Abstract
Laccases have potential applications in industrial, biotechnological, and environmental set ups. Development of cost effective and efficient production technologies has gained significant attention in recent years. To enhance the laccase production from Rheinheimera sp. (Gram negative) using submerged fermentation (SmF) and from Lysinibacillus sp. (Gram positive) using solid-state fermentation (SSF), the inducing effect of various flavonoid-rich agro-industrial residues was investigated. Peels of citrus fruits, soybean meal, tofu dreg, lignin monomers, and lingo-cellulosic waste, used tea leaves and peels of onion and kiwi, paper, and dying industry effluents were tested as inducers. In SmF, 0.1% of soybean meal, tofu dreg, and powdered orange peel were best, enhancing the laccase production 2.57-, 2.11-, and 2.05-fold, respectively. In SSF, 10 mg (w/w) of used tata acti green tea leaves per 5 g of wheat bran, 1% pulp and paper industry effluent (agro based), and 1% wine made from Sygium cumini enhanced the laccase production 2.69-, 2.61-, and 2.09-fold, respectively. These results suggest the utilization of these flavonoid and phenolic-rich waste materials to be potential enhancers of industrially important laccase production.
Keywords: Bacterial laccase, Rheinheimera sp., Lysinibacillus sp., Flavonoid-rich agro-industrial residues, Submerged fermentation, Solid-state fermentation
Introduction
Laccases (benzenediol: oxygen oxidoreductase; EC1.10.3.2) have received much attention in recent years because of their broad range of specificity and multifunctional nature. They are found extensively in plants and fungi. However, only fungal laccases have been the major concern for biotechnological applications due to their higher redox potential and the lesser number of known bacterial laccases. Nevertheless, bacterial laccases have the advantage of stability over a wide range of temperature and pH, easy handling, and early production, e.g., Streptomyces, Bacillus halodurans, and Gamma-proteobacterium. Laccases have been reported for various applications in industrial, biotechnological, and environmental fields such as delignification (Gonzalo et al. 2016), bioremediation (Mendez et al. 2016), saccharification, ethanol fermentation (Moreno et al. 2016), and pulp biobleaching (Singh et al. 2008). The ever-increasing application of this industrially important enzyme demands efficient and cost-effective large-scale production using inexpensive raw material as substrate. In India, 960 million tons of solid waste is generated every year from domestic and agriculture industries and is allowed to degrade in soil resulting in environmental pollution problems. The energy and environment crises of world have led the focus on strategies employing utilization of bio-based products to meet the ecological and economic requirements such as the valorization of agro-industry waste as growth media for biotechnologically useful microorganisms and their enzymes production, biofuel production, extraction of useful products, and valuable animal feed (Taurisano et al. 2014). 40–60% reduction in total production cost of industries can be achieved utilizing agro-industrial residues as a raw material rather than expensive synthetic media. Fungal laccase production using lignocellulosic wastes as substrate has been extensively studied (Ire and Ahuekwe 2016). The present work focused on enhancing bacterial laccase production using low cost and abundantly available flavonoid-rich agro-industrial residues at low concentrations in submerged and solid-state fermentation.
Materials and methods
Microorganisms
Cellulase-free alkalophilic laccase producing bacteria viz. Rheinheimera sp. (Gene bank accession no. FJ 645062) (Virk et al. 2012) and Lysinibacillus sp. (Gene bank accession no. KU 553348) (Kaur et al. 2016) were isolated previously in our laboratory from industrial waste water and sewage samples.
Inoculum preparation
Inoculum was prepared by growing the organisms in 20 mL of M162 medium (Degryse et al. 1978) containing M162 Buffer [(10×) g/L: CaSO4·2H2O, 0.4; MgCl2·6H2O, 2.0; nitrilotriacetic acid, 1.0; 0.01 M ferric citrate solution, 5 mL; micronutrient solution (g/L: H2SO4, 5 mL; MnSO4·H2O, 2.28; ZnSO47H2O, 5; H3BO3, 5; CuSO4. 5H2O, 0.25; Na2MO4·2H2O, 0.25; CoCl2·6H2O, 0.45), 1 mL] and (g/L: yeast extract, 2; tryptone, 2) at 37 °C for 16 h at 150 rpm.
