Abstract
Background
In sub-Saharan Africa couple HIV testing and counseling (CHTC) has been associated with substantial increases in safe sex, especially when at least one partner is HIV-infected. However, this relationship has not been characterized in an Option B+ context.
Setting
The study was conducted at the antenatal clinic at Bwaila District Hospital in Lilongwe, Malawi in 2016 under an Option B+ program.
Methods
Ninety heterosexual couples with an HIV-infected pregnant woman (female-positive couples) and 47 couples with an HIV-uninfected pregnant woman (female-negative couples) were enrolled in an observational study. Each couple member was assessed immediately before and one month after CHTC for safe sex (abstinence or consistent condom use in the last month). Generalized estimating equations were used to model change in safe sex before and after CHTC and to compare safe sex between female-positive and female-negative couples.
Results
Mean age was 26 years among women and 32 years among men. Before CHTC, safe sex was comparable among female-positive couples (8%) and female-negative couples (2%) (RR: 3.7, 95% CI: 0.5, 29.8). One month after CHTC, safe sex was higher among female-positive couples (75%) than among female-negative couples (3%) (RR: 30.0, 95% CI: 4.3, 207.7). Safe sex increased substantially after CTHC for female-positive couples (RR 9.6, 95% CI: 4.6, 20.0), but not for female-negative couples (RR: 1.2, 95% CI: 0.1, 18.7).
Conclusion
Engaging pregnant couples in CHTC can have prevention benefits for couples with an HIV-infected pregnant woman, but additional prevention approaches may be needed for couples with an HIV-uninfected pregnant woman.
Keywords: HIV, pregnancy, couples, condom, prevention, counseling, communication
Introduction
In 2011, Malawi adopted Option B+, a test-and-treat approach to the prevention of mother to child transmission (PMTCT).1 Under Option B+, women routinely test for HIV during pregnancy, and those who test HIV-positive are eligible to start immediate lifelong antiretroviral therapy (ART).2 Option B+ has been brought to scale nationwide, helping many women learn their HIV statuses and initiate ART.2-5 However, failure to engage male partners in PMTCT programs has been associated with worse maternal and infant outcomes,6-8 as well as missed opportunities for men to learn their own HIV statuses and access HIV prevention and treatment.
Couple HIV testing and counseling (CHTC) is a service in which two couple members learn their HIV statuses together from a trained counselor. Following CHTC, couples in which one or both partners are HIV-infected often increase consistent condom use in the following month and sustain this over time.9-11 CHTC has also been associated with lowered HIV incidence.12 However, these assessments were conducted prior to the “treatment as prevention” (TasP) paradigm, the understanding that sexual transmission is negligible when an HIV-infected partner is adherent to ART and virally suppressed.13 Within a TasP context, some couples may select abstinence or consistent condom as a temporary “bridge” strategy in the months prior to viral suppression. Understanding the impact of CHTC on immediate consistent condom use or abstinence within a TasP program, like Option B+, is important.
Additionally, although the effect of CHTC on consistent condom use is well-documented, the dyadic context in which behavior change occurs is not, especially within heterosexual couples in sub-Saharan Africa.14-17 In this formative work, we used Karney's dyadic framework to explore how proximal relationship contextual factors, including trust, intimacy, satisfaction, communication, HIV communication, and relationship power influence HIV behaviors.14 Specifically, it is not known how CHTC influences the relationship context or how this context differs between couples with and without an HIV-infected woman. Finally, it is not known how these factors relate to consistent condom use and abstinence among heterosexual sub-Saharan Africa couples.
Within Malawi's Option B+ program, we assess whether CHTC is associated with safe sex among heterosexual couples with HIV-infected women (female-positive couples) and couples with HIV-uninfected women (female-negative couples), as well as whether relationship factors are associated with safe sex. Understanding these questions has implications for CHTC targeting within an Option B+ TasP program.
Methods
Study Setting
The study was conducted from December 2015-August 2016 at the antenatal clinic (ANC) at Bwaila District Hospital, a high-volume urban maternity hospital in Lilongwe, Malawi. During this period, approximately 1200 pregnant women presented each month for a first antenatal visit. Consistent with Malawi's Option B+ program, all women without a documented HIV status were tested for HIV at their first antenatal visit using opt-out procedures, and newly diagnosed women were offered same-day ART through the Option B+ program. During this period antenatal HIV prevalence was 11%, with approximately half of these HIV-infected women already on ART and half initiating ART. Approximately 10-15% of these pregnant women present with male partners for CHTC, and the rest receive individual HTC.18 CHTC was received from a trained counselor and consisted of couple pre-test counseling, simultaneous testing and return of test results, and couple post-test counseling. Couples with at least one HIV-infected person received messages about antiretroviral therapy initiation, care-seeking and adherence, and HIV-discordant couples received messages about TasP. All couples received messages about consistent condom use, but the rationales differed: transmission prevention for HIV-discordant couples, reinfection prevention for HIV-concordant positive couples, and prevention of future transmission for HIV-concordant negative couples.
Study Participants and Procedures
During the study period, all women with HIV-positive test results were approached in ANC and screened for eligibility. These HIV-infected pregnant women were eligible if they tested HIV-positive at their initial ANC visit, were ≥ 18 years old, had a current male sexual partner, had not received CHTC at that ANC visit, were willing to invite a partner for CHTC, believed they would both be in Lilongwe for ≥2 months, and were interested in study participation. Eligible women interested in participation provided informed consent
Each woman was given one invitation for a male partner to present to the clinic for important family health information. Women provided their partners' phone numbers, when available, and could elect to have the clinic call their partners right away or to wait for one week. Because this was a study of couples, data were only collected from women who presented with a partner. These couples which had an HIV-infected pregnant woman were referred to as “female-positive couples.”
