cultured in McCoy’s 5A medium, and FSa cells in RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Denville Scientific Inc., Metuchen, NJ), penicillin (100 units/ml) and streptomycin (100 μg/ml) (Invitrogen Life Technologies) as described in detail elsewhere (Miller et al. 2016). All cell cultures were maintained at 37 °C in a humidified environment containing 5% CO2. For all cell survival experiments, cells were grown to confluence and maintained in confluence for three days. One day prior to all experiments the cells were refed with fresh medium.
Drugs
Metformin (1,1-Dimethylbiguanide hydrochloride) and N-acetyl-L-cysteine (NAC) were dissolved in phosphate-buffered saline (PBS) immediately prior to use at concentrations of 500 mM and 100 mM respectively, and sterilized using a 0.22 μm syringe filter according to the methods described elsewhere (Miller et al. 2014; Miller et al. 2016). Emodin (1,3,8-Tri-hydroxy-6-methylanthraquinone) and Rotenone were dissolved in DMSO at concentrations of 100 mM immediately prior to use. Each drug was obtained from Sigma-Aldrich, St. Louis, MO. Cells were treated with 5 mM metformin for 30 min or 1 h, 5 mM metformin for 30 min and then 10 mM NAC or 50 μM emodin were added for an additional 30 min as previously described (Miller et al. 2016), or 1 μM rotenone for 1 h prior to radiation exposure.
Irradiation Conditions
For in vitro studies, tumor cells were irradiated with a single 4 Gy dose. In vivo irradiations of SA-NH and FSa tumors were performed on tumor-bearing mice that were placed in lead-shielded cylindrical clear-plastic holders. Their unshielded tumor-bearing right hind legs were secured in place with surgical tape during irradiation and exposed to a single 10 Gy dose. All irradiations were performed using a Philips RT250 x-ray generator with a 1mm copper filter operating at 15 mA and 250 kVp at a dose rate of 1.33 Gy/min.
Cell Survival Assay
Immediately following each treatment protocol, cell survival was determined using an in vitro colony forming assay. Cells were counted, diluted, and known numbers seeded into 100-mm tissue culture dishes to allow the development of 100–200 colonies per dish. Colonies of SA-NH and SA-NH+mIκBα1 or FSa cells were fixed and stained 10 and 14 days later, respectively. Each experiment was repeated three times.
Animals and Tumor Models
Female C3H mice, 8–11 weeks old (Harlan Laboratories, Indianapolis, IN), were housed in a specific pathogen free barrier facility at The University of Chicago. Experiments were carried out with The University of Chicago Animal Care and Use Committee (IACUC) approval, ACUP number 70672. The University of Chicago has an Animal Welfare Assurance on file with the Office of Laboratory Animal Welfare (A3523-01) and conducts its reviews in accordance with United States Public Health Service (USPHS) regulations and applicable federal and local laws. For the tumor growth delay experiments, mice were housed up to five per cage under standard conditions (12 h light and12 h dark at 48% relative humidity and a constant temperature of 22 °C). The right hind legs of mice were injected subcutaneously with either 5 ×106 SA-NH or 3 × 106 FSa cells in a volume of 0.1ml and were monitored daily. Total numbers of mice in each experimental group from two separate experiments ranged from 7 to 16 animals.
Tumor Growth Delay Measurements
Treatment and measurements began when tumors grew to a minimum volume of 250 mm3 as measured in three dimensions (length along the right hind leg, width at its widest point, and the height of the tumor measured from the femur to the highest point of the tumor). Tumor-bearing mice in the control group were loaded into the animal holding chamber with their hind legs taped to the chamber for the same amount of time that the tumor-bearing mice in the radiation groups were secured. Animals in all experimental groups were sacrificed when tumors exceeded an approximate volume of 1000 mm3.
Statistical Analysis
Means and standard errors were calculated for all data points from at least three independent experiments. Pairwise comparisons of cell survival between each of the experimental conditions were performed using a Student’s two-tailed t-test (SigmaPlot software11.0, SPSS, Chicago, IL). Mean tumor volumes over time from 7 days on were compared among groups using repeated measures analysis of covariance (ANCOVA) assuming a quadratic model and accounting for separate variances in each group. Post-hoc pairwise comparisons among groups were also done using the Tukey-Kramer method (Kramer 1956). Absolute tumor growth delay values in the metformin treated groups was defined as the median time in days for the tumors to grow to greater than 1000 mm3 minus the median time in days for 10 Gy only tumors to grow to this size (Schimming et al. 1999). Since some tumors did not reach 1000 mm3, time to reach 1000 mm3 was compared between groups using a Kaplan-Meier curve and the log rank test.
