Abstract
Although the majority of low grade, early stage endometrial cancer patients will have good survival outcomes with surgery alone, those patients who do recur tend to do poorly. Optimal identification of the subset of patients who are at high risk of recurrence and would benefit from adjuvant treatment has been difficult. The purpose of this study was to evaluate the impact of somatic tumor mutation on survival outcomes in this patient population. For this study, low grade was defined as endometrioid FIGO grades 1 or 2, while early stage was defined as endometrioid stages I or II (disease confined to the uterus). Next-generation sequencing was performed using panels comprised of 46–200 genes. Recurrence-free and overall survival was compared across gene mutational status in both univariate and multivariate analyses. 342 patients were identified, 245 of which had endometrioid histology. For grade 1–2, stage I–II endometrioid endometrial cancer patients, age (HR 1.07, 95% CI 1.03–1.10), CTNNB1 mutation (HR 5.97, 95% CI 2.69–13.21), and TP53 mutation (HR 4.07, 95% CI 1.57–10.54) were associated with worse recurrence-free survival on multivariate analysis. When considering endometrioid tumors of all grades and stages, CTNNB1 mutant tumors were associated with significantly higher rates of grade 1–2 disease, lower rates of deep myometrial invasion, and lower rates of lymphatic/vascular space invasion. When both TP53 and CTNNB1 mutations were considered, presence of either TP53 mutation or CTNNB1 mutation remained a statistically significant predictor of recurrence-free survival on multivariate analysis and was associated with a more precise confidence interval (HR 4.69, 95% CI 2.38–9.24). Thus, mutational analysis of a 2 gene panel of CTNNB1 and TP53 can help to identify a subset of low grade, early stage endometrial cancer patients who are at high risk of recurrence.
INTRODUCTION
Endometrial cancer is the most common gynecologic malignancy, with an estimated 60,050 new cases in 2016 (1). The vast majority of endometrial cancers have endometrioid histology and are diagnosed at an early stage (2). Treatment primarily consists of surgical management, and five year survival is 69–88% for FIGO stage I–II disease (2). However, a subset of these patients will have poor outcomes, and determining which patients are at highest risk for a recurrence of their disease—and would, therefore, benefit most from adjuvant treatment or more extensive surgical staging—has been challenging.
Prior research has sought to identify clinical and/or pathological risk factors that place patients with seemingly lower risk endometrial cancer at higher risk of recurrence. In the Gynecologic Oncology Group (GOG) 99 trial, the GOG considered the “high-intermediate risk” group to be based on deep myometrial invasion, histologic grade 2 or 3 disease, or lymphatic/vascular space invasion (3). Depending on the patient’s age, the presence of either one, two, or three of those factors dictated whether adjuvant therapy was recommended. Similarly, in the Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC) trials, the “high-intermediate risk” group was considered to be those patients with at least two of the following characteristics: deep myometrial invasion, age greater than 60, and grade 3 histology (4, 5). Both criteria have been implemented in subsequent clinical trials and are used in clinical practice. However, despite these and other proposed algorithms (6–9), the appropriate criteria for allocating early endometrial cancer patients to adjuvant treatment remains uncertain. Balancing concerns about over-treatment with the reality that recurrences still occur in this population and are largely incurable reinforces the need for better risk-prediction strategies.
With the advent of the Cancer Genome Atlas (TCGA) and the greater clinical laboratory incorporation of next-generation sequencing, one proposed strategy for improved risk-stratification has been through the use of molecular biomarkers. The purpose of this study was to evaluate the impact of somatic tumor mutation on recurrence-free survival in this patient population.
MATERIALS AND METHODS
Patient selection
We performed a retrospective analysis of endometrial cancer patients at the University of Texas MD Anderson Cancer Center who had had genomic profiling of their endometrial cancer performed since the year 2000. Patients were included if they had endometrial cancer that was histologically-confirmed by pathologists at MD Anderson Cancer Center and had undergone molecular assessment of their tumor at our institution. Molecular evaluation of mutational status was performed using either a next-generation sequencing panel of 46 or 50 genes (10) in a clinical molecular diagnostics lab or a next-generation sequencing panel of 200 genes (11) in a research setting.
Patients who had neoadjuvant treatment were excluded from analyses of tumor grade, myometrial invasion, lymphatic/vascular invasion, and tumor size. Patients were excluded from survival analyses if they did not have surgical treatment of their endometrial cancer, had a concurrent cancer diagnosis requiring adjuvant treatment, or had a prior cancer diagnosis with a recurrence of disease after treatment for endometrial cancer was initiated. Patients with progressive disease were excluded from recurrence-free survival analyses, but included in other analyses.
Data collection
Demographic information was obtained by a review of the electronic medical record. Operative reports, clinic notes, and pathology reports were reviewed for acquisition of clinical information. Patients who received neoadjuvant chemotherapy were considered to be inevaluable for tumor grade, myometrial invasion, lymphatic/vascular space invasion, or tumor size. Tumor stage was determined using the FIGO Staging System, and stage was determined using surgical reports, imaging studies, pathology reports, and clinical findings when appropriate. Date of recurrence was defined as the first clinical identification of recurrent disease, either by imaging or tissue diagnosis. Due to the large number of patients who sought at least part of their care at an outside institution, outside records were used when available. When outside records were not available for review, patient-reported information was used. When exact dates were unavailable, dates were estimated based on available records and patient report, with the default date designated to be the first day of the reported month or year. Data were censored at August 28, 2015. Study data were collected and managed using the REDCap electronic database (12). The study was approved by the University of Texas MD Anderson Cancer Center’s institutional review board (Protocol LAB01-718).