Laccase production by solid-state fermentation (SSF) using Lysinibacillus sp
0.5 mL of this inoculum was mixed with 4.5 mL of M162 buffer (pH 7.0) and added to 5 g of sterile wheat bran (substrate moisture ratio of 1:1) in a 250 mL flask and incubated at 37 °C for 72 h with intermittent shaking and ≥95% humidity. The fermented substrate was suspended in 50 mL of 0.02% Tween 80, shaken at 160 rpm for 30 min and filtered. The obtained solution was centrifuged at 10,000×g for 15 min at 4 °C and the supernatant was used as crude enzyme preparation.
Laccase production by submerged fermentation (SmF) using Rheinheimera sp
0.2 mL of this inoculum was added to 20 mL of M162 medium and incubated at 37 °C, 150 rpm for 48 h. The culture supernatant was obtained by centrifugation at 10,000×g, 4 °C for 10 min and was used as crude extracellular enzyme.
Enzyme assay
Laccase activity was determined using 2 mM guaiacol, at 55 °C in 0.1 M phosphate buffer (pH 6.5) (Bains et al. 2003). The change in absorbance due to oxidation of substrate in the reaction mixture was monitored for 10 min of incubation at 465 nm (ε = 48,000 M−1 cm−1). Enzyme units were expressed in nkat (nmoles of substrate converted/second/mL of enzyme).
Results and discussion
Strategies such as mutagenesis (Verma et al. 2016), co-cultivation (Gupta et al. 2014), solid-state fermentation, and utilization of aromatic inducers have been carried out to enhance the production of laccases. However, increasing demand in industries offers potential opportunities for economical and sustainable utilization of agro-industrial residues for laccase production. Natural agriculture residues viz. banana leaves, orange peels (Ire and Ahuekwe 2016), wheat bran (Selvaraj et al. 2015), tomato pomace, cotton stalks, peanut, and shells sawdust (Daassi et al. 2016) have been used as substrates for enhanced production of fungal laccases.
Rheinheimera sp. (Gram negative) produced cellulase-free alkalophilic laccase that was reported previously from our laboratory for eco-friendly deinking of old newspaper at optimum pH 8 and 55 °C without any mediator supplementation (Virk et al. 2013). Gram-positive bacterium Lysinibacillus sp. (Kaur et al. 2016) produced extracellular, thermo-alkali stable laccase and was reported from our laboratory for biobleaching of kraft pulp and deinking of old newspaper at pH 9 and 70 °C (Gupta et al. 2014). These promising potential applications of laccases in deinking and biobleaching warranted their economical overproduction. Therefore, this study emphasizes on enhanced production of this industrially relevant enzyme utilizing cheaper agro-industrial residues as inducers.
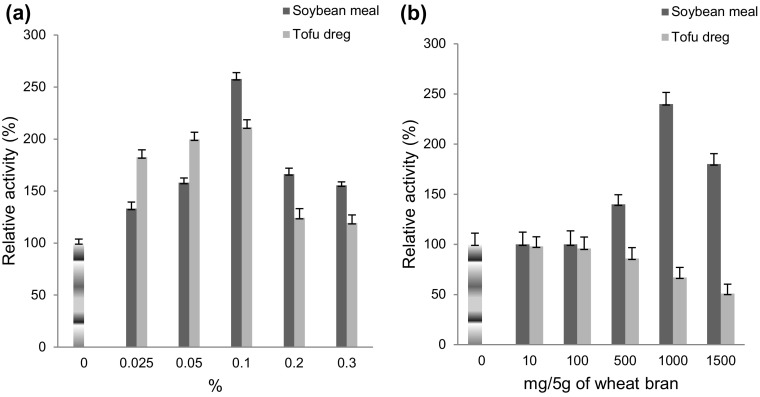
Effect of soybean meal and tofu dreg (okara)
Laccase production was maximally induced at 0.1% soybean meal (2.57-fold) and tofu dreg (2.11-fold) in SmF (Fig. 1a) whereas in SSF, 1000 mg soybean meal enhanced the laccase production (2.39-fold) by Lysinibacillus sp. (Fig. 1b). Inhibitory effect of tofu dreg supplementation was observed in SSF. Such inducing effect may be due to tannins and amino acids (tryptophan, phenylalanine, methionine, leucine, and lysine) rich soybean meal. Different positioning of COOH and NH2 groups of amino acids may enhance the laccase gene expression. 8.05-fold (Gao et al. 2013) and 37.9-fold (Gonzalez et al. 2013) increase in laccase production was reported from Trichoderma harzianum and T. pubescens, respectively, using soybean meal as substrate.
Fig. 1.