RESULTS
In Vitro Toxicity of Metformin
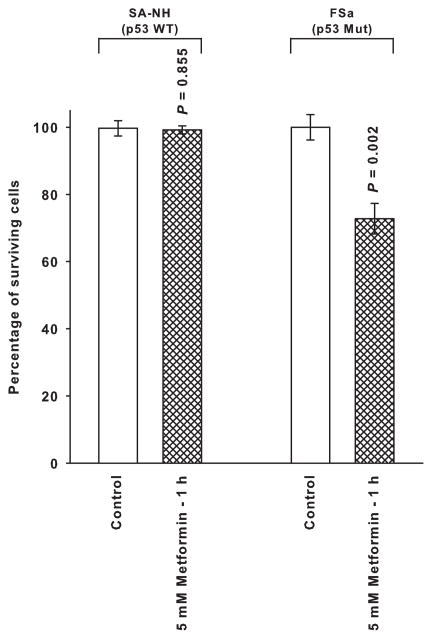
Presented in Fig. 1 is a toxicity assessment for a 1 h exposure of metformin on p53 wild type SA-NH and p53 mutant FSa cells. Metformin at a dose of 5 mM exhibited no toxic effect on SA-NH cells, but reduced cell survival of FSa cells by over 20%.
Figure 1.
The toxicity profile for metformin in p53 wild type (WT) SA-NH murine sarcoma and p53 mutant (Mut) FSa fibrosarcoma cells exposed to a 5 mM concentration for 1 h. Each experiment was performed three times and error bars represent the standard error of the mean (SEM). P values comparing the survival of metformin treated cells with those of their corresponding untreated controls are presented for comparison.
Effects of Metformin on Radiation Response of SA-NH and FSa under In Vitro Conditions
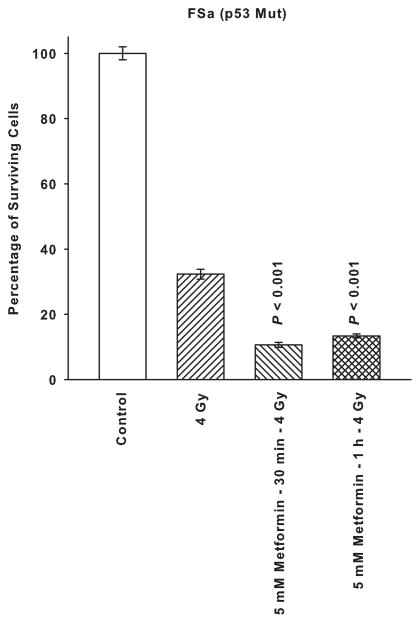
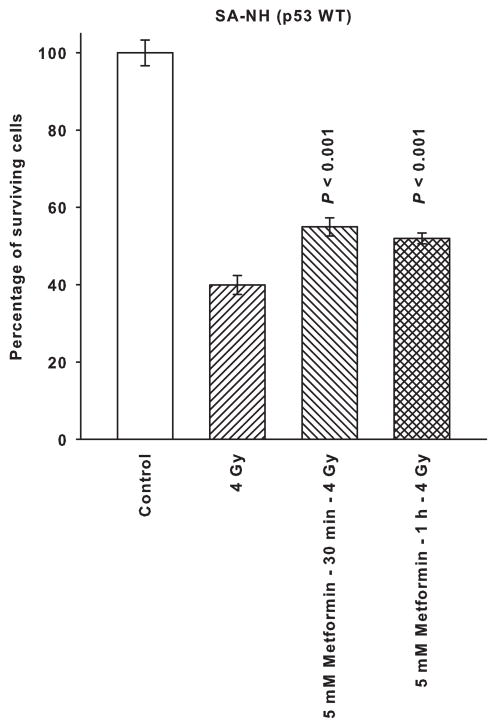
SA-NH and FSa cells were exposed to a dose of 4 Gy alone or following a 30 min or 1 h treatment with 5 mM of metformin. As described in Fig. 2, metformin significantly enhanced resistance of SA-NH cells to ionizing radiation following either a 30 min or 1 h exposure (P < 0.001). In contrast, a 5 mM dose of metformin significantly reduced survival of FSa cells following both a 30 min and 1 h exposure (P < 0.001). The overall reduction in survival of FSa cells induced by metformin following irradiation presumably reflects both a direct cytotoxicity as described in Fig. 1, as well as a radiation sensitizing component.
Figure 2.
The radiation response modifying effects of a 5 mM dose of metformin on SA-NH and FSa tumor cells following an exposure of 30 min or 1 h administered prior to irradiation with a single 4 Gy dose. Each experiment was repeated three times and error bars represent the SEM. P values comparing cell survival at 4 Gy only to those following treatment with metformin are presented for comparison.
Effect of NFκB Activation on Metformin’s Modification of Radiation Response
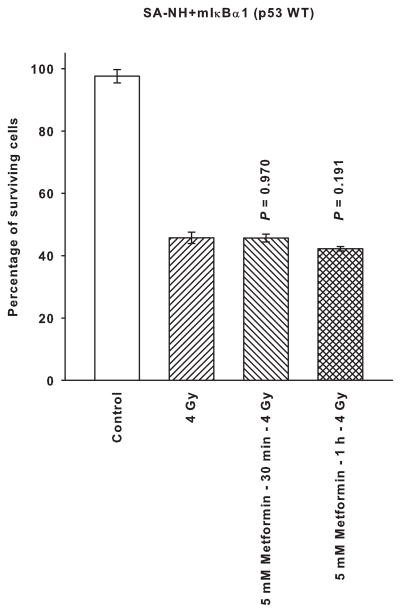
To assess the potential role of NFκB activation on metformin’s ability to affect radiation response, p53 wild type cells were stably transfected with a mutant IκBα vector and a resulting clone was isolated and designated SA-NH+mIκBα1. The effects of radiation with and without exposure to metformin on this clone are presented in Fig. 3. Under conditions under which NFκB activation is inhibited, metformin was ineffective in altering radiation response indicating the requirement for an intact NFκB signaling pathway for this process to occur. Metformin exposure times of 30 min and 1 h were equally ineffective in altering radiation response.
