Abstract
Keratinocyte migration is critical to reepithelialization during wound repair. The motility response is promoted by growth factors, cytokines, and cytokines produced in the wound bed, including those that activate the epidermal growth factor (EGF) receptor. The Alu-Leu-Arg-negative CXC chemokine interferon-inducible protein 9 (IP-9; also known as CXCL11, I-TAC, beta-R1, and H-174) is produced by keratinocytes in response to injury. As keratinocytes also express the receptor, CXCR3, this prompted us to examine the role and molecular mechanism by which IP-9 regulates keratinocyte motility. Unexpectedly, as CXCR3 liganding blocks growth factor-induced motility in fibroblasts, IP-9 alone promoted motility in undifferentiated keratinocytes (37 ± 6% of the level of the highly motogenic EGF) as determined in a two-dimensional in vitro wound healing assay. IP-9 even enhanced EGF-induced motility in undifferentiated keratinocytes (116 ± 5%; P < 0.05 compared to EGF alone), suggesting two separate mechanisms of action. IP-9-increased motility and -decreased adhesiveness required the intracellular protease calpain. The increases in both motility and calpain activity by IP-9 were blocked by pharmacological and molecular inhibition of phospholipase C-β3 and chelation of calcium, which prevented an intracellular calcium flux. Molecular downregulation or RNA interference-mediated depletion of μ-calpain (calpain 1) but not M-calpain (calpain 2) blocked IP-9-induced calpain activation and motility. In accord with elimination of IP-9-induced de-adhesion, RNA interference-mediated depletion of calpain 1 but not calpain 2 prevented cleavage of the focal adhesion component focal adhesion kinase and disassembly of vinculin aggregates. In comparison, EGF-induced motility of the same undifferentiated keratinocytes requires the previously described extracellular signal-regulated kinase to the M-calpain pathway. These data demonstrate that while both EGF- and IP-9-induced motility in keratinocytes requires calpain activity, the isoform of calpain triggered depends on the nature of the receptor for the particular ligand. Interestingly, physiological nonapoptotic calcium fluxes were capable of activating μ-calpain, implying that the calcium requirement of μ-calpain for activation is attained during cell signaling. This is also the first demonstration of differential activation of the two ubiquitous calpain isoforms in the same cell by different signals.
Regulated cell motility is critical to wound healing (25, 35). During repair, tissue deposition and remodeling by the immigrant fibroblasts and keratinocytes result in the regeneration of an intact skin barrier and functional organ. The cells from the remaining epidermal and dermal layers must proliferate and migrate to repopulate the nascent wound. The basal keratinocytes undergo a transition that enables such repopulation while the provisional matrix is invaded by fibroblasts as the first step in regenerating the future dermal layer. The numerous growth factors present throughout repair, including high levels of epidermal growth factor (EGF) receptor (EGFR) ligands such as HB-EGF and transforming growth factor alpha, are thought to promote these mitogenic and motogenic responses (26, 35, 45, 50, 60). However, the process of cell repopulation is limited late in the process of healing to prevent fibroplasia and excess matrix deposition. Late in the repair process, members of the Alu-Leu-Arg (ELR)-negative family of CXC chemokines appear (13, 42). It has been proposed that the migration of fibroblasts and keratinocytes is controlled by the waves of these growth factors and chemokines produced throughout wound repair (9, 35, 42, 44, 60). This would include signals to promote as well as inhibit cell migration.
Active cell locomotion requires the coordination of a number of cellular processes that should be common among cell types (18, 28). Thus, as numerous external signals can modulate cell motility, two key questions are which biochemical pathway is actuated to promote migration during regeneration and whether these differ between cell types. Any such signaling pathway needs to affect key biophysical processes. During cell migration, tail de-adhesion may be rate-limiting; in experimental models, failure to detach limits cell motility (23, 44). Activation of calpain (EC 3.4.22.17), an intracellular limited protease, is required for integrin-mediated tail de-adhesion on moderately and highly adhesive substrata (30, 37) and for growth factor-induced motility (17, 43). This intracellular protease is a key switch, as calpain inhibitors convert EGFR-mediated signals from cell motility to matrix contractility (1). Thus, calpain activators appear to shift a wide range of cells to motility-permissive adhesion regimens, while inhibitory signals for calpain block productive locomotion. As such, we proposed that keratinocyte motility was dependent on calpain activity.
This requirement for calpain activity provides a target for regulating cell motility (18). Confounding any analysis, two calpain isoforms with seemingly identical target specificities are present in practically all cells (48). In vitro, calpain 1 (μ-calpain) is activated at nearly micromolar concentrations of calcium; calpain 2 (M-calpain) requires millimolar calcium levels. While calcium fluxes have been postulated to regulate μ-calpain, this has yet to be demonstrated conclusively in living cells (5), and the signaling cascade that triggers this isoform during cell locomotion remain undefined (30). Furthermore, the physiologically relevant activators of M-calpain are unknown since intracellular calcium levels fail to reach the nearly millimolar concentrations required in vitro (22). Still, we know that plasma membrane-localized M-calpain is activated subsequent to growth factor signaling by direct extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAP kinase) phosphorylation (12, 16, 17, 44). However, what signals and respective intracellular signaling pathways operate during reepithelialization by keratinocytes remains an open question (42).
In an initial exploration (43, 44), we reported that ELR-negative CXC chemokines, present during the process of wound repair (13, 42), can block fibroblast motility by preventing activation of M-calpain and subsequent de-adhesion, demonstrating that this might be a physiologically operative pathway. Of this family of chemokines, interferon-inducible protein 10 (IP-10) appears to be produced by the neovasculature deep in the dermis (13). A related ELR-negative CXC chemokine, IP-9, also called beta-R1 (40), H174 (24), and I-TAC (10), is produced by basal keratinocytes in response to immune-mediated injuries (52). Previously, we demonstrated that IP-9 is a wound response factor (42).
These ELR-negative CXC chemokines were originally found as modulators of cells of the hematopoietic lineage, but chemokine receptors have been found on endothelial and epithelial cells (36, 38, 44, 47, 63). The ELR-negative members of the CXC family of chemokines, all of which bind to a common CXCR3 receptor (2, 14, 27), inhibit endothelial cell proliferation, migration (21, 31, 51), and fibroblast migration (44). These appear to act dominantly over promitogenic and promotility chemokines and growth factors (43, 44). IP-9 is a wound response factor situated in the right place to limit fibroblast repopulation and promote the remodeling phase (42). However, with IP-9 being produced by keratinocytes in or near the wound bed, the situation for the reepithelializing keratinocytes is of great interest; would this chemokine, which serves to “mature” the dermis, also have the collateral effect of slowing reepithelialization?
Here we report that keratinocyte motility is promoted by both EGF and IP-9 through their activation of calpains. Furthermore, IP-9 does not block EGF-induced motility in keratinocytes, opposite to its effects on fibroblasts. Keratinocyte motility induced by EGF requires the pathway previously described in fibroblasts, which culminates in activation of the calpain 2 isoform, M-calpain (17). In undifferentiated keratinocytes, IP-9 also activated calpain, though it was the μ-calpain isoform and not the M-calpain isoform that was both triggered and required. This chemokine activation occurred through a phospholipase Cβ (PLCβ)-mediated calcium flux in distinction to the ERK MAP kinase signaling cascade that growth factors utilize to activate M-calpain (17). These two pathways converge at cell-diminished cell adhesion to substratum as mirrored in vinculin aggregate disassembly and cleavage of the focal adhesion kinase (FAK). This is the first demonstration, to our knowledge, of different isoforms of calpain being activated by distinct signals in the same cell to accomplish the same phenotypic end point, cell migration. We also show for the first time that IP-9 increased the intracellular calcium flux and resulted in triggering of the μ-calpain isoform, in turn resulting in productive cell motility.
MATERIALS AND METHODS
Materials.
Human neonatal foreskin epidermal keratinocytes (HEKn) were obtained from Cascade Biologicals, Inc. (Portland, Oreg.). IP-9 and IP-10 were purchased from Peprotech (Rock Hill, N.J.). Human recombinant EGF was obtained from Collaborative Biomedical Products (Bedford, Mass.). Calpain inhibitor I ALLN (calpain inhibitor 1), ET-18-OCH3, propranolol, and clasto-lactacystin β-lactone were obtained from Biomol (Plymouth Meeting, Pa.). Calpain inhibitor IV (Z-Leu-Leu-Y-fluoromethyl ketone [FMK]) and BAPTA-AM (1,2-bis[o-aminophenoxy]ethane-N,N,N′,N′-tetraacetic acid) were purchased from Calbiochem (La Jolla, Calif.). PD98059 was obtained from New England BioLabs (Beverly, Mass.). The pharmacological doses presented herein were established in prior publications (17, 42, 43) or empirically (data not shown). Boc-LM-CMAC (t-butoxycarbonyl-Leu-Met-chloromethylaminocoumarin) was obtained from Molecular Probes (Eugene, Oreg.). Purified porcine M- and μ-calpains were obtained from Calbiochem. Casein was purchased from Sigma (St. Louis, Mo.). The other reagents were from Sigma Chemical Corporation.
Keratinocyte cultures.
Human primary epidermal keratinocytes (HEKn) were grown in serum-free medium with human EGF (10.2 ng/ml), hydrocortisone (0.18 μg/ml), bovine pituitary extract (0.2%, vol/vol), bovine insulin (5 μg/ml), and transferrin (5 μg/ml) (EpiLife medium, Cascade Biologicals) containing a low calcium concentration (0.06 mM) to maintain a dedifferentiated, proliferative, and migratory state. This state of the keratinocytes will be referred to as undifferentiated to distinguish them from polarized keratinocytes noted upon a switch to high calcium (0.37 mM) (42). Cells were allowed to become quiescent in EpiLife medium without EGF (or bovine pituitary extract or insulin) for 48 h prior to testing. All primary epithelial cultures were used at passage 2 to 9, as this preceded any culture-induced or in vitro aging changes in phenotype, as confirmed empirically.
HaCaT cells, a spontaneously immortalized but not transformed human keratinocyte cell line (4), has been used as a model for human keratinocytes. HaCaT cells was kindly provided by N. Fusenig, German Cancer Research Center (Heidelberg, Germany). The cells were maintained as monolayer cultures in Dulbecco's modified Eagle's medium (Life Technologies, Gaithersburg, Md.), supplemented with 10% fetal calf serum (Life Technologies) along with 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM minimal essential medium, nonessential amino acids, and the antibiotics penicillin (100 U/ml) and streptomycin (100 μg/ml). Subconfluent cells were passaged with a 1:8 split ratio at 3-day intervals with 0.25% trypsin with 0.25 mM EDTA. Cells were allowed to become quiescent for 48 h with 0.1% dialyzed fetal calf serum prior to experiments.
Cell migration assay.