We also enrolled “female-negative couples,” those with an HIV-uninfected pregnant woman. Due to staffing limitations, we sought to enroll only one female-negative couple, for every two female-positive couples enrolled. HIV-uninfected women were selected from ANC using frequency matching based on age categories of HIV-infected women: 18-19, 20-24, 25-29, 30-34, and 35-39 years. Each potential HIV-uninfected woman was screened using the same eligibility criteria as HIV-infected women. If they consented, they were provided with the same invitation, and their partners were traced using the same procedures as female-positive couples.
Once a couple presented together, they each had two visits: a first visit on the day they first presented together and a second visit one month later. At the first visit, the two partners initially met separately with same-sex interviewers; the man provided informed consent, and the man and the woman participated in separate interviewer-administered questionnaires about demographics, relationship characteristics and dynamics, and HIV care-seeking and sexual behaviors. Afterwards, couples received CHTC and were offered condoms—typically 20-30, but more if they requested them or reported more than 30 coital acts per month. Condoms were received from study staff, rather than the clinic's pharmacy, to minimize logistics and avoid stock-outs. Other procedures were consistent with national CHTC protocols.
At the second visit, each participant completed a second, similar interviewer-administered questionnaire. Participants who returned without their partners at the second visit were still interviewed.
Data were collected on Android tablets using Open Data Kit software and uploaded to an encrypted web-based server. Data were downloaded bi-weekly and stored on a secure server.
Measures
The primary outcome of interest was safe sex, assessed at both the first study visit (pre-CHTC) and second study visit (post-CHTC). At both visits, male and female participants were asked how many sexual encounters they had with their study partner in the last month, and, of these, how many were protected with a condom. We divided responses into four categories for each couple: abstinence (no sexual activity), no condom use (0% of encounters protected), inconsistent condom use (1-99% of encounters protected), and consistent condom use (100% of encounters protected). For modeled analyses, we created a variable “safe sex” which dichotomized consistent condom use or abstinence (safe sex) versus no or inconsistent-condom use (unsafe sex). As sensitivity analyses, we also explored “any condom use” (inconsistent and consistent condom use versus non-condom use) and “consistent condom use” (consistent condom use versus all other categories). If couple responses differed, the riskier behavior was used. If only one couple member presented, only that response was used.
Relationship characteristics (perceptions about the relationship) were assessed from each partner separately before and after CHTC using scales with validated psychometric properties used previously in sub-Saharan Africa.15,19,20
Commitment and intimacy were assessed using sub-scales from the Sternberg Triangular Love Scale.21 The seven-item commitment sub-scale measured relationship stability and longevity with items such as, “my relationship with my partner is permanent.” The five-item intimacy sub-scale measured closeness with items like, “my partner and I have a mutual understanding of each other.” Both used five-point Likert responses (strongly disagree-strongly agree) and were reported as means with a high score of 5 and higher values indicating better relationships.
The eight-item Dyadic Trust scale addressed issues of perceived honesty, reliance, and fairness on a five-point Likert scale (strongly disagree-strongly agree) with questions such as “my partner is sincere in his/her promises.22” Higher values corresponded to greater trust with a total possible summed score of 40.
Couples Satisfaction Index assessed perceived happiness, comfort, and reward,23 with items such as “how rewarding is your relationship?” Higher values corresponded to greater satisfaction with a total possible summed score of 20.
Three relationship dynamics (interaction patterns between couples) that were measured included communication, HIV communication, and sexual relationship power. The latter two scales included questions related to HIV and sexual behavior.
Communication was measured using three questions from the Communication Patterns Questionnaire-Short Form,24,25 which addressed how couples handled relationship challenges. For example, “When issues arise, we try to discuss them.” Items were evaluated on a four-point Likert scale (very unlikely-very likely) with higher values indicating better communication. Items were summed for a total possible score of 12.
HIV communication was assessed with a scale developed by the study team using items from other HIV communication scales,26 adding newer prevention modalities (e.g. medical male circumcision), and removing contextually irrelevant items (e.g. injection drug use). The scale contained 12 items for female-negative couples and 13 items for female-positive couples. One example was, “In the last month, have you had a conversation about HIV prevention?” Responses were recorded as yes or no and were averaged for a total score of 1; higher values indicated greater communication.
Sexual relationship power was evaluated using the 15-question relationship control subscale of the Sexual Relationship Power Scale. 27 This scale was developed to assess relationship power in women but has been used in multiple samples of men with comparable psychometric properties.28 The subscale assessed whether a sexual partner exhibited controlling behaviors. For example, “my partner will get violent if I ask to use a condom.” Items were evaluated on a four-point Likert scale (strongly disagree-strongly agree) and reported as means. Higher scores corresponded to greater relationship power with a maximum score of 4.
Scale items are presented in Appendix 1. On all scales, in descriptive analyses, we considered values >80% to be high, 50%-79% to be moderate, <50% to be low.
For dyadic models, we calculated the mean score of each measure for both couple members, as well as the difference between couple members (female minus male values). If a couple member did not present for visit two, his or her baseline value was used.