Figure 3.
The absence of a radiation response modifying effect following a 30 min or 1 h exposure of SA-NH cells stably transfected with a mutant IκBα (SA-NH+mIκBα1) to a 5 mM dose of metformin is described. Each experiment was repeated three times and error bars represent the SEM. P values comparing cells treated with 5 mM of metformin for 30 min or 1 h prior to irradiation and cells exposed to 4 Gy only are presented for comparison.
Effects of Metformin in Combination with NAC or Emodin as Compared to Rotenone on Radiation Response
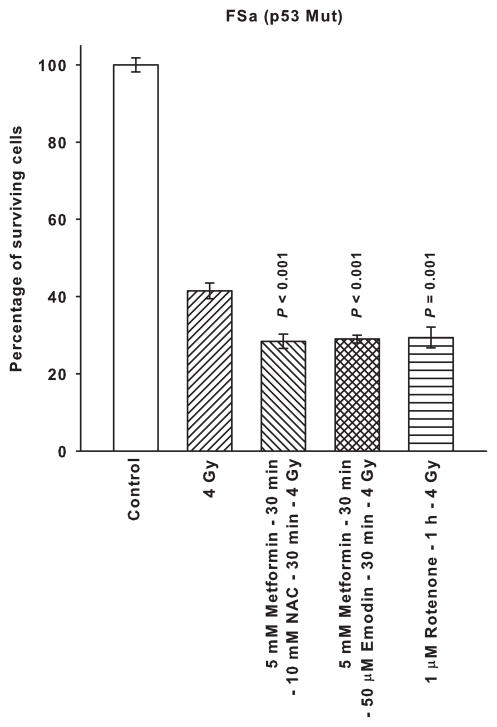
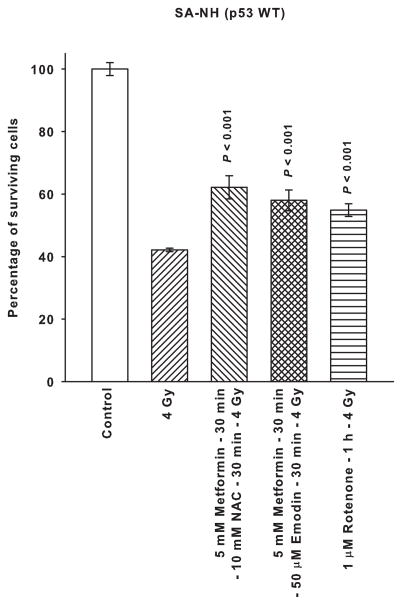
Presented in Fig. 4 is a comparison of metformin administered in combination with the free radical scavenger NAC or the free radical generator emodin along with the mitochondrial complex I inhibitor rotenone on affecting radiation response of SA-NH and FSa tumor cells. Neither NAC nor emodin altered the magnitude of the protective effect exerted by metformin on SA-NH cells. Rotenone’s effects on enhancing SA-NH cell survival following 4 Gy was essentially of the same magnitude as described for metformin alone in Fig. 2. In contrast, metformin combined with NAC or emodin and rotenone alone each sensitized FSa cells by a similar magnitude as evidenced by the comparable survival levels.
Figure 4.
The radiation response modifying effects of 5 mM metformin administered for 30 min followed by a 30 min exposure to 10 mM of the free radical scavenger N-acetyl-cysteine (NAC) or 50 μM of the free radical generating drug Emodin are contrasted to those exerted by a 1 h exposure to the mitochondrial complex I inhibitor Rotenone (1 μM) using p53 WT SA-NH and p53 Mut FSa. Surviving fractions were compared to corresponding radiation only treated cells. Each experiment was repeated three times and error bars represent the SEM. P values comparing radiation with metformin plus NAC, metformin plus Emodin, and Rotenone to that of radiation only are presented for comparison.
