Abstract
Autoimmune liver diseases are conditions of low prevalence that comprise the triad of autoimmune hepatitis, primary biliary cholangitis (cirrhosis) and primary sclerosing cholangitis and their poorly characterised overlapping syndromes. Diagnostic autoantibodies are associated with autoimmune hepatitis and primary biliary cholangitis but not with primary sclerosing cholangitis. Autoantibodies are useful disease markers that facilitate early diagnosis of autoimmune hepatitis and primary biliary cholangitis and allow for therapeutic intervention to prevent progression to liver cirrhosis and associated complications. Adult onset type 1 autoimmune hepatitis is associated with F-actin reactive smooth muscle autoantibody, antinuclear autoantibody in 60% of patients, and autoantibody to SLA/LP in 15–20%. Juvenile onset type 2 autoimmune hepatitis is associated with LKM-1 and LC-1 autoantibodies. Primary biliary cholangitis is associated with a mitochondria-associated autoantibody designated M2 in >90% of patients and with disease-specific antinuclear autoantibodies in 50% that bind to antigens in the nuclear core complex and in multiple nuclear dots. Autoantibodies to the nuclear core complex target gp210, nucleoporin p62 and nuclear lamin B receptor. Autoantibodies to multiple nuclear dots target Sp100 and PML antigens. Liver autoantibodies in asymptomatic patients with normal liver function may precede the subsequent development of overt autoimmune liver disease. For routine diagnostic immunology laboratories, initial screening for liver autoantibodies by immunofluorescence remains the method of choice with confirmation for reactivity with their target antigen by enzyme-linked immunosorbent assay (ELISA) or line blot when required.
Introduction
Autoimmune liver diseases comprising the triad of autoimmune hepatitis, primary biliary cholangitis (PBC) (cirrhosis) and primary sclerosing cholangitis and their overlap syndromes are uncommon. The nomenclatures for primary biliary cirrhosis have recently been changed to PBC largely because patients with this disease do not necessary have cirrhosis at the time of clinical presentation.1 The prevalence of autoimmune hepatitis varies from 0.1 to 1.9/100 000 among Caucasian populations2 and that of PBC is similar at 2.3/10 000.3 Nonetheless early diagnosis is essential because if untreated, the diseases progress to liver cirrhosis and death from liver failure, whereas early therapeutic intervention by immunosuppression for autoimmune hepatitis4 and by ursodeoxycholic acid (UDCA) for PBC5 can control disease progression. Liver autoantibodies play a key role in early identification of these diseases as they may occur in asymptomatic subjects before the development of overt disease.5
Diagnostic autoantibodies for autoimmune hepatitis
Codified criteria for the diagnosis of autoimmune hepatitis have been developed by the International Autoimmune Hepatitis group.6 The criteria comprise compatible liver histopathology including interphase hepatitis, elevated serum IgG, liver autoantibodies, elevated serum transaminases and negative serology for viral hepatitis. Interphase hepatitis is characterised by lymphocytic infiltration with or without plasma cells with associated hepatocyte cell death (piecemeal necrosis) at parenchymal-connective tissue junctions (interphases) around portal tracts.