Immunohistochemistry
When tissue was available for tumors in which a CTNNB1 mutation was detected, immunohistochemistry to detect localization of β-catenin protein was performed using formalin-fixed, paraffin-embedded sections as previously detailed (clone 14, dilution 1:500; BD Biosciences, San Jose, CA) (13). When possible, the same paraffin block/mirror image block that was used for sequencing was also used for immunohistochemistry. Presence or absence of nuclear staining was evaluated and percentage of tumor demonstrating nuclear staining was recorded. Presence of membrane staining in other epithelial cells served as an internal positive control. Immunohistochemistry assessment was performed by a trained gynecologic pathologist (RRB).
Statistical analysis
Summary statistics were used to describe the demographic and clinical characteristics of the patients. Fisher’s exact, chi-squared, Kruskal-Wallis, and Wilcoxon rank-sum test were used comparing demographic and clinical characteristics of the patients between CTNNB1 and TP53 mutation statuses. Recurrence-free survival and overall survival were estimated using the Kaplan-Meier product-limit estimator, and then a log-rank test was conducted to compare CTNNB1 and TP53 mutation status. Overall survival was measured from the date of surgery to the date of last contact or death. Patients alive were censored at the date of last contact or clinic visit. Recurrence-free survival was measured from the date of surgery to the earliest date of the last clinic visit, date of first recurrence, or date of death. Patients alive and recurrence-free were censored at the date of last contact or clinic visit. Stata v14.2 (College Station, TX) was used to conduct statistical analysis.
RESULTS
Patient Characteristics
342 endometrial cancer patients met our criteria for evaluation. Clinical and pathology characteristics are listed in Table 1. The mean age of our endometrial cancer cohort was 60.6 years, and the majority of patients had tumors with endometrioid histology, grade 2, and FIGO stage I or II at diagnosis. PTEN was the most frequent mutation (45%), followed by PIK3CA (39%) and ARID1A (38%); all mutations that were present in at least 10% of the patients in our cohort are listed in Table 1.
Table 1.
Clinical and pathology characteristics for the entire cohort and for the endometrioid tumors only.
| Characteristic | All Endometrial Cancer (n = 342) |
Endometrioid Histology (n = 245) |
Mixed Endometrioid & Non-Endometrioid Histology (n = 50) |
Non-Endometrioid Histology (n = 47) |
|---|---|---|---|---|
|
| ||||
| Age in years, mean (SD) | 60.6 (11.7) | 59.2 (11.7) | 63.1 (10.3) | 65.3 (12.1) |
|
| ||||
| Body mass index in kg/m2, mean (SD)a | 33.8 (10.5) | 35.0 (10.7) | 32.6 (9.5) | 28.9 (8.8) |
|
| ||||
| Race, n (%) | ||||
| White | 245 (72%) | 174 (71%) | 36 (72%) | 35 (74%) |
| Black | 28 (8%) | 12 (5%) | 8 (16%) | 8 (17%) |
| Hispanic | 54 (16%) | 47 (19%) | 4 (8%) | 3 (6%) |
| Asian | 14 (4%) | 11 (5%) | 2 (4%) | 1 (2%) |
| Other | 1 (0%) | 1 (0%) | 0 (0%) | 0 (0%) |
|
| ||||
| Histology, n (%) | ||||
| Endometrioid | 245 (72%) | N/A | N/A | N/A |
| Mixed Endometrioid and Non-Endometrioid | 50 (15%) | |||
| Non-Endometrioid | 47 (14%) | |||
|
| ||||
| Grade for pure endometrioid tumors, n (%)b | ||||
| 1 | N/A | 30 (13%) | N/A | N/A |
| 2 | 161 (67%) | |||
| 3 | 48 (20%) | |||
|
| ||||
| Myometrial invasion, n (%)c | ||||
| < 50% | 183 (58%) | 141 (60%) | 25 (57%) | 17 (44%) |
| ≥ 50% | 134 (42%) | 93 (40%) | 19 (43%) | 22 (56%) |
|
| ||||
| LVSI, n (%)d | ||||
| No | 138 (45%) | 116 (50%) | 13 (32%) | 9 (24%) |
| Yes | 170 (55%) | 114 (50%) | 28 (68%) | 28 (76%) |
|
| ||||
| Tumor size in cm, mean (SD)e | 4.8 (3.2) | 4.5 (3.2) | 5.2 (2.9) | 6.2 (3.6) |
|
| ||||
| Stage, n (%)f | ||||
| I or II | 210 (63%) | 173 (72%) | 24 (49%) | 13 (28%) |
| III or IV | 126 (38%) | 68 (28%) | 25 (51%) | 33 (72%) |
|
| ||||
| Mutations, n (%) | ||||
| PTEN | 154 (45%) | 132 (54%) | 17 (34%) | 5 (11%) |
| PIK3CA | 135 (39%) | 101 (41%) | 22 (44%) | 12 (26%) |
| ARID1Ag | 86 (38%) | 68 (42%) | 13 (36%) | 5 (19%) |
| PIK3R1g | 53 (24%) | 43 (27%) | 9 (25%) | 1 (4%) |
| TP53 | 73 (21%) | 32 (13%) | 19 (38%) | 22 (47%) |
| KRAS | 66 (19%) | 52 (21%) | 9 (18%) | 5 (11%) |
| CTNNB1 | 60 (18%) | 53 (22%) | 6 (12%) | 1 (2%) |
| FGFR2 | 32 (9%) | 28 (11%) | 4 (8%) | 0 (0%) |
340 patients were included in BMI assessment for the overall cohort; 2 patients did not have either a height or weight recorded at the time of their initial evaluation at the University of Texas MD Anderson Cancer Center.