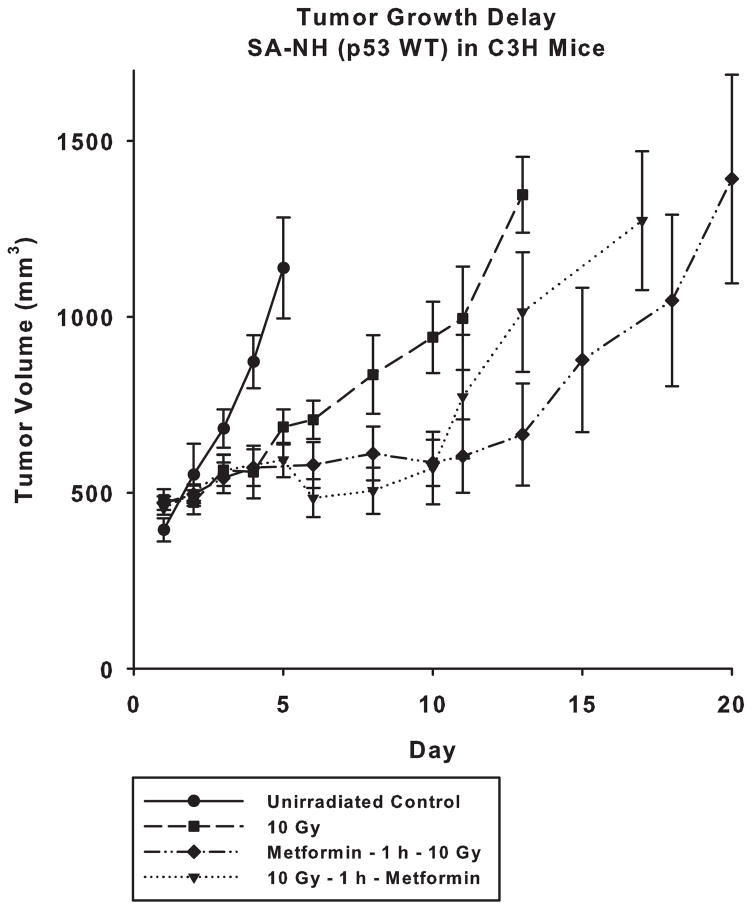
Effect of Metformin on the Radiation Response of SA-NH Assayed by Tumor Growth Delay
In contrast to metformin’s enhancement of radiation resistance of SA-NH treated under in vitro conditions, SA-NH tumors were significantly sensitized to a 10 Gy dose of ionizing radiation when tumor bearing mice were injected with a 250 mg/kg dose of metformin either 1 h before (P < 0.05), or following irradiation (P = 0.05 at day 10). Presented in Fig. 5 are tumor growth delay plots describing the increased sensitivity of SA-NH tumors to ionizing radiation when treated with metformin. Administering metformin 1 h prior to exposure to ionizing radiation was more effective, a delay of 9 days (P = 0.001), than if given 1 h afterwards, a delay of 3.5 days (P = 0.015), in extending the time required for tumors in each of the groups to achieve a comparable size.
Figure 5.
Tumor growth delay studies demonstrating the effects of a single 10 Gy dose alone or in combination with a 250 mg/kg dose of metformin administered i.p. either 1 h prior to or following irradiation of SA-NH tumors grown in the flanks of C3H mice. Treatment of tumors began when they reached an average size of approximately 250 mm3 and growth measurements ended after they exceeded 1000 mm3 in size. An untreated control group was also monitored for comparison. Each metformin plus radiation treated tumor group is compared to the radiation only group. Mean growth times and SEM were determined. Mean tumor volumes over time were compared among groups using repeated measures analysis of covariance (ANCOVA) assuming a quadratic model and accounting for separate variances in each group. A post-hoc pairwise comparison among groups was performed using the Tukey-Kramer method. Time to reach 1000 mm3 in size in each group was estimated using a Kaplan-Meier curve and compared between groups using the log rank test.
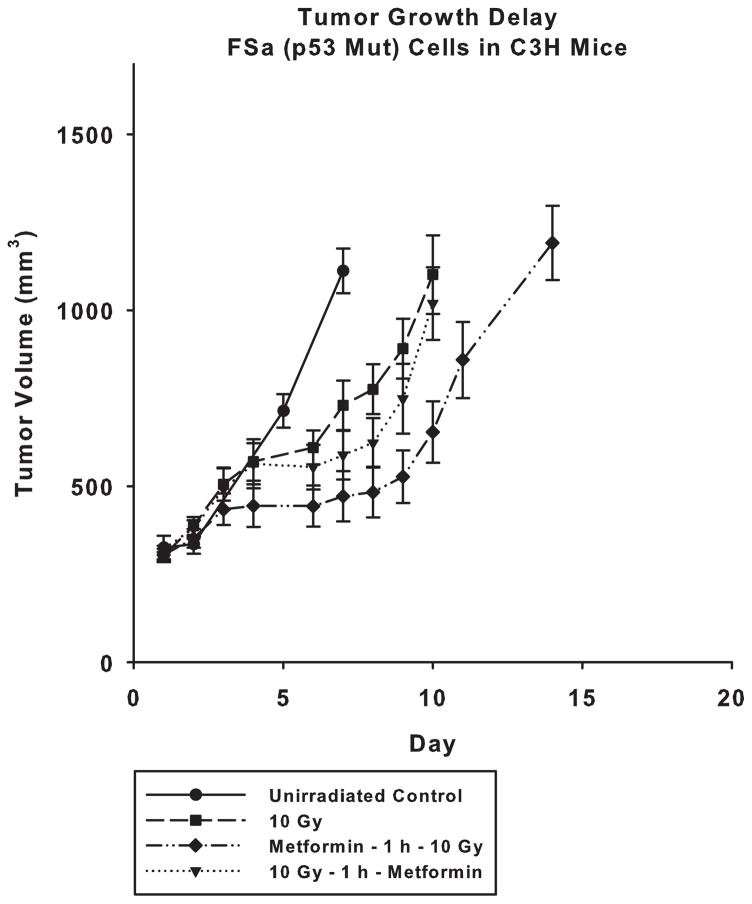
Effect of Metformin on the Radiation Response of FSa Assayed by Tumor Growth Delay
As described in Fig. 6, FSa tumors exposed to metformin at a dose of 250 mg/kg 1 h before radiation exposure continued to exhibit a sensitizing response consistent with that observed under in vitro conditions (P < 0.05 comparing curves over time and at days 7–14), and an increase of 4 days of growth (P = 0.013) to achieve a comparable size with tumors only exposed to 10 Gy. When exposed to metformin following exposure to ionizing radiation, FSa tumors failed to exhibit a significant sensitization response, P > 0.05. Only the pre-irradiation treatment with metformin was observed to be effective in significantly sensitizing FSa tumors to the effects of a 10 Gy exposure.