Effect of soybean meal and tofu dreg on laccase production from a Rheinheimera sp. in SmF using M162 medium and b Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C. Control 100% activity was 60.50 ± 11.3 nkat/mL (SmF) and 455.23 ± 9.9 nkats/g (SSF)
Tofu dreg (a byproduct generated during soymilk production processes) is a rich source of fiber, protein, isoflavones (genistein and daidzein), lignans, phytosterols, saponins, and phytates (Mateos-Aparicio et al. 2010) was utilized as a nitrogen source for high yield laccase production from Copings comatus, Hypsizygus marmoreus, Lentinus edodes, and Pleurotus eryngii (Fang et al. 2008). Twofold increases in laccase production were observed using okara and wheat (8:2) combination as substrate from mixed culture of Aspergillus niger, T. viride, S. cerevisiae, and Candida utilis (Pan et al. 2004).
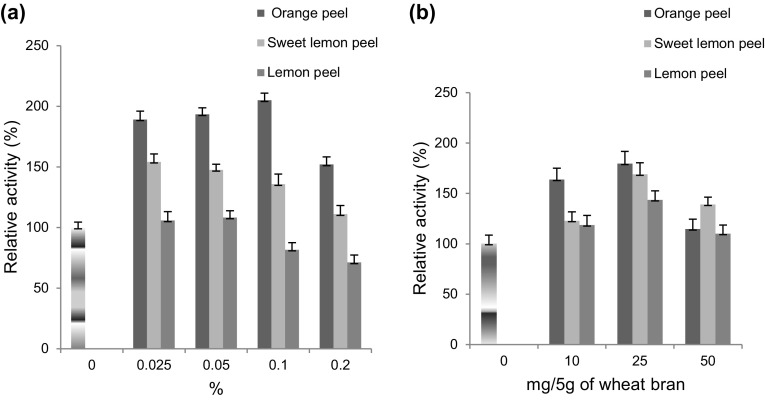
Effect of powdered peels of citrus fruit
Supplementation of 0.1% and 25 mg of powdered orange peel enhanced the laccase production 2.05-fold in SmF (Fig. 2a) and 1.79-fold in SSF (Fig. 2b), respectively. However, lemon peel supplementation decreased laccase production in SmF. This enhancement might be due to the presence of various phenolics and flavonoids such as hesperidine, narirutin, naringin, isonaringin, and eriocitrin (Adham 2015). Ire and Ahuekwe (2016) reported threefold enhancements in Pleurotus ostreatus laccase production using grounded orange peel as substrate.
Fig. 2.
Effect of powdered peel of citrus fruits (orange, sweet lemon and lemon) on laccase production from a Rheinheimera sp. in SmF using M162 medium and from b Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C. Control 100% activity was 40.3 ± 8.5 nkat/mL (SmF) and 276.33 ± 9.5 nkats/g of substrate (SSF)
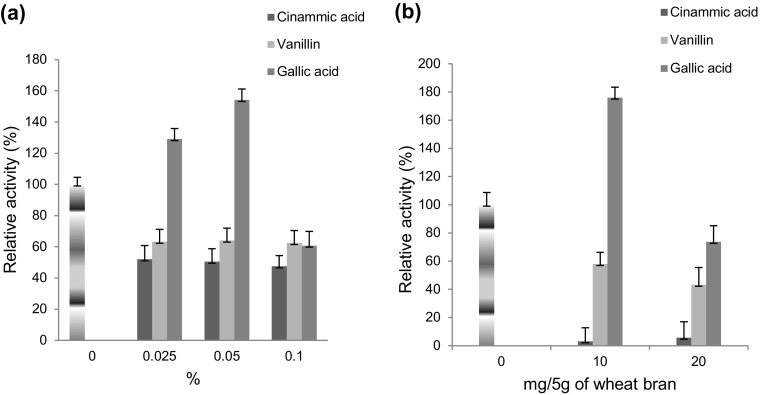
Effect of lignin monomers
Gallic acid (3,4,5-trihydroxy benzoic acid) induced the laccase production 1.54-fold in SmF (Fig. 3a) and 1.75-fold in SSF (Fig. 3b). The aromatic phenolic compounds have been widely used for enhancing the laccase production. However, stimulating and inhibiting effect of these compounds differentiated on the basis of substrate specificity of laccases. 1.69- and 1.61-fold enhancement in laccase production from Fusarium incarnatum was reported by Chhaya and Gupte (2013) using gallic acid and vanillin, respectively. Whereas, in this study, vanillin (4-hydroxy, 3-methoxy benzoic acid) and cinnamic acid (2E−3-phenylprop-2-enoic acid) inhibited the laccase production in both SmF and SSF.