Statistical Methods
Fisher exact tests were used to evaluate differences between baseline demographic characteristics of case and control participants separately by gender. We also explored frequencies of abstinence, no condom use, inconsistent condom use, and condom use by men and women from female-positive and female-negative couples. Correlation between couple members was calculated using intra-class correlation coefficients (ICC).
We modeled changes in relationship factors and sexual behavior pre- and post-CHTC at the couple level using generalized estimating equations. These models included one record per dyad pre-HTC and one record per dyad post-CHTC. In models comparing changes in relationship factors, we used an identity link and normal distribution to estimate changes in means and 95% confidence intervals (CIs). In models comparing changes in safe sex, we used a log link and binomial distribution to estimate risk ratios (RRs) and 95% CIs. All models used exchangeable correlation matrices and robust variance estimates to account for correlation between time periods.29 All unadjusted models contained a terms for pre-/post-CHTC, couple status (female-positive/female-negative), and their interaction. All adjusted models contained a term for age category to account for frequency matching and relationship length, a known confounder; additional covariates were selected using backward elimination. All covariates associated with the exposure or outcome were included in a full model and eliminated if they did not change the primary coefficient of interest, pre-/post-CHTC, by more than 15%.
We ran a second set of models to assess the association between baseline relationship factors and safe sex post-CHTC using generalized linear methods. We modeled RRs and 95% CIs using modified Poisson regression with robust variance estimates. Models were adjusted for age category, relationship length, and couple HIV status. Analyses were performed using SAS version 9.4 (Cary, North Carolina, USA).
Ethics
The study received approval from both the National Health Science Research Committee in Malawi and the University of North Carolina at Chapel Hill Institutional Review Board. All participants provided informed consent.
Results
Population
Three-hundred and eighteen HIV-infected women were screened; 202 (64%) were eligible (Figure 1). The main reasons for ineligibility were not having a current male sexual partner (N=30, 9%) or not having a male partner in Lilongwe (N=72, 23%). Few lacked interest in participation (N=15, 5%). Ninety-seven HIV-uninfected women were screened, and, of these, 92 (95%) were eligible. Few lacked a current sexual partner (N=3, 3%), and none had a partner living outside of Lilongwe. Few lacked interest in participation (N=3, 3%).
Figure 1. Consort Diagram.
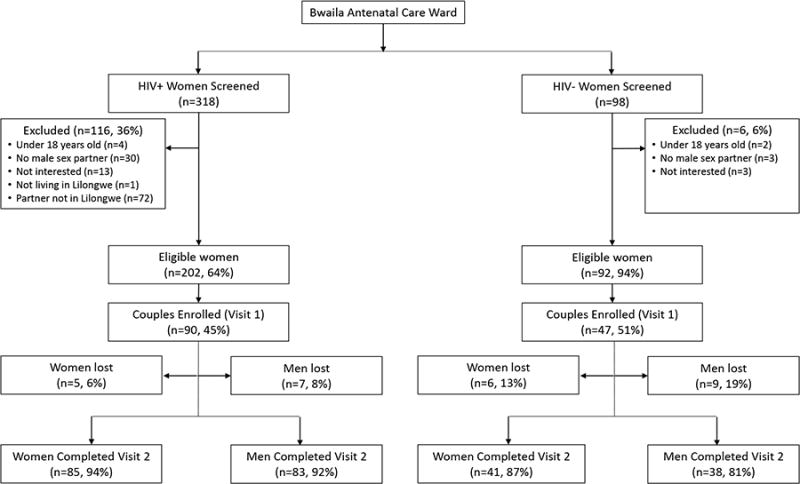
Figure 1 depicts the proportion of women screened, eligible, enrolled, and retained. Women may have been excluded for more than one reason.
Of those eligible, participation rates were comparable among HIV-infected (45%) and HIV-uninfected women (51%) (p=0.3) (Figure 1). Ninety female-positive couples and 47 female-negative couples enrolled, exceeding the enrollment target by two. The remaining eligible couples failed to present; additional information on these couples is not available. Most couples (121, 88%) had both members present, five (4%) had only the woman present, and 11 (8%) had neither member present.
The mean age among women was 26 years. The mean age among men was 32 years. Median relationship duration was four years (IQR: 2-7 years). 99% were married and 98% believed their study partner fathered the current pregnancy. Median gestational age at enrolment was five months (IQR: 5-6 months).
HIV status pre- and post-CHTC
Most women (72%) had tested for HIV prior to their antenatal visit. Of these women, 9% had received an HIV-positive result prior to their antenatal visit. Most men (69%) had also tested for HIV before study enrollment. Of these men, 13 (14%) had received an HIV-positive result previously (Table 1), and five reported currently being on ART. Thus, for most HIV-infected women (84/90, 93%) and HIV-infected men (56/69, 81%), the HIV-positive diagnosis was new.