Classification
Autoimmune hepatitis is divided into type 1 and type 2, distinguished by autoantibody profile and by age of onset, with type 1 in adults, and type 2 in children, but with indistinguishable clinical presentation. Patients who are asymptomatic at presentation have a good prognosis and may not require immunosuppressive therapy. On the other hand, cirrhosis on initial liver biopsy carries a poor prognosis.7 DRB1*04:01 positivity has been identified in association with a favourable clinical outcome.8
F-actin-specific smooth muscle autoantibody, antinuclear autoantibody and autoantibody to SLA/LP segregates with type 1 autoimmune hepatitis
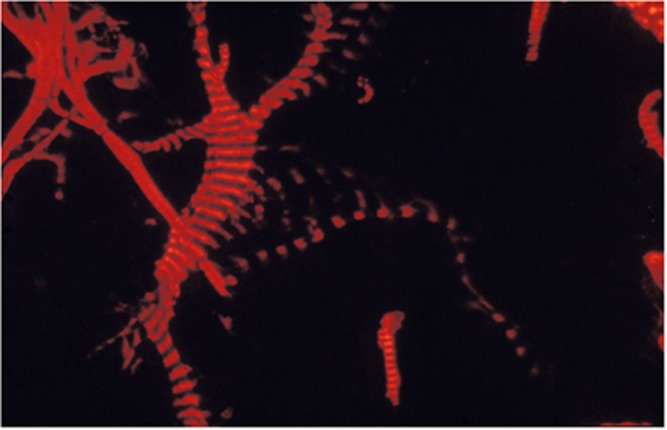
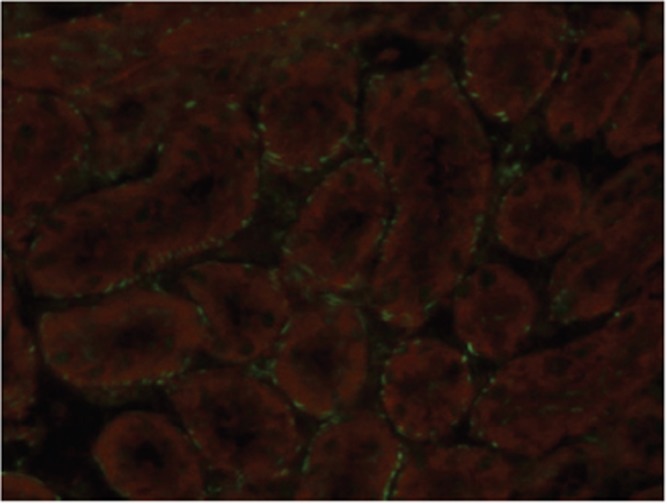
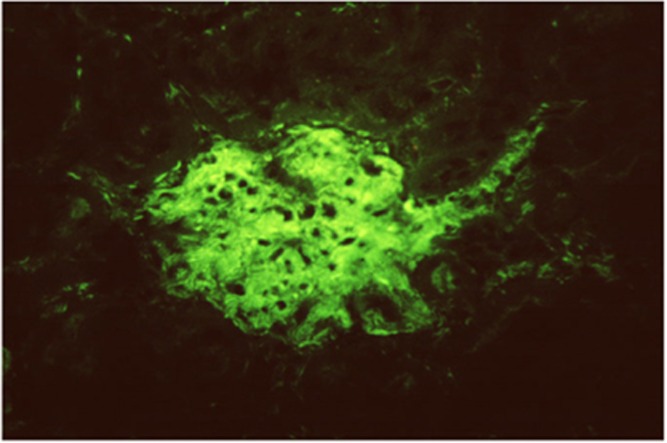
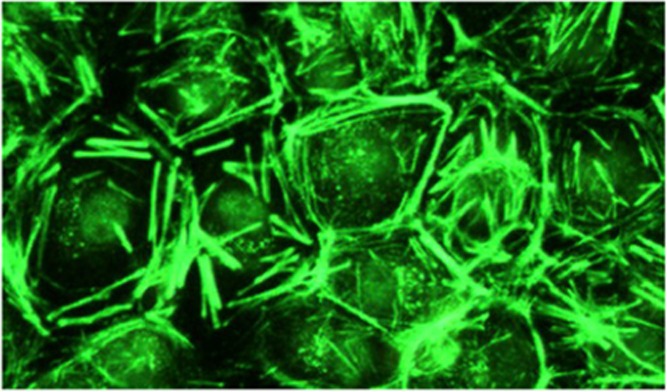
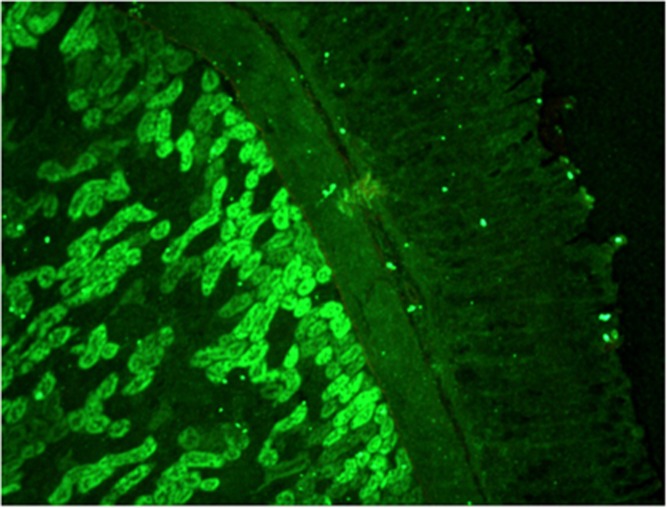
Smooth muscle antibody with specificity for F-actin microfilaments is the prototype autoantibody that segregates with type 1 autoimmune hepatitis and a serological marker for histological and biochemical disease activity.9 It is found in about 60% of patients.10 Designated smooth muscle autoantibody because of its reactivity with smooth muscle11, 12 has since been found to react with F-actin microfilaments in skeletal muscle13 (Figure 1), cardiac muscle14 and non-muscle cells that include gastric parietal cells15 and brain synapses.16 Specificity for actin was first demonstrated by Gabbiani et al.17 by immunoabsorbtion with platelet actin. In routine diagnostic laboratories, smooth muscle autoantibody is recognised by the immunofluorescence (IF) staining of the gastric muscularis externa, muscularis mucosa and smooth muscle fibres that extend from the muscularis mucosa into the lamina propria. F-actin-specific smooth muscle autoantibody is recognised by the additional characteristic pattern of IF staining of contractile fibrils around renal tubules (Figure 2) and designated as ‘SMA-T’ autoantibodies, frequently