239 patients were included in the endometrioid grade assessment; 6 received neoadjuvant chemotherapy.
317 patients were included in the myometrial invasion assessment for the overall cohort; 16 received neoadjuvant, 9 did not have information available.
308 patients were included in the LVSI assessment for the overall cohort; 16 received neoadjuvant chemotherapy, 18 patients did not have information available.
300 patients were included in the tumor size assessment for the overall cohort; 16 received neoadjuvant chemotherapy, 26 did not have accurate tumor size information available.
336 patients were included in the stage assessment for the overall cohort; 6 patients did not have clinical, pathology, or radiological information available for stage assessment.
225 patients were included in the analyses for the overall cohort for both ARID1A and PIK3R1.
Characteristics were then evaluated by each histology type (endometrioid, mixed histology with both endometrioid and non-endometrioid components, and non-endometrioid). For the endometrioid cohort (n=245), the mean age was slightly younger at 59.2 years, and a larger proportion of tumors were grade 1 or 2 (designated as low grade). The endometrioid cohort remained predominantly stage I or II, which we designated early stage in this analysis as these are patients with tumors that are confined to the uterus and cervix and who therefore are less likely to receive adjuvant systemic treatment. PTEN remained the most frequent mutation (54%), followed by ARID1A (42%) and PIK3CA (41%). For all subsequent analyses, the cohort was limited to only those patients with endometrioid histology, as patients with non-endometrioid or mixed endometrioid/non-endometrioid endometrial carcinomas typically receive more aggressive systemic therapy due to worsened outcomes in these subsets.
Survival Analyses of Low Grade, Early Stage Tumors
Because high grade (grade 3) and advanced stage (stage III-IV) are both known to be associated with recurrence of disease and worse survival outcomes in general, these patients often receive more aggressive adjuvant therapy, frequently including systemic treatment in the setting of advanced stage disease (2). For this reason, the survival analyses were limited to patients with low grade and early stage tumors in order to better identify patients who would be higher-risk within an otherwise lower-risk cohort. On univariate analyses of common clinical and pathology characteristics, as well as the most common somatic mutations in our cohort, only age at diagnosis (HR 1.03, 95% CI 1.01–1.06, p = 0.005), CTNNB1 exon 3 mutation (HR 2.06, 95% CI 1.15–3.69, p = 0.02), and TP53 mutation (2.49, 95% 1.05–5.90, p = 0.04) were associated with significantly worse recurrence-free survival. Kaplan-Meier curves for CTNNB1 and TP53 mutation are shown in Figures 1A and 1B. BMI, tumor size, the receipt of adjuvant therapy, the presence of lymphatic/vascular space invasion, the presence of deep myometrial invasion, or any of the other mutations listed in Table 1 were not significantly associated with recurrence-free survival (data not shown). Univariate analyses for overall survival showed only age at diagnosis to have a statistically significant impact (HR 1.08, 95% CI 1.04–1.11, p < 0.001).
Figure 1.
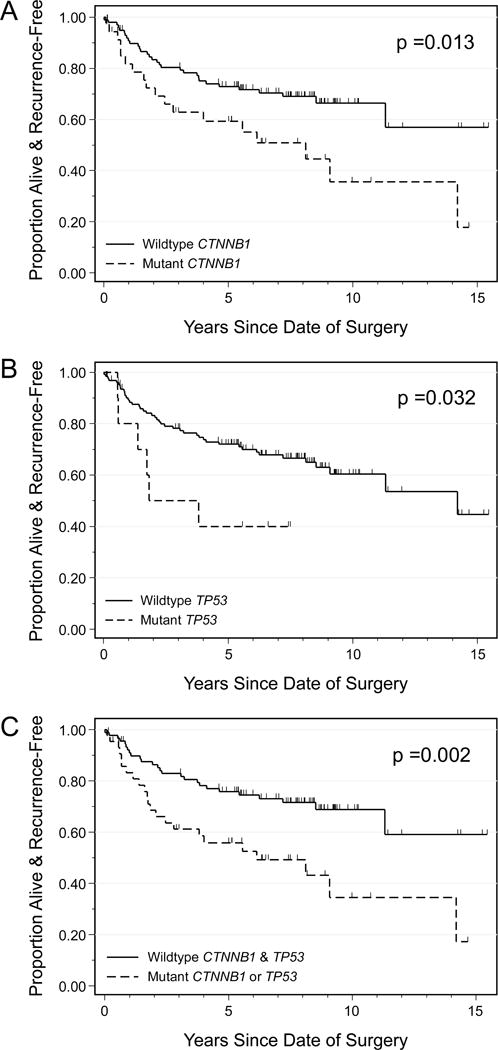
Recurrence-free survival for endometrioid endometrial cancer patients, grade 1–2 and stage I–II, by CTNNB1 mutation status (A), TP53 mutation status (B), and combined CTNNB1 and TP53 mutation (C). Presence of mutation in CTNNB1 or TP53 is associated with worse recurrence-free survival in this subset of endometrial cancer patients.
Multivariate analysis for recurrence-free survival for this low grade, early stage endometrioid cohort was next performed. The multivariate analysis included clinical and pathology characteristics which have previously been shown to be associated with survival outcomes and somatic mutations with p < 0.2 on the univariate survival analyses (CTNNB1 and TP53). Variables ultimately included were age at diagnosis, BMI, myometrial invasion, lymphatic/vascular space invasion, tumor size, adjuvant treatment, CTNNB1 mutation, and TP53 mutation (Table 2). CTNNB1 was found to have the highest hazard ratio in this multivariable analysis, with a hazard ratio of 5.97 (95% CI 2.69–13.21). Other statistically significant variables included TP53 mutation with an HR 4.07 (95% CI 1.57–10.54), and age at diagnosis with an HR 1.07 (95% CI 1.03–1.10). Of note, only 13 of 148 patients with grade 1 or 2 and stage I or II tumors had a mutation in TP53, and thus the utility of TP53 mutation as biomarker of recurrence may be limited. By comparison, CTNNB1 mutation is present in 26% of these same patients.