Table 1. Baseline Characteristics of Enrolled Persons.
| F+ Females (N=90) | F-Females (N=47) | Fisher's Exact Test | F+ Males (N=90) | F- Males (N=46)* | Fisher's Exact Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| N | (%) | N | (%) | p-value | N | (%) | N | (%) | p-value | |
| HIV Status | ||||||||||
| HIV-positive | 90 | (100%) | 0 | (0%) | 67 | (74%) | 2 | (4%) | ||
| HIV-negative | 0 | (0%) | 47 | (100%) | 22 | (24%) | 43 | (93%) | ||
| Unknown | 0 | (0%) | 0 | (0%) | < 0.001 | 1 | (1%) | 1 | (2%) | < 0.001 |
| Age | ||||||||||
| 18-24 | 40 | (44%) | 21 | (45%) | 13 | (14%) | 7 | (15%) | ||
| 25-29 | 26 | (29%) | 14 | (30%) | 22 | (24%) | 14 | (30%) | ||
| 30-34 | 20 | (22%) | 9 | (19%) | 25 | (28%) | 9 | (20%) | ||
| 35-40 | 4 | (4%) | 3 | (6%) | 0.9 | 30 | (33%) | 16 | (35%) | 0.7 |
| Education | ||||||||||
| None | 8 | (9%) | 5 | (11%) | 9 | (10%) | 1 | (2%) | ||
| Primary incomplete | 28 | (31%) | 16 | (34%) | 20 | (22%) | 6 | (13%) | ||
| Primary completed | 15 | (17%) | 6 | (13%) | 20 | (22%) | 6 | (13%) | ||
| Secondary incomplete | 24 | (27%) | 10 | (21%) | 14 | (16%) | 5 | (11%) | ||
| Secondary completed | 15 | (17%) | 10 | (21%) | 0.9 | 27 | (30%) | 28 | (61%) | 0.01 |
| Floor | ||||||||||
| Dirt or dung | 13 | (14%) | 7 | (15%) | 22 | (24%) | 8 | (17%) | ||
| Cement or tile | 67 | (74%) | 38 | (81%) | 68 | (76%) | 38 | (83%) | ||
| Other | 10 | (11%) | 2 | (4%) | 0.4 | 0 | (0%) | 0 | (0%) | 0.4 |
| Hunger in the last month | ||||||||||
| No | 77 | (86%) | 44 | (94%) | 60 | (67%) | 39 | (85%) | ||
| Yes | 13 | (14%) | 3 | (6%) | 0.3 | 30 | (33%) | 7 | (15%) | 0.04 |
| Employment | ||||||||||
| Not employed | 76 | (84%) | 34 | (72%) | 3 | (3%) | 1 | (2%) | ||
| Self-employed | 7 | (8%) | 6 | (13%) | 44 | (49%) | 21 | (46%) | ||
| Employed | 7 | (8%) | 7 | (15%) | 0.2 | 43 | (48%) | 24 | (52%) | 0.9 |
| HTC history | ||||||||||
| Never tested | 25 | (28%) | 14 | (30%) | 29 | (32%) | 12 | (26%) | ||
| Previously negative | 59 | (66%) | 33 | (70%) | 45 | (50%) | 32 | (70%) | ||
| Previously positive | 6 | (7%) | 0 | (0%) | 0.2 | 11 | (12%) | 2 | (4%) | 0.1 |
| Missing | 0 | 0 | 5 | 0 | ||||||
| Trimester | ||||||||||
| 1st trimester | 5 | (6%) | 2 | (4%) | ||||||
| 2nd trimester | 69 | (77%) | 38 | (81%) | ||||||
| 3rd trimester | 16 | (18%) | 7 | (15%) | 0.9 | |||||
| Primiparous | ||||||||||
| No | 75 | (83%) | 38 | (81%) | ||||||
| Yes | 15 | (17%) | 9 | (19%) | 0.8 | |||||
One record was lost.
Ninety-nine percent of female-positive couples and 98% of female-negative couples accepted CHTC. Following CHTC, 67 female-positive couples were classified as HIV-concordant positive (74%), 22 as HIV-discordant (24%), and one as unknown (1%). Following CHTC, 43 female-negative couples were classified as HIV-concordant negative (93%), 2 as HIV-discordant (4%), and one as unknown (2%). Both HIV-infected men knew they were HIV-infected prior to CHTC and neither were on ART.
Relationship Traits pre- and post-CHTC
Before CHTC, couples reported high levels of relationship commitment (mean=4.6/5) and intimacy (mean=4.6/5), but only moderate levels of trust (mean=32/40) and satisfaction (mean=13/20) (Table 2). For all characteristics, means were lower for female-positive couples than female-negative couples at baseline and follow-up (Appendix 2). Post-CHTC, trust increased modestly for female-positive couples, but other characteristics remained at similar levels (Table 2). Before CHTC, general communication was high (mean=9.9/12) and HIV communication was moderate (mean=0.7/1). Self-report was lower for female-positive couples than female-negative couples (Appendix 2). Relationship power was moderate (mean=2.7/4) and there were no differences between female-positive couples and female-negative couples (Appendix 2). Post-CHTC, HIV communication increased significantly for female-positive and female-negative couples and relationship power increased for female-positive couples; general communication did not increase (Table 2).All characteristics and dynamics had very good to excellent reliability pre- and post-CHTC (Chronbach's alpha=0.70-0.93).
Table 2. Relationship Characteristics of Dyads before and after CHTC.