An in vitro wound healing assay was performed in keratinocytes plated in tissue culture dishes (seeded at 40,000 per cm2) (6). At 80% confluence the cells were allowed to become quiescent in EpiLife medium without EGF, bovine pituitary extract, and insulin or in Dulbecco's modified Eagle's medium with 0.1% fetal bovine serum for 48 h. The undifferentiated keratinocytes (HEKn) and HaCaT cells were then treated or not with EGF (1 nM) and/or IP-9 (50 ng/ml) and incubated at 37οC for 24 h. Photographs were taken at 0 and 24 h, and the relative distance traveled by the cells at the acellular front was determined by computer-assisted image analysis; markings on the plate ensured measurement of the same site for the photographs. The distance migrated was then expressed as a percentage of the EGF-induced distance within each experiment, allowing direct interexperiment comparisons.
Cell de-adhesion assay.
The cell substratum adhesiveness of undifferentiated keratinocytes was quantitated with an inverted centrifugation assay (62). Cells were plated at a concentration of 103 cells per ml in a 24-well plate. Cells were allowed to become quiescent for 24 h and then treated with antisense oligonucleotides specific for M-calpain and μ-calpain for 8 h along with EGF and IP-9 to deplete the existing calpain. Cells were then washed and incubated for a further 12 to 14 h in the presence of antisense oligonucleotides but not EGF and IP-9 to allow recovery from EGF and IP-9 to allow recovery from EGF and IP-9 exposure but prevent de novo calpain synthesis (17). After 14 h the cells were again stimulated or not with IP-9 for 2 h and/or EGF for 30 min. Wells were completely filled with EpiLife supplemented with 1% bovine serum albumin and 25 mM HEPES (pH 7.4) and then sealed with enzyme-linked immunosorbent assay sealing tape (Corning, Cambridge, Mass,) and centrifuged inverted for 10 min at 2,920 × g at 37οC with a Beckman CS6R plate centrifuge. Before and after the centrifugation, the number of cells on the plates was counted under phase-contrast microscopy.
Calpain activity assays.
Calpain activity was determined with a variety of assays to detect calpain isoforms (casein zymography) and activation status in living cells (Boc fluorescence) and cell extracts (MAP2 fluorescence).
Casein (7.5 mg/ml) was copolymerized with 30% acrylamide-bisacrylamide (wt/vol), with 5× and 2× HEPES-imidazole buffer and 200 mM EGTA as the separating gel and 30% acrylamide-bisacrylamide (wt/vol) and 5× HEPES-imidazole buffer as the stacking gel was poured into Minigel casts (Bio-Rad, Hercules, Calif.) (11). Ammonium persulfate (10%, wt/vol) and TEMED (tetramethylethylenediamine) were used to catalyze the polymerization. The casein gels were run with a buffer containing 1× HEPES-imidazole buffer, 5 mM EDTA, and 20 mM 2-mercaptoethanol for 15 min at 4°C. Samples were lysed in 25 mM MOPS (morpholinepropanesulfonic acid; pH 7.5)-10 mM EGTA-10 mM EDTA-5 mM 2-mercaptoethanol with 5 μg/ml of calpain inhibitor I to downregulate endogenous calpain production. An equal volume of sample buffer (25 mM Tris [pH 6.8], 25 mM EDTA, 50% glycerol, 50 mM 2-mercaptoethanol, 0.5% [wt/vol] bromophenol blue) was added to the sample. Equal amounts of protein (approximately 40 μg of sample) were loaded for each condition. Samples were loaded and the gels were run at 125 V for 3 h in the cold. The gel was then removed and incubated in 20 mM MOPS (pH 7.5)-5 mM 2-mercaptoethanol with or without calcium (5 mM) for 24 h at ambient temperature. To visualize the areas of calpain proteolysis, the gel was stained with Coomassie blue.
For all the live cell experiments, we used IP-9 (50 ng/ml), EGF (1 nM), calpain inhibitor 1 (5 μg/ml), PD98059 (2 μM), ET-18-OCH3 (100 nM), Z-LLY-FMK (10 μM), propranolol (100 μM), Boc-LM-CMAC (50 μM), and BAPTA-AM (5 μM); cells were incubated at 37οC (17). The concentrations of inhibitors were determined empirically as the minimal dose required to inhibit that specific target without altering cell viability or inhibiting nontarget pathways; the doses of most of the inhibitors are from published experimental studies. For the inhibitors for which the optimal dose was not available, dosage studies were performed, and reported here are the optimal concentrations which did not affect the cell shape and morphology but were sufficient to inhibit calpain activity.
The calpain activity in individual living cells was detected with a Boc-LM-CMAC assay for in vivo proteolysis. In brief, keratinocytes were plated on glass coverslips. The cells were allowed to become quiescent at 50% confluence for 48 h and then treated in the presence or absence of IP-9, calpain inhibitor 1, ET-18-OCH3, and/or EGF for 2 h, 1 h, 30 min, and 10 min, respectively. BAPTA-AM, a cell-permeating acetoxymethyl (AM) ester derivative of 1,2-bis(O-aminophenoxy)-ethane-N,N,N,N-tetraacetic acid, is an intracellular calcium chelator and was used to determine the requirement of intracellular calcium for induced calpain activity. BAPTA-AM was loaded for 30 min prior to Boc-LM-CMAC and in the presence or absence of EGF and/or IP-9. All cells were loaded with Boc-LM-CMAC for 20 min prior to mounting on glass slides. The treated and control cells were then observed for CMAC fluorescence with an Olympus fluorescent microscope (model BX40, Olympus M-NUA filter). Representative images of each slide were captured with a SPOTII charge-coupled device camera (Diagnostic Instruments, Sterling Heights, Mich.). The image exposure settings were identical within each experiment (i.e., for no-EGF and EGF treatment) but did vary slightly between experiments; thus, one can directly compare fluorescence intensity within an experiment but not between experiments. Cell shape did not vary appreciably between treatments and controls when analyzed for cross-sectional area (data not shown). Images shown are representative of three or more separate experiments.
A second assay of calpain activity used is the hydrolysis of microtubule-associated protein 2 (MAP2)-dichlorotriazinylaminofluorescein (DTAF) by cell lysates (17, 57). Briefly, MAP2 (Cytoskeleton, Denver, Colo.) was conjugated with DTAF by incubation in a pH 8.5 PIPES (piperazine ethanesulfonic acid) buffer for 30 min at 4°C. Labeled MAP2 was isolated by size exclusion column chromatography and dialyzed against pH 7.5 HEPES buffer overnight. Cells were grown to confluence and allowed to become quiescent for 48 h. After a 2-h treatment with IP-9, 30 min with calpain inhibitor I, and/or 5 min with EGF, cells were washed twice with ice-cold phosphate-buffered saline and lysed in 20 nM HEPES (pH 7.4)-10% glycerol-0.1% Triton X-100-500 mM sodium chloride-1 mM sodium vanadate. After removing the cell debris by centrifugation, 0.9 μg of DTAF-labeled MAP2 and 2 μl of 0.1 mM CaCl2 were added to 100 μl of lysate. Fluorescence was immediately measured with an AmincoBowman Series II spectrofluorometer (Spectronic Instruments Inc., Rochester, N.Y.) at excitation and emission wavelengths of 490 and 520 nm, respectively, for 3 min at room temperature.
Immunoblotting.
Undifferentiated keratinocytes were treated with IP-9 (50 ng/ml), EGF (1 nM), or forskolin (25 μM) for 2 h, 10 min, and 15 min, respectively, as determined for optimal effects empirically and in prior efforts (42, 43). HaCat cells were treated with IP-9 (200 ng/ml) and EGF (10 nM) for 30 min and calpain inhibitor 1 (5 μM) for 30 min to determine the cleavage of the calpain substrate FAK. Cells were then lysed and proteins separated on sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with anti-phospho-ERK antibody (New England Biolabs) or anti-FAK (clone 77; BD Biosciences Pharmingen, San Diego, Calif.) before visualizing with alkaline phosphatase-conjugated secondary antibody and nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) substrate (Promega, Madison, Wis.).
Calpain antisense downregulation.
The isoform-specific role of calpain activity in cell migration with and without EGF and IP-9 treatment was determined by downregulating M-calpain and μ-calpain with antisense phosphothiorate oligonucleotides (17). Cells were treated with 20 μM oligonucleotide (Life Technologies) throughout the cell motility assay. The oligonucleotides were designed to hybridize with the initiation codon for each isoform (5′-ATGCCCGCCATGCTGCGT-3′) for M-calpain (calpain 2) and (5′-ATCTCCTCCGACATCCTG-3′) for μ-calpain (calpain 1).
To determine the efficacy of downregulation of calpain isoforms, the Boc-LM-CMAC assay was used. Cells were treated or not with EGF and/or IP-9, and the calpain levels were then analyzed under conditions similar to those seen during the productive locomotive phase of the motility experiment. In brief, cells were treated with EGF, IP-9 and oligonucleotides for 8 h to deplete the existing calpain. Cells were then incubated for a further 12 to 14 h in the presence of oligonucleotide but not EGF and IP-9 to allow recovery from EGF and IP-9 exposure but prevent de novo calpain synthesis. After 12 h the cells were again stimulated or not with IP-9 for 2 h and/or EGF for 10 min, followed by treatment with Boc-LM-CMAC for 20 min, and calpain activity was assessed.
siRNA transfections.
Short interfering RNA (siRNA) duplexes (siRNAs) were synthesized and purified by IDT (Coralville, Iowa). The siRNA sequence for targeting human M-calpain (GenBank accession number NM_001748) was M-calpain siRNA (5′-GGCAUUAGAAGAAGCAGGUTT). The siRNA sequence for targeting human μ-calpain (GenBank accession number X04366) was done with two sequences of μ-calpain siRNA (5′-GCCAGGAAGGCAGCUUUCGC-3′ and 5′-GAGGAGAUUGACGAGAACTT). Green fluorescent protein (GFP) siRNA(5′-GACCCGCGCCGAGGUGAAGTT-3′) was used as a negative control.
Transfection of siRNAs was performed with the manufacturer's protocol for Lipofectamine 2000 (Invitrogen). Briefly, 4 μl of 20 μM siRNA was mixed with 200 μl of Opti-MEM; 4 μl of Lipofectamine 2000 was diluted in 200 μl of Opti-MEM and incubated at room temperature for 5 min. After the incubation, the diluted Lipofectamine 2000 was combined with the diluted siRNA and then incubated for an additional 20 min at room temperature. Total 400 μl of siRNA-Lipofectamine 2000 complexes was applied to each well of cultured HaCaT keratinocytes at ≈70% confluence in a six-well plate. The cells were stimulated with EGF or IP-9 during the entire time of motility and activation studies. The cells were analyzed 48 h after siRNA transfection by immunoblotting with calpain antibodies specific for M-calpain (Santacruz Biologicals, Santacruz, Calif.) and μ-calpain (Biomol) or FAK (BD Biosciences Pharmigen), to assess calpain activity with Boc-LM-CMAC assay, by immunofluorescence to visualize vinculin aggregates, and to determine cell motility and adhesiveness.
