Abstract
Dysregulated bile acids (BAs) are closely associated with liver diseases and attributed to altered gut microbiota. Here, we show that the intrahepatic retention of hydrophobic BAs including deoxycholate (DCA), taurocholate (TCA), taurochenodeoxycholate (TCDCA), and taurolithocholate (TLCA) were substantially increased in a streptozotocin and high fat diet (HFD) induced nonalcoholic steatohepatitis-hepatocellular carcinoma (NASH-HCC) mouse model. Additionally chronic HFD-fed mice spontaneously developed liver tumors with significantly increased hepatic BA levels. Enhancing intestinal excretion of hydrophobic BAs in the NASH-HCC model mice by a 2% cholestyramine feeding significantly prevented HCC development. The gut microbiota alterations were closely correlated with altered BA levels in liver and feces. HFD-induced inflammation inhibited key BA transporters, resulting in sustained increases in intrahepatic BA concentrations. Our study also showed a significantly increased cell proliferation in BA treated normal human hepatic cell lines and a down-regulated expression of tumor suppressor gene CEBPα in TCDCA treated HepG2 cell line, suggesting that several hydrophobic BAs may collaboratively promote liver carcinogenesis.
Keywords: bile acids, gut microbiota, inflammation, proliferation, liver carcinogenesis
Introduction
Liver cancer is the fifth most common cancer in men (~554,000 cases) and the ninth in women (~228,000 cases) globally.1 About 39,230 new cases of liver cancer (28,410 in men and 10,820 in women) were predicted in 2016 in the USA.2 Hepatocellular carcinoma (HCC) accounting for over 90% of primary liver cancer, mainly occurs in Asia and Africa, while its incidence is steadily increased in United States over the past thirty years.3 In addition to the chronic liver injury resulting from hepatic inflammation, increasing evidence suggests that other intrahepatic and systemic factors are likely to play significant roles in the process of liver injury and the subsequent carcinogenesis.4 Evidences have demonstrated that some of bile acids (BAs) may act as promoters for hepatocarcinogenesis.5, 6 BAs, particularly those with high hydrophobicity, can readily induce DNA damage and frequent apoptosis, and, after repeated exposure, may select for cells with reduced apoptosis capability.7 Increasing evidence obtained recently supports the view that certain BA species may be carcinogens to humans.8, 9
Primary BAs, cholate (CA) and chenodeoxycholate (CDCA), are produced in the liver from cholesterol, and conjugated predominantly with taurine and/or glycine to form bile salts before secretion into the bile canaliculi. In the intestine, BAs are metabolized by intestinal anaerobic bacteria to secondary BAs, deoxycholate (DCA) and lithocholate (LCA).10 Importantly, accumulation of BAs within hepatocytes can cause cytotoxicity by inducing mitochondrial dysfunction and formation of reactive oxygen species (ROS), ultimately leading to apoptosis or necrosis. Generally, the more hydrophobic BA species (e.g., unconjugated secondary > conjugated primary) have greater cytotoxic effects.11 Several studies have shown that taurochenodeoxycholate (TCDCA), glycochenodeoxycholate (GCDCA), glycocholate (GCA) and DCA induced ROS and cell apoptosis in isolated rat hepatocytes and human hepatoma cells.12
The nuclear receptor farnesoid X receptor (FXR; gene symbol NR1H4) is the primary hepatic BA sensor studied most extensively for its regulation of numerous genes involved in BA homeostasis.16 Both male and female mice lacking expression of FXR and exhibiting increased BA pool spontaneously develop liver tumors.6, 13 Lowering BA pool in these mice by a 2% cholestyramine feeding significantly reduces the malignant lesions.6 Small hetero-dimer partner (SHP) is another nuclear receptor negatively modulating BAs. Fxr−/− Shp−/− double-knockout mice which have chronically elevated BA levels also spontaneously develop liver tumors.5 DCA presented a strong hepatocarcinoma-promoting activity in the mouse model pretreated with diethylnitrosamine.8 Exposure of hepatocytes to DCA induced expression of inflammatory genes which were associated with the development of cancer.14 Taurine-conjugated LCA (TLCA) is capable to impair BA flow and to induce cholestasis.15 Taurocholate (TCA) is found to down-regulate gluconeogenic genes and induce inflammatory gene expression in hepatocytes.14 As further evidence for an important role of the microbiota in the metabolism of BAs, mice grown under germ-free conditions or treated with antibiotics have altered hepatic gene expression patterns with changes in cholesterol, steroid and BA synthesis pathways as well as in conjugated (especially taurine conjugated) BA signatures in multiple body compartments.16 These studies indicate that the dysbiosis or elevated BA levels may produce substantial hepatic toxicities including a variety of cellular disorders, leading to sustained liver injury. Most importantly, our study on BAs showed that TCA, TCDCA, GCA, and GCDCA were the most abundant BAs in blood and significantly increased in patients with cirrhosis and HCC.17, 18
On the basis of these findings, we hypothesized that intrahepatic accumulation of BAs critically induces sustained hepatocellular injuries responsible for the subsequent development of fibrosis and malignancy. We used a streptozotocin-high fat diet (STZ-HFD) induced nonalcoholic steatohepatitis (NASH)-HCC mouse model,19 which is highly relevant to human liver disease progression from steatosis to NASH, fibrosis, and finally HCC and nearly 100% of mice in the model group developed HCC. The hypothesis was further verified in chronic HFD-fed mice and human normal hepatic cell lines and tumor cell lines.
Materials and Methods
Mice and diet
Experimental 1
A NASH- HCC C57BL/6J mice model induced by STZ coupled with HFD was developed19. Pathogen-free 14-day pregnant C57BL/6J mice were purchased from CLEA Japan (Tokyo, Japan) and the new born male mice were divided into two groups: control group and model (STZ-HFD) group. The mice in control group was housed without any treatment and fed normal diet (CE-2 from CLEA Japan Inc., composed of 12 kcal% fat, 29 kcal% protein, 59 kcal% carbohydrates). The mice in STZ-HFD were subjected to a single subcutaneous injection of 200 μg STZ (Sigma, MO, USA) at 2 days after birth and fed with HFD (HFD32 from CLEA japan Inc., STZ-FHD group) ad libitum after 4 weeks of age for 16 weeks (Fig. 1A). During the experiment, the body weight of all animals was recorded once a week. At week 6, 8, 12, and 20, 6 mice in each group were euthanized and liver, plasma, and fecal samples were collected.