Fig. 3.
Effect of lignin monomers (cinammic acid, vanillin, and gallic acid) on laccase production from a Rheinheimera sp. in SmF using M162 medium and from b Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C. Control 100% activity was 51.65 ± 8.5 nkat/mL (SmF) and 190 ± 10.3 nkats/g of substrate (SSF)
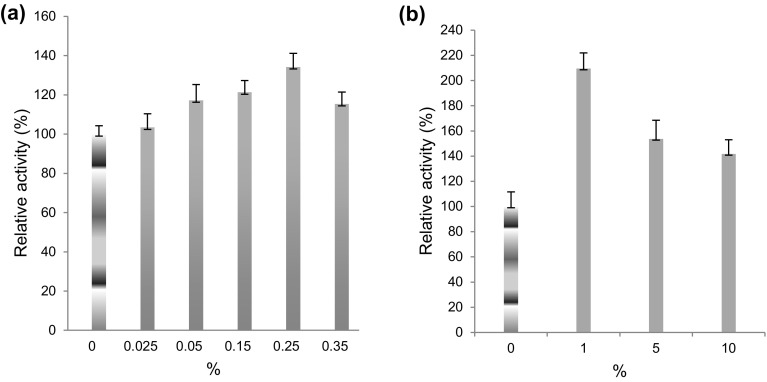
Effect of wine
In SSF, 2.09-fold enhancement in laccase production was observed using 1% of wine (Fig. 4a), while in SmF, only 1.34-fold increment was observed using 0.25% of wine (Fig. 4b). The increase might be due to tannins, anthocyanins, and phenolic content of wine (Hosu et al. 2014). However, no study is known until now observing inducive effect of wine on laccase production.
Fig. 4.
Effect of 1 year matured Syzygium cumini (Jamun Seed) wine having alcohol content of 10% (v/v) on laccase production from a Rheinheimera sp. in SmF using M162 medium and from b Lysinibacillus sp. in SSF uses wheat bran as substrate at 37 °C. Control 100% activity was 46.32 ± 6.8 nkat/mL (SmF) and 264.09 ± 7.4 nkats/g of substrate (SSF)
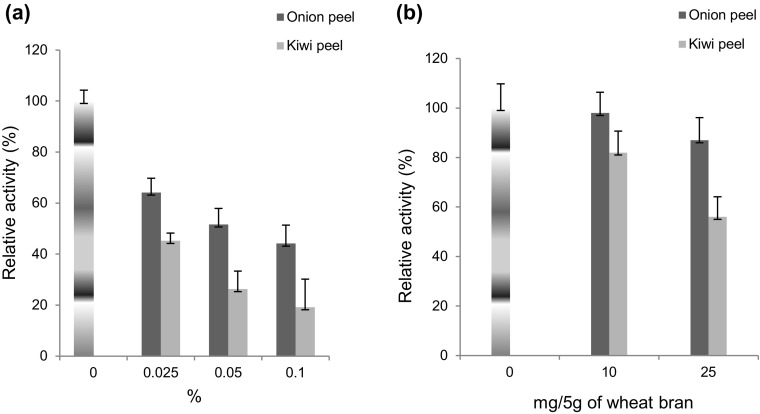
Effect of powdered peels of onion and kiwi
Inhibition of laccase production was observed in both submerged (Fig. 5a) and solid-state fermentation (Fig. 5b) using powdered peels of onion and kiwi. High concentrations of organic acids in kiwi might be providing acidic environment (pH 3.5–5.0) to bacterial culture which is not suitable for their growth and enzyme production. However, 5.2-fold increment in T. hirsute laccase production was reported by pre-treatment of kiwi peels maintaining the cultures pH in between 5.0 and 5.5 suitable for fungal laccase production. Onion peels contain phenolic compounds, such as quercetin and flavonoids. However, in this study, decrease in laccase production was observed which may be due to the presence of organosulfur compounds such as cycloalliin or sulfanilamide. Organosulfur compounds can freely penetrate the cell membranes and are known to inhibit the growth of bacteria by forming complexes with cellular materials (Wang 2014).
Fig. 5.