Figure 6.
Tumor growth delay studies demonstrating the effects of a single 10 Gy dose alone or in combination with a 250 mg/kg dose of metformin administered i.p. either 1 h prior to or following irradiation of FSa tumors grown in the flanks of C3H mice. Treatment of tumors began when they reached an average size of approximately 250 mm3 and growth measurements ended after they exceeded 1000 mm3 in size. An untreated control group was also monitored for comparison. Each metformin plus radiation treated tumor group is compared to the radiation only group. Mean growth times and SEM were determined. Mean tumor volumes over time were compared among groups using repeated measures analysis of covariance (ANCOVA) assuming a quadratic model and accounting for separate variances in each group. A post-hoc pairwise comparison among groups was performed using the Tukey-Kramer method. Time to reach 1000 mm3 in size in each group was estimated using a Kaplan-Meier curve and compared between groups using the log rank test.
DISCUSSION
The transcription factor p53 is known to be an important mediator in the regulation of reactive oxygen species (ROS), antioxidant status, and mitochondrial respiration in cells (Sablina et al. 2005, Milicevic et al. 2014, Budanov et al. 2014). It facilitates protection against oxidative stress through the upregulation and expression of anti-oxidant genes including copper zinc superoxide dismutase (Sod1), manganese superoxide dismutase (Sod2), glutathione peroxidase (GPx1), aldehyde dehydrogenase 4 and catalase (Sinthupibulyakit et al. 2010, Sablina et al. 2005, Milicevic et al. 2014, Budanov et al. 2014). Malignant cells, in contrast to normal cells, are characterized by an elevated level of ROS which has been implicated in the process of carcinogenesis and which are kept under control through the action of these anti-oxidant proteins activated by wild type p53 (Weinberg et al. 2015, Turrens 2003, Solaini et al. 2011). This element of protection from the effects of elevated ROS, however, is compromised and absent in p53 mutant tumor cells, making them more susceptible to the therapeutic effects of agents that can exacerbate ROS production and elevate the oxidative environment of the cell. Also linked to deficits in p53 function is the impairment of the cytochrome c oxidase, e.g. mitochondrial complex IV, which is important in mitochondrial respiration (Matoba et al. 2006, Zhou et al. 2003).
Metformin is an inhibitor of mitochondrial complex I, which not only affects the production of ROS, but also inhibits mitochondrial respiration (Wheaton et al. 2014, Andrzejewski et al. 2014, Weinberg et al. 2015, Algire et al. 2012, Schockel et al. 2015, Cheng et al. 2016). It is not surprising therefore, that metformin would express a direct toxic effect on p53 mutant cells such as demonstrated in Fig. 1 for FSa fibrosarcoma cells. In contrast to the isogenic p53 null human colon carcinoma cell line HCT116 (−/−), which was earlier reported to be sensitive to the toxicity of metformin, p53 mutant FSa cells carry only a substitution of methionine for valine at position 169 suggesting that complete loss of function of p53 is not required for this toxic effect to be expressed (Buzzai et al. 2007, Storozhuk et al. 2013, Schimming et al. 1999). If both complex I and IV are compromised through the action of metformin and p53 mutational status, mitochondrial respiration would be significantly inhibited leading to cell death and an associated reduction in cell survival as described by the response of FSa as compared to SA-NH to metformin in Fig. 1.
Metformin’s ability to affect radiation response in cells reflects complex processes that are modified directly or indirectly as a result of p53 mutational status. As discussed above, malignant as compared to normal cells express an elevated intracellular ROS environment that is controlled through the action of elevated anti-oxidant enzymes such as the superoxide dismutases, glutathione peroxidase and catalase. This balance in p53 wild type malignant cells allows for cell growth and a control of the oxidative environment. Metformin, through its inhibition of mitochondrial complex I and associated mitigation of ROS production, as well as its anti-oxidant effects on NADPH enzymes Nox 2 and 4 (Cheng et al. 2015, Bost et al. 2012), has exhibited radio-protective effects on a variety of normal and p53 wild type tumor model systems irradiated under in vitro conditions. Consistent with our earlier report, metformin administered 30 min or 1 h prior to radiation exposure significantly protected p53 wild type SA-NH tumor cells (see Fig. 2) (Miller et al. 2014). FSa cells exposed under these same conditions were sensitized rather than protected by metformin. The overall response of FSa reflects both the direct toxicity of metformin on these cells, as well as an enhanced sensitization to 4 Gy of ionizing radiation. Metformin’s effects on mitigating radiation sensitivity are not limited solely to its anti-oxidant properties, but appears to also be affected by NFκB-mediated survival pathways as evidenced by the lack of a radiation sensitivity modifying effect in SA-NH cells stably transfected with a mutant IκBα vector that inhibits NFκB activation (Murley et al. 2004). Since p53 mutations are known to promote cancer progression by augmenting NFκB activation in the context of chronic inflammation and enhance both proinvasive and prosurvival activities, inhibition of NFκB activity in p53 mutant tumor cells should exhibit as profound effect or more than was observed in the p53 WT SA-NH tumor model (Cooks et al. 2013; Bellazzo et al., 2015). Prosurvival pathways associated with p53 mutational status and NFκB activation play a determinant role in metformin’s effects on radiation response.