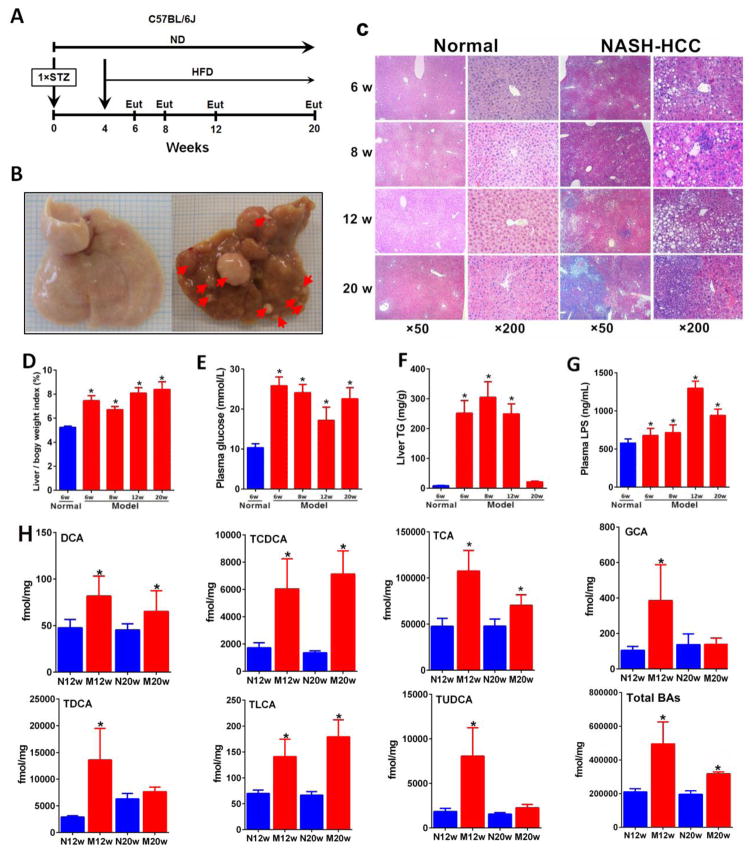
Figure 1.
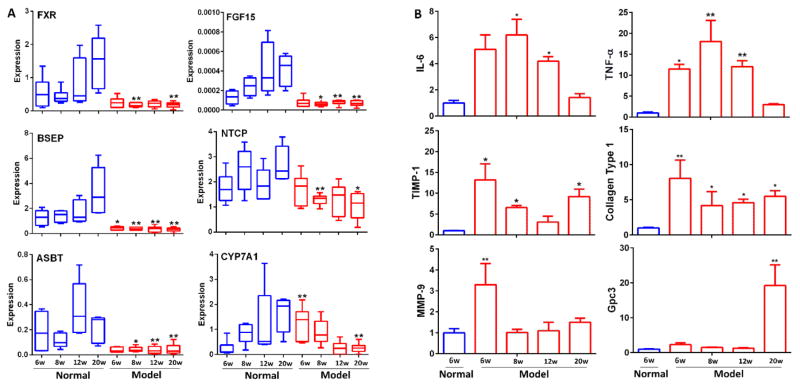
Pathophysiological features of NASH–HCC model. (A) Timeline of the experimental procedure (n=24 per group). Eut, euthanasia; ND, normal diet. (B) Representative macroscopic photographs of livers. Arrowheads indicate HCCs. (C) H&E stained liver sections from normal and NASH-HCC mice at weeks 6, 8, 12 and 20. Original magnifications × 50 and ×200, respectively. (D) Liver to body weight index (%) at week 6, 8, 12 and 20. (E) Fasting plasma glucose at week 6, 8, 12 and 20. (F) Liver TG at week 6, 8, 12 and 20. (G) Plasma LPS at week 6, 8, 12 and 20. (H) BAs, DCA, TDCA, TLCA, GCA, TCA, TCDCA, TUDCA and total BAs were significantly increased in liver. * p<0.05, ** p<0.01, ***p<0.001 vs. normal (n=6) (Mean ± SE).
Experimental 2
In addition to Experimental 1, we fed C57BL/6J male mice with HFD alone to observe the liver carcinogenesis and to further confirm that BAs will promote liver carcinogenesis. Two groups of mice were included: (1) control; (2) HFD. We sacrificed mice at different time points and at week 58, we observed HCC formation in HFD-fed mice.
Experimental 3
We found that the BA levels were significantly increased in hepatic carcinogenesis in Experimental 1; we repeated the NASH-HCC mice model to see whether the BA-binding resin, cholestyramine, can attenuate/prevent liver carcinogenesis. Three groups of mice were included: (1) control (n=9); (2) model (STZ-HFD, n=30) and (3) STZ-HFD-BA resin (n=30) group, mice were fed with HFD diet containing 2% cholestyramine. All the procedures are the same as in Experimental 1. At week 20, the mice were euthanized and liver, plasma, and fecal samples were collected (Fig. 3A).
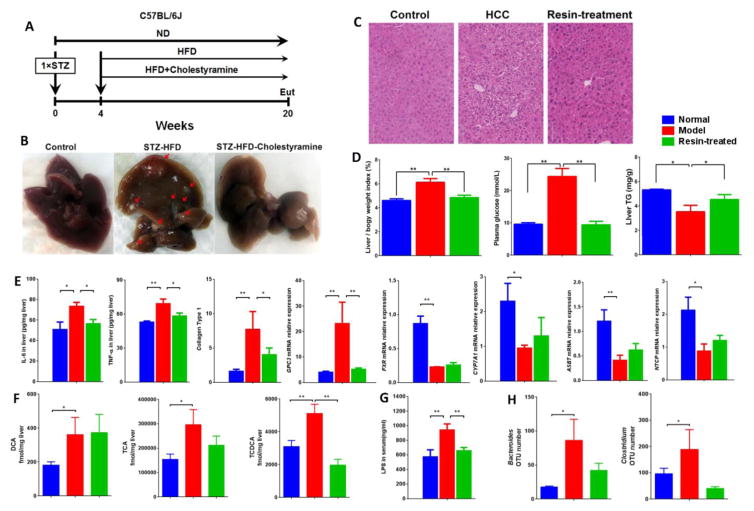
Figure 3.
(A) Timeline of the experimental procedure (n=9 for ND group and n=8 for intervention group). Eut, euthanasia; ND, normal diet. (B) Representative macroscopic photographs of livers. Arrowheads indicate HCCs. C, H&E stained liver sections from normal, NASH-HCC and NASH-HCC-cholestyramine mice at week 20. Bar = 70 μm. (D) Liver to body weight (%), fasting plasma glucose and liver TG levels at week 20. (E) Levels of inflammatory markers, liver tumor markers and mRNA expression of BA transporters at week 20. (F) Levels of DCA, TCA and TCDCA in liver at different groups. (G) Plasma LPS at week 20. (H) OUT numbers of Bacteroides and Clostridium in different groups at week 20. * p<0.05, ** p<0.01 vs. normal (n=9) (Mean ± SD).
All animal procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86–23, revised 1985).
Measurement of serum ALT and AST
The levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by FUJI DRI-CHEM 7000 (Fujifilm, Tokyo, Japan).