Effect of powdered peel of onion and kiwi on laccase production from a Rheinheimera sp. in SmF using M162 medium and from b Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C. Control 100% activity was 38.24 ± 7.4 nkat/mL (SmF) and 110.23 ± 8.5 nkats/g (SSF)
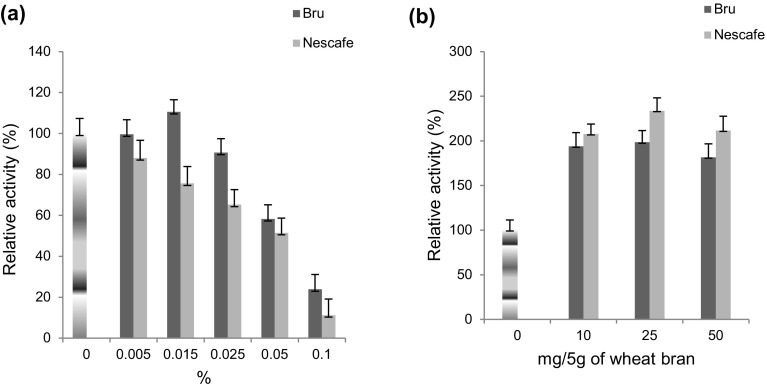
Effect of coffee
In SSF, 25 mg of nescafe and bru enhanced the production 2.33- and 1.98-fold (Fig. 6b), respectively, whereas, in SmF, only 1.10-fold of increment was observed with 0.015% of bru (Fig. 6a). No inducing effect of nescafe was observed in SmF on laccase production. Enhancement might be due to polyphenolics and tannins such as chlorogenic acid, epicatechin, catechin, protocatechuic acid, and ferulic acid in coffee. Enhancement of 91.89-fold (SSF) and 79.83-fold (SmF) in T. pubescens laccase production was reported using coffee husk as substrate medium (Gonzalez et al. 2013). Similar findings were also observed by Niladevi et al. (2007) from S. psammoticus using coffee pulp.
Fig. 6.
Effect of coffee on laccase production from a Rheinheimera sp. in SmF using M162 medium and from b Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C. Control 100% activity was 33.21 ± 9.3 nkat/mL (SmF) and 98.95 ± 14.2 nkats/g of substrate (SSF)
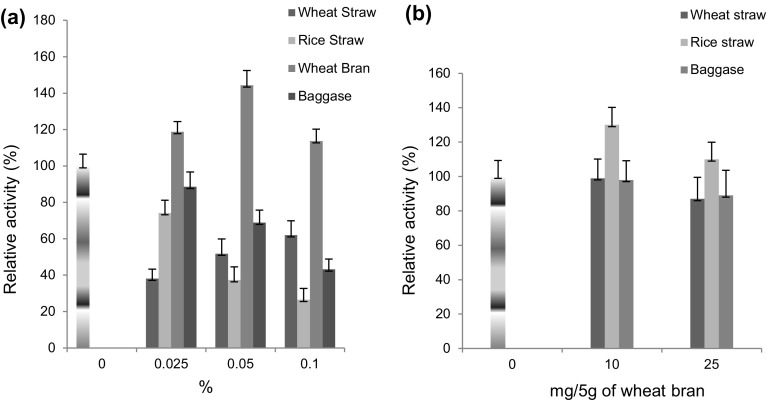
Effect of agro residues
The results showed 1.44- and 1.30-fold enhancement in laccase production using 0.05% wheat bran in SmF (Fig. 7a) and 10 mg rice straw in SSF (Fig. 7b), respectively.
Fig. 7.
Effect of agro residues (wheat straw, rice straw, wheat bran, and baggase) on laccase production from a Rheinheimera sp. in SmF using M162 medium and from b Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C. Control 100% activity was 47.75 ± 7.5 nkat/mL (SmF) and 60 ± 9.8 nkats/g (SSF)
Wheat bran is a byproduct from milling industry constituted of 58% nonstarch polysaccharides, 19% starch, 3% lignin, and 0.4–1.0% ferulic acid supporting the production of lignolytic enzymes. Stimulatory effect of wheat bran on laccase production was also studied using Coriolopsis gallica (7.1-fold) (Kenkebashvili et al. 2012) and Psathyrella candolleana (2.5-fold) (Fu et al. 2013). A limited work has been reported on utilization of rice straw, although it contains 17% of lignin. Rice straw was utilized by Mahmoud et al. (2013) and Niladevi et al. (2007) as a substrate for S. lydicus and S. psammoticus laccase production, respectively.