PLC-β3 antisense downregulation.
The role of PLC-β3 in transmitting IP-9 signals was elucidated by downregulating PLC-β3 with two antisense phosphothiorate oligonucleotide; the sequences of the oligonucleotides were 5′-CTGGGCGCCCGCCATGGCCCG-3′ and 5′-GTACAAGAAGAAGCCATTGGG-3′. Cells were treated with 20 μM oligonucleotide (Life Technologies) for 48 h, after which the cells were stimulated with IP-9 or EGF for 2 h or 10 min, respectively. This was followed by treatment with Boc-LM-CMAC for 20 min, and calpain activity was assessed. Antisense downregulation of PLC-γ1 was used as control for the experiments (8). The extent of downregulation was assessed by immunoblotting with a primary antibody specific for PLC-β3 (Santa Cruz Biologicals).
Intracellular calcium measurements.
Fluo-3-AM (1-[2-amino-5-(2,7-dichloro-6-hydroxy-3-oxy-9-xanthenyl) phenoxy]-2-[2-amino-5-methylphenoxy]ethane-N,N,N′,N′-tetraacetic acid was used as the membrane-permeable acetoxymethyl ester as described (53). HEKn and HaCaT cells were grown to 75% confluence in a cover glass slide and allowed to become quiescent for 24 h. After 24 h, cells were incubated with IP-9 (50 ng/ml) for 2 h or platelet-derived growth factor (100 ng/ml) for 30 min and BAPTA-AM (20 μM) for 40 min prior to loading of 10 μM fluo-3 AM for 1 h, allowing cell esterases to release the membrane-impermeant free calcium-binding polycarboxylic acids. After washing with phosphate-buffered saline several times, cells were placed in HEPES buffer (10 mM) for HEKn or for HaCaT cells in Dulbecco's modified Eagle's medium without sodium bicarbonate, 10 mM HEPES, 10% fetal calf serum, along with 1% streptomycin and adjusting the pH to 7.3. Following this, the fluorescence was measured as a function of time at 525 nM with excitation at 488 nM. Twelve images were captured at 10 seconds apart with 200 millisecond fluorescence integration at 100× magnification with a inverted confocal microscope.
Experimental analysis.
Background fluorescence was subtracted and fluorescence was calculated as a function of time for individual cells over the entire measurement period. The number of cells analyzed was 18 (12 frames) for different treatments along with nontreated cells. Six cells each from three different experiments gave similar findings.
Vinculin staining and image acquisition.
HaCaT keratinocytes were seeded at 8,000 cells per well on eight-well chamber slides, and the cells were allowed to become quiescent for 48 h once they reached subconfluence. Prior to fixing the cells with freshly prepared 4% paraformaldehyde (vol/vol), the cells were treated with EGF (10 nM) or IP-9 (50 ng/ml) for 30 min and 60 min, along with calpain inhibitor 1. Transfections with M-calpain siRNA and μ-calpain siRNA were accomplished as above with the modification that cells were seeded on a glass coverslip in a six-well plate. After the transfection and staining for vinculin, the coverslips were mounted onto a glass slide to view focal adhesions. In brief, after fixing and the initial steps of blocking with 5% bovine serum albumin and washing with Tris-buffered saline with Tween 20 (TBST), the cells were incubated for 1 h with monoclonal mouse vinculin clone HVIN-1 V-1931 (1:400) obtained from Sigma. After washing three times with TBST, the cells were incubated with Alexa fluorescein isothiocyanate (1:100)-conjugated goat anti-mouse immunoglobulin G along with propidium iodide (30 μg/ml) for nuclear staining, followed by mounting the cells with Gelvatol (Aldrich Chemical, Milwaukee, Wis,).
With the confocal laser-scanning microscope (LSM 510, Zeiss, Jena, Germany),double-labeled immunofluorescence was examined. Illumination was provided by argon (458, 477, 488, and 514 nm, 30 mW), HeNe (543 nm, 1 mW), and HeN (633 nm, 5 mW) lasers. Digital images were captured with LSM 510 3.2 software (Zeiss). The acquired eight-bit images at a resolution of 1,240 pixels was processed with Adobe Photoshop 7.0.1 software (Adobe Systems, San Jose, Calif.).
RESULTS
IP-9, a wound response protein, promotes keratinocyte motility.
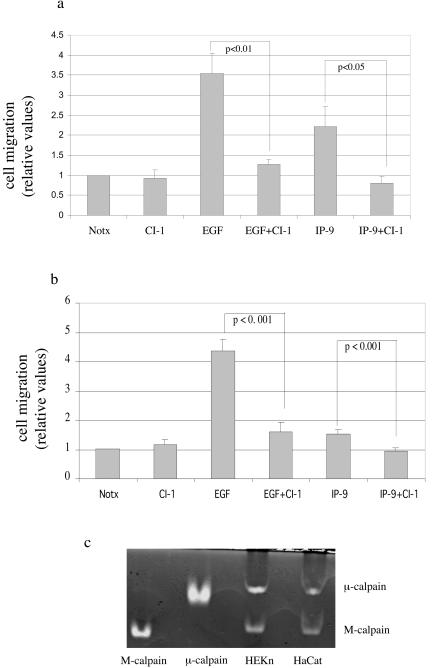
CXCR3-binding ligands (IP-9, IP-10, and PF4) limit growth factor-induced fibroblast motility by preventing M-calpain-mediated rear de-adhesion (42-44). However, we had earlier reported that IP-9 enhances the motility of undifferentiated keratinocytes (42). This is evident from IP-9 exposure leading to increased motility of the challenged keratinocytes in both the absence of EGF and its presence. Our previous work demonstrated that inhibition of calpain by the pancalpain inhibitor calpain inhibitor 1 (ALLN) (5 μg/ml) prevented induction of motility (Fig. 1a) and de-adhesion by either EGF or IP-9.
FIG. 1.
IP-9 expressed in wounded keratinocytes induces motility of undifferentiated keratinocytes (HEKn cells) (a) and immortalized keratinocytes (HaCaT cells) (b) expressing both calpain isoforms (c). (a) Early-passage human keratinocytes were tested for induced motility in an in vitro wound healing assay. Cells were treated with EGF (1 nM) or IP-9 (50 ng/ml) and a pharmacological inhibitor of calpain, calpain inhibitor 1 (5 μM), throughout the assay. The values are shown as the ratio of EGF (1 nM)-induced cell motility. The values are means ± standard error of the mean of three independent studies each performed in triplicate. Statistical analysis was performed by Student's t test. (b) Human immortalized keratinocytes, HaCaT cells, were treated with EGF (1 nM) or IP-9 (50 ng/ml) and calpain inhibitor 1 (5 μM) throughout the assay. The values are mean ± standard error of the mean of three independent studies, each performed in triplicate. Statistical analysis was performed by Student's t test. (c) Casein zymography demonstrates that both HEKn and HaCaT keratinocytes possess potential calpain activity from both ubiquitous isoforms. Proteins (approximately 40 μg) from both HEKn and HaCaT keratinocytes were electrophoresed into a casein gel and subsequently incubated in buffer containing 20 mM MOPS, pH 7.5-5 mM 2-mercaptoethanol and calcium (5 mM). Shown here are the Coomassie blue-stained casein gels. Purified porcine M- and μ-calpains (1 μg each) served as controls. Images are representative of three separate experiments. The data presented in part a are independently derived but similar to those published before(42); the data are provided herein for context.
The HaCaT cell line, which has been used as a model for human keratinocytes (4), remains adherent to glass in a calcium-free external environment, offering a convenient tool for investigating calcium-related signal transduction. Since we have used human immortalized keratinocytes (HaCaT) to perform calcium flux measurements, certain key experiments are repeated to validate the use of HaCaT cells in this study. We demonstrate the similar effects of EGF and IP-9 and calpain inhibitor 1 in HaCaT cells (Fig. 1b). However, these data of IP-9 inducing motility in keratinocytes leads to the conundrum of how CXCR3 ligands may initiate diametrically opposite cell responses in different cell types.
IP-9 induces calpain activity in undifferentiated keratinocytes.
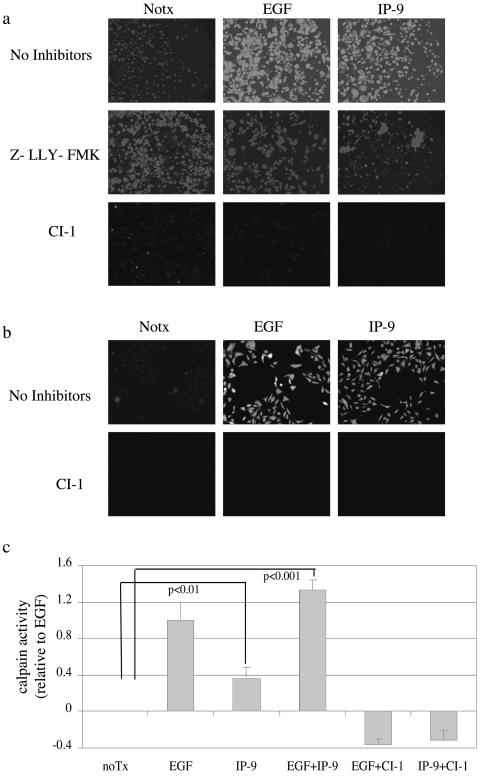
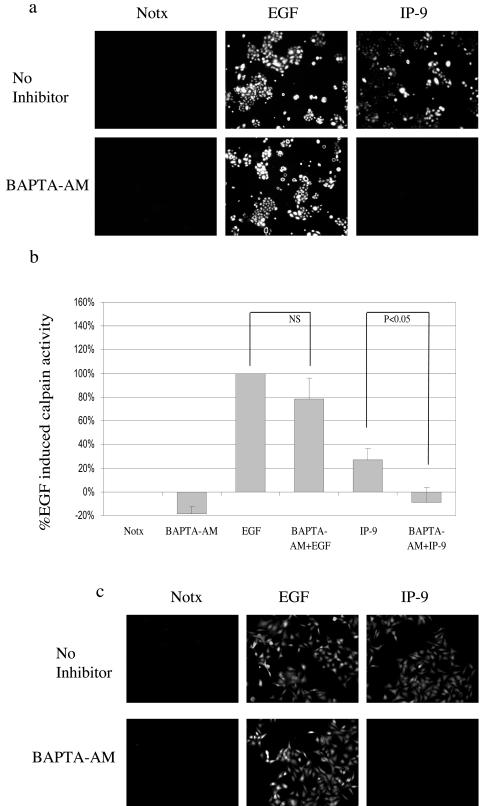
The two main isoforms of calpain, M- and μ-calpain, are defined by their in vitro sensitivity to calcium for activation(48). Both of these isoforms are expressed in HEKn and HaCaT keratinocytes (Fig. 1c). Thus, the signals initiated by IP-9 (and EGF) might regulate either isoform, as the earlier study utilized agents that inhibit both isoforms (42) (Fig. 1). Calpain activity was assessed by the Boc-LM-CMAC assay, which provides an in vivo measurement under physiological conditions, since it identifies cells in which calpain is activated (41). EGF increased calpain activity more than twofold compared to nontreated HEKn and HaCaT cells (Figs. 2a and 2b), in concordance with what we measured in other epithelial cells (32). Calpain activity was also increased in IP-9-treated cells compared to basal activity in nontreated cells. Increased activation of calpain was also noted in the ex vivo MAP2 cleavage assay, which was used to confirm the Boc-LM-CMAC fluorescence (Fig. 2c). The Boc fluorescence induced by either EGF or IP-9 was due to calpain activity as determined by inhibition by the pharmacological inhibitors calpain inhibitor 1 (ALLN) (5 μg/ml) (Fig. 2a and 2b) and calpain inhibitor IV (Z-LLY-FMK) (10 μM) (Fig. 2a). It must be noted that both Boc-LM-CMAC and MAP2 assays determine only relative calpain activation and thus comparison of absolute activity cannot be made between M-and μ-calpain-mediated cleavage. These data demonstrate that EGF and IP-9 can induce calpain activity in both undifferentiated keratinocytes (HEKn) and immortalized HaCaT cells.