with staining of the mesangial cells of renal glomeruli (SMA-G) (Figure 3) together with staining of blood vessels of renal blood vessels (SMA-V). The original subclassification of smooth muscle antibody into SMA-V, SMA-G and SMA-T introduced by Botazzo remains useful to this day.18 The classic ‘picket-fence’ staining around renal tubules of SMA-T is diagnostic when it is clearly visualised (Figure 2). However this pattern of staining may be difficult to identify with low-titre autoantibodies; hence it is prudent to confirm SMA-T autoantibody by using as substrate cultures of cell lines, which then display characteristic ‘actin cables’ by immunofluorescence19 (Figure 4). Despite its initial promise, an ELISA developed for F-actin20 may generate false positive results in sera that are classical SMA-V and not SMA-T.19 Thus, while the ELISA may be used for screening, it is not suitable for confirmation (Figure 4). One possible explanation may be the depolymerisation of filamentous F-actin to monomeric globular G-actin as antibody to G-actin is not specific for type 1 autoimmune hepatitis. In contrast to SMA-T autoantibody directed against F-actin microfilaments, SMA-V autoantibody is typically directed to vimentin intermediate filaments that can be seen with a variety of viral infections.21, 22, 23 Smooth muscle autoantibody is thus a heterogeneous set of autoantibodies that react with various molecular targets of the cytoskeleton.24
Figure 1.
F-actin-specific SMA-T smooth muscle antibody binds skeletal muscle striations by immunofluorescence.
Figure 2.
F-actin-specific SMA-T smooth muscle antibody binds by immunofluorescence contractile fibrils around renal tubules in a ‘picket-fence’ pattern.
Figure 3.
F-actin-specific SMA-G smooth muscle antibody gives immunofluorsence staining of the mesangium of renal glomeruli.
Figure 4.
F-actin-specific SMA-T smooth muscle antibody gives immunofluorescence staining of ‘actin cables’ in a rat epithelial cell line.