Effect of effluents
An increase of 1.29-fold (SmF) and 2.61-fold (SSF) was observed using 0.15 and 1% optimized concentration of agro-based effluent (effluent from paper mill using 100% wheat straw agro pulp). 1.41 (SmF) and 1.40-fold (SSF) enhancement in laccase production was observed using 0.25 and 1% of textile mill effluent. This inducive effect may be due to the presence of phenolics, aliphatic, and lignin-related compounds. Inducive effect of textile effluent and distillery effluent has been observed on marine fungus and Bacillus sp. laccase production (Kaushik and Thakur 2014). Wood-based effluent (Table 1) was from paper mill using hard wood pulp composed of eucalyptus (70–80%), poplar (50–60%), and bamboo (10–15%).
Table 1.
Effect of effluents on laccase production from Rheinheimera sp. in SmF using M162 medium and from Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C
| Effluent | Fermentation | Concentration range tested | Optimum concentration | Fold increase |
|---|---|---|---|---|
| Agro based (Kuantum Paper Mill, Hoshiarpur, India) | SmF (%) | 0.025–0.25 | 0.15 | 1.29 ± 3.0 |
| SSF (mg/5 g wheat bran) | 1–10 | 1 | 2.61 ± 8.9 | |
| Wood based (Kuantum Paper Mill, Hoshiarpur, India) | SmF (%) | 0.025–0.25 | 0.05 | 1.28 ± 3.0 |
| SSF (mg/5 g wheat bran) | 1–2 | 1 | 1.09 ± 7.8 | |
| Paper based (Shreyans Industry Limited, Ropar, India) | SmF (%) | 0.15–0.35 | 0.25 | 1.05 ± 5.6 |
| SSF (mg/5 g wheat bran) | 0.005–6 | 1 | 1.1 ± 11.8 | |
| Textile based (Khurana Textile Mill, Ludhiana, India) | SmF (%) | 0.15–0.35 | 0.25 | 1.41 ± 8.6 |
| SSF (mg/5 g wheat bran) | 0.005–6 | 1 | 1.40 ± 7.87 |
Effect of used tea leaves
Used tea leaves (Table 2) were added to 20 mL M162 medium (SmF) and 5 g of wheat bran (SSF) medium at different concentrations to examine the inducing effect on laccase production. 0.015% of used Assam tea leaves enhanced the production by 1.87-fold in SmF, whereas, in SSF, increment of 2.69-fold was observed using 10 mg of Tata acti green tea. Supplementation of flavonoids and polyphenolic-rich used tea leaves enhanced the laccase production in both SSF and SmF. However, inhibition was observed using Lipton tea, Brooke bond green tea, Taj mahal green tea, and Tata taza tea in SmF that might be due to distinctive nature and concentration of polyphenolics in different tea leaves. Our previous work (Gupta et al. 2014) reported 18.9% enhancement in laccase production using tea leaves in a co-cultivated culture of B. halodurans FNP 135 and Bacillus sp.
Table 2.
Effect of used tea leaves on laccase production from Rheinheimera sp. in SmF using M162 medium and from Lysinibacillus sp. in SSF using wheat bran as substrate at 37 °C
| Used tea leaves | Fermentation | Concentration range tested | Optimum concentration | Fold increase |
|---|---|---|---|---|
| Brooke bond black tea | SmF (%) | 0.005–0.05 | 0.005 | 1.18 ± 7.8 |
| SSF (mg/5 g wheat bran) | 10–25 | 10 | 1.33 ± 11.6 | |
| Taj mahal black tea | SmF (%) | 0.005–0.05 | 0.015 | 1.10 ± 3.2 |
| SSF (mg/5 g wheat bran) | 10–50 | 25 | 1.55 ± 8.5 | |
| Tata acti tea | SmF (%) | 0.005–0.1 | 0.025 | 1.09 ± 8.6 |
| SSF (mg/5 g wheat bran) | 10–50 | 10 | 2.69 ± 9.5 | |
| Twinning tea | SmF (%) | 0.005–0.1 | 0.025 | 1.02 ± 7.3 |
| SSF (mg/5 g wheat bran) | 10–50 | 50 | 1.54 ± 8.8 | |
| Assam tea | SmF (%) | 0.005–0.05 | 0.015 | 1.87 ± 7.3 |
| SSF (mg/5 g wheat bran) | 10–50 | 50 | 1.49 ± 9.8 | |
| Lipton green tea | SSF (mg/5 g wheat bran) | 10–25 | 25 | 2.12 ± 12.5 |
| Lipton black tea | SSF (mg/5 g wheat bran) | 10–25 | 10 | 1.10 ± 13.4 |
| Brooke bond green tea | SSF (mg/5 g wheat bran) | 10–25 | 25 | 2.0 ± 7.8 |
| Taj mahal green tea | SSF (mg/5 g wheat bran) | 10–25 | 10 | 1.59 ± 12.3 |
| Tata taza tea | SSF (mg/5 g wheat bran) | 10–50 | 10 | 2.40 ± 14.6 |
Utilization of agro-industrial residues as substrate has been widely studied for fungal laccase production (Selvaraj et al. 2015). This study reports that agro-industrial residues can be used at low concentration as inducers for laccase production as high concentration results in inhibition of bacterial laccase production.