Table 2.
Multivariate analysis for recurrence-free survival in patients with grade 1–2, stage I–II endometrioid endometrial cancer (n=125)a.
| Variable | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Age at diagnosis | 1.07 | 1.03–1.10 | < 0.001 |
| BMI | 1.00 | 0.96–1.03 | 0.83 |
| Myometrial invasion ≥ 50% | 0.80 | 0.35–1.83 | 0.59 |
| LVSI | 1.84 | 0.84–4.03 | 0.13 |
| Tumor size | 0.95 | 0.80–1.11 | 0.50 |
| Adjuvant treatmentb | 0.80 | 0.37–1.72 | 0.80 |
| TP53 mutation | 4.07 | 1.57–10.54 | 0.004 |
| CTNNB1 mutation | 5.97 | 2.69–13.21 | <0.001 |
125 patients had the above information available and were included in the analysis
Adjuvant treatment was treated as a time-dependent covariate
Characterization of CTNNB1 Mutant Patient Cohort
In addition to significantly worse recurrence-free survival and overall survival, patients with CTNNB1 somatic mutations have other unique characteristics compared to patients with wildtype tumors (Table 3). Patients with tumors harboring CTNNB1 mutation were younger (age 61 vs. 53, p < 0.001). Despite the worse prognosis, endometrial carcinomas with CTNNB1 mutation showed higher rates of low grade tumors (76% vs. 92%, p < 0.001), lower rates of lymphatic/vascular space invasion (54% vs. 33%, p = 0.003), and lower rates of deep myometrial invasion (43% vs. 27%, p = 0.04). There were no significant differences in race, BMI, or tumor size between the mutant and wildtype group. Tumors with CTNNB1 mutation were also significantly less likely to have KRAS mutation, TP53 mutation, and FGFR2 mutation. Despite the fact that CTNNB1 mutation co-segregates with factors that otherwise would be expected to be associated with good outcomes (younger age, lower tumor grade, less myometrial invasion, lower incidence of lymphatic/vascular space invasion, and lower frequency of co-TP53 mutation), presence of this mutation is associated with significantly worse recurrence-free survival. CTNNB1 mutation does not appear to be altering the location of metastasis/recurrence, as patients with mutant and wildtype tumors have comparable incidences of extra-vaginal spread when only patients who had a recurrence of their disease are considered (68% of recurrences for wildtype group vs. 60% of recurrences for mutant group; p = 0.61).
Table 3.
Clinical and pathology characteristics of patients with endometrioid endometrial cancer, stratified by CTNNB1 mutation status.
| Characteristic |
CTNNB1 Wildtype (n = 192) |
CTNNB1 Mutant (n = 53) |
p-value |
|---|---|---|---|
|
| |||
| Age in years, mean (SD) | 60.9 (11.5) | 52.9 (10.2) | < 0.001 |
|
| |||
| Body mass index in kg/m2, mean (SD)a | 34.6 (10.7) | 36.3 (10.7) | 0.19 |
|
| |||
| Race, n (%) | 0.60 | ||
| White | 139 (72%) | 35 (66%) | |
| Black | 10 (5%) | 2 (4%) | |
| Hispanic | 35 (18%) | 12 (23%) | |
| Asian | 7 (4%) | 4 (8%) | |
| Other | 1 (1%) | 0 (0%) | |
|
| |||
| Grade, n (%)b | < 0.001 | ||
| 1 | 15 (8%) | 15 (29%) | |
| 2 | 128 (68%) | 33 (63%) | |
| 3 | 44 (24%) | 4 (8%) | |
|
| |||
| Myometrial invasion, n (%)c | 0.04 | ||
| < 50% | 106 (57%) | 35 (73%) | |
| ≥ 50% | 80 (43%) | 13 (27%) | |
|
| |||
| Lymphovascular space invasion, n (%)d | 0.01 | ||
| No | 85 (46%) | 31 (67%) | |
| Yes | 99 (54%) | 15 (33%) | |
|
| |||
| Tumor size in cm, mean (SD)e | 4.6 (3.0) | 4.1 (3.8) | 0.18 |
|
| |||
| Stage, n (%)f | 0.56 | ||
| I or II | 134 (71%) | 39 (75%) | |
| III or IV | 55 (29%) | 13 (25%) | |
|
| |||
| Mutations | |||
| KRAS | 47 (24%) | 5 (9%) | 0.02 |
| PIK3CA | 82 (43%) | 19 (36%) | 0.37 |
| TP53 | 30 (16%) | 2 (4%) | 0.02 |
| PTEN | 98 (51%) | 34 (64%) | 0.09 |
| FGFR2 | 26 (14%) | 2 (4%) | 0.05 |
| ARID1Ag | 60 (44%) | 8 (30%) | 0.15 |
| PIK3R1g | 37 (27%) | 6 (22%) | 0.58 |
243 patients were included in BMI assessment for the endometrioid cohort; 2 patients did not have either a height or weight recorded at the time of their initial evaluation at the University of Texas MD Anderson Cancer Center.
239 patients were included in the grade assessment for the endometrioid cohort; 6 received neoadjuvant chemotherapy.
234 patients were included in the myometrial invasion assessment for the endometrioid cohort; 6 received neoadjuvant, 5 did not have invasion information available.