The association of anti-oxidant properties exhibited by metformin and its subsequent modifying effects on radiation response prompted the use of other ROS modifying agents, the free radical scavenger NAC and the free radical generator drug emodin, to be administered in conjunction with metformin. Rotenone, a potent inhibitor of mitochondrial complex I, was used to investigate its comparative effectiveness with metformin as a radiation modifying agent. Surprisingly, neither NAC nor emodin under conditions shown to be effective in altering the very low radiation dose adaptive response in SA-NH and FSa tumor models (Miller et al. 2016) were observed to affect metformin’s radiation sensitivity modifying effectiveness (see Fig. 4). Furthermore, rotenone treatment effectively mimicked the effectiveness of metformin used alone in protecting SA-NH but sensitizing FSa tumor cells. Neither NAC nor emodin has been reported to adversely affect mitochondrial complex I, while rotenone’s effects are primarily attributed to its ability to inhibit this process (Green et al. 2011, Srinivas et al. 2007, Miller et al. 2016). These data suggest that NAC and emodin treatment are insufficient to alter significantly metformin’s effectiveness by enhancing or inhibiting ROS production that results following the inhibition of mitochondrial complex I. This may reflect the inherent efficiency and levels of anti-oxidant enzymes present in the p53 wild type SA-NH cells, along with the associated prosurvival pathways that results in enhanced radiation resistance as compared to the deficit of these processes expressed in the p53 mutant FSa tumor cells. It has been reported that while metformin and rotenone both are effective in inhibiting mitochondrial complex I, they do so by acting on different sites of the complex (Wheaton et al. 2014). Mitochondrial complex I produces superoxide which is then converted quickly to hydrogen peroxide by superoxide dismutase activity. Metformin, in contrast to rotenone, has been reported not to increase these ROS species, while rotenone stimulates such ROS production. The lack of modifying effects of NAC and emodin on metformin’s ability to alter radiation response, along with the similarity between rotenone and metformin as radiation response modifiers for tumor cells differing in p53 status irradiated under relative homogenous aerated conditions in vitro, suggests that the primary process being affected is mitochondrial metabolism along with its downstream consequences.
Tumor growth delay studies were performed to further characterize the radiation survival modifying effects of metformin under more environmentally complex conditions. Metformin was observed to significantly sensitize SA-NH cells regardless if it was administered 1 h prior to or following exposure to ionizing radiation (See Fig. 5). This is in contrast to the protective properties expressed when irradiation and metformin exposure was performed under in vitro conditions as shown in Fig. 2. While SA-NH cells grown under in vitro conditions were uniformly well oxygenated and supplied with ample nutrients for growth, these cells grown in the flank of mice as solid tumors were exposed to heterogeneous environmental conditions including both oxygen and nutrient deprivation. Impairment of mitochondrial respiration by metformin under these conditions would result in greater stress and enhanced metabolically associated cell killing. In particular, metformin is effective in decreasing blood glucose levels. Consequently, as a result of limited glucose availability, mitochondrial ATP production would be inhibited resulting in subsequent cell sensitization and death (Weinberg et al. 2015). An alternative but not mutually exclusive hypothesis has proposed that inhibition of mitochondrial complex I would also result in a reduced rate of oxygen consumption in these cells resulting in an overall increase in oxygen diffusion and subsequent tumor oxygenation, e.g. a reduction in the degree of heterogeneity in oxygen tension experienced by a greater proportion of the tumor cells during irradiation (Zannella et al. 2013). This in turn could also result in an overall sensitization of the tumor to ionizing radiation. Under these in vivo conditions of heterogeneous environmental factors, metformin was also found to significantly sensitize FSa tumors when administered prior to exposure to ionizing radiation (see Fig. 6). Surprisingly, metformin was relatively ineffective in sensitizing FSa cells when administered following irradiation. Since defects in the cytochrome c oxidase mitochondrial complex IV have been associated with p53 mutant malignant cells, it may be that the metabolic stress affecting mitochondrial respiration in FSa cells immediately following irradiation was too severe to observe additional damage induced by the post-radiation treatment with metformin.