FIG. 2.
IP-9 and EGF activate calpain in undifferentiated keratinocytes. Undifferentiated keratinocytes (HEKn cells) (a) and immortalized keratinocytes (HaCaT cells) (b) were tested for calpain activity following stimulation by IP-9 (50 ng/ml) for 120 min or EGF (1 nM) for 10 min by intracellular cleavage of the synthetic substrate Boc-LM-CMAC and subsequent fluorescence, as shown by a representative experiment of three to five experiments. (a) The calpain inhibitor calpain inhibitor 1 (5 μg/ml) and the second calpain inhibitor Z-LLY-FMK (10 μM) were added for 30 min prior to the addition of ligand. (c) The involvement of calpain was confirmed by ex vivo MAP2 cleavage in HEKn cells as described. Shown is MAP2 fluorescence over unstimulated cells of two experiments performed in triplicate. Statistical analysis was performed by Student's t test. The Boc-LM-CMAC fluorescence is not cell size dependent, and the addition of the substrate Boc-LM-CMAC to the cells does not change cell size or shape significantly.
IP-9 activates μ-calpain.
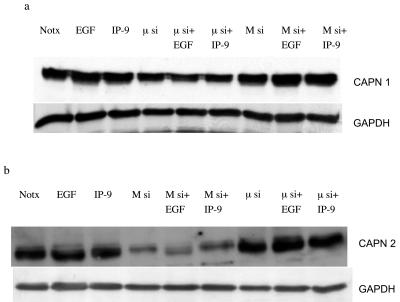
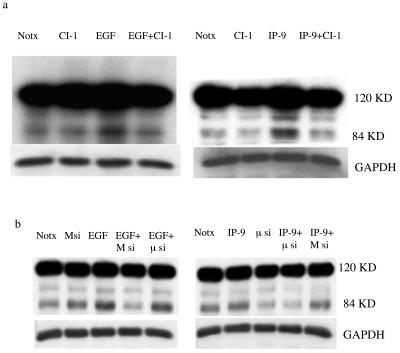
As we observed that calpain plays a central role in IP-9- and EGFR-induced migration of undifferentiated keratinocytes, the next step was to decipher the isoform of calpain which is involved in the signaling pathway. Two ubiquitous calpain isoforms are present in keratinocytes (Fig. 1c), which led us to question which one IP-9 activates. Undifferentiated HaCaT cells were transfected with small interfering RNAs (siRNAs) that specifically target M- or μ-calpain. Immunoblotting demonstrated that M-calpain was reduced by over two-thirds (to 30 ± 10%, n = 3) and μ-calpain by half (to 42 ± 9%; n = 4) (Fig. 3). Independently, the calpain isoforms were targeted by specific antisense constructs, and these downregulated their cognate proteins by about half each (data not shown), consistent with previous findings (17). In this situation, both isoforms were downregulated in the absence of exogenous ligand, suggesting that the transfection process itself resulted in calpain turnover. Regardless of the mode of calpain downregulation, these methods could downregulate each calpain isoform independently, enabling us to determine which isoform was required for each ligand.
FIG. 3.
Molecular interventions downregulate ubiquitous calpains in an isoform-specific manner. siRNAs targeted against either μ-calpain (a) or M-calpain (b) were transfected into HaCaT cells, and the protein level of the isoforms was determined 48 h later by isoform-specific immunoblotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) staining demonstrates equal cell loading. Shown is one of up to four similar immunoblots for each analysis.
In these keratinocytes, molecular elimination reduced potential calpain activity, as demonstrated by BOC. Antisense or siRNA to M-calpain limited EGF-induced calpain activity as expected (Fig. 4); BOC fluorescence was down to less than 40% of untreated levels as determined by densitometry. In contrast to EGF-induced calpain activity, IP-9-induced calpain activity in keratinocytes was limited by antisense or siRNA to μ-calpain but not M-calpain. Thus, the loss of stimulated calpain activity in cells reflected the diminished calpain protein in an isoform-specific manner.
FIG. 4.
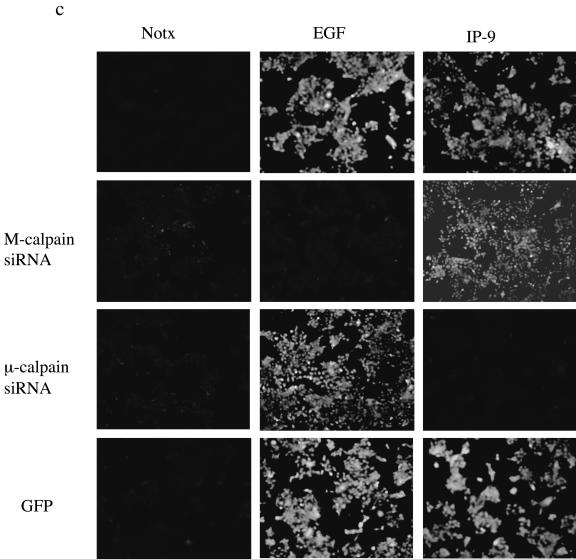
IP-9 requires μ-calpain for calpain activity. (a and b) IP-9 activates μ-calpain, whereas EGF triggers M-calpain. Undifferentiated primary keratinocytes (HEKn cells) were treated with IP-9 (50 ng/ml) or/and EGF (1 nM) in the presence or absence of antisense oligonucleotides (20 μM) to M- and μ-calpain or a scrambled oligonucleotide for 8 h to deplete endogenous calpain (17). IP-9 and EGF were removed, and the oligonucleotides were replenished for an additional 12 h. Cells were then again stimulated with IP-9 or EGF for 120 min and 10 min, respectively, and calpain cleavage of the substrate Boc-LM-CMAC was observed. Representative experiment images (of three experiments) are shown in a and quantified in b. Values are means ± standard errors of the means of two independent studies, each performed in duplicate. Statistical analysis was performed by Student's t test. (c) siRNA elimination of μ-calpain limits IP-9 induction of BOC fluorescence in HaCaT cells; 48 h after transfection with isoform-specific siRNA or GFP-targeted siRNA, HaCaT cells were exposed to IP-9 or EGF for 120 min and 10 min, respectively, and calpain cleavage of the substrate Boc-LM-CMAC was observed. Representative experiment images (of two experiments) are shown.
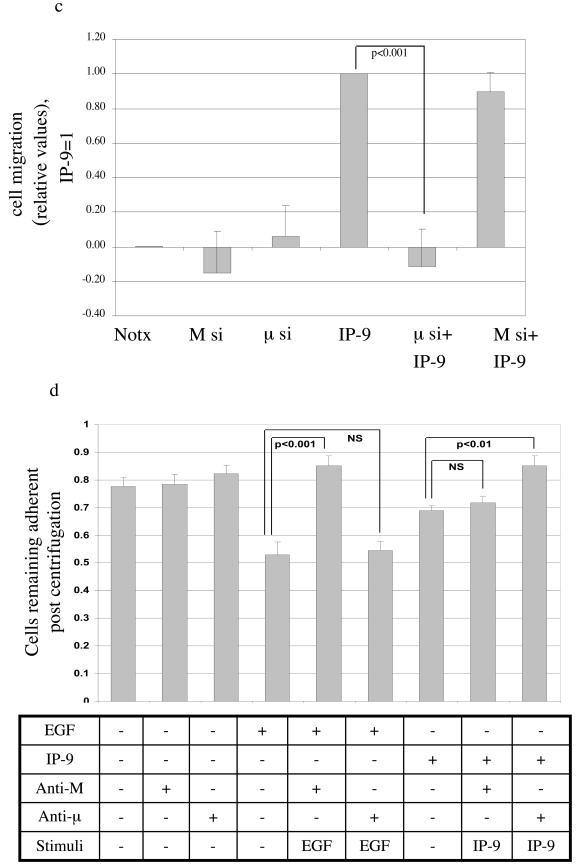
IP-9-induced keratinocyte migration and de-adhesion requires μ-calpain.