230 patients were included in the LVSI assessment for the endometrioid cohort; 6 received neoadjuvant chemotherapy, 9 patients did not have LVSI information available.
222 patients were included in the tumor size assessment for the endometrioid cohort; 6 received neoadjuvant chemotherapy, 17 did not have accurate tumor size information available.
241 patients were included in the stage assessment for the endometrioid cohort; 4 patients did not have clinical, pathology, or radiological information available for stage assessment.
162 patients were included in the analyses for the endometrioid cohort for both ARID1A and PIK3R1.
TP53 Mutation Characterization
In the univariate and multivariate recurrence-free survival analyses, presence of a TP53 mutation was also associated with significantly worse survival. Therefore, we also stratified the baseline clinical and pathology characteristics for the endometrioid cohort by TP53 mutation status (Table 4). Patients with tumors harboring TP53 mutation were more likely to have grade 3 tumors (50% vs. 15%, p < 0.001), but there were no significant differences in age at diagnosis, BMI, race, deep myometrial invasion, lymphatic/vascular space invasion, tumor size, or stage at diagnosis. Endometrial carcinomas with TP53 mutation were also less likely to have a PTEN co-mutation (38% vs. 56%, p <0.05), but no other correlations with other frequent somatic mutations were seen.
Table 4.
Clinical and pathology characteristics of patients with endometrioid endometrial cancer, stratified by TP53.
| Characteristic |
TP53 Wildtype (n = 213) |
TP53 Mutant (n = 32) |
p-value |
|---|---|---|---|
|
| |||
| Age in years, mean (SD) | 59.2 (11.4) | 59.1 (13.7) | 0.98 |
|
| |||
| Body mass index in kg/m2, mean (SD)a | 34.9 (10.5) | 35.8 (12.3) | 0.99 |
|
| |||
| Race, n (%) | > 0.99 | ||
| White | 150 (70%) | 23 (75%) | |
| Black | 11 (5%) | 1 (3%) | |
| Hispanic | 41 (19%) | 6 (19%) | |
| Asian | 10 (5%) | 1 (3%) | |
| Other | 1 (0%) | 0 (0%) | |
|
| |||
| Grade, n (%)b | < 0.001 | ||
| 1 | 27 (13%) | 3 (9%) | |
| 2 | 148 (71%) | 13 (41%) | |
| 3 | 32 (15%) | 16 (50%) | |
|
| |||
| Myometrial invasion, n (%)c | 0.51 | ||
| < 50% | 124 (61%) | 17 (55%) | |
| ≥ 50% | 79 (39%) | 14 (45%) | |
|
| |||
| Lymphovascular space invasion, n (%)d | 0.16 | ||
| No | 104 (52%) | 12 (39%) | |
| Yes | 95 (48%) | 19 (61%) | |
|
| |||
| Tumor size in cm, mean (SD)e | 4.4 (2.9) | 5.1 (4.4) | 0.89 |
|
| |||
| Stage, n (%)f | 0.59 | ||
| I or II | 152 (72%) | 21 (68%) | |
| III or IV | 58 (28%) | 10 (32%) | |
|
| |||
| Mutations | |||
| KRAS | 46 (22%) | 6 (19%) | 0.71 |
| PIK3CA | 87 (41%) | 14 (44%) | 0.76 |
| CTNNB1 | 51 (24%) | 2 (6%) | 0.02 |
| PTEN | 120 (56%) | 12 (38%) | < 0.05 |
| FGFR2 | 23 (11%) | 5 (16%) | 0.42 |
| ARID1Ag | 64 (44%) | 4 (27%) | 0.28 |
| PIK3R1g | 38 (26%) | 5 (33%) | 0.53 |
243 patients were included in BMI assessment for the endometrioid cohort; 2 patients did not have either a height or weight recorded at the time of their initial evaluation at the University of Texas MD Anderson Cancer Center.
239 patients were included in the grade assessment for the endometrioid cohort; 6 received neoadjuvant chemotherapy.
317 and 234 patients were included in the myometrial invasion assessment for the endometrioid cohort; 6 received neoadjuvant, 5 did not have invasion information available.
230 patients were included in the LVSI assessment for the endometrioid cohort; 6 received neoadjuvant chemotherapy, 9 patients did not have LVSI information available.
222 patients were included in the tumor size assessment for the endometrioid cohort; 6 received neoadjuvant chemotherapy, 17 did not have accurate tumor size information available.
241 patients were included in the stage assessment for the endometrioid cohort; 4 patients did not have clinical, pathology, or radiological information available for stage assessment.
162 patients were included in the analyses for the endometrioid cohort for both ARID1A and PIK3R1.
Analyses Using Combination of CTNNB1 and TP53 Mutation
TP53 mutation was present in only 9% of grade 1 or 2 and stage I or II endometrioid carcinomas, while CTNNB1 mutation was present in 26% of this same subset. Thus, TP53 has limited utility as a single biomarker in the low grade, early stage endometrial cancer patients. Of note, only one patient had a tumor with a mutation in both CTNNB1 and TP53, suggesting that these mutations occur in relatively distinct subsets of patients. We therefore sought to evaluate the association of the presence of CTNNB1 or TP53 mutation for prediction of recurrence-free survival in order to potentially increase the number of patients captured by molecular evaluation. On a univariate recurrence-free survival evaluation, the presence of either a TP53 mutation or a CTNNB1 mutation was associated with a significantly worse recurrence-free survival (p = 0.002) (Figure 1C). We then evaluated a multivariate model which included the same variables as our previous multivariable model, with the exception of a new combination variable which encompassed the presence of a CTNNB1 or TP53 mutation compared with having neither mutation. In this model, the combination variable of CTNNB1 or TP53 mutation remained statistically significant, with an HR 4.69 (95% CI 2.38–9.24) (Table 5). Age at diagnosis was the only other variable with a significant association with recurrence-free survival (HR 1.06, 95% CI 1.03–1.09).