Histological Evaluation
Liver tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin blocks, and processed by routine hematoxylin and eosin (H&E) staining. Individual NAFLD activity score was calculated.
Measurement of BAs
The BA levels in plasma, liver, and feces were quantitatively measured by ultra-performance liquid chromatography triple quadrupole mass spectrometry (UPLC-TQMS) according to our previously reported protocol.18, 20 All separations were performed with an ACQUITY BEH C18 column (1.7 μm, 100 mm × 2.1 mm internal dimensions) (Waters, Milford, MA) (Supporting Information Table 1). Data acquisition was performed using MassLynx version 4.1 and BA quantification were performed using the TargetLynx applications manager version 4.1 (Waters Corp., Milford, MA). For details, see Methods in Supporting Information.
Gut microbiota characterization
The diversity of bacteria was characterized by using a bacterialtag-encoded FLX 16S rDNA amplicon pyrosequencing (bTEFAP) approach as previously reported.21, 22 For details, see Methods in Supporting Information.
Real-time quantitative polymerase chain reaction
DNA and RNA were simultaneously extracted using homogenized tissue lysates in RLT Plus and the AllPrep DNA/RNA Mini kit (Qiagen, Valencia, CA) following manufacturer’s protocol. RNA integrity was assessed using 1μl of RNA on the RNA Nano 6000 chips and the 2100 Agilent Bioanalyzer. Expression of target mRNA was measured in triplicate by the comparative cycle threshold method on the Applied Biosystems 7900 FAST Real Time PCR Systems (Applied Biosystems). The forward and reverse primers were purchased from Integrated DNA Technologies (Coralville, IA) (Supporting Information Table 2). Target gene expression was normalized to ACTB levels and the relative expression of the target genes was calculated using the “dCT” a.k.a Comparative Ct approach.23
Measurement of TG, IL-6 and TNF-α in the liver
The levels of triglyceride (TG), interleukin-6 (IL-6) and tumor necrosis factor (TNF-α) in the liver were measured using Elisa kits from BlueGene Biotech, Shanghai, China.
Measurement of LPS in plasma, liver and feces
The levels of lipopolysaccharides (LPS) in plasma, liver and feces were determined using a mouse LPS Elisa kit (BlueGene Biotech, Shanghai, China) according to the manufacturer’s protocol.
Statistical analysis
All statistical analyses were calculated using GraphPad Prism (version 6.0; GraphPad Software, San Diego, USA) and SPSS 22.0 (IBM SPSS, USA). Data are expressed as mean ± SEM. The differences between the groups in BA measurements were analyzed by t tests with Holm-Sidak method for multiple comparisons correction. We regarded p values of < 0.05 as significant. Spearman correlation analysis was made to evaluate the interactions between gut microbiota and BA levels in liver and feces, giving a value ranging from 1.0 (maximum positive correlation) to −1 (maximum anticorrelation) and 0 (no correlation).
Cell culture and BA treatment
Normal human hepatic cell lines WRL-68 and THLE-2 were purchased from Sigma. For BA treatment, cells were harvested, rinsed twice in PBS, resuspended in sugar-free DMEM (Life Technologies) supplemented with 10% charcoal-stripped FBS and seeded into 6-well plate at a density of 5 × 104/well. We set the glucose (Glc, Sigma) at 27.5 mM and Oleic acid (OA, Sigma) at 0.3 mM to mimic high glucose and fat conditions in vitro.24, 25 Each of the 5 BAs, CA, TLCA, TCA, TCDCA, GCDCA, DCA, or LCA was added into the media at concentrations of 5 μM, 50 μM, 100 μM or 200 μM. After 14 days treatment, normal medium (DMEM-F12 containing 10% FBS) were added into each well to replace the spent media. Cell proliferation and anchorage-independent growth assays were performed in the normal medium. Additionally, western blot assay was performed to analyze the alteration of oncoprotein c-myc expression.
Human cancer cell line, HepG2, was purchased from Sigma and maintained in DMEM-F12 (Life Technologies) supplemented with 10% fetal bovine serum (FBS, Life Technologies). For BA treatment, cells were harvested, rinsed twice with PBS, seeded in sugar-free DMEM (Life Technologies) supplemented with 10% charcoal-stripped FBS and 6 mM glucose. Each of the 5 BAs, CA, UDCA, TCDCA, DCA, or LCA was added into the media at a concentration of 100 μM. The spent media was replaced by fresh normal medium (DMEM-F12 containing 10% FBS) 14 days later. And then cells were used for cell proliferation assay and western blot analysis for the tumor suppressor protein CEBPα
Primary antibodies for c-myc (Santa Cruz), CEBPα (Santa Cruz) and Actin (Li-cor) were purchased and used with a diluted concentration as indicated by the manufacturers.
Results
Hepatic BAs were significantly increased in STZ-HFD induced NASH-HCC mice
Pathological phenotypes of steatosis, NASH, fibrosis, and HCC were successfully developed in male C57BL/6J mice. Neonatal mice injected subcutaneously with STZ induced mild islet inflammation and islet destruction. Four weeks after birth, STZ-primed mice were given with HFD, which resulted in sequential histological changes from fatty liver, to NASH, fibrosis, and HCC (Fig. 1A). Notably, all HFD-fed mice developed HCC (Fig. 1B). H&E staining (Fig. 1C) showed fatty liver, but no inflammatory foci at 6 week, fatty liver with moderate inflammatory infiltrate include neutrophils, lymphocytes and monocytes, and ballooning degeneration of hepatocytes at week 8, chronic fibrosis at week 12 and HCC at week 20 with increased NAFLD activity score (Supporting Information Table 3).
Body weight gain was observed with the aggravated liver injury in STZ-HFD mice (Supporting Information Table 3). The liver weight was significantly increased in STZ-HFD group compared to normal mice, especially at fibrosis and HCC stages (Fig. 1D and Supporting Information Table 3). Fasting plasma glucose and liver TG were significantly higher in the STZ-HFD group (Fig. 1E and 1F). Mild elevation of serum ALT and AST were detectable in STZ-HFD mice (Supporting Information Table 3). The endotoxin (lipopolysaccharides, LPS) levels in plasma, liver and feces were markedly increased in STZ-HFD group compared to controls (Fig. 1G and Supporting Information Fig. 1A).
Importantly, the hepatic levels of TCA, DCA, GCA, TDCA, TLCA, TUDCA, TCDCA, and total BAs were substantially increased in the model mice at week 12 and 20 (Fig. 1H). The hepatic accumulation of BAs occurred at liver fibrosis stage while fecal BAs were depleted at the same stage, and gradually increased at HCC stage (Supporting Information Fig. 2). Notably, among all the BA species, TCDCA was the most significantly increased species in the liver of model mice compared to the controls. In addition to these BAs, LCA was increased in plasma (Supporting Information Fig. 2A) and feces (Supporting Information Fig. 2B) in model mice.