| F+ Couples (N=90) | F- Couples (N=47) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean Couple Score (SD) | Change in Mean Score (95% CI) | Mean Couple Score (SD) | Change in Mean Score (95% CI) | |||||
| Pre-CHTC | Post-CHTC | Unadjusted | Adjusted* | Pre-CHTC | Post-CHTC | Unadjusted | Adjusted* | |
| Commitment (Max=5) | 4.6 (0.4) | 4.5 (0.6) | -0.09 (-0.21, 0.02) | -0.08 (-0.20, 0.03) | 4.8 (0.4) | 4.8 (0.3) | 0.03 (-0.09, 0.15) | 0.02 (-0.10, 0.14) |
| Intimacy (Max=5) | 4.5 (0.4) | 4.5 (0.5) | -0.01 (-0.12, 0.10) | 0.01 (-0.10, 0.11) | 4.7 (0.3) | 4.7 (0.3) | 0.05 (-0.06, 0.17) | 0.04 (-0.07, 0.15) |
| Trust (Max=40) | 30.1 (5.8) | 31.8 (6.2) | 1.69 (0.40, 2.98) | 1.79 (0.53, 3.05) | 34.2 (4.2) | 34.3 (3.8) | 0.17 (-1.21, 1.56) | 0.06 (-1.34, 1.46) |
| Satisfaction (Max=20) | 12.8 (1.8) | 13.0 (1.9) | 0.14 (-0.34, 0.62) | 0.21 (-0.26, 0.67) | 14.3 (1.0) | 14.3 (1.3) | -0.04 (-0.45, 0.37) | -0.01 (-0.42, 0.40) |
| Communication (Max=12) | 9.5 (0.9) | 9.7 (1.1) | 0.18 (-0.09, 0.46) | 0.20 (-0.07, 0.47) | 10.6 (0.9) | 10.9 (1.0) | 0.31 (-0.10, 0.72) | 0.33 (-0.09, 0.74) |
| HIV Communication (Max=1) | 0.7 (0.2) | 0.9 (0.1) | 0.20 (0.16, 0.24) | 0.21 (0.17, 0.25) | 0.7 (0.1) | 0.8 (0.1) | 0.09 (0.05, 0.12) | 0.08 (0.05, 0.12) |
| Relationship power (Max=4) | 2.7 (0.4) | 2.9 (0.4) | 0.21 (0.11, 0.31) | 0.22 (0.12, 0.31) | 2.8 (0.4) | 2.8 (0.4) | 0.02 (-0.09, 0.13) | 0.002 (-0.11, 0.11) |
SD=Standard Deviation, CI=Confidence Interval
Adjusted for female age category, relationship length, time period, and female HIV status
Sexual Behavior pre- and post-CHTC
Before CHTC, nearly all couple members reported engaging in unprotected sex in the last month (Figure 2). Among individual participants in female-positive couples, 79% reported unprotected sex, 9% reported inconsistent condom use, 4% reported consistent condom use, and 8% reported abstinence. This distribution was similar between female-positive couples who were HIV-discordant and female-positive couples who were HIV-concordant (p=0.4) (Figure 2). Among individual participants in female-negative couples, 97% reported unprotected sex, 1% reported inconsistent condom use, 0% reported consistent condom use, and 2% reported abstinence. The ICC was 0.39 (95% CI: 0.24, 0.52). At the dyadic level, 8% of female-positive couples and 2% of female-negative couples reported safe sex (p=0.3).
Figure 2. Sexual Behavior Before and after CHTC.
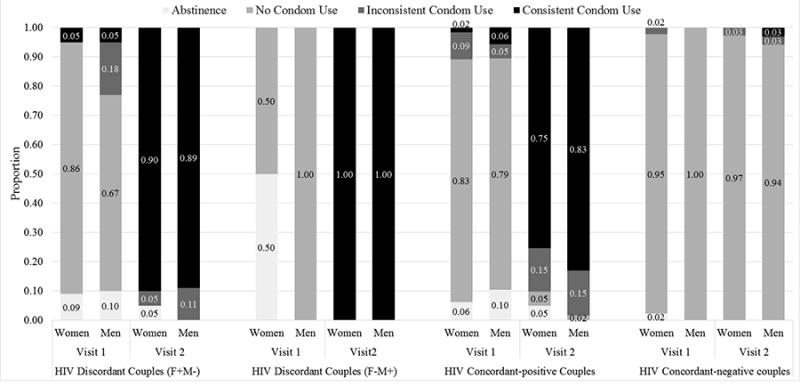
Figure 2 depicts the proportion of men and women reporting abstinence, no condom use, inconsistent condom use, and consistent condom use pre-CHTC and post-CHTC based on couple HIV status. Individual-level data are reported.
After CHTC, substantial behavior change was observed by female-positive couples (Figure 2). Among individual participants in female-positive couples, 2% reported unprotected sex, 13% reported inconsistent condom use, 82% reported consistent condom use, and 3% reported abstinence. Distributions were similar among female-positive HIV-discordant and HIV-concordant couples (p=0.2) (Figure 2). Among individual participants in female-negative couples, 94% reported unprotected sex, 3% reported inconsistent condom use, 4% reported consistent condom use, and 0% reported abstinence. The ICC was 0.77 (95% CI: 0.68, 0.84). Both female-negative HIV-discordant couples reported consistently using condoms post-CHTC. At the dyadic level, 75% of female-positive couples and 3% of female-negative couples reported safe sex (p<0.0001).
The final adjusted model assessing safe sex pre- and post-CHTC included female age category and relationship duration; no other baseline characteristics remained after backwards elimination. Before CHTC, female-positive couples and female-negative couples had similar probabilities of reporting safe sex (RR, 3.7, 95% CI: 0.5, 29.8). After CHTC, female-positive couples were 30.0 times more likely to report safe sex than female-negative couples (95% CI: 4.3, 207.7). Female-negative couples were as likely to report safe sex post-CHTC as pre-CHTC (RR: 1.1, 95% CI: 0.1, 18.7). Female-positive couples were ten times more likely to report safe sex post-CHTC than pre-CHTC (RR: 9.6, 95% CI: 4.6, 20.0). They were also 31.9 (95% CI: 4.5, 225.4) times more likely to report consistent condom use and 20.7 (95% CI: 5.3, 80.5) times more likely to report any condom use.