Autoantibody to soluble liver antigen/liver pancreas (SLA/LP) is an additional, more recent and less frequent diagnostic marker for type 1 autoimmune hepatitis25 that, occurring either alone or together with autoantibody to Ro52, carries a poor prognosis.8, 26, 27 The target antigen has been identified as UGA suppressor tRNA-associated protein,28 a serine tRNA protein complex implicated in cotranslational incorporation of selenocystine into cells29 It is present in 15–20% of patients.30 SLA/LP autoantibodies are major risk factors for a poor short- and long-term outcome. These patients are in need of high surveillance. The presence of anti-SLA autoantibody conferred 3.1-fold increased risk of hepatic death in AIH patients. The remission rates were comparable between anti-SLA seropositive and seronegative AIH patients, while anti-SLA positivity was associated with nearly two-fold increased risk of relapse after drug withdrawal. Human leukocyte antigen (HLA) allele DR3 was positively associated with anti-SLA autoantibody.26
Antinuclear autoantibody giving specked or homogeneous nuclear staining by immunofluorescence along with autoantibodies to nucleoli31 frequently segregates with F-actin-specific SMA-T autoantibody and is found in about 60% of patients.10 The presence of Lupus Erythematosus (LE) cells was the basis for the outdated nomenclature of ‘Lupoid Hepatitis’.32 The designation’chronic active hepatitis’ first applied to a novel liver disease in the 1950s was replaced by the designation ‘Autoimmune Hepatitis’ in 1965.33
Autoantibody to LKM-1 and LC-1 segregates with type 2 autoimmune hepatitis
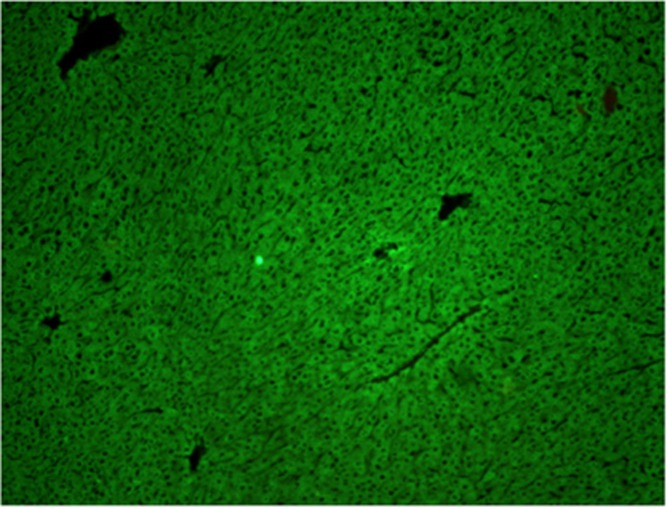
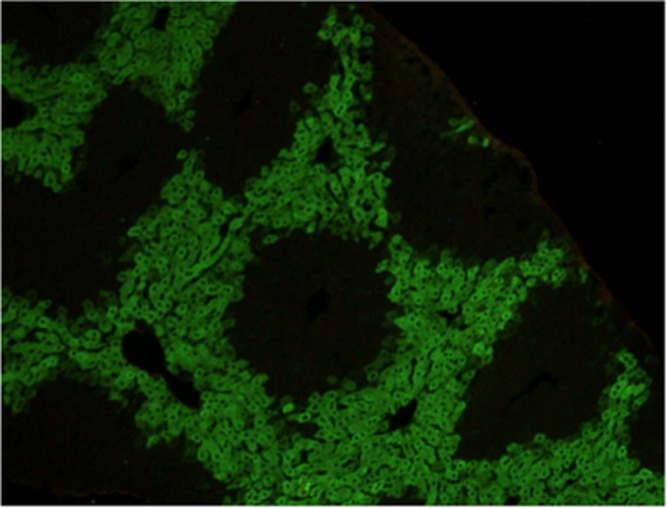
Autoantibody to Liver Kidney Microsomes-1 (LKM1) is the signature antibody of type 2 autoimmune hepatitis. Its major target antigen is Cytochrome P4502D6 (CYP2D6).34, 35 Anti-LKM autoantibody gives characteristic staining of the proximal renal tubules (Figure 5) and hepatocytes (Figure 6) by immunofluorescence. LKM-1 autoantibody is also found in up to 10% of patients with hepatitis C virus infections
Figure 5.