Interestingly, the primary BAs, CA and CDCA, in feces were decreased in fibrosis phase (week 12) in model group as compared to controls (Supporting Information Fig. 3) while in plasma and liver, their levels were increased. Total fecal BAs were slightly lower in model group than in controls at fibrosis stage, while at HCC stage, its level increased in plasma and liver at fibrosis and HCC stages (Supporting Information Fig. 3).
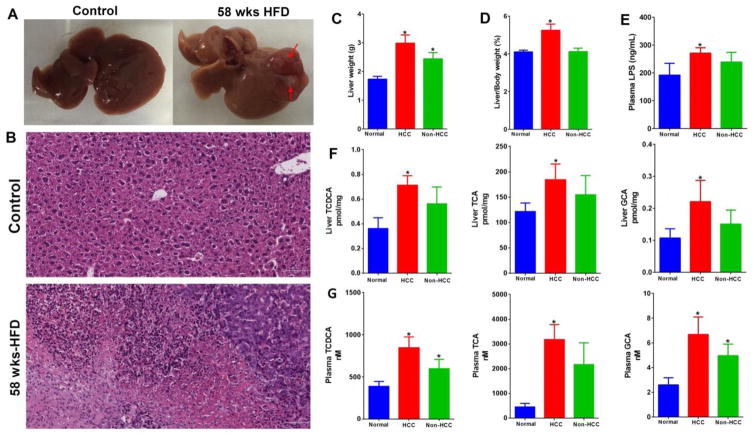
Liver tumor was developed in chronic HFD-fed mice
That significantly increased BAs in liver were observed in STZ-HFD treated mice leads to the hypothesis that HFD-induced high concentrations of BAs and their retention in liver may act as tumor promoters. Since STZ was classified as potential carcinogen by International Agency for Research on Cancer, we explored if HFD alone will develop liver cancer, although no HCC was observed in male mice treated with STZ alone for 20 weeks.19 In this long-term HFD intervention study, liver tumor was observed in more than half (6 out of 11) of the HFD-fed mice at week 58 (Fig. 2A and 2B). Liver weight (Fig. 2C) and liver to body weight ratio (Fig. 2D) was markedly increased in HFD-fed induced HCC mice. LPS levels in plasma, liver and feces were all significantly increased in HCC mice (Fig. 2E and Supporting Information Fig. 1B). Notably, liver and plasma BAs, TCA, GCA and TCDCA were increased in all mice fed with HFD but with statistical significance in those mice developed HCC as compared to normal (Fig. 2F and 2G).
Figure 2.
Pathophysiological features of HFD-induced HCC mice (n=8 for control group and n=11 for HFD-fed group). (A) Representative macroscopic photographs of livers. Arrowheads indicate HCCs. (B) H&E stained liver sections from normal and HFD-fed mice at week 58. Bar = 72 μm. (C) Liver weight (g). (D) Liver to body weight (%). (E) Plasma LPS levels. (F) Levels of GCA, TCDCA and TCA in liver. (G) Levels of GCA, TCDCA and TCA in plasma. * p<0.05, vs. normal (n=8) (Mean ± SEM).
Enhancing intestinal excretion of hydrophobic BAs can prevent HCC development
HFD-induced accumulation of hepatic BAs was shown to promote liver tumor. To ascertain the role of BAs in HCC development, we repeated the STZ-HFD mice model and reduced the amount of BAs in mice with oral administration of cholestyramine (Fig. 3A). We observed that compared with the STZ-HFD model group, cholestyramine feeding significantly reduced the incidence and size of liver malignant lesions (Fig. 3B). The histology from STZ-HFD was normalized by cholestyramine treatment, which was supported by the reversed pathological features in blood and liver tissue (Fig. 3C and 3D). After cholestyramine administration, the levels of IL-6, TNF-α, collagen Type 1, and the expressions of cancer-related genes Glypican-3 (Gpc3) were normalized (Fig. 3E). Altered BA transporters (Fig. 3E) as well as BAs, mainly DCA, TCA, and TCDCA, in liver (Fig. 3F) and plasma (Supporting Information Fig. 4) were attenuated after cholestyramine intervention. Disruption of gut microbiota and the LPS levels in plasma, liver and feces in response to STZ-HFD intervention were also recovered by cholestyramine intervention (Fig. 3G and 3H and Supporting Information Fig. 1C and 5).
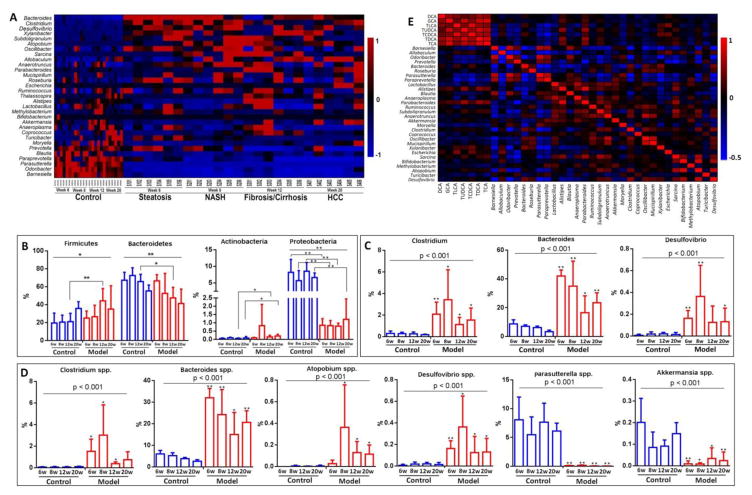
Gut microbiota was significantly altered and correlated with altered BAs during hepatocarcinogenesis
Our metagenomics data showed that the gut microbiota was altered significantly in mice with STZ-HFD intervention at week 6, 8, 12, and 20 (Fig. 4A–4D and Supporting Information Figs. 6–8), consistent with our recently paper on fecal microbiota analysis on NASH-HCC mice22. The abundance of specific microbes changed with the liver disease progression. The relative abundance of OTUs (%) in the fecal Firmicutes and Antinobacteria were significantly increased in model group, while Bacteroides and Proteobacteria were significantly decreased when compared to controls at the phylum level (Fig. 4B). The genus level of Clostridium, Bacteroides, and Desulfovibrio were significantly increased in model group (Fig. 4C). The relative abundance of OTUs (%) in the fecal Clostridium spp., Bacteroides spp., Atopobium app., and Desulfovibrio spp. were significantly increased, while Paasutterella spp. and Akkermansia spp were significantly decreased in model group compared to control group at the species level (Fig. 4D). All these bacteria are reported to be involved in the BA deconjugation, dehydroxylation, and BA degradation to CO2 and H2O.10, 26–28
Figure 4.