Metformin offers the promise of not only selectively sensitizing malignant cells to the toxic effects of radiation and chemotherapy, but also of serving as an effective chemopreventive and radio-protective agent in protecting dose limiting normal tissues from tumorigenesis and cell killing (Song et al. 2012, Miller et al. 2014). Complicating its repositioning as an effective cancer therapeutic agent is the diversity of effects attributed to its use. It has been described as both a ROS inducer and scavenger (Cheng et al. 2015, Algire et al. 2012, Picone et al. 2015), and its properties appear to be significantly affected by the p53 mutational status of the target cells (Muaddi et al. 2013, Buzzai et al. 2007). At the core of its activity is its ability to inhibit mitochondrial complex I and alter glucose availability for glycolytic respiration, thus altering downstream metabolic processes associated with subsequent cell growth, survival and death in both malignant and non-malignant cells.
Acknowledgments
GRANT SUPPORT
This work was supported in part by the DOE Low Dose Program/Project Grant DE-413 SC0001271 awarded to Dr. Grdina. Dr. Grdina was also supported by NIH NCI R01-CA132998.
Biographies
Jeffrey S. Murley, PhD is a Research Associate, Associate Professor in the Department of Radiation and Cellular Oncology at The University of Chicago. His research interests include: Adaptive responses induced by very low doses of ionizing radiation and the intracellular signaling pathways involved in these responses.
Richard C. Miller, PhD is a Research Associate, Associate Professor in the Department of Radiation and Cellular Oncology at The University of Chicago. His research interests include: Adaptive responses induced by very low doses of ionizing radiation and in vivo tumor responses following low dose radiation exposure.
Raziye Rana Senlik is an Undergraduate Student/Research Technician.
Alfred W. Rademaker, PhD is a Professor of Preventive Medicine (Biostatistics) at Northwestern University Feinberg School of Medicine. His research interests include: Early phase cancer studies, diagnostic and screening studies, study design and sample size determination.
David J. Grdina, PhD is a Professor in the Department of Radiation and Cellular Oncology at The University of Chicago. His research interests include: Adaptive responses induced by very low doses of ionizing radiation or thiol containing cytoprotective drugs, combining carcinogenic therapies and anti-carcinogenic drugs.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
Dr. David J. Grdina and Dr. Jeffrey S. Murley are minority equity partners in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. Dr. Richard C. Miller has no conflict of interest to disclose. Ms. Raziye Rana Senlik has no conflict of interest to disclose. Dr. Alfred W. Rademaker has no conflict of interest to disclose. The authors alone are responsible for the content and writing of the paper.
References
- Algire C, Moiseeva O, Deschenes-Simard X, Amrein L, Petruccelli L, Birman E, Viollet B, Ferbeyre G, Pollak MN. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res. 2012;5(4):536–43. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- Andrzejewski S, Gravel S-P, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metabol. 2014;2:1–14. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellazzo A, Di Minin G, Collavin L. Cytoplasmic gain-of-function mutant p53 contributes to inflammation-associated cancer. Molec Cell Oncol. 2015;2(4):e1002719-1–e1002719-3. doi: 10.1080/23723556.2014.1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Ben-Sahra I, Tanti J-F. Prevention of mutagenesis: New potential mechanisms of metformin action in neoplastic cells. Cancer Prev Res. 2012;5(4):503–6. doi: 10.1158/1940-6207.CAPR-12-0085. [DOI] [PubMed] [Google Scholar]
- Boudreau HE, Casterline BW, Burke DJ, Leto TL. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-β-mediated migration of human lung and breast epithelial cells. Brit J Cancer. 2014;110:2569–82. doi: 10.1038/bjc.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV. The role of tumor suppressor p53 in the antioxidant defense and metabolism. Subcell Biochem. 2014;86:337–58. doi: 10.1007/978-94-017-9211-0_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cells growth. Cancer Res. 2007;67(14):6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Cheng G, Lanza-Jacoby S. Metformin decreases growth of pancreatic cancer cells by decreasing reactive oxygen species: role of NOX4. Biochem Biophys Res Comm. 2015;465(1):41–46. doi: 10.1016/j.bbrc.2015.07.118. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zielonka J, Ouari O, Lopez M, McAllister D, Boyle K, Barrios CS, Weber JJ, Johnson BD, Hardy M, Dwinell MB, Kalyanaraman B. Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. Cancer Res. 2016;76(13):3904–15. doi: 10.1158/0008-5472.CAN-15-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rozenfeld N, Harpaz N, Itzkowitz S, Harris CC, Rotter V, Gorgoulis VG, Oren M. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–46. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves AP, Maximo V, Lima J, Singh KK, Soares P, Videira A. Involvement of p53 in cell death following cell cycle arrest and mitotic catastrophe induced by rotenone. Biochim et Biophys Acta. 2011;1813:492–99. doi: 10.1016/j.bbamcr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ML, Singh AV, Ruest LB, Pisano MM, Prough RA, Knudsen TB. Differential programming of p53-deficient embryonic cells during rotenone block. Toxicol. 2011;290:31–41. doi: 10.1016/j.tox.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri H, Collin F, Bonnefont-Rousselot D, Legrand A, Jore D, Gardes-Albert M. Radical-induced oxidation of metformin. Eur J Biochem. 2004;271:4745–4752. doi: 10.1111/j.1432-1033.2004.04438.x. [DOI] [PubMed] [Google Scholar]