LKM-1 antibody shows staining of proximal renal tubules by immunofluorescence.
Figure 6.
LKM-1 antibody shows staining of hepatocytes by immunofluorescence.
Autoantibody to LC-1 stains the hepatocytes by immunofluorescence but spares cells around the central vein (Figure 7).36 The target antigen has been identified as formiminotransferase cyclodeaminase,37, 38 a 62 kDa cytosolic protein. LC-1 antibody is found together with LKM autoantibody in 30% of cases, and in 10% of cases it is the sole autoantibody. In contrast to autoantibody to LKM, anti-LC-1 autoantibody parallels liver disease activity.39 Its presence is associated with a unfavourable clinical course and a more rapid disease progression40
Figure 7.
LC-1 antibody showing staining of hepatocytes but not those around the central vein.
Diagnostic autoantibodies for PBC
PBC is a progressive disease of insidious onset resulting in destruction of epithelial cells of small intrahepatic bile ducts leading to cholestasis and cirrhosis. As with autoimmune hepatitis, early diagnosis is essential as it can be controlled by UDCA.5 It is characterised by elevated serum alkaline phosphatase, diagnostic autoantibodies to mitochondria and PBC-specific anti-nuclear autoantibodies and liver histopathology of granulomas around the bile ducts41, 42
Autoantibodies to mitochondria, designated M2 diagnostic for PBC are found in 95–98% of patients. The cDNA encoding the target antigen was molecularly cloned by Gershwin et al.43 and identified as the inner lipoyl domain of the E2 subunit of pyruvate dehydrogenase complex.44 In routine diagnostic laboratories, it is identified by distinctive immunofluorescence staining of distal renal tubules, gastric parietal cells and liver hepatocytes. In doubtful instances, the presence of the antibody can be confirmed by line blots with the target M2 antigen.
Autoantibodies to PBC-specific anti-nuclear autoantibody
PBC-specific anti-nuclear autoantibody (ANA) comprises antibody to the nuclear pore complex that targets gp210 and nucleoporin p6245, 46 as well as antibody to multiple nuclear dots that target Sp100 and PML, and are found in about 50% of patients with PBC.47 PML is a transformation and cell growth suppressing protein expressed in promyelocytic leukaemia cells that is co-localised with Sp100 in nuclear dots.48 Sp100 may function as a nuclear hormone transcriptional coactivator.49 Autoantibody to the nuclear pore complex that gives punctate nuclear rim immunofluorescence staining accounts for about 25% of PBC-specific ANA,50 binds to the lamin B receptor51 and to gp210 that binds preferentially to the N terminus of gp21052 and is a poor prognostic marker.53 Autoantibody to centromeres, although not specific to PBC may also be found in this disease.54 PBC-specific ANA may be particularly useful in M2-negative primary biliary cirrhosis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is a cholestatic liver disease characterised by inflammation and fibrosis of both intrahepatic and extrahepatic bile ducts that leads to multifocal bile duct strictures and eventually to cirrhosis55. Although a number of autoantibodies of low prevalence and low titres have been reported in primary sclerosing cholangitis, none are diagnostic of this disease.56 Inflammatory bowel disease is associated in up to 80% of patients.57 pANCA (anti-neutrophil cytoplasmic antibody) has been proposed as a marker of ulcerative colitis and primary sclerosing cholangitis,58 but it has been questioned whether the association is an epiphenomenon.59 There is no effective treatment besides liver transplantation.