Conclusion
The inducing effect of various agro-industrial residues was investigated on laccase production from two different bacteria: Rheinheimera sp. (SMF) and Lysinibacillus sp. (SSF). This study assesses the utilization of agro-industrial residues as cost effective and easily available laccase inducers. Among all residues, soybean meal was the best laccase inducer for both Rheinheimera sp. and Lysinibacillus sp. Utilization of these residues not only enhanced laccase production but may also help reduce pollution problems caused due to improper disposal of industrial wastes. These results suggest the utilization of flavonoid and phenolic-rich waste materials as potential enhancers of industrially important laccase production.
Acknowledgements
This work was supported by the Department of Biotechnology, Government of India, New Delhi (Grant No. BT/PR9734/BCE/8/1058/2013).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- Adham AN. Comparative antimicrobial activity of peel and juice extract of citrus fruits growing in Kurdistan/Iraq. Am J Microbiol Res. 2015 [Google Scholar]
- Bains J, Capalash N, Sharma P. Laccase from a non-melanogenic, alkalotolerant gamma proteobacterium JB isolated from industrial wastewater drained soil. Biotechnol Lett. 2003;25:1155–1159. doi: 10.1023/A:1024569722413. [DOI] [PubMed] [Google Scholar]
- Chhaya U, Gupte A. Possible role of laccase from Fusarium incarnatum UC-14 in bioremediation of bisphenol A using reverse micelles system. J Hazard Mater. 2013 doi: 10.1016/j.jhazmat.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Daassi D, Zourari- Mechici H, Frikha F, Mechichi T. Sawdust waste as a low-cost support-substrate for laccases production and adsorbent for azo dyes decolorization. J Environ Health Sci Eng. 2016 doi: 10.1186/s40201-016-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalo G, Colpa DI, Habib MHM, Fraaije MW. Bacterial enzymes involved in lignin degradation. J Biotechnol. 2016;236:110–119. doi: 10.1016/j.jbiotec.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Degryse E, Glansdorff N, Pierard A. A comparative analysis of thermophilic bacteria belonging to the genus Thermus. Arch Microbiol. 1978;117:189–196. doi: 10.1007/BF00402307. [DOI] [PubMed] [Google Scholar]
- Fang J, Cong-qiang LI, Li-xia KE. Preliminary study on using bean dregs powder as nitrogen sources in submerged culture of mushroom. Edible Fungi China. 2008;27:50–52. [Google Scholar]
- Fu K, Fu S, Zhan H, Zhou P, Liu M, Liu H. A newly isolated wood-rot fungus for laccase production in submerged cultures. Bioresources. 2013;8:1385–1397. [Google Scholar]
- Gao H, Chu X, Wang Y, Zhou F, Zhao K, Mu Z, Liu Q. Media optimization for laccase production by Trichoderma harzianum ZF-2 using response surface methodology. J Microbiol Biotechnol. 2013;23:1757–1764. doi: 10.4014/jmb.1302.02057. [DOI] [PubMed] [Google Scholar]
- Gonzalez JC, Medina SC, Rodriguez A, Osma JF, Almeciga-Diaz CJ, Sanchez OF. Production of Trametes pubescens laccase under submerged and semi-solid culture conditions on agro-industrial wastes. PLoS ONE. 2013 doi: 10.1371/journal.pone.0073721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Garg S, Capalash N, Gupta N, Sharma P. Production of thermo-alkali stable laccase and xylanase by co-culturing of Bacillus sp. and B. halodurans for biobleaching of waste paper. Bioprocess Biosyst Eng. 2014 doi: 10.1007/s00449-014-1340-0. [DOI] [PubMed] [Google Scholar]
- Hosu A, Cristea VM, Cimpoiu C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in romanian red wines: prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014;150:113–118. doi: 10.1016/j.foodchem.2013.10.153. [DOI] [PubMed] [Google Scholar]