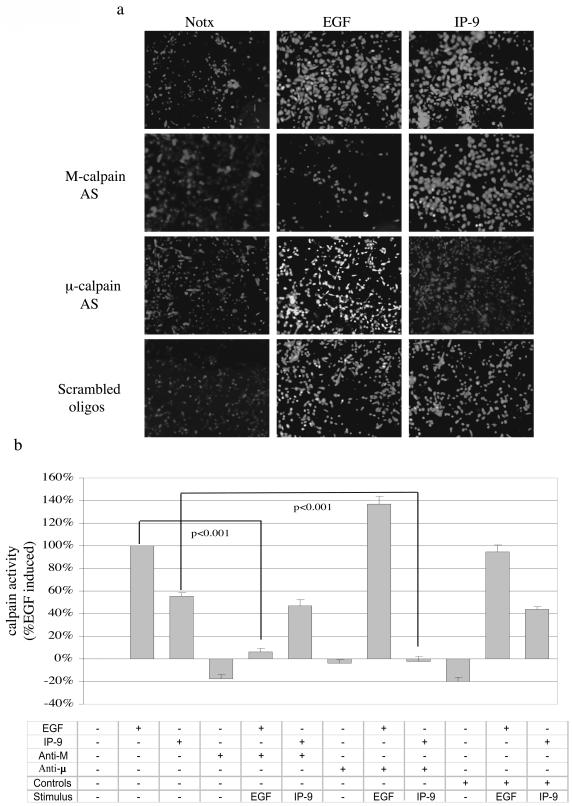
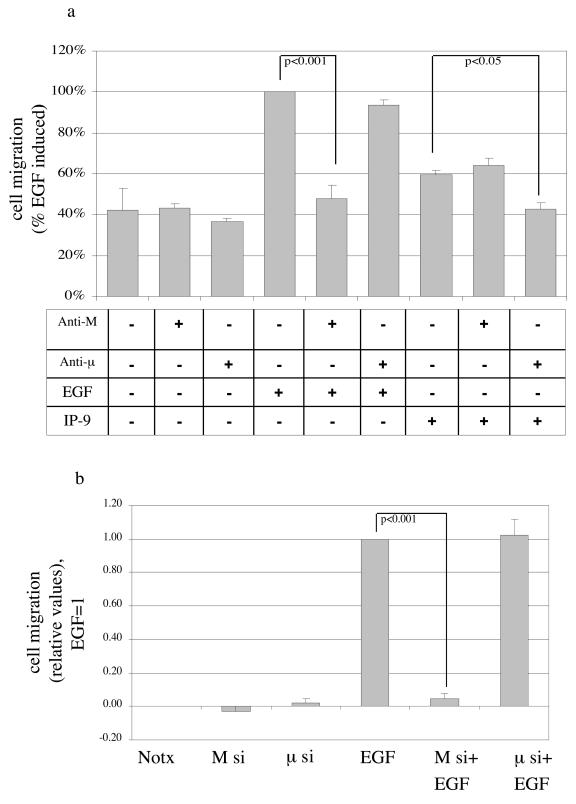
The isoform required for induced migration could be determined by molecular elimination. IP-9-induced motility was limited by the antisense oligonucleotide or siRNA to μ-calpain (Fig. 5). The antisense oligonucleotide or siRNA against M-calpain eliminated EGF-induced motility but had no significant effect on IP-9-induced motility. That this is consistent with the proposed mode of activation is demonstrated by the effects on de-adhesion. Downregulation of M-calpain but not μ-calpain blocked EGF-induced de-adhesion and that of μ-calpain but not M-calpain inhibited IP-9 actions (Fig. 5d). The specificity of the molecular downregulation is confirmed by the reciprocal effects of the antisense oligonucleotides or siRNA on either IP-9 or EGF effects, but not both. These data demonstrate that in keratinocytes, CXCR3 and EGFR activate separate calpain isoforms and these contribute to motility via decreased adhesiveness.
FIG. 5.
IP-9 requires μ-calpain for cell migration and cell de-adhesion. (a) Antisense downregulation of μ-calpain but not M-calpain blocks IP-9-induced motility of HEKn keratinocytes in an in vitro wound healing assay. Cells were treated in the presence or absence of EGF (1 nM) or IP-9 (50 ng/ml) in the presence of antisense oligonucleotides directed against the initiation codon regions of M- and μ-calpain or a scrambled oligonucleotide (not shown) for 24 h. Cell motility was calculated as a percentage of EGF-induced responses in the absence of oligonucleotide exposure. Values are means ± standard errors of the means of three independent studies, each performed in triplicate. (b and c) siRNA downregulation of μ-calpain but not M-calpain blocks IP-9-induced motility and siRNA downregulation of M- calpain but not μ-calpain blocks EGF induced motility of HaCaT cells; 48 h after transfection, HaCaT cells were exposed to EGF or IP-9 in an in vitro wound healing assay. The values are shown as the ratio of EGF (1 nM)-induced cell motility. Values are means ± standard errors of the means of three independent studies, each performed in duplicate. (d) Antisense downregulation of μ-calpain blocks IP-9 induced de-adhesion and antisense downregulation of M-calpain blocks EGF induced deadhesion in undifferentiated keratinocytes. Cells were treated with EGF (1 nM) and IP-9 (50 ng/ml) and antisense oligonucleotides specific for M-calpain or μ-calpain for 8 h, recovered in the presence of antisense oligonucleotides for 14 h, and then stimulated with IP-9 or EGF for 2 h and 30 min, respectively. Values are calculated as a percentage of precentrifugation cells remaining adherent. Values are means ± standard errors of the means of two independent studies, each performed in duplicate. Statistical analyses were performed with Student's t test.
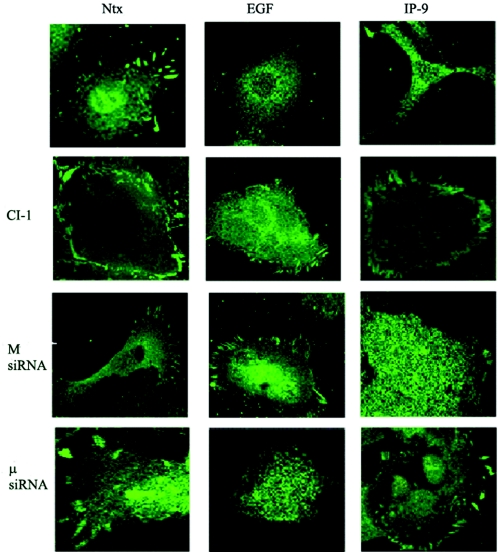
The reduction in adhesiveness to the substratum induced by EGF coincides with loss of focal adhesions in fibroblasts (62). As such, we determined whether EGF and IP-9 caused disassembly of such structures in these keratinocytes. EGF and IP-9 stimulation reduced the number of vinculin aggregates, which represent adhesion plaques to the substratum (Fig. 6). Pharmacological inhibition of calpain eliminated the EGF- and IP-9-mediated loss of focal adhesion plaques. Vinculin dispersion required different calpain isoforms depending on whether triggered by EGF or IP-9, as siRNA directed towards M-calpain resulted in retained vinculin aggregates in EGF-treated cells, and siRNA directed towards μ-calpain showed more intense vinculin concentrations when cells were stimulated with IP-9 (Fig. 6).
FIG. 6.
IP-9 and EGF induce disassembly of vinculin aggregates. HaCaT keratinocytes were exposed to EGF or IP-9 for 60 min prior to fixing and staining for vinculin by immunofluorescence (a time course demonstrated loss of aggregates starting by 30 min; data not shown). One subset of cells was exposed to calpain inhibitor 1 during the factor exposure. In another series of experiments, the keratinocytes were treated with isoform-specific siRNA to target M- or μ-calpain independently. Vinculin staining was imaged by confocal microscopy, and representative cells are shown (three independent experiments were performed for each inhibitory challenge).
This reduction in adhesion plaques should be reflected by cleavage of known targets of calpains. Herein, we probed FAK, as calpain-mediated cleavage yields a band with an approximate size of 84 kDa. Both EGF and IP-9 induced a time-dependent appearance of this cleavage product, peaking at about 30 to 60 min (data not shown), that was diminished in the presence of calpain inhibitor 1 (Fig. 7a). The appearance of the FAK cleavage product was also reduced by targeting the cells with isoform-specific siRNA (Fig. 7b), with μ-calpain- but not M-calpain-directed constructs interfering with IP-9-induced FAK cleavage. In sum, these data support the model that, in keratinocytes, IP-9 utilizes μ-calpain to reduce the adhesiveness necessary during cell locomotion.
FIG. 7.
IP-9-induced FAK cleavage is μ-calpain-dependent. EGF (10 nM) and IP-9 (200 ng/ml) stimulation resulted in the appearance of a ≈84-kDa cleavage product of FAK as detected by immunoblotting. The appearance of this fragment was maximal at 30 to 60 min (shown are 30-min challenges). Concomitant treatment with calpain inhibitor 1 (a) or isoform-specific siRNA (b) reduced the level of this cleavage product of FAK. Shown is one of three similar immunoblots for each situation.
IP-9-induced calpain activity in undifferentiated keratinocytes requires calcium mobilization.
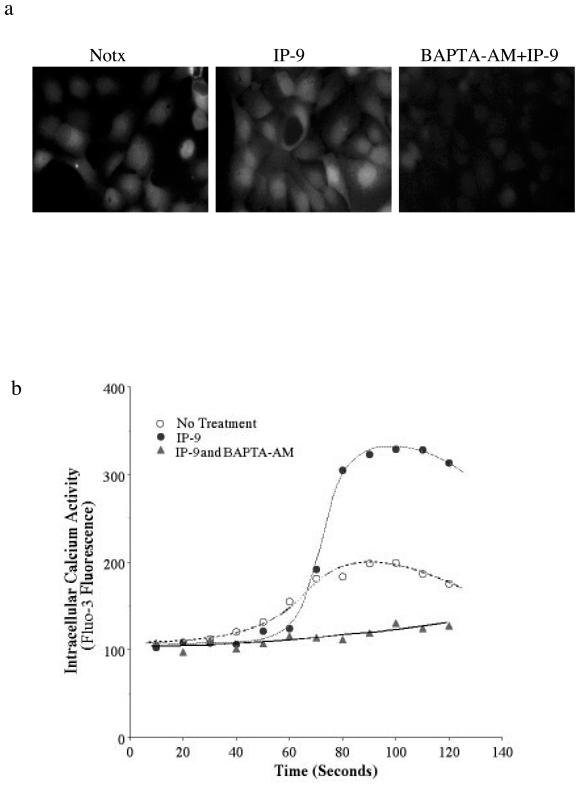
As IP-9 activates the more calcium-sensitive isoform, μ-calpain (calpain 1), we determined whether this is secondary to calcium mobilization. The calcium flux achieved may result in highly localized calcium increases (i.e., in sparks or puffs) that can reach the micromolar concentration needed to trigger μ-calpain. First, we established that IP-9 stimulation results in a cellular calcium transient. Surprisingly, although Ca2+ transients were seen, they involved the entire cell in virtually all cases rather than isolated puffs or sparks (Fig. 8). Exposure of fluo-3-loaded HaCaT cells to IP-9 elicited calcium transients within 30 s. BAPTA-AM was used as the calcium chelating agent to prevent such fluxes. The chelation of intracellular calcium by 20 μM BAPTA-AM eliminated these transients when induced by IP-9 (see the movie in the supplemental material). We were able to reproduce the same results in HEKn with fluo-3 (data not shown).
FIG. 8.
Calcium flux elicited by IP-9 was inhibited by intracellular calcium chelator BAPTA-AM. (a) Intracellular calcium concentration rose in response to IP-9 stimulation in HaCaT cells. The frames shown were taken 60 s after addition of IP-9 or diluent (Notx); BAPTA-AM was added 40 min prior to IP-9. HEKn cells demonstrated a similar, BAPTA-AM-quenchable rise in calcium concentration upon IP-9 exposure (data not shown). (b) Representative graph of a single cell trace of fluorescence showing average intracellular calcium in control cells (middle line) and calcium traces in specific cells showing transients in IP-9-stimulated cells (top line). No calcium fluxes were observed when IP-9 was added along with BAPTA-AM (bottomline). The mean intracellular calcium concentration of all IP-9-treated cells was greater on average from the control cells (see panel a and also the movie in the supplemental material), but the average rise in calcium concentration is not provided due to expected temporal offsets of the peaks in intracellular calcium concentration. Shown is a representative experiment of three, each done in triplicate, for both HaCaT and HEKn cells.