There were no meaningful associations between mean relationship factors pre-CHTC and safe sex post-CHTC. Similarly, there were no meaningful associations in female-male differences between relationship factors pre-CHTC and safe sex post-CHTC (Table 3).
Table 3. Associations between Baseline Relationship Characteristics and Safe Sex at Follow-Up.
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| RR | (95% CI) | RR | (95% CI) | |
| Commitment | ||||
| Mean | 0.72 | (0.49, 1.04) | 0.96 | (0.72, 1.28) |
| Difference | 0.81 | (0.62, 1.05) | 1.01 | (0.83, 1.24) |
| Intimacy | ||||
| Mean | 0.77 | (0.54, 1.10) | 1.08 | (0.79, 1.46) |
| Difference | 0.74 | (0.61, 0.91) | 0.90 | (0.78, 1.04) |
| Trust | ||||
| Mean | 0.98 | (0.95, 1.01) | 1.02 | (0.99, 1.04) |
| Difference | 0.99 | (0.97, 1.01) | 1.00 | (0.98, 1.01) |
| Satisfaction | ||||
| Mean | 0.90 | (0.84, 0.97) | 1.03 | (0.94, 1.12) |
| Difference | 1.03 | (0.97, 1.10) | 0.99 | (0.94, 1.04) |
| Communication | ||||
| Mean | 0.85 | (0.71, 1.01) | 1.07 | (0.92, 1.26) |
| Difference | 0.97 | (0.89, 1.06) | 0.93 | (0.87, 1.00) |
| HIV communication | ||||
| Mean | 0.62 | (0.23, 1.64) | 1.40 | (0.68, 2.89) |
| Difference | 1.58 | (0.85, 2.93) | 1.28 | (0.80, 2.02) |
| Relationship Power | ||||
| Mean | 1.24 | (0.84, 1.84) | 1.27 | (0.98, 1.64) |
| Difference | 0.93 | (0.73, 1.18) | 1.02 | (0.86, 1.22) |
RR=risk ratio, CI=confidence interval
Mean: Average of female and male scores at visit 1
Difference: Female score - male score at visit 1
Adjusted for female age category, relationship length, and female HIV status
Discussion
In an Option B+ program in Lilongwe, Malawi, safe sex was negligible for all participants before CHTC. One month after CHTC, safe sex remained low in female-negative couples but was adopted by three quarters of female-positive couples. Greater HIV communication also occurred post-CHTC by both female-positive and female-negative couples.
Even though ART adherence leads to viral suppression and very low transmissibility, viral suppression can take months to achieve. During these months, additional HIV prevention measures are needed, especially in HIV-discordant couples. During the brief period between screening and CHTC, few couples adopted consistent condom use. But following CHTC and condom provision, condom uptake was nearly universal by HIV-discordant couples, suggesting CHTC with condom provision could protect most HIV-discordant couples. Condom use may also be important at later periods, as HIV-infected persons and their partners may not be able to accurately gauge viral suppression.30,31
Substantial behavior change was not observed for couples who were HIV-concordant negative and may have perceived sexual behavior with their study partner to be safe following CHTC. This observation is not surprising, as partners only remain at risk for HIV acquisition from outside partners, a substantial driver of new infections in the region.32,33 Understanding which HIV-uninfected women are at highest risk for HIV acquisition is critical as HIV incidence among pregnant women is high and may account for a substantial share of new pediatric HIV cases.34,35 Understanding which HIV-uninfected women are at highest risk for HIV acquisition is the focus of a future analysis. These select women may be good candidates for antiretroviral pre-exposure prophylaxis.
Approximately half of the eligible couples presented to the clinic and participated in the study. In this population, comparisons between presenters and non-presenters are not possible, as information was not collected from women who did not return with a partner. However, in a similar study assessing CHTC uptake at this site, fear of partner anger, violence, and abandonment were associated with lower presentation.36 We speculate that similar fears may have inhibited couples from presenting. Couples who presented for CHTC may have stronger, more equitable, healthier relationships, than those who did not present. Similarly, more than 90% of participants reported for follow-up, but those who did not may have had riskier behaviors than those who did. With respect to baseline characteristics, such as age, relationship duration, and education level, this study population is quite similar to the larger antenatal population, but we would not expect results to generalize beyond the antenatal population.
Relationship traits were not associated with safe sex. This contrasts with observations among men who have sex with men (MSM) in the US. In MSM couples, positive relationship characteristics with primary partners, including intimacy, commitment, and attachment have been associated with higher rates of unprotected anal intercourse with primary partners.38-40 Similar findings have been observed in South Africa, where couple intimacy and HIV-specific social support have been associated with increased unprotected sexual acts.41 Couples in different contexts may reach different decisions when balancing closeness (through unprotected sex) and HIV protection (through protected sex).
All measures in this study are subject to information bias. In particular, sexual behavior is subject to socially desirable reporting, which could have been differential with respect to HIV status. However, we believe a substantial share of the self-reported behavior change was valid. First, very few female-positive couples reported consistent condom use at baseline, suggesting HIV-infected persons were willing to report unprotected sex, even after HIV diagnosis. Either sexual behavior changed or truth-telling behavior changed. Next, we observed that female-negative couples did not report consistent condom use, even though they also received messages about consistent condom use. Study staff confirmed that nearly all female-positive couples took condoms, but most female-negative couples did not. Next, we observed strong correlation in self-report between couple members, especially at follow-up. Finally, in similar populations in the region, self-reported consistent condom is strongly associated with lower HIV and other STIs, the most convincing observation.42,43
To understand long-term impacts of CHTC, following larger samples for longer periods with biological outcomes is critical. However, as Option B+ and other TasP programs are brought to scale in sub-Saharan Africa, offering CHTC and distributing condoms can play an important role in facilitating couple communication and consistent condom use prior to viral suppression.44 These observations add to a large body of work highlighting the critical role of couple-based approaches to HIV prevention.