Disrupted gut homeostasis during hepatocarcinogenesis. (A) Heatmap showed significantly altered gut microbiota during hepatic carcinogenesis. The relative abundance of OTUs (%) in the fecal firmicutes, bacteroidetes, actinobacteria, and proteobacteria at the phylum level (B), the fecal clostridium, bacteroides, and desulfovibrio at the genus level (C), and (D) the fecal clostridium spp., bacteroides spp., atopobium app., desulfovibrio spp., paasutterella spp. and akkermansia spp. at the species level. (E) Correlations between gut microbiota and liver BAs. *, p < 0.05, **, p < 0.01, compared to controls.
Spearman correlation analysis between the gut microbiota changes and BA concentrations in liver in Fig. 4E showed that TCDCA was significantly negatively correlated with Barnesiella (r=−0.31, p=0.033), Odoribacter (r=−0.29, p=0.047), Parasutterella (r=−0.34, p=0.019), and Paraprevotella (r=−0.48, p=0.001), and was significantly positively correlated with Xylanibacter (r=0.40, p=0.05) and Escherichia (r=0.41, p=0.004). TCA was correlated with Parabacteroides (r=0.46, p=0.001), Alistipes (r=0.40, p=0.005), and Sarcina (r=0.30, p=0.038). GCA was correlated with Alistipes (r=0.34, p=0.017) and Parabacteroides (r=0.30, p=0.036). TLCA was correlated with Parasutterella (r=−0.30, p=0.036), Paraprevotella (r=−0.42, p=0.003), Parabacteroides (r=0.29, p=0.043), and Escherichia (r=0.50, p=0.000). TDCA was correlated Alistipes (r=0.51, p=0.000) and Parabacteroides (r=0.58, p=0.000).
The fecal primary BAs, CDCA was positively correlated with Oribacterium (r=0.31, p=0.03) and slackia (r=0.38, p=0.009) abundance and CA was positively correlated with stenotrophomonas (r=0.53, p=0.0001) and was negatively correlated with parabacteroides (r=−0.29, p=0.046) abundance. On the other hand, Bacteroides was positively correlated with LCA (r=0.60, p=8.6E–06), DCA (r=0.41, p=0.005), TLCA (r=0.37, p=0.012) and TDCA (r=0.38, p=0.008). There was also a significant positive correlation between Clostridium and DCA (r=0.31, p=0.032) and LCA (r=0.64, p=1.1E–06), GLCA (r=0.46, p=0.001), and GDCA (r=0.48, p=0.001). Allobaculum was negatively correlated with TCDCA (r=−0.51, p=0.000), GCA (r=−0.55, p=0.000), TLCA (r=−0.32, p=0.027), TUDCA (r=−0.59, p=0.000) and TCA (r=−0.62, p=0.000). Parasutterella was negatively correlated with DCA (r=−0.38, p=0.009) and LCA (r=−0.69, p=8.6E–08). There was a significant negative correlation between faecalibacterium and DCA (r=−0.39, p=0.008). LCA was also significantly negatively correlated with barnesiella (r=−0.57, p=3.3E–05) and odoribacter (r=−0.58, p=4.8E–05) (Supporting Information Table 4).
BAs accumulation down-regulated the genes involved in BA transport and synthesis
Gene expression analysis showed that a down-regulation of genes involved in BA transport and synthesis in the liver (Fig. 5A and Supporting Information Fig. 9). Notably, hepatic FXR expression in mouse liver was significantly decreased in the mouse model with NASH and fibrosis, suggesting a mechanism of down-regulating hepatic efflux transporters, thus leading to increased BA accumulation in hepatocytes and BA-induced liver injury. As evidenced by a significantly down-regulated BSEP at weeks 6, 8, 12, and 20, and upregulated CYP7A1 at week 6, lead to a marked increase of hepatic BA retention in the pathological development of HCC. This is consistent with the observation of abnormally high BA levels in the liver of the mouse model at weeks 6, 8, 12, and 20. All these indicated that HFD -induced fatty liver leads to a dysregulated BA synthesis and transport in the liver with significantly inhibited hepatic FXR and BSEP expressions. Expression of the uptake transporter for BAs, the sodium-taurocholate cotransporting polypeptide (NTCP), the apical sodium dependent bile acid transporter (ASBT) and the SHP was suppressed by STZ-HFD treatment.
Figure 5.
(A) The mRNA expression of genes in normal group and STZ-HFD intervention group with quantitative real-time polymerase chain reaction (qRT-PCR) analysis at week 6, 8, 12 and 20. (B) The mRNA expression of genes in normal group (6w) and STZ+HFD intervention group with quantitative real-time polymerase chain reaction (qRT-PCR) analysis at week 6, 8, 12 and 20. Each measure was performed three times in duplicates, and is expressed as mean ± SEM. *P<0.05, **P<0.01 vs. control group.
Expression of cytokines was significantly increased in liver carcinogenesis
The expressions of pro-inflammation-related genes such as IL-6 and TNF-α were increased in steatosis and steatohepatitis phases, the expressions of fibrosis-related genes, TIMP metallopeptidase inhibitor 1 (TIMP-1) and collagen Type 1, were increased prior to histological evidence of collagen deposition, and the expressions of cancer-related genes (matrix metallopeptidase 9 (MMP-9) and Gpc3 were increased at the HCC stage (Fig. 5B). All these results suggest that STZ-HFD-induced inflammation and oxidative stress inhibited key BA transporters, resulting in increased intrahepatic BA concentrations (Fig. 1), which promote several important proinflammatory, fibrosis, and cancer markers.