We determined whether the calcium transients were necessary for IP-9-induced calpain activity by challenging the keratinocytes in the presence of BAPTA-AM. Quantitative measurement of the HEKn keratinocytes showed that BAPTA-AM completely eliminated IP-9-induced calpain activity (to −9 ± 12%; P < 0.05) (Fig. 9). HaCaT cells produced identical results (Fig. 9c). EGF-induced calpain activation was unperturbed by BAPTA-AM, consistent with the mechanism of ERK-directed phosphorylation of M-calpain as noted in fibroblasts (16). These data demonstrate that increased calcium is necessary for calpain activity induced by IP-9 in undifferentiated keratinocytes.
FIG. 9.
Intracellular calcium is required for IP-9-induced calpain activity. Undifferentiated keratinocytes were treated in the presence or absence of IP-9 (50 ng/ml) for 120 min or EGF (1 nM) for 10 min and visualized (a) and quantitated (mean ± standard error of the mean of >15 cells/experiment) (b) for calpain activity. BAPTA-AM, a membrane-permeant acetoxymethyl ester of an intracellular calcium chelator, was used to determine the requirement of intracellular calcium for IP-9-induced calpain activity. BAPTA-AM (5 μM) was loaded 30 min prior to Boc-LM-CMAC and in the presence or absence of EGF and IP-9. This experiment was performed three times. Statistical analysis was performed by Student's t test. (c) A similar experiment was performed in human immortalized keratinocytes (HaCaT cells) to reproduce the results obtained with undifferentiated keratinocytes.
IP-9-induced calpain activity was downstream of PLC-β3.
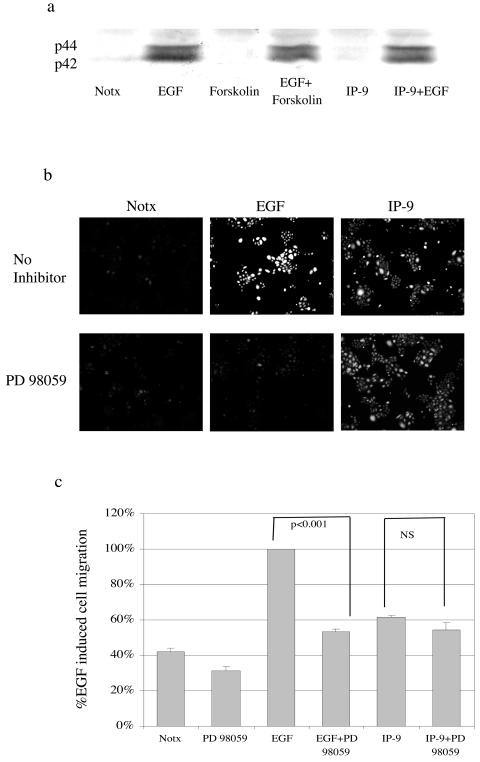
EGFR-mediated M-calpain activation occurs via the ERK MAP kinase in fibroblasts (16). To probe this pathway, we found that EGF triggers ERK activation, as demonstrated by phospho-ERK (Fig. 10a). IP-9 neither activated ERK nor inhibited EGF-induced ERK phosphorylation, suggesting that IP-9's activation of calpain occurs via a separate pathway. This was demonstrated directly by the failure of the MEK1 inhibitor PD98059 to block IP-9-induced calpain activity and cell migration (Fig. 10b and 10c). That EGF-induced events were prevented demonstrates that the ERK pathway is the intermediary in EGFR-mediated keratinocyte motility, similar to fibroblast motility. These data demonstrate that IP-9 does not also utilize the ERK signaling pathway in addition to other signaling pathways.
FIG. 10.
ERK is required for EGF-induced cell migration. a) EGF (1 nM, 10 min) but not IP-9 (50 ng/ml, 120 min) induces ERK MAP kinase in undifferentiated keratinocytes. Forskolin (25 μM, 15 min prior to ligand) served as an independent activator of cyclic AMP. Activation of ERK was determined by immunoblotting equal protein lysates for phospho-ERK. (b) Pharmacological inhibition of MAP kinase kinase with PD98059 (2 μM, 30 min prior to ligand) prevents EGF-induced calpain cleavage of the Boc-LM-CMAC substrate. Images are representative of three experiments. (c) PD98059 (2 μm) blocked cell migration in undifferentiated keratinocytes induced by EGF (1 nM) but not IP-9 (50 ng/ml) (n = 3). Statistical analysis was performed by Student's t test.
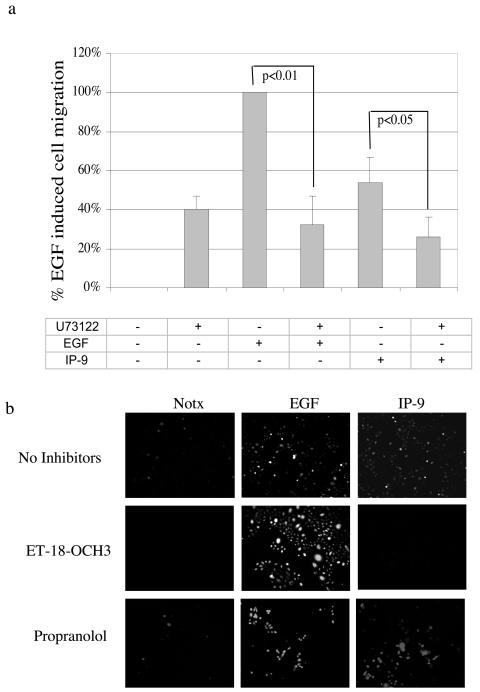
One potential pathway to calcium mobilization and subsequent activation of μ-calpain would be via phospholipases. The seven-transmembrane G protein-coupled receptor family, of which CXCR3 is a member, stimulates PLCβ with the possibility of subsequent inositol 1,4,5-triphosphate (IP3)-mediated calcium mobilization, presumably inducing μ-calpain activity. Thus, we inhibited the first part of such a pathway. We found that the pan-PLC inhibitor U-73122 blocked IP-9-induced motility as did calpain inhibitor 1 (Fig. 11a). Similar inhibition of motility was noted in the presence of antisense (but not control sense) oligonucleotides to PLC-β3 (data not shown). U-73122 also inhibited EGF-induced motility, despite the fact that PLC blockade had no effect on EGFR-mediated calpain activity. This is not unexpected, as EGFR-mediated motility requires PLCγ signaling to enable cytoskeletal reorganization (59). The need for PLC signaling is not simply permissive, as basal haptokinetic motility was unaffected by U-73122, similar to what has been found for fibroblasts (8).
FIG. 11.
IP-9-induced cell migration requires PLC-dependent calpain activation. (a) Pharmacological inhibition of all PLC isoforms by U-73122 (2 μM) blocked both EGF- and IP-9-induced cell motility in undifferentiated keratinocytes. The values are shown as ratios of the EGF (1 nM)-induced cell motility. The values are means ± standard errors of the means of six independent studies, each performed in triplicate. Statistical analysis was performed by Student's t test. (b) Undifferentiated keratinocytes were tested for calpain activity following stimulation by IP-9 (50 ng/ml) for 120 min or EGF (1 nM) for 10 min. The pan-PLC inhibitor ET-18-OCH3 (100 nM) and the phospholipase D inhibitor propranolol (100 μM) (as a nonspecific control in addition to molecular downregulation) were added for 30 min prior to the addition of ligand. Calpain activity was monitored by intracellular cleavage of the synthetic substrate Boc-LM-CMAC and subsequent fluorescence. Shown is a representative experiment of three to five experiments.
Examining calpain activation, the pan-PLC inhibitor ET-18-OCH3 blocked IP-9-induced (to −10 ± 7%) but not EGF-induced calpain activity (Fig. 11b). We used ET-18-OCH3 in this assay instead of U-73122 because intrinsic U-73122 fluorescence interferes with Boc-LM-CMAC measurement (data not shown). Propranolol (100 μM), which blocks phospholipase D-mediated diacylglycerol formation by inhibiting phosphatidate phosphohydrolase, did not inhibit IP-9- or EGF-induced calpain activity in undifferentiated keratinocytes (Fig. 11b). This suggested that PLC signaling was involved but did not define the isoform.
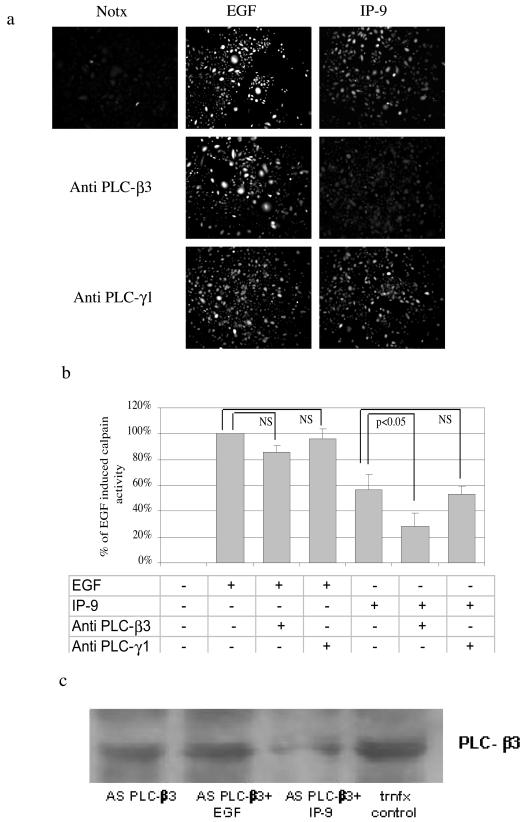
The requirement for PLC-β3 signaling was demonstrated by molecular downregulation (Fig. 12a and b). Antisense oligonucleotides downregulate PLC-β3 levels to less than half in the presence of IP-9 stimulation (Fig. 12c). Such a stimulation-dependent downregulation is not unprecedented for long-half-life proteins, as antisense downregulation of M-calpain also requires costimulation by EGF (17). These antisense oligonucleotides to PLC-β3 but not those against PLC-γ1 inhibited calpain activation (Fig. 12a). The PLC-γ1 antisense oligonucleotide was functional, as it inhibited EGF-induced motility secondary to preventing cytoskeletal reorganization (7) (data not shown). Thus, the concordance of the pharmacological and molecular interventions points to PLC-β3 as being required for IP-9-induced calpain activation.
FIG. 12.