Table 5.
Multivariate analysis for recurrence-free survival in patients with grade 1–2, stage I–II endometrioid endometrial cancer evaluating a combination of CTNNB1 and TP53 mutations.a
| Characteristic | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age at diagnosis | 1.06 | 1.03 – 1.09 | < 0.001 |
| BMI | 1.00 | 0.96 – 1.03 | 0.87 |
| Myometrial invasion (≥ 50%) | 0.86 | 0.39 – 1.90 | 0.72 |
| LVSI | 1.83 | 0.84 – 3.99 | 0.13 |
| Tumor size | 0.95 | 0.81 – 1.12 | 0.57 |
| Adjuvant therapyb | 0.78 | 0.37 – 1.65 | 0.51 |
| CTNNB1 or TP53 mutation | 4.69 | 2.38 – 9.24 | < 0.001 |
125 patients had the above information available and were included in the analysis
Adjuvant treatment was treated as a time-dependent covariate
Immunohistochemistry
Of the 60 tumors with CTNNB1 mutation, 50 were able to be evaluated for β-catenin immunohistochemistry. Of these, 42 (84%) demonstrated nuclear expression. The proportion of the tumor with nuclear staining ranged from 5–60%. Eleven of 42 tumors (26%) had nuclear expression in at least 30% of the tumor. All tumors, even those with no nuclear expression, had cytoplasmic protein expression.
DISCUSSION
Our findings demonstrate a significantly decreased recurrence-free survival for patients with low grade, early stage endometrioid endometrial cancers whose tumors harbored a CTNNB1 or TP53 mutation and showed that this reduction persisted on a multivariate analysis. The effect of these mutations on overall survival is more uncertain, likely due in part to the longer clinical course associated with this subset of endometrial cancer patients. CTNNB1 mutation was associated with worse overall survival on multivariate but not univariate analysis, and TP53 mutation had no effect on overall survival in either the univariate or multivariate analyses. From a practical standpoint, although both biomarkers were useful independently, incorporation of both TP53 and CTNNB1 mutation information led to more precise estimates of recurrence risk than either alone. Further, use of either individual or combination evaluation was associated with a higher hazard ratio than any other clinical or pathology finding in their respective multivariate analyses and specifically was higher than commonly relied upon histologic characteristics such as deep myometrial invasion and lymphatic/vascular space invasion.
Following the publication of the Cancer Genome Atlas’s (TCGA) endometrial cancer data, we have a more thorough understanding of the genomics of endometrial cancer. The TCGA analysis revealed high rates of PI3K/AKT pathway mutations, as well as KRAS, CTNNB1, and ARID1A mutations within endometrioid tumors (14). Tumors with CTNNB1 mutation were predominantly contained within the microsatellite-stable, copy-number low endometrioid cluster (14). A recent reanalysis of TCGA data limited to the 271 tumors with endometrioid histology, excluding the non-endometrioid serous carcinomas, found that those patients whose tumors had activation of the Wnt/β-catenin pathway activation had worse overall survival even when compared to other low grade cohorts (15). Higher expression of Cyclin D1 and Myc, two genes known to be activated by Wnt/β-catenin pathway activation, were associated with worse survival (15). CTNNB1 mutation is one mechanism that can activate this pathway; the TCGA cluster with Wnt/β-catenin pathway activation and CTNNB1 mutation had the lowest number of other concurrent mutations. Similarly, in our patient cohort the group with CTNNB1 mutation had a significantly lower incidence of concurrent KRAS and TP53 mutations. In support of the idea that CTNNB1 mutation is a driver, rather than passenger, in endometrial carcinogenesis, exon 3 deletion of the CTNNB1 gene in a murine model led to upregulation of the Wnt/beta-catenin pathway and the development of endometrial hyperplasia, a precursor to endometrioid-type endometrial carcinoma (16). In a different mouse model, activation of uterine targeted β-catenin and loss of PTEN resulted in endometrial adenocarcinoma that was earlier in onset and more aggressive than in mice with PTEN loss alone (17). At this point, we do not know whether CTNNB1 gene mutation or Wnt/β-catenin pathway activation as measured by upregulation of pathway genes is a more powerful prognostic indicator. However, sequencing of hotspot mutations in CTNNB1 is a less technically challenging assay for the clinical molecular diagnostics laboratory and as it is a dichotomous variable has less challenges with setting cut offs.
Several prior studies have evaluated the impact of CTNNB1 mutation in endometrial cancer. A case control study of 47 stage IA grade 1 endometrial cancer patients found a nine times higher odds of CTNNB1 mutation in tumors of those patients who recurred compared to those who did not, with no differences in odds of KRAS or PIK3CA mutation (18). Alternatively, a 2012 study by Byron et al. evaluated disease-free survival and overall survival in 386 cases of stage I or II endometrioid endometrial cancer, and found no difference based on CTNNB1 mutation status (19). In contrast to our study, however, these data included all grades of tumors in the early stage analysis. When our data included all grades of endometrioid tumors, we similarly did not find CTNNB1 to be statistically significantly associated with recurrence-free survival. This observation further underscores the importance of considering CTNNB1 mutation within the context of low grade, early stage tumors, as there is likely to be limited utility of this assessment within the very heterogeneous cohort of all endometrial cancers. Two other studies also evaluated a more heterogeneous endometrial cancer patient population, including high grade and/or late stage tumors, and had conflicting results in terms of survival outcomes. Both of these studies used immunohistochemistry as a surrogate for mutation status (20, 21). The prevalence of mutant tumors based on immunohistochemistry in the study by Athanassidou et al. was significantly higher than that previously described in the literature for endometrial carcinomas, suggesting that there may be a discordance in β-catenin immunohistochemical staining pattern and presence of mutation as defined by sequencing. Although the majority of tumors in our study demonstrated nuclear expression of β-catenin protein, 16% did not. Furthermore, only 24% of tumors had nuclear expression in 30% or more of the tumor cells. This pattern of nuclear expression is consistent with that reported previously (22–24). Therefore, for endometrial cancer, it is unclear if immunohistochemistry can act as an effective surrogate to CTNNB1 gene sequencing.