Overlap syndromes
Some patients have a hepatitic and cholestatic profile in association with autoantibodies that segregate with autoimmune hepatitis and PBC. These are a poorly characterised set of patients.60, 61 Nonetheless It has been reported that 2–19% of patients with primary biliary cirrhosis and 7–14% of patients with primary sclerosing cholangitis have features that overlap with autoimmunue hepatitis.61
Predictive value of diagnostic autoantibodies for asymptomatic autoimmune liver diseasess
Autoantibodies in asymptomatic subjects may precede overt autoimmune disease by many years. This is certainly the case for autoantibodies associated with asymptomatic PBC patients followed up for 19 years.62 Liver biopsies of asymptomatic subjects with mitochondria antibody and normal liver function has shown the histological hallmarks of PBC.63 Autoantibodies to F-actin-specific smooth muscle antibody may also be found in asymptomatic subjects with normal liver function.64 A 12-year follow-up has reported the rare occurrence of development of overt autoimmune hepatitis in asymptomatic subjects with F-actin antibody and normal liver function.65
Concluding remarks
F-actin reactive smooth muscle autoantibody, antinuclear autoantibody and autoantibody to SLA/LP are diagnostic for type 1 autoimmune hepatitis, while LKM-1 and LC-1 autoantibody are diagnostic for type 2 autoimmune hepatitis. M2 mitochondria autoantibody, nuclear pore complex autoantibody directed to gp210, nucleoporin p62 and nuclear lamin B receptor and autoantibody to multiple nuclear dots that target Sp100 and PML antigens are diagnostic for PBC. Table 1 provides a summary of diagnostic autoantibodies in autoimmune hepatitis and PBC. There are no diagnostic antibodies for primary sclerosing cholangitis.
Table 1. Diagnostic autoantibodies in autoimmune hepatitis and primary biliary cholangitis.
| Type 1 autoimmune hepatitis | F-actin reactive smooth muscle autoantibody |
| Anti-nuclear autoantibody | |
| SLA/LP autoantibody | |
| Type 2 autoimmune hepatitis | LKM-1 autoantibody |
| LC-1 autoantibody | |
| Primary biliary cholangitis | M2 mitochondria autoantibody |
| Nuclear core complex autoantibody directed to gp230, nucleoporin p62 and nuclear lamin B receptor | |
| Muliple nuclear dots autoantibody directed to sp100 and PML |
Footnotes
The author declare no conflict of interest.
References
- Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K et al. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. J Hepatol 2015; 63: 1285–1287. [DOI] [PubMed] [Google Scholar]
- Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis 2002; 6: 635–647. [DOI] [PubMed] [Google Scholar]
- Triger DR, Berg PA, Rodes J. Epidemiology of primary biliary cirrhosis. Liver 1984; 4: 195–200. [DOI] [PubMed] [Google Scholar]
- Mackay IR, Weiden S, Ungar B. Treatment of active chronic hepatitis and lupoid hepatitis with 6-mercaptopurine and azothioprine. Lancet 1964; 1: 899–902. [DOI] [PubMed] [Google Scholar]
- Mayo MJ. Natural history of primary biliary cirrhosis. Clin Liver Dis 2008; 12: 277–288 viii. [DOI] [PubMed] [Google Scholar]
- Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51: 2193–2213. [DOI] [PubMed] [Google Scholar]
- Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology 2005; 42: 53–62. [DOI] [PubMed] [Google Scholar]
- Kirstein MM, Metzler F, Geiger E, Heinrich E, Hallensleben M, Manns MP et al. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology 2015; 62: 1524–1535. [DOI] [PubMed] [Google Scholar]
- Couto CA, Bittencourt PL, Porta G, Abrantes-Lemos CP, Carrilho FJ, Guardia BD et al. Antismooth muscle and antiactin antibodies are indirect markers of histological and biochemical activity of autoimmune hepatitis. Hepatology 2014; 59: 592–600. [DOI] [PubMed] [Google Scholar]