BAs initiate the malignant transformation of normal hepatic cells and inhibit tumor suppressor gene CEBPα in HepG2 cell line
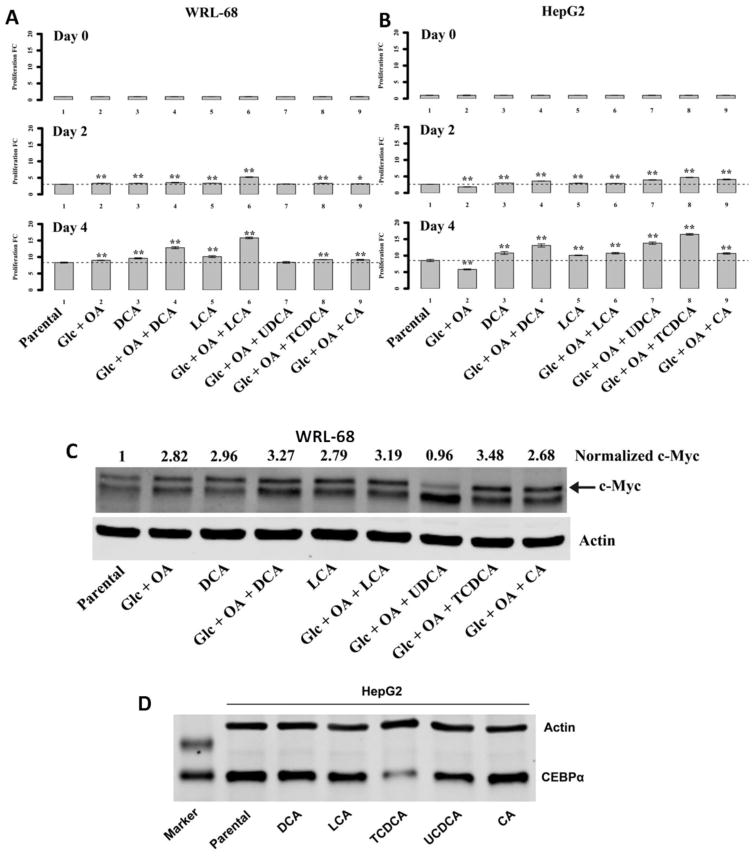
We further tested our hypothesis by investigating the tumor promoting effects of BAs, particularly DCA, LCA, TLCA, TCDCA, TCA, CA, UDCA and GCDCA in cultured normal human liver cells with high glucose and fatty acid concentrations. We found DCA, LCA, CA and TCDCA treatment in high glucose (27.5 mM) and oleic acid (0.3 mM) concentrations significantly promoted WRL-68 (Fig. 6A) and THLE-2 proliferation (Supporting Information Fig. 10), as well as HepG2 cells (Fig. 6B). However, TLCA, TCA and GCDCA treatment had very little influence on the proliferation of WRL-68 cell (Supporting Information Fig. 11). These results showed that DCA, LCA or TCDCA are able to accelerate the growth rate of normal hepatic cells in a high glucose and high fat microenvironment, which may lead to malignant transformation of hepatocytes.
Figure 6.
Proliferation assay for WRL-68 (A) and HepG2 (B) treated with BAs with or without high glucose and oleic acid. (C) DCA, LCA and TCDCA can upregulate the expression of oncoprotein c-myc in WL-68 cells and (D) Expression of CEBPα was reduced by TCDCA treatment in HepG2 cells.
DCA or LCA treatment under high glucose and high fat condition didn’t enhance anchorage-independent growth of WRL-68 as compared with parental cells but were significantly increased as compared to those treated with high glucose and high fat (Supporting Information Fig. 12).
Notably, DCA, LCA and TCDCA increased the expression of oncoprotein c-myc in WRL-68 cells (Fig. 6C) and TCDCA reduced the expression of tumor suppressor gene, CEBPα in HepG2 cells (Fig. 6D).
Discussion
Gut microbiota is being increasingly recognized as an important factor connecting obesity, diabetes, fatty liver disease, and HCC.8, 29 However the definitive mechanisms underlying gut microbiota-mediated development of fatty liver disease and its progression to HCC are still lacking. Our study points to a liver-BA-microbiota metabolic axis as an intrinsic link between gut microbiota and obesity-related liver carcinogenesis.
Using a unique NASH-HCC mouse model, we showed significant accumulation of a panel of hydrophobic and thus, cytotoxic BAs in liver, following the significantly altered gut microbial compositions during liver disease progression. There are strong and bidirectional interactions between BAs and the gut microbiota through biochemical transformation of BA by microbiota (deconjugation, dehydroxylation, dehydrogenation) and, at the same time, through the antimicrobial effects of BAs.30 The study by Sayin et al.31 provided evidence that gut microbiota is a major determinant of bile hydrophobicity in a FXR-dependent manner. Similarly, the interactions between FXR and BAs are also bidirectional, where FXR controls BA synthesis, transport, and metabolism in liver and intestine, and BAs activate FXR. FXR contributes to the maintenance of BA homeostasis and reduction of BA toxicity. In rat liver, FXR activation causes the expressional up-regulation of BSEP and SHP. SHP interferes with the transcription genes that control BA synthesis, Cyp7a1 and Cyp8b1. SHP also affects NTCP expression, the sodium dependent BA uptake carrier from portal vein to liver. In the terminal ileum FXR activation causes the down-regulation of the ileal BA-binding protein and up-regulation of OSTα/β, intestinal BA transporters essential for BA reabsorption. The decreased expression of FXR, BSEP, SHP, and then the Cyp7a1, Cyp8b1, NTCP, and OSTα expression in our results explains the accumulation of BAs in liver due to the decreased pump rate from liver to bile and increased reabsorption of BAs from the portal vein.
The key secondary BA, DCA, was first proposed to be a carcinogen in 1940 by Cook et al.,32 based on induction of tumors in mice when injected. Our results, together with the previous observation that high fat consumption resulted in higher fecal DCA concentrations in healthy male volunteers (ages 20–60),33 suggest that DCA may contribute to at least certain aspects of HCC development. BAs, particularly, GCA, TCA, GCDCA, TCDCA, GDCA, DCA, and TDCA are all implicated as etiologic agents in cancer of gastrointestinal tract.34 Continuous exposure to high levels of BAs may induce mutations and aberrant proliferation, as evidenced by enhanced proliferation of normal human cell lines WRL-68 and THLE-2 or HepG2 cells after exposure to DCA, LCA, or TCDCA. The transcription factor c-myc mediates important biological effects including cell growth, proliferation, loss of differentiation and apoptosis35 and overexpression of c-myc has been observed in human HCC.36 Reports showed that BAs such as DCA and CDCA can be a potent inducer of c-myc in an acid environment,37 and a more recent study further demonstrated that DCA and CDCA under acidic conditions increased human telomerase reverse transcriptase (hTERT) expression in human gastric cancer cells by activation of c-myc transcription.38 Elevated levels of serum and hepatic BAs along with an increased expression of the IL-1β and elevated β-catenin and its target gene c-myc were observed in the Fxr-null mice, which spontaneously develop hepatocellular lesions, adenomas and carcinomas at 12 months of age.39
The liver with high BA levels in model mice shows inflammation, steatohepatitis, fibrosis, and apoptosis. Inflammation is known to stimulate cell death and increase cell turnover, thus promoting liver tumorigenesis.40 As expected, STZ intervention and continuous HFD stimulates oxidative stress and inflammation, as evidenced by continuously increased levels of TNF-α, MMP-9, Timp-1, IL-6, Collagen Type 1 and Gpc-3, a marker for HCC,41 as shown in other reports.6, 19
We fed C57BL/6J mice with HFD for 58 weeks to determine if HFD-induced BA overload can induce the liver tumor formation. The long-term feeding of HFD can induce liver tumor in mice in which significantly increased TCDCA, TCA and GCA in plasma and liver were also observed. TCDCA treatment to HepG2 cells markedly increased the cell proliferation and reduced the expression CEBPα, suggesting that BAs alone may have the tumor promoting effects. CEBPα is a tumor suppressor protein which is neutralized or reduced in HCC.42 Studies showed that the elevated expression of CEBP inhibits liver carcinogenesis in the CEBP knockin mice in which CEBP is expressed from alpha-fetoprotein.43 It was reported that BAs activate the gene encoding SHP via a functional FXR site in its promoter where SHP can bind to CEBPα.44 Additionally, the primary hepatic BA sensor, FXR also inhibits the expression of gankyrin, a small proteasome subunit that mediates the downregulation of tumor suppressor proteins such as p53, HNF4α and CEBPα in the development of HCC.45
Oral administration of cholestyramine significantly reduced the levels of IL-6, TNF-α, and the expressions of collagen Type 1 and Gpc3 and can prevent tumorigenesis in the mice, which gives new impetus to therapeutic efforts to reduce elevated BA levels or to counteract the pro-inflammatory and pro-carcinogenic toxicity of intestinal BAs.