The presence of miRNAs has also been shown to be associated with lymph node metastases (25). As data have linked specific miRNAs to the Wnt/b-catenin pathway (26, 27), these miRNAs may represent another viable option for identifying higher risk tumors, or further elucidating mechanisms for the worse prognoses of these patients. A disadvantage of this approach is that currently there are no clinical-grade assays for miRNA assessment.
Less research has delineated the epidemiologic or clinical characteristics of low grade tumors with TP53 mutation. Prevalence rates of TP53 mutation have been reported to be about 10–20% in endometrioid endometrial cancer (20, 28, 29), with the majority occurring in grade 3 endometrioid tumors (30–33). A 2012 reanalysis of a subset of the PORTEC-2 trial population found that 9 of 48 patients with low risk or high intermediate risk EC (all with grade 1–2 and stage I disease) had a TP53 mutation based on increased levels on immunohistochemistry analysis and demonstrated TP53 to be the single most significant prognostic factor on multivariate disease-free survival analysis. Although the prevalence of TP53 mutation was higher than in our current study, the overall association with disease recurrence mirrors the findings presented in our current research.
In our current study, neither myometrial invasion nor lymphatic/vascular space invasion were associated with recurrence-free survival in our multivariate analysis of low grade, early stage patients, which is contrary to the high-intermediate risk criteria currently in use (3, 5). Interestingly, CTNNB1 and TP53 were not only both associated with recurrence-free survival, but had significantly elevated hazard ratios on par with those seen with tumor grade in prior studies of early stage endometrial cancers (5, 9). We suspect the reason that myometrial invasion, lymphatic/vascular invasion, and tumor size were not independent predictors in this retrospective cohort may have been related to common adjuvant treatment decision-making strategies within this retrospective cohort, as providers may have already taken these variables into account and thereby decreased these patients’ risks for recurrence. However, this finding further highlights the importance of the patient population identified by CTNNB1 and TP53 assessment, as these patients are not being captured by current risk-prediction algorithms. In fact, patients with endometrial carcinomas with CTNNB1 mutation are significantly more likely to have tumors with pathological characteristics commonly associated with lower clinical risk of recurrence (lower FIGO grade, less incidence of deep myometrial invasion, and less incidence of lymphatic/vascular space invasion). This seeming paradox highlights that pathological variables traditionally used to assess recurrence risk may not be optimal clinical benchmarks.
This study adds to the growing body of literature that suggests that molecular testing may be able to inform treatment decision making for endometrial cancer patients and highlights a subgroup of endometrial cancer patients whose optimal treatment strategies remain uncertain. Several strategies have been proposed (14, 34, 35), most recently using data from PORTEC. McAlpine et al. proposed an approach, using POLE sequencing, mismatch repair protein immunohistochemistry, and p53 immunohistochemistry, in which the two TCGA subgroups with higher grade endometrioid and serous carcinomas could be separated into two distinct groups based on survival differences, with the POLE mutant group having significantly better survival. Similarly, we propose that assessment of CTNNB1 and TP53 mutation status can help to stratify the two TCGA groups with the lower grade endometrioid carcinomas into prognostic groups. In the PORTEC study, an algorithm was proposed in which tumors were stratified using TP53 mutation, microsatellite instability, and POLE mutation, and those that remained following the three prior evaluations (34). Within this broad fourth category, CTNNB1 mutation was found to be associated with increased risk of distant recurrence and thus the authors advocated for its evaluation in risk-stratification evaluation (34). Including Myers et al., PORTEC, and our current study, there are now three published studies in three distinct endometrial cancer patient populations that CTNNB1 tumor mutation was associated with recurrence of disease. Thus, we believe that use of CTNNB1 sequencing as a prognostic should be studied in prospective clinical trials. Furthermore, as our current study also found a significant rate of recurrences outside of the vagina, these findings suggest that vaginal brachytherapy alone in patients with CTNNB1 mutant tumors may be insufficient for recurrence-prevention.
With the growing clinical availability of molecular testing, including molecular information along with the usual pathology and clinical data in treatment planning algorithms is becoming a more realistic goal. Our current data suggest that even these patients with low grade, early stage disease may benefit from molecular profiling of their endometrial cancers. Prospective clinical trials are needed to better characterize the value of adjuvant treatment strategies in otherwise low risk patients with high risk mutations, with the ultimate goal of incorporating molecular information into routine endometrial cancer treatment algorithms.