- Bayraktar Y, Bayraktar M, Gurakar A, Hassanein TI, Van Thiel DH. A comparison of the prevalence of autoantibodies in individuals with chronic hepatitis C and those with autoimmune hepatitis: the role of interferon in the development of autoimmune diseases. Hepatogastroenterology 1997; 44: 417–425. [PubMed] [Google Scholar]
- Toh BH. Smooth muscle autoantibodies and autoantigens. Clin Exp Immunol 1979; 38: 621–628. [PMC free article] [PubMed] [Google Scholar]
- Johnson GD, Holborow EJ, Glynn LE. Antibody to smooth muscle in patients with liver disease. Lancet 1965; 2: 878–879. [DOI] [PubMed] [Google Scholar]
- Chaponnier C, Kohler L, Gabbiani G. Fixation of human anti-actin autoantibodies on skeletal muscle fibres. Clin Exp Immunol 1977; 27: 278–284. [PMC free article] [PubMed] [Google Scholar]
- Toh BH, Clarke FM, Ceredig R. Reaction of human smooth muscle autoantibody with skeletal muscle, cardiac muscle, and thymic myoid cells. Clin Immunol Immunopathol 1978; 9: 28–36. [DOI] [PubMed] [Google Scholar]
- Ceredig R, Toh BH. Reaction of human smooth muscle autoantibody with gastric parietal cells: a pitfall in the diagnosis of parietal cell autoantibody. J Clin Pathol 1977; 30: 666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh BH, Gallichio HA, Jeffrey PL, Livett BG, Muller HK, Cauchi MN et al. Anti-actin stains synapses. Nature 1976; 264: 648–650. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Lamelin JP, Vassalli P, Majno G, Bouvier CA et al. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to ‘nonmuscular’ cells. Am J Pathol 1973; 72: 473–488. [PMC free article] [PubMed] [Google Scholar]
- Bottazzo GF, Florin-Christensen A, Fairfax A, Swana G, Doniach D, Groeschel-Stewart U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol 1976; 29: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh BH, Taylor R, Pollock W, Dearden S, Gill CC, Buchner C et al. ‘Actin-reactive’ discriminated from ‘non-actin-reactive’ smooth muscle autoantibody by immunofluorescence reactivity with rat epithelial cell line. Pathology 2010; 42: 463–469. [DOI] [PubMed] [Google Scholar]
- Granito A, Muratori L, Muratori P, Pappas G, Guidi M, Cassani F et al. Antibodies to filamentous actin (F-actin) in type 1 autoimmune hepatitis. J Clin Pathol 2006; 59: 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki P, Linder E, Virtanen I, Stenman S. Human smooth muscle autoantibodies reacting with intermediate (100A) filaments. Nature 1977; 268: 240–241. [DOI] [PubMed] [Google Scholar]
- Toh BH, Yildiz A, Sotelo J, Osung O, Holborow EJ, Kanakoudi F et al. Viral infections and IgM autoantibodies to cytoplasmic intermediate filaments. Clin Exp Immunol 1979; 37: 76–82. [PMC free article] [PubMed] [Google Scholar]
- Brown C, Pedersen J, Underwood JR, Gust I, Toh BH. Autoantibodies to intermediate filaments in acute viral hepatitis A, B and non-A, non-B are directed against vimentin. J Clin Lab Immunol 1986; 19: 1–4. [PubMed] [Google Scholar]
- Toh BH. Anti-cytoskeletal autoantibodies: diagnostic significance for liver diseases, infections and systemic autoimmune diseases. Autoimmunity 1991; 11: 119–125. [DOI] [PubMed] [Google Scholar]
- Ballot E, Homberg JC, Johanet C. Antibodies to soluble liver antigen: an additional marker in type 1 auto-immune hepatitis. J Hepatol 2000; 33: 208–215. [DOI] [PubMed] [Google Scholar]
- Chen ZX, Shao JG, Shen Y, Zhang J, Hua Y, Wang LJ et al. Prognostic implications of antibodies to soluble liver antigen in autoimmune hepatitis: a PRISMA-compliant meta-analysis. Medicine 2015; 94: e953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano-Loza AJ, Shums Z, Norman GL, Czaja AJ. Prognostic implications of antibodies to Ro/SSA and soluble liver antigen in type 1 autoimmune hepatitis. Liver Int 2012; 32: 85–92. [DOI] [PubMed] [Google Scholar]