PLC-β3signaling is required for IP-9 induced calpain activation and motility. Undifferentiated keratinocytes were grown in the presence or absence of antisense oligonucleotides (20 μM) to PLC-β3 (or PLC-γ1) for 48 h in quiescence medium. After this time, cells were stimulated with IP-9 (50 ng/ml) or EGF (1 nM) for 120 min or 10 min, respectively, and calpain cleavage of the substrate Boc-LM-CMAC was ob served. Representative experiment images (of three experiments) are shown (a) and quantified (mean ± standard error of the mean of >15 cells/experiment) (b). Statistical analysis was performed by Student's t test. (c) PLC-β3 levels were assessed by immunoblotting cells exposed to specific (antisense PLC-β3) or irrelevant (trnfx control) oligonucleotides. Shown is a representative of at least three such immunoblots. trnfx, transfection.
DISCUSSION
Herein, we define the signaling pathways by which IP-9 induces motility in undifferentiated keratinocytes. We report the use of two different isoforms of calpain (M- and μ-calpain) in the same cell by two distinct classes of ligands (EGF and IP-9). We have shown previously that IP-9 and IP-10 (which bind to the same CXCR3) promote migration of undifferentiated keratinocytes (42) while inhibiting EGF-induced migration in fibroblasts (43). Herein, we define the molecular basis for this differential signaling by reporting that in undifferentiated keratinocytes, IP-9 activates μ-calpain downstream of a PLC-β3-mediated calcium flux, while EGF utilizes the same ERK to M-calpain cascade that it triggers in other cell types. These two pathways appear to converge at the disassembly of adhesion plaques. These findings highlight the cell type-specific nature of signaling pathways and divergent phenotypic outcomes even from the same ligand-receptor pair.
The present investigation shows that two signaling pathways might be operative during migration of undifferentiated keratinocytes, the PLCβ pathway through which IP-9-induced motility occurs and the ERK MAP kinase signaling cascade pathway which actuates EGF-induced cell migration. These parallel paths to calpain activation appear to occur in isolation from each other. Unlike EGF, in undifferentiated keratinocytes IP-9 did not activate the ERK MAP kinase pathway (Fig. 9). There are reports of the related IP-10 activating ERK MAP kinase in astrocytes (61) but not in T cells (54); however, this activation might not be a direct effect but due to secondary autocrine signaling (39), as it occurs in ovarian cancer cells through an EGFR autocrine loop (58).
In fibroblasts, we find that IP-9 acts similarly to its related ELR-negative CXC chemokines that share the CXCR3 by inhibiting EGF-induced M-calpain activation (data not shown) (42). Interestingly, the positive motogenic effect of IP-9 in undifferentiated keratinocytes occurs via activation of the μ-calpain isoform. This activation pathway is independent of the EGFR signaling pathway which occurs through ERK activating M-calpain (17). IP-9 is additive with EGF, and IP-9 signaling is not blocked by inhibitors of MAP kinase kinase or downregulation of M-calpain. To our knowledge this is the first report showing the stimulation of cell migration by a growth factor (EGF) and by a chemokine (IP-9) in undifferentiated keratinocytes at least in part via activation of different isoforms of calpain, M- and μ-calpain.
We herein demonstrate that IP-9 stimulates intracellular calcium flux, which results in the activation of μ-calpain. Our data do not point to whether this is from internal stores or influx through the membrane, though the latter would be favored on the grounds that it is only in calcium puffs that one approaches the nearly micromolar concentration of calcium that activates μ-calpain in vitro (3, 22, 48). There has been debate as to whether such physiological transients can activate cellular calpains, at least in nonischemic situations (20, 49). For growth factor induction of M-calpain activity, the answer seems clearly no (16). However, even the nearly to low micromolar calcium concentrations in puffs and sparks are less than the published pCa of the purified enzyme. Thus, cofactors have been invoked as reducing the calcium requirement (33, 56). Alternately, a low level of activation, coupled with rapid autolysis, may be an attenuation mechanism that prevents excess activation. At low micromolar calcium concentrations, μ-calpain would be activated at ≈5% of its total activity but still significantly more active than at the approximately 200 nM calcium present in the cytosol of unstimulated cells (34, 46). As proteolytic signal transduction is not reversible, any cell molecular cascades would be considered to be amplified or persistent. Obviously, the two modes of μ-calpain activation, cofactors and limited activation, are not mutually exclusive and will require further experimentation.
The use of μ-calpain for motility-associated de-adhesion has a number of implications. First, IP-9 activation of calpain may occur throughout a greater volume of the cytosol than that induced by growth factors. EGFR-mediated activation of M-calpain occurs in the periplasma membrane space at or near where EGF binds to its receptor (19), which, coupled with rapid autodegradation of activated calpain (49), likely limits actions to the adhesion plaque itself. On the other hand, IP-9-mediated μ-calpain activation has not been localized and could occur throughout the cytosol; the intermediaries IP3 and calcium are freely diffusible. Still, activation of μ-calpain could be localized by the source of the calcium flux, as domain III of the molecules has C2-like phosphoinositide binding properties (15), though the translocation to the membrane may occur after calcium influx (55). Thus, whether M- and μ-calpain share physiological targets would depend on the sites of activation or activity.
At least some of the same targets are shared, as both calpains lead to FAK cleavage and vinculin aggregate disassembly in these keratinocytes (Fig. 6 and 7). Even if μ-calpain was activated in the periplasma membrane space secondary to influx, this might occur over a broader expanse of membrane, as the modes of enabling calcium channels could allow broader distribution than the tightly linked EGFR-ERK-M-calpain pathway (16, 19). One last point to be made is that these diverse signals for motility, one via a classical receptor with intrinsic tyrosine kinase activity and the other through a classic seven-transmembrane G protein-coupled receptor, converge at the penultimate biochemical step of calpain activation. Thus, inhibition of motility would best be accomplished by targeting these convergent nodes, calpains or their substrates, rather than targeting upstream inducers or signaling pathways.
Our findings unravel a phenomenon wherein one chemokine inhibited cell migration in dermal fibroblasts while promoting migration in keratinocytes. These opposite cell behaviors induced by a soluble chemokine would be required for appropriate wound healing (29). Fibroblasts from the dermis and keratinocytes from the epidermis enter the wound and migrate (45), but the cells migrating into the wound should be limited at the appropriate time to prepare the wound bed for the remodeling phase. Fibroblasts underlying the healed margin wouldfunctionally need to be in the remodeling phase, for which migration is minimized. Inhibition of rear detachment channels the motility-associated contractile forces into matrix contraction (1).
The ELR-negative CXC chemokines would accomplish this phenotypic change, with IP-10 being produced by the neovascular endothelial cells (31) and IP-9 by the basal keratinocytes. However, as IP-9 is soluble and diffusible, it would reach and affect the adjacent migrating keratinocytes that are required for reepithelialization. We predicted that these cells might be resistant to the antimotility effects; however, these keratinocytes respond to IP-9 by increased migration, presumably accomplishing this by altering G protein isoform use. Due to the complexity of the biological process of repair and the fact that the signaling pathways occur via ubiquitous enzymes that have pleiotropic roles (i.e., PLCβ and calcium fluxes), such future studies lie beyond the present scope. Despite this caveat, one ends up with an elegant system in which one signal, IP-9, might serve to synchronize the separate dermal and epidermal compartments during wound repair.
Supplementary Material
Acknowledgments
These studies were supported by grants from the National Institutes of Health and National Institute of General Medical Sciences (GM063569, GM69668, and AR47700) and by the Department of Veterans Affairs.
We thank the members of the Wells lab for their suggestions and comments, particularly R. Bodnar for help in completing the confocal microscopy studies. We thank N. Fusenig, German Cancer Research Center (Heidelberg, Germany), for the kind gift of HaCaT cells; all inquiries concerning these cells should be directed to him.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allen, F. D., C. F. Asnes, P. Chang, E. L. Elson, D. A. Lauffenburger, and A. Wells. 2002. EGF-induced matrix contraction is modulated by calpain. Wound Repair Regeneration 10:67-76. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature 392:565-568. [DOI] [PubMed] [Google Scholar]
- 3.Bootman, M. D., P. Lipp, and M. J. Berridge. 2001. The organisation and functions of local Ca2+ signals. J. Cell Sci. 114:2213-2222. [DOI] [PubMed] [Google Scholar]
- 4.Boukamp, P., R. T. Petrussevka, D. Breitkreutz, J. Hornung, and A. Markham. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri, P., S. M. Colles, D. S. Damron, and L. M. Graham. 2003. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arteriosclerosis Thrombosis Vascular Biol. 23:218-223. [DOI] [PubMed] [Google Scholar]
- 6.Chen, P., K. Gupta, and A. Wells. 1994. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J. Cell Biol. 124:547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, P., J. Murphy-Ullrich, and A. Wells. 1996. A role for gelsolin in actuating EGF receptor-mediated cell motility. J. Cell Biol. 134:689-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, P., H. Xie, M. C. Sekar, K. B. Gupta, and A. Wells. 1994. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but MAP kinase activity is not sufficient for induced cell movement. J. Cell Biol. 127:847-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, R. A. F. 1996. The molecular and cellular biology of wound repair, 2nd ed. Plenum Press, New York, N.Y.