- Ire FS, Ahuekwe E. Production of fungal laccase using orange peelings as substrate by submerged static fermentation. Br Microbiol Res J. 2016;15:1–19. doi: 10.9734/BMRJ/2016/27257. [DOI] [Google Scholar]
- Kaur K, Singh G, Gupta V, Caplash N, Sharma P. Impact of phosphate and other medium components on physiological regulation of bacterial laccase production. Biotechnol Prog. 2016 doi: 10.1002/btpr.2408. [DOI] [PubMed] [Google Scholar]
- Kaushik G, Thakur IS. Production of laccase and optimization of its production by Bacillus sp. using distillery spent wash as inducer. Bioremediat J. 2014;18:28–37. doi: 10.1080/10889868.2013.834869. [DOI] [Google Scholar]
- Kenkebashvili N, Elisashvili V, Wasser SP. Effect of carbon, nitrogen sources and copper concentration on the ligninolytic enzyme production by Coriolopsis gallica. J Waste Convers Bioprod Biotechnol. 2012;1:22–27. [Google Scholar]
- Mahmoud MG, Rifaat HM, El Sayed OH, El Beih FM, Selim MS. Effect of inducers and process parameters on laccase production by locally isolated marine Streptomyces lydicus from Red Sea, Egypt. Int J ChemTech Res. 2013;5:15–23. [Google Scholar]
- Mateos-Aparicio I, Redondo-Cuenca A, Villanueva-Suárez MJ. Isolation and characterisation of cell wall polysaccharides from legume by-products: okara (soymilk residue), pea pod and broad bean pod. Food Chem. 2010;122:339–345. doi: 10.1016/j.foodchem.2010.02.042. [DOI] [Google Scholar]
- Mendez AD, Fraile PG, Carro L, Rivas R. Screening for bacterial laccases with potential bioremediation application. N Biotechnol. 2016 [Google Scholar]
- Moreno AD, Ibarra D, Mialon A, Ballesteros M. A bacterial laccase for enhancing saccharification and ethanol fermentation of steam-pretreated biomass. Fermentation. 2016 [Google Scholar]
- Niladevi KN, Sukumaran RK, Prema P. Utilization of rice straw for laccase production by Streptomyces psammoticus in solid-state fermentation. J Ind Microbiol Biotechnol. 2007 doi: 10.1007/s10295-007-0239-z. [DOI] [PubMed] [Google Scholar]
- Pan T, Zhang D, Zhao C, Li K. Study on microbial protein production by mixed culture solid state fermentation on soybean waste. Chem Bioeng. 2004;6:35–41. [Google Scholar]
- Selvaraj B, Sanjeevirayar A, Rajendran A. Laccase production using mixed substrates containing lignocellulosic materials by Pleurotus ostreatus in submerged liquid culture. Int J ChemTech Res. 2015;7:355–368. [Google Scholar]
- Singh G, Ahuja N, Batish M, Capalash N, Sharma P. Biobleaching of wheat straw-rich soda pulp with alkalophilic laccase from gamma-proteobacterium JB: optimization of process parameters using response surface methodology. Bioresour Technol. 2008 doi: 10.1016/j.biortech.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Taurisano V, Anzelmo G, Poli A, Di donato P. Re-use of agro-industrial waste: recovery of valuable compounds by eco-friendly techniques. Int J Perform Eng. 2014;10:419–425. [Google Scholar]
- Verma A, Dhiman K, Shirkot P. Hyper-production of laccase by Pseudomonas putida LUA 15.1 through mutagenesis. J Microbiol. 2016 [Google Scholar]
- Virk AP, Capalash N, Sharma P. A alkalophilic laccase from Rheinheimera sp. isolate: production and biobleaching of kraft pulp. Biotechnol Prog. 2012;28:1426–1431. doi: 10.1002/btpr.1619. [DOI] [PubMed] [Google Scholar]
- Virk AP, Puri M, Gupta V, Capalash N, Sharma P. Combined enzymatic and physical deinking methodology for efficient eco-friendly recycling of old newsprint. PLoS ONE. 2013 doi: 10.1371/journal.pone.0072346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L (2014) The antibacterial effect of secondary compounds in plants. 50th annual southeastern michigan junior science and humanities symposium. Wayne State University, Detroit