Supplementary Material
Acknowledgments
We would like to thank Mary Kacheyo for providing CHTC and the Lighthouse Trust and Lilongwe District Health Office for their support. We thank all participants.
Footnotes
Conflicts of Interest and Sources of Funding: The study and NER were supported by the National Institute of Mental Health (K99MH104154-01A1). LAG was supported by the National Institute of Child Health and Human Development (4T32HD052468-09). AW was supported by the Doris Duke International Clinical Research Fellowship.
References
- 1.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011 Jul 16;378(9787):282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV--Malawi, July 2011-September 2012. MMWR Morbidity and mortality weekly report. 2013 Mar 1;62(8):148–151. [PMC free article] [PubMed] [Google Scholar]
- 3.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. Aids. 2014 Feb 20;28(4):589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas AD, Tenthani L, Msukwa MT, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi's option B+ programme: an observational cohort study. The lancet HIV. 2016 Apr;3(4):e175–182. doi: 10.1016/S2352-3018(16)00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenthani L, Haas AD, Egger M, et al. Brief Report: HIV Testing Among Pregnant Women Who Attend Antenatal Care in Malawi. Journal of acquired immune deficiency syndromes. 2015 Aug 15;69(5):610–614. doi: 10.1097/QAI.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg NE, Mtande TK, Saidi F, et al. Recruiting Male Partners for Couple HIV Counseling and Testing in Malawi's Option B+ Program: A Randomized Controlled Trial. 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. Journal of acquired immune deficiency syndromes. 2011 Jan 1;56(1):76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalembo FW, Zgambo M, Mulaga AN, Yukai D, Ahmed I. Association between Male Partner Involvement and the Uptake of Prevention of Mother-to-Child Transmission of HIV (PMTCT) Interventions in Mwanza District, Malawi: A Retrospective Cohort Study. PloS one. 2013;8(6):e66517. doi: 10.1371/journal.pone.0066517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg NE, Pettifor AE, Bruyn GD, et al. HIV Testing and Counseling Leads to Immediate Consistent Condom Use Among South African Stable HIV-Discordant Couples. Journal of acquired immune deficiency syndromes. 2013 Feb 1;62(2):226–233. doi: 10.1097/QAI.0b013e31827971ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran S, Mishra S, Condie N, Pickles M. How do HIV-negative individuals in sub-Saharan Africa change their sexual risk behaviour upon learning their serostatus? A systematic review. Sexually transmitted infections. 2016 Aug 17; doi: 10.1136/sextrans-2015-052354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denison JA, O'Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990--2005. AIDS and behavior. 2008 May;12(3):363–373. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg NE, Hauser BM, Ryan J, Miller WC. The effect of HIV counselling and testing on HIV acquisition in sub-Saharan Africa: a systematic review. Sexually transmitted infections. 2016 Aug 16; doi: 10.1136/sextrans-2016-052651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karney BR, Hops H, Redding CA, Reis HT, Rothman AJ, Simpson JA. A framework for incorporating dyads in models of HIV-prevention. AIDS and behavior. Dec 2010;14(Suppl 2):189–203. doi: 10.1007/s10461-010-9802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darbes LA, van Rooyen H, Hosegood V, et al. Uthando Lwethu ('our love'): a protocol for a couples-based intervention to increase testing for HIV: a randomized controlled trial in rural KwaZulu-Natal, South Africa. Trials. 2014;15(1):64. doi: 10.1186/1745-6215-15-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Bassel N, Gilbert L, Witte S, Wu E, Hunt T, Remien RH. Couple-based HIV prevention in the United States: advantages, gaps, and future directions. Journal of acquired immune deficiency syndromes. Dec 2010;55(Suppl 2):S98–101. doi: 10.1097/QAI.0b013e3181fbf407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. Aids. 2005 May 20;19(8):807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 18.Mphonda S, Rosenberg NE, Kamanga E, et al. Assessment of Peer-Based and Structural Strategies for Increasing Male Participation in an Antenatal Setting in Lilongwe, Malawi. African Journal of Reproductive Health, Special Edition on HIV/AIDS. 2013 in press. [PMC free article] [PubMed] [Google Scholar]
- 19.Conroy AA. Gender, power, and intimate partner violence: a study on couples from rural Malawi. Journal of interpersonal violence. 2014 Mar;29(5):866–888. doi: 10.1177/0886260513505907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conroy AA, McGrath N, van Rooyen H, et al. Power and the association with relationship quality in South African couples: Implications for HIV/AIDS interventions. Social science & medicine. 2016 Mar;153:1–11. doi: 10.1016/j.socscimed.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackers M, Davis MH. Intimacy, passion, and commitment in adult romantic relationships: A test of the triangular theory of love. Journal of Social and Personal Relatoinships. 1992;9(1):21–50. [Google Scholar]
- 22.Larzelere RE, Huston TL. The Dyadic Trust Scale: Toward Understanding Interpersonal Trust in Close Relationships. Journal of Marriage and the Family. 1980;42(3):10. [Google Scholar]