- 10.Cole, K. E., C. A. Strick, T. J. Paradis, K. T. Ogborne, M. Loetscher, R. P. Gladue, W. Lin, J. G. Boyd, B. Moser, D. E. Wood, B. G. Sahagan, and K. Neote. 1998. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 187:2009-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croall, D. E., K. Moffett, and H. Hatch. 2002. Casein zymography of calpains using a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-imidazole buffer. Anal. Biochem. 304:129-132. [DOI] [PubMed] [Google Scholar]
- 12.Cuevas, B. D., A. N. Abell, J. A. Witowsky, T. Yujiri, N. L. Johnson, K. Kesavan, M. Ware, P. L. Jones, S. A. Weed, R. L. DeBiasi, Y. Oka, K. L. Tyler, and G. L. Johnson. 2003. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO J. 22:3346-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelhardt, E., A. Toksoy, M. Goebeler, S. Debus, E. B. Brocker, and R. Gillitzer. 1998. Chemokines IL-8, GROα, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am. J. Pathol. 153:1849-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farber, J. M. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukocyte Biol. 61:246-257. [PubMed] [Google Scholar]
- 15.Gil-Parrado, S., O. Popp, T. A. Knoch, S. Zahler, F. Bestvater, M. Felgentrager, A. Holloschi, A. Fernandez-Montalvan, E. A. Auerswald, H. Fritz, P. Fuentes-Prior, W. Machleidt, and E. Spiess. 2003. Subcellular localization and in vivo subunit interactions of ubiquitous μ-calpain. J. Biol. Chem. 278:16336-16346. [DOI] [PubMed] [Google Scholar]
- 16.Glading, A., R. J. Bodnar, I. J. Reynolds, H. Shiraha, L. Satish, D. A. Potter, H. C. Blair, and A. Wells. 2004. Epidermal growth factor activates m-calpain (calpain 2), at least in part, by ERK-mediated phosphorylation. Mol. Cell. Biol. 24:2499-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glading, A., P. Chang, D. A. Lauffenburger, and A. Wells. 2000. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J. Biol. Chem. 275:2390-2398. [DOI] [PubMed] [Google Scholar]
- 18.Glading, A., D. A. Lauffenburger, and A. Wells. 2002. Cutting to the chase: calpain proteases in cell migration. Trends Cell Biol. 12:46-54. [DOI] [PubMed] [Google Scholar]
- 19.Glading, A., F. Uberall, S. M. Keyse, D. A. Lauffenburger, and A. Wells. 2001. Membrane proximal ERK signaling is required for M-calpain activation downstream of EGF receptor signaling. J. Biol. Chem. 276:23341-23348. [DOI] [PubMed] [Google Scholar]
- 20.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731-801. [DOI] [PubMed] [Google Scholar]
- 21.Gupta, S. K., and J. P. Singh. 1994. Inhibition of endothelial cell proliferation by platelet factor-4 involves a unique action on S phase progression. J. Cell Biol. 127:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirose, K., S. Kadowaki, M. Tanabe, T. Takeshima, and M. Iino. 1999. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science 284:1527-1530. [DOI] [PubMed] [Google Scholar]
- 23.Huttenlocher, A., S. P. Palecek, Q. Lu, W. Zhang, R. L. Mellgren, D. A. Lauffenburger, M. H. Ginsburg, and A. F. Horwitz. 1997. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272:32719-32722. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs, W., T. Mikkelsen, R. Smith, K. Nelson, M. L. Rosenblum, and E. C. Kohn. 1997. Inhibitory effects of CAI in glioblastoma growth and invasion. J. Neuro-Oncol. 32:93-101. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe, A. T., W. R. Heymann, and N. Lawrence. 1999. Epidermal maturation arrest. Dermatol. Surg. 25:900-903. [DOI] [PubMed] [Google Scholar]
- 26.Kiritsky, C. P., A. B. Lynch, and S. E. Lynch. 1993. Role of growth factors in cutaneous wound healing: a review. Crit. Rev. Oral Biol. Med. 4:729-760. [DOI] [PubMed] [Google Scholar]
- 27.Lasagni, L., M. Francalanci, F. Annunziato, E. Lazzeri, S. Giannini, L. Cosmi, C. Sagrinati, B. Mazzinghi, C. Orlando, E. Maggi, F. Marra, S. Romagnani, M. Serio, and P. Romagnani. 2003. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC and acts as functional receptor for platelet factor 4. J. Exp. Med. 197:1537-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauffenburger, D. A. 1996. Making connections count. Nature 383:390-391. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence, W. T., and R. F. Diegelmann. 1994. Growth factors in wound healing. Clin. Dermatol. 12:157-169. [DOI] [PubMed] [Google Scholar]
- 30.Lokuta, M. A., P. A. Nuzzi, and A. Huttenlocher. 2003. Calpain regulates neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA 100:4006-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luster, A. D., S. M. Greenberg, and P. Leder. 1995. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J. Exp. Med. 182:219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamoune, A., J.-H. Luo, D. A. Lauffenburger, and A. Wells. 2003. m-Calpain as a target for limiting prostate cancer invasion. Cancer Research. 63:4632-4640. [PubMed] [Google Scholar]
- 33.Melloni, E., M. Averna, F. Salamino, B. Sparatore, R. Minafra, and S. Pontermoli. 2000. Acyl-CoA-binding protein is a potent m-calpain activator. J. Biol. Chem. 275:82-86. [DOI] [PubMed] [Google Scholar]
- 34.Moldoveanu, T., C. M. Hosfield, D. Lim, L. S. Elce, Z. Jia, and P. L. Davies. 2002. A Ca2+ switch aligns the active site of calpain. Cell 108:649-660. [DOI] [PubMed] [Google Scholar]
- 35.Nanney, L. B., S. Paulsen, M. K. Davidson, N. L. Cardwell, J. S. Whitsitt, and J. M. Davidson. 2000. Boosting epidermal growth factor receptor expression by gene gun transfection stimulates epidermal growth in vivo. Wound Repair Regeneration 8:117-127. [DOI] [PubMed] [Google Scholar]
- 36.Neote, K., J. Y. Mak, L. F. Kolakowski, and T. J. Schall. 1994. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood 84:44-52. [PubMed] [Google Scholar]
- 37.Palecek, S., A. Huttenlocher, A. F. Horwitz, and D. A. Lauffenburger. 1998. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J. Cell Sci. 111:929-940. [DOI] [PubMed] [Google Scholar]
- 38.Peiper, S. C., Z. X. Wang, K. Neote, A. W. Martin, H. J. Showell, M. J. Conklyn, K. Ogborne, T. J. Hadley, Z. H. Lu, and J. Hesselgesser. 1995. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J. Exp. Med. 181:1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prenzel, N., E. Zwick, H. Daub, M. Leserer, R. Abraham, C. Wallasch, and A. Ullrich. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884-888. [DOI] [PubMed] [Google Scholar]
- 40.Rani, M. R., G. R. Foster, S. Leung, D. Leaman, G. R. Stark, and R. M. Ransohoff. 1996. Characterization of beta-R1, a gene that is selectively induced by interferon β (IFN-β) compared with IFN-α. J. Biol. Chem. 271:22878-22884. [DOI] [PubMed] [Google Scholar]
- 41.Rosser, B. G., S. P. Powers, and G. J. Gores. 1993. Calpain activity increases in hepatocytes following addition of ATP. Demonstration by a novel fluorescent approach. J. Biol. Chem. 268:23593-23600. [PubMed] [Google Scholar]
- 42.Satish, L., D. Yager, and A. Wells. 2003. ELR-negative CXC chemokine IP-9 as a mediator of epidermal-dermal communication during wound repair. J. Investig. Dermatol. 120:1110-1117. [DOI] [PubMed] [Google Scholar]
- 43.Shiraha, H., A. Glading, J. Chou, Z. Jia, and A. Wells. 2002. Activation of m-calpain (calpain II) by epidermal growth factor is limited by PKA phosphorylation of m-calpain. Mol. Cell. Biol. 22:2716-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiraha, H., K. Gupta, A. Glading, and A. Wells. 1999. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J. Cell Biol. 146:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer, A. J., and R. A. F. Clark. 1999. Cutaneous wound healing. N. Engl. J. Med. 341:738-746. [DOI] [PubMed] [Google Scholar]
- 46.Smith, S. D., Z. Jia, K. K. Huynh, A. Wells, and J. S. Elce. 2005. Glutamate substitutions at a PKA consensus site are consistent with inactivation of calpain by phosphorylation. FEBS Lett., 542:115-118. [DOI] [PubMed]
- 47.Soejima, K., and B. J. Rollins. 2001. A functional IFN-gamma-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J. Immunol. 167:6576-6582. [DOI] [PubMed] [Google Scholar]
- 48.Sorimachi, H., S. Ishura, and K. Suzuki. 1997. Structure and physiological function of calpains. Biochem. J. 328:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorimachi, H., and K. Suzuki. 2001. The structure of calpain. J. Biochem. 129:653-664. [DOI] [PubMed] [Google Scholar]
- 50.Steenfos, H. H. 1993. Growth factors and wound healing. Scand. J. Plastic Reconstructive Surg. Hand Surg. 28:95-105. [DOI] [PubMed] [Google Scholar]
- 51.Strieter, R. M., S. L. Kunkel, D. A. Arenberg, M. D. Burdick, and P. J. Polverini. 1995. Interferon γ-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem. Biophys. Res. Commun. 210:51-57. [DOI] [PubMed] [Google Scholar]
- 52.Tensen, C. P., J. Flier, E. M. vanderRaaij-Helmer, S. Sampat-Sardjoepersad, R. C. vanderSchors, R. Leurs, R. J. Scheper, D. M. Boorsma, and R. Willemze. 1999. Human IP-9: A keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). J. Investig. Dermatol. 112:716-722. [DOI] [PubMed] [Google Scholar]
- 53.Teti, A., H. C. Blair, P. Schlesinger, M. Grano, A. Zambonin-Zallone, A. J. Kahn, S. L. Teitelbaum, and K. A. Hruska. 1989. Extracellular protons acidify osteoclasts, reduce cytosolic calcium, and promote expression of cell-matrix attachment structures. J. Clin. Investig. 84:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tilton, B., L. Oberlin, P. Loetscher, F. Baleux, I. Clark-Lewis, and M. Thelen. 2000. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase b and extracellular signal-regulated kinase 2 activation in T lymphocytes. J. Exp. Med. 192:313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tompa, P., Y. Emori, H. Sorimachi, K. Suzuki, and P. Friedrich. 2001. Domain III of calpain is a Ca+2-regulated phospholipid-binding domain. Biochem. Biophys. Res. Commun. 280:1333-1339. [DOI] [PubMed] [Google Scholar]
- 56.Tompa, P., Z. Mucsi, G. Orosz, and P. Friedrich. 2002. Calpastatin subdomains A and C are activators of calpain. J. Biol. Chem. 277:9022-9026. [DOI] [PubMed] [Google Scholar]
- 57.Tompa, P., E. Schad, A. Baki, A. Alexa, J. Batke, and P. Friedrich. 1995. An ultrasensitive, continuous fluorometric assay for calpain activity. Anal. Biochem. 228:287-293. [DOI] [PubMed] [Google Scholar]
- 58.Venkatakrishnan, G., R. Salgia, and J. E. Groopman. 2000. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J. Biol. Chem. 275:6868-6875. [DOI] [PubMed] [Google Scholar]
- 59.Ware, M. F., A. Wells, and D. A. Lauffenburger. 1998. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in matrix-dependent manner. J. Cell Sci. 111:2423-2432. [DOI] [PubMed] [Google Scholar]
- 60.Wells, A., K. Gupta, P. Chang, S. Swindle, A. Glading, and H. Shiraha. 1998. Epidermal growth factor receptor-mediated motility in fibroblasts. Microsc. Res. Technique 43:395-411. [DOI] [PubMed] [Google Scholar]
- 61.Xia, M. Q., B. J. Bacskai, R. B. Knowles, S. X. Qin, and B. T. Hyman. 2000. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. J. Neuroimmunol. 108:227-235. [DOI] [PubMed] [Google Scholar]
- 62.Xie, H., M. A. Pallero, D. Gupta, P. Chang, M. F. Ware, W. Witke, D. J. Kwiatkowski, D. A. Lauffenburger, J. E. Murphy-Ullrich, and A. Wells. 1998. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCγ signaling pathway. J. Cell Sci. 111:615-624. [DOI] [PubMed] [Google Scholar]
- 63.Yue, T. L., X. Wang, C. P. Sung, B. Olson, P. J. McKenna, J. L. Gu, and G. Z. Feuerstein. 1994. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circulation Res. 75:1-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.