Based on these results, we determined that BAs could collaboratively function to promote hepatic carcinogenesis through several mechanisms (Supporting Information Fig. 13). The first mechanism is that BAs slowly induce metabolic transformation of hepatocytes. Our results showed that secondary BAs and TCDCA are able to accelerate the growth rate of normal hepatic cells in a high glucose and high fat microenvironment. This is not a sufficient evidence for their tumor promoting effects. However, if we take into consideration apoptosis-inducing effect of secondary BAs, which have been extensively reported, it becomes clear that the chronic exposure to excessive amounts of BAs leads to increased and selective growth of hepatocytes that are resistant to apoptosis. The continued renewal of the hepatocytes exposed to high levels of BAs leads to continued decrease of apoptotic cells, increase of hepatocytes that are apoptosis resistant and/or cells with unrepaired DNA damage.
The second mechanism is the BA-induced cholestatic liver injury due to long-term exposure to excessive amounts of hepatic BAs which leads to increased oxidative stress that can lead to DNA damage, mitochondrial damage and disruption of cell membranes in hepatocytes, ultimately leading to HCC via mechanisms of increasing ROS levels, activating Ras and NF-κB.46 Our results collectively showed that a HFD-induced liver damage resulted from increased secondary BAs production in intestine and increased circulation to and retention in liver due to down-regulated BSEP.
The loss of FXR anti-inflammatory effect in liver is another mechanism of BA-promoted liver tumorigenesis. Hepatic FXR exerts anti-inflammatory activity by inhibiting activation of the pro-inflammatory transcription factor NF-κB.47 However, such inhibition is also bi-directional and mediated by BAs. Under inflammatory conditions, elevated pro-inflammatory cytokines, TNF-α and IL-6, modulate FXR-α2 expression with concurrent decreases in BSEP expression48 and reduce bile canalicular contraction, leading to BA accumulation in hepatocytes. On the other hand, increased hepatic BA concentration can stimulate secretion of pro-inflammatory cytokines, TNF-α, and IL-1β from Kupffer cells (hepatic resident macrophages) that activate TNF receptor signaling and the mitogen-activated protein kinase (MAPK)/JNK pathway.49 Activated NF-κB in the liver inhibits hepatic FXR signaling and this in turn down-regulates SHP. It is known that Fxr−/−Shp−/− mice develop spontaneous liver tumors when exposed to chronically elevated BAs.5
Additionally, as our results show, BA-induced increase in LPS production in intestine may also represent an important factor contributing to obesity-related hepatic carcinogenesis. LPS has been implicated as important cofactors in the pathogenesis of liver injury and has been shown to promote hepatic fibrosis. In the pathogenesis of chronic inflammation and autoimmune diseases, dysregulated intestinal BAs may be a causal factor for increased absorption of bacterial LPS,50 thereby promoting systemic inflammation in the organism. These processes are also bi-directionally regulated, as an increase in LPS levels involves a decrease in BA excretion and bile flow leading to further increased intestinal absorption of bacterial LPS. Our results showed that genes involved in inflammation and oxidative stress were attenuated after cholestyramine intervention together with the lowered BA accumulation in liver, indicating an improved liver pathology.
Taken together, the BA-promoted liver carcinogenesis is a complex process involving multiple mechanisms, multiple metabolic organs (liver, bile, intestine, and gut microbiome) and collaborative actions among different BA species. Combining published data with our findings, it is clear that HFD-induced or obesity-related liver carcinogenesis is mediated by altered gut microbiota which results in sustained retention of high concentrations of hepatic BAs. Enhancing the intestinal excretion of hydrophobic BAs or regaining a BA homeostasis will be an attractive therapeutic strategy. These new findings in the study are paving the way for development of better biomarkers and therapies in liver-BA-gut microbiota axis.
Supplementary Material
Novelty and Impact.
Dysregulated bile acids (BAs) are closely associated with liver diseases and attributed to altered gut microbiota. Here, we show that high fat diet-induced liver carcinogenesis is mediated by altered gut microbiota which results in sustained retention of high concentrations of hepatic BAs. Enhancing the intestinal excretion of hydrophobic BAs significantly prevent HCC development. The findings suggest that several hydrophobic BAs may collaboratively promote liver carcinogenesis and regaining a BA homeostasis will be an attractive therapeutic strategy.
Acknowledgments
This study was financially supported by the National Institutes of Health Grant 1U01CA188387-01A1, National Natural Science Foundation of China (Nos. 81573873, 8153000502 and 81273728), the Natural Science Foundation of Shanghai, China (14ZR1441400), the Program for Budgeted Scientific Research Project of Shanghai Municipal Education Commission, China (I3YZ044), and the E-institutes of Shanghai Municipal Education Commission, China (E03008).
The authors thank the Genomics Shared Resource at University of Hawaii Cancer Center (supported by NCI grant CA071789-15) for performing the RT-qPCR assays for this study.
Abbreviations used
- BAs
bile acids
- DCA
deoxycholate
- TCA
taurocholate
- TCDCA
taurochenodeoxycholate
- TLCA
taurolithocholate
- STZ
streptozotocin
- HFD
high fat diet
- NASH-HCC
nonalcoholic steatohepatitis-hepatocellular carcinoma
- HCC
hepatocellular carcinoma
- CA
cholate
- CDCA
chenodeoxycholate
- LCA
lithocholate
- GCDCA
glycochenodeoxycholate
- GCA
glycocholate
- FXR
farnesoid X receptor
- SHP
small hetero-dimer partner
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- H&E
hematoxylin and eosin
- UPLC-TQMS
ultra-performance liquid chromatography triple quadrupole mass spectrometry
- TG
triglyceride
- IL-6
interleukin-6
- TNF-α
tumor necrosis factor
- LPS
lipopolysaccharides
- OTUs
operational taxonomic units
- NTCP
sodium-taurocholate cotransporting polypeptide
- ASBT
apical sodium dependent bile acid transporter
- TIMP-1
TIMP metallopeptidase inhibitor 1
- MMP-9
matrix metallopeptidase 9
- Gpc3
Glypican-3
Footnotes
Conflict of interest. The authors declare that they have no conflict of interest.