- 23.Funk JL, Rogge RD. Testing the ruler with item response theory: increasing precision of measurement for relationship satisfaction with the Couples Satisfaction Index. Journal of family psychology : JFP : journal of the Division of Family Psychology of the American Psychological Association. 2007 Dec;21(4):572–583. doi: 10.1037/0893-3200.21.4.572. [DOI] [PubMed] [Google Scholar]
- 24.Christensen A, Heavey CL. Gender and social structure in the demand/withdrawal pattern of marital conflict. Journal of personality and social psychology. 1990;59(1):73. doi: 10.1037//0022-3514.59.1.73. [DOI] [PubMed] [Google Scholar]
- 25.Futris TG, Campbell K, Nielsen RB, Burwell SR. The communication patterns questionnaire--short form: a review and assessment. The Family. 2010 [Google Scholar]
- 26.Widman L, Choukas-Bradley S, Noar SM, Nesi J, Garrett K. Parent-Adolescent Sexual Communication and Adolescent Safer Sex Behavior: A Meta-Analysis. JAMA pediatrics. 2016 Jan;170(1):52–61. doi: 10.1001/jamapediatrics.2015.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulerwitz J, Gortmaker SL, Jong WD. Measuring sexual relationship power in HIV/STD research. Sex Roles. 2000;42:637–660. [Google Scholar]
- 28.McMahon JM, Volpe EM, Klostermann K, Trabold N, Xue Y. A systematic review of the psychometric properties of the Sexual Relationship Power Scale in HIV/AIDS research. Archives of sexual behavior. 2015 Feb;44(2):267–294. doi: 10.1007/s10508-014-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 30.Conroy AA, Gamarel KE, Neilands TB, et al. Partner Reports of HIV Viral Suppression Predict Sexual Behavior in Serodiscordant Male Couples. Journal of acquired immune deficiency syndromes. 2016 Oct 01;73(2):e31–33. doi: 10.1097/QAI.0000000000001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conroy AA, Gamarel KE, Neilands TB, Dilworth SE, Darbes LA, Johnson MO. Relationship Dynamics and Partner Beliefs About Viral Suppression: A Longitudinal Study of Male Couples Living with HIV/AIDS (The Duo Project) AIDS and behavior. 2016 Jul;20(7):1572–1583. doi: 10.1007/s10461-016-1423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellan SE, Fiorella KJ, Melesse DY, Getz WM, Williams BG, Dushoff J. Extra-couple HIV transmission in sub-Saharan Africa: a mathematical modelling study of survey data. Lancet. 2013 May 4;381(9877):1561–1569. doi: 10.1016/S0140-6736(12)61960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray R, Ssempiija V, Shelton J, et al. The contribution of HIV-discordant relationships to new HIV infections in Rakai, Uganda. AIDS. 2011 Mar 27;25(6):863–865. doi: 10.1097/QAD.0b013e3283448790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS medicine. 2014 Feb;11(2):e1001608. doi: 10.1371/journal.pmed.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. Journal of acquired immune deficiency syndromes. 2012 Apr 1;59(4):417–425. doi: 10.1097/QAI.0b013e3182432f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg NE, Mtande TK, Saidi F, et al. Recruiting male partners for couple HIV testing and counselling in Malawi's option B+ programme: an unblinded randomised controlled trial. The lancet HIV. 2015 Nov;2(11):e483–491. doi: 10.1016/S2352-3018(15)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leddy A, Chakravarty D, Dladla S, de Bruyn G, Darbes L. Sexual communication self-efficacy, hegemonic masculine norms and condom use among heterosexual couples in South Africa. AIDS care. 2016;28(2):228–233. doi: 10.1080/09540121.2015.1080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoff CC, Campbell CK, Chakravarty D, Darbes LA. Relationship-Based Predictors of Sexual Risk for HIV Among MSM Couples: A Systematic Review of the Literature. AIDS and behavior. 2016 Dec;20(12):2873–2892. doi: 10.1007/s10461-016-1350-9. [DOI] [PubMed] [Google Scholar]
- 39.Hoff CC, Chakravarty D, Beougher SC, Neilands TB, Darbes LA. Relationship characteristics associated with sexual risk behavior among MSM in committed relationships. AIDS patient care and STDs. 2012 Dec;26(12):738–745. doi: 10.1089/apc.2012.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darbes LA, Chakravarty D, Neilands TB, Beougher SC, Hoff CC. Sexual risk for HIV among gay male couples: a longitudinal study of the impact of relationship dynamics. Archives of sexual behavior. 2014 Jan;43(1):47–60. doi: 10.1007/s10508-013-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darbes LA, McGrath NM, Knight L, et al. IAS. Melbourne, Australia: 2014. Relationship factors are associated with increased unprotected sex in heterosexual couples in KwaZulu-Natal, South Africa. [Google Scholar]
- 42.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. The Journal of infectious diseases. 2012 Feb 1;205(3):358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magaret AS, Mujugira A, Hughes JP, et al. Effect of Condom Use on Per-act HSV-2 Transmission Risk in HIV-1, HSV-2-discordant Couples. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Feb 15;62(4):456–461. doi: 10.1093/cid/civ908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karita E, Nsanzimana S, Ndagije F, et al. Evolution of Couples Voluntary Counseling and Testing for HIV in Rwanda: From Research to Public Health Practice. Journal of acquired immune deficiency syndromes. 2016 Jul 21; doi: 10.1097/QAI.0000000000001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