Acknowledgments
Financial Support: NIH Research Training Grant (KCK) T32 CA101642; NIH SPORE in Uterine Cancer (RRB) NIH 2P50 CA098258; The Red and Charline McCombs Institute Center for Global Cancer Early Detection (RRB); NIH through MD Anderson’s Cancer Center Support Grant (BMF and DU) CA016672
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 3.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 5.Creutzberg CL, Nout RA, Lybeert ML, et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e631–8. doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–46. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigsby PW, Perez CA, Kuten A, et al. Clinical stage I endometrial cancer: prognostic factors for local control and distant metastasis and implications of the new FIGO surgical staging system. Int J Radiat Oncol Biol Phys. 1992;22:905–11. doi: 10.1016/0360-3016(92)90786-h. [DOI] [PubMed] [Google Scholar]
- 8.Lurain JR, Rice BL, Rademaker AW, et al. Prognostic factors associated with recurrence in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol. 1991;78:63–9. [PubMed] [Google Scholar]
- 9.Rydzewski NR, Strohl AE, Donnelly ED, et al. Receipt of vaginal brachytherapy is associated with improved survival in women with stage I endometrioid adenocarcinoma of the uterus: A National Cancer Data Base study. Cancer. 2016;122:3724–31. doi: 10.1002/cncr.30228. [DOI] [PubMed] [Google Scholar]
- 10.Meric-Bernstam F, Johnson A, Holla V, et al. A decision support framework for genomically informed investigational cancer therapy. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, Meric-Bernstam F, Zhao H, et al. Clinical actionability enhanced through deep targeted sequencing of solid tumors. Clin Chem. 2015;61:544–53. doi: 10.1373/clinchem.2014.231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in β-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–27. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research N. Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Patel L, Mills GB, et al. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong JW, Lee HS, Franco HL, et al. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Zee M, Jia Y, Wang Y, et al. Alterations in Wnt-beta-catenin and Pten signalling play distinct roles in endometrial cancer initiation and progression. J Pathol. 2013;230:48–58. doi: 10.1002/path.4160. [DOI] [PubMed] [Google Scholar]
- 18.Myers A, Barry WT, Hirsch MS, Matulonis U, Lee L. beta-Catenin mutations in recurrent FIGO IA grade I endometrioid endometrial cancers. Gynecol Oncol. 2014;134:426–7. doi: 10.1016/j.ygyno.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Byron SA, Gartside M, Powell MA, et al. FGFR2 point mutations in 466 endometrioid endometrial tumors: relationship with MSI, KRAS, PIK3CA, CTNNB1 mutations and clinicopathological features. PloS One. 2012;7:e30801. doi: 10.1371/journal.pone.0030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nout RA, Bosse T, Creutzberg CL, et al. Improved risk assessment of endometrial cancer by combined analysis of MSI, PI3K-AKT, Wnt/beta-catenin and P53 pathway activation. Gynecol Oncol. 2012;126:466–73. doi: 10.1016/j.ygyno.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Athanassiadou P, Athanassiades P, Grapsa D, et al. The prognostic value of PTEN, p53, and beta-catenin in endometrial carcinoma: a prospective immunocytochemical study. Int J Gynecol Cancer. 2007;17:697–704. doi: 10.1111/j.1525-1438.2007.00845.x. [DOI] [PubMed] [Google Scholar]
- 22.Machin P, Catasus L, Pons C, et al. CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol. 2002;33:206–12. doi: 10.1053/hupa.2002.30723. [DOI] [PubMed] [Google Scholar]
- 23.Saegusa M, Hashimura M, Yoshida T, et al. beta-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001;84:209–17. doi: 10.1054/bjoc.2000.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saegusa M, Okayasu I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001;194:59–67. doi: 10.1002/path.856. [DOI] [PubMed] [Google Scholar]
- 25.Canlorbe G, Wang Z, Laas E, et al. Identification of microRNA expression profile related to lymph node status in women with early-stage grade 1–2 endometrial cancer. Mod Pathol. 2016;29:391–401. doi: 10.1038/modpathol.2016.30. [DOI] [PubMed] [Google Scholar]
- 26.Aprelikova O, Palla J, Hibler B, et al. Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumor cell motility. Oncogene. 2013;32:3246–53. doi: 10.1038/onc.2012.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravo M, Cordella A, Rinaldi A, et al. Small non-coding RNA deregulation in endometrial carcinogenesis. Oncotarget. 2015;6:4677–91. doi: 10.18632/oncotarget.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int. 2010;2010:162363. doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelsen IB, Stefansson I, Akslen LA, Salvesen HB. Pathologic expression of p53 or p16 in preoperative curettage specimens identifies high-risk endometrial carcinomas. Am J Obstet Gynecol. 2006;195:979–86. doi: 10.1016/j.ajog.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 30.Graesslin O, Chantot-Bastaraud S, Lorenzato M, et al. Fluorescence in situ hybridization and immunohistochemical analysis of p53 expression in endometrial cancer: prognostic value and relation to ploidy. Ann Surg Oncol. 2008;15:484–92. doi: 10.1245/s10434-007-9712-1. [DOI] [PubMed] [Google Scholar]
- 31.Kihana T, Hamada K, Inoue Y, et al. Mutation and allelic loss of the p53 gene in endometrial carcinoma. Incidence and outcome in 92 surgical patients. Cancer. 1995;76:72–8. doi: 10.1002/1097-0142(19950701)76:1<72::aid-cncr2820760110>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Jeon YT, Kang S, Kang DH, et al. Cyclooxygenase-2 and p53 expressions in endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1538–42. [PubMed] [Google Scholar]
- 33.Semczuk A, Marzec B, Skomra D, et al. Allelic loss at TP53 is not related to p53 protein overexpression in primary human endometrial carcinomas. Oncology. 2005;69:317–25. doi: 10.1159/000089764. [DOI] [PubMed] [Google Scholar]
- 34.Stelloo E, Nout RA, Osse EM, et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin Cancer Res. 2016;22:4215–24. doi: 10.1158/1078-0432.CCR-15-2878. [DOI] [PubMed] [Google Scholar]
- 35.Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113:299–310. doi: 10.1038/bjc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]