References
- 1.GLOBOCAN 2012 CANCER FACT SHEET. Liver Cancer Incidence and Mortality Worldwide in 2012 Summary. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society, Inc; [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Liver Cancer. Atlanta: American Cancer Society; http://www.cancer.org/acs/groups/cid/documents/webcontent/003114-pdf.pdf. [Google Scholar]
- 5.Anakk S, Bhosale M, Schmidt VA, Johnson RL, Finegold MJ, Moore DD. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 2013;5:1060–9. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–7. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 7.Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World Journal of Gastroenterology. 2012;18:4985–93. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res-Rev Mutat. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. Journal of lipid research. 2006;47:241–59. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World journal of gastroenterology: WJG. 2009;15:1677–89. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang Y, Han SI, Mitchell C, Gupta S, Studer E, Grant S, Hylemon PB, Dent P. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology. 2004;40:961–71. doi: 10.1002/hep.20385. [DOI] [PubMed] [Google Scholar]
- 13.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–6. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–86. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–16. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- 16.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4523–30. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng X, Qi X, Cao Y, Su M, Xu LX, Yen Y, Liu P, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics. 2011;10:25. doi: 10.1074/mcp.M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Xie G, Zhao A, Zheng X, Huang F, Wang Y, Yao C, Jia W, Liu P. Serum Bile Acids Are Associated with Pathological Progression of Hepatitis B-Induced Cirrhosis. Journal of Proteome Research. 2016;15:1126–34. doi: 10.1021/acs.jproteome.5b00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, Arumugam S, Watanabe K, Ichida T, Asakura H, Yoneyama H. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013;46:141–52. doi: 10.1007/s00795-013-0016-1. [DOI] [PubMed] [Google Scholar]
- 20.Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Z, Zeisel SH, Jia W. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. Faseb J. 2013;27:3583–93. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG, Edrington TS. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) Bmc Microbiol. 2008:8. doi: 10.1186/1471-2180-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie G, Wang X, Liu P, Wei R, Chen W, Rajani C, Hernandez BY, Alegado R, Dong B, Li D, Jia W. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget. 2016 doi: 10.18632/oncotarget.8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Chanda D, Zhang Y, Choi HS, Chiang JY. Glucose stimulates cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. J Lipid Res. 2010;51:832–42. doi: 10.1194/jlr.M002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui W, Chen SL, Hu KQ. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2010;2:95–104. [PMC free article] [PubMed] [Google Scholar]
- 26.Hylemon PB, Harder J. Biotransformation of monoterpenes, bile acids, and other isoprenoids in anaerobic ecosystems. FEMS microbiology reviews. 1998;22:475–88. doi: 10.1111/j.1574-6976.1998.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiang JY. Bile acids: regulation of synthesis. Journal of lipid research. 2009;50:1955–66. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philipp B. Bacterial degradation of bile salts. Applied microbiology and biotechnology. 2011;89:903–15. doi: 10.1007/s00253-010-2998-0. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 30.Islam KBMS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile Acid Is a Host Factor That Regulates the Composition of the Cecal Microbiota in Rats. Gastroenterology. 2011;141:1773–81. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–35. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Cook JW, Kennaway EL, Kennaway NM. Production of Tumours in Mice by Deoxycholic Acid. Nature. 1940;145:627. [Google Scholar]
- 33.Rafter JJ, Child P, Anderson AM, Alder R, Eng V, Bruce WR. Cellular toxicity of fecal water depends on diet. The American journal of clinical nutrition. 1987;45:559–63. doi: 10.1093/ajcn/45.3.559. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009 Jul 21;15(27):3329–40. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nevzorova YA, Hu W, Cubero FJ, Haas U, Freimuth J, Tacke F, Trautwein C, Liedtke C. Overexpression of c-myc in hepatocytes promotes activation of hepatic stellate cells and facilitates the onset of liver fibrosis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832:1765–75. doi: 10.1016/j.bbadis.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nature genetics. 2002;31:339–46. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 37.Tselepis C, Morris CD, Wakelin D, Hardy R, Perry I, Luong QT, Harper E, Harrison R, Attwood SEA, Jankowski JAZ. Upregulation of the oncogene c-myc in Barrett’s adenocarcinoma: induction of c-myc by acidified bile acid in vitro. Gut. 2003;52:174–80. doi: 10.1136/gut.52.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Zhou P, Sun X, Zheng J, Wei G, Zhang L, Wang H, Yao J, Lu S, Jia P. Acidified bile acids increase hTERT expression via c-myc activation in human gastric cancer cells. Oncology reports. 2015;33:3038–44. doi: 10.3892/or.2015.3908. [DOI] [PubMed] [Google Scholar]
- 39.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–6. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 41.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 42.Iakova P, Timchenko L, Timchenko NA. Intracellular signaling and hepatocellular carcinoma. Seminars in cancer biology. 2011;21:28–34. doi: 10.1016/j.semcancer.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan EH, Hooi SC, Laban M, Wong E, Ponniah S, Wee A, Wang ND. CCAAT/enhancer binding protein alpha knock-in mice exhibit early liver glycogen storage and reduced susceptibility to hepatocellular carcinoma. Cancer Res. 2005;65:10330–7. doi: 10.1158/0008-5472.CAN-04-4486. [DOI] [PubMed] [Google Scholar]
- 44.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. Journal of Lipid Research. 2009;50:1509–20. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J, Zhao K, Zheng L, Xu Z, Gong W, Chen S, Shen X, Huang G, Gao M, Zeng Y, Zhang Y, He F. Upregulation of microRNA-122 by farnesoid X receptor suppresses the growth of hepatocellular carcinoma cells. Molecular cancer. 2015;14:163. doi: 10.1186/s12943-015-0427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pean N, Doignon I, Tordjmann T. Bile acids and liver carcinogenesis: TGR5 as a novel piece in the puzzle? Clinics and research in hepatology and gastroenterology. 2013;37:226–9. doi: 10.1016/j.clinre.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–43. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Song XL, Valanejad L, Vasilenko A, More V, Qiu X, Chen WK, Lai YR, Slitt A, Stoner M, Yan BF, Deng RT. Bile salt export pump is dysregulated with altered farnesoid X receptor isoform expression in patients with hepatocellular carcinoma. Hepatology. 2013;57:1530–41. doi: 10.1002/hep.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyake JH, Wang SL, Davis RA. Bile acid induction of cytokine expression by macrophages correlates with repression of hepatic cholesterol 7alpha-hydroxylase. The Journal of biological chemistry. 2000;275:21805–8. doi: 10.1074/jbc.C000275200. [DOI] [PubMed] [Google Scholar]
- 50.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology letters. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.