- Koritzinsky M. Metformin: A novel biological modifier of tumor response to radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(2):454–64. doi: 10.1016/j.ijrobp.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12:309–10. [Google Scholar]
- Matoba S, Kang J-G, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–53. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- Milicevic Z, Kasapovic J, Gavrilovic L, Milovanovic Z, Bajic V, Spremo-Potparevic B. Mutant p53 expression and antioxidant status deficiency in breast cancer. EXCLI Journal. 2014;13:691–708. [PMC free article] [PubMed] [Google Scholar]
- Miller RC, Murley JS, Grdina DJ. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat Res. 2014;181:464–70. doi: 10.1667/RR13672.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RC, Murley JS, Rademaker AW, Woloschak GE, Li JJ, Weichselbaum RR, Grdina DJ. Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiation sensitivity. Free Radic Biol Med. 2016;99:110–19. doi: 10.1016/j.freeradbiomed.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muaddi H, Chowdhury S, Vellanki R, Zamiara P, Koritzinsky M. Contributions of AMPK and p53 dependent signaling to radiation response in the presence of metformin. Radiother Oncol. 2013;108:446–50. doi: 10.1016/j.radonc.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Murley JS, Kataoka Y, Cao D, Li JJ, Oberley LW, Grdina DJ. Delayed radioprotection by NFκB-mediated induction of Sod2 (MnSOD) in SA-NH tumor cells after exposure to clinically used thiol-containing drugs. Radiat Res. 2004;162:536–46. doi: 10.1667/rr3256. [DOI] [PubMed] [Google Scholar]
- Pervin S, Tran L, Urman R, Braga M, Parveen M, Li SA, Chaudhuri G, Singh R. Oxidative stress specifically downregulates survivin to promote breast tumour formation. Brit J Cancer. 2013;108:848–58. doi: 10.1038/bjc.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone P, Nuzzo D, Caruana L, Messina E, Barera A, Vasto S, Di Carlo M. Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-κB activation: Use of insulin to attenuate metformin’s effect. Biochim et Biophys Acta. 2015;1853:1046–59. doi: 10.1016/j.bbamcr.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nature Medicine. 2005;11(12):1306–13. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimming R, Mason KA, Hunter N, Weil M, Kishi K, Milas L. Lack of correlation between mitotic arrest or apoptosis and antitumor effect of docetaxel. Cancer Chermother Pharm. 1999;43:165–72. doi: 10.1007/s002800050879. [DOI] [PubMed] [Google Scholar]
- Schockel L, Glasauer A, Basit F, Bitschar K, Truong H, Erdmann G, Algire C, Hägebarth A, Willems PH, Kopitz C, Koopman WJ, Héroult M. Targeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growth. Cancer Metabol. 2015;3(11):1–16. doi: 10.1186/s40170-015-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinthupibulyakit C, Ittarat W, St Clair WH, St Clair DK. p53 protects lung cancer cells against metabolic stress. Int J Oncol. 2010;37:1575–81. doi: 10.3892/ijo_00000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HD, Crane CH, Garrett CR, Eng C, Chang GJ, Skibber JM, Rodriguez-Bigas MA, Kelly P, Sandulache VC, Delclos ME, Krishnan S, Das P. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2013;2(1):99–107. doi: 10.1002/cam4.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaini G, Sgarbi G, Baracca A. Oxidative phosphorylation in cancer cells. Biochimica Biophysica Acta. 2011;1807:534–42. doi: 10.1016/j.bbabio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Song CW, Lee H, Dings RPM, Williams B, Powers J, Dos Santos T, Choi BH, Park HJ. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;362:1–7. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of Emodin action: Transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27(5):591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G, Tsakiridis T. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021–32. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Xu Y, Wang X, Han W, Pan H, Xiao M. Metformin: A novel bu controversial drug in cancer prevention and treatment. Molec Pharmaceutics. 2015;12:3783–91. doi: 10.1021/acs.molpharmaceut.5b00577. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(2): 335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nature Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu G, Budinger SGR, Chandel NS. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014;2242:1–18. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannella V, Dal Pra A, Muaddi H, McKee T, Stapleton S, Sykes J, Glicksman R, Chaib S, Zamiara P, Milosevic M, Wouters BG, Bristow RG, Koritzinsky M. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res. 2013;19(24):5741–50. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Zhang T, Fan J, Wu K, Guan Z, Wang X, Li L, Hsieh JT, He D, Guo P. Metformin sensitizes prostate cancer cells to radiation through EGFR/p-DNA-PKcs in vitro and in vivo. Radiat Res. 2014;181:641–49. doi: 10.1667/RR13561.1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Storr SJ, Johnson K, Green AR, Rakha EA, Ellis IO, Morgan DAL, Martin SG. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget. 2014;5(24):12936–49. doi: 10.18632/oncotarget.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Kachhap S, Singh KK. Mitochondrial impairment in p53-deficient human cancer cells. Mutagenesis. 2003;18(3):287–9. doi: 10.1093/mutage/18.3.287. [DOI] [PubMed] [Google Scholar]