- Wies I, Brunner S, Henninger J, Herkel J, Kanzler S, Meyer zum Buschenfelde KH et al. Identification of target antigen for SLA/LP autoantibodies in autoimmune hepatitis. Lancet 2000; 355: 1510–1515. [DOI] [PubMed] [Google Scholar]
- Gelpi C, Sontheimer EJ, Rodriguez-Sanchez JL. Autoantibodies against a serine tRNA-protein complex implicated in cotranslational selenocysteine insertion. Proc Natl Acad Sci USA 1992; 89: 9739–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeres M, Herkel J, Czaja AJ, Wies I, Kanzler S, Cancado EL et al. Establishment of standardised SLA/LP immunoassays: specificity for autoimmune hepatitis, worldwide occurrence, and clinical characteristics. Gut 2002; 51: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneally D, Mackay IR, Toh BH. Antinucleolar autoantibodies demonstrated by monolayers of human fibroblasts in sera from patients with systemic lupus erythematosus, progressive systemic sclerosis and chronic active hepatitis. J Clin Lab Immunol 1984; 14: 13–16. [PubMed] [Google Scholar]
- Mackay IR. Historical reflections on autoimmune hepatitis. World J Gastroenterol 2008; 14: 3292–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay IR, Toh BH. Autoimmune hepatitis: the way we were, the way we are today and the way we hope to be. Autoimmunity 2002; 35: 293–305. [DOI] [PubMed] [Google Scholar]
- Manns MP, Johnson EF, Griffin KJ, Tan EM, Sullivan KF. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450db1. J Clin Invest 1989; 83: 1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatselis NK, Zachou K, Koukoulis GK, Dalekos GN. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico-laboratory and histological characteristics. World J Gastroenterol 2015; 21: 60–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini E, Abuaf N, Cavalli F, Durand V, Johanet C, Homberg JC. Antibody to liver cytosol (anti-LC1) in patients with autoimmune chronic active hepatitis type 2. Hepatology 1988; 8: 1662–1666. [DOI] [PubMed] [Google Scholar]
- Muratori L, Sztul E, Muratori P, Gao Y, Ripalti A, Ponti C et al. Distinct epitopes on formiminotransferase cyclodeaminase induce autoimmune liver cytosol antibody type 1. Hepatology 2001; 34: 494–501. [DOI] [PubMed] [Google Scholar]
- Lapierre P, Hajoui O, Homberg JC, Alvarez F. Formiminotransferase cyclodeaminase is an organ-specific autoantigen recognized by sera of patients with autoimmune hepatitis. Gastroenterology 1999; 116: 643–649. [DOI] [PubMed] [Google Scholar]
- Muratori L, Cataleta M, Muratori P, Lenzi M, Bianchi FB. Liver/kidney microsomal antibody type 1 and liver cytosol antibody type 1 concentrations in type 2 autoimmune hepatitis. Gut 1998; 42: 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesierska-Gadek J, Penner E, Battezzati PM, Selmi C, Zuin M, Hitchman E et al. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology 2006; 43: 1135–1144. [DOI] [PubMed] [Google Scholar]
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005; 353: 1261–1273. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology 2009; 50: 291–308. [DOI] [PubMed] [Google Scholar]
- Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol 1987; 138: 3525–3531. [PubMed] [Google Scholar]
- Wang J, Budamagunta MS, Voss JC, Kurth MJ, Lam KS, Lu L et al. Antimitochondrial antibody recognition and structural integrity of the inner lipoyl domain of the E2 subunit of pyruvate dehydrogenase complex. J Immunol 2013; 191: 2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesierska-Gadek J, Hohenuer H, Hitchman E, Penner E. Autoantibodies against nucleoporin p62 constitute a novel marker of primary biliary cirrhosis. Gastroenterology 1996; 110: 840–847. [DOI] [PubMed] [Google Scholar]
- Wesierska-Gadek J, Klima A, Komina O, Ranftler C, Invernizzi P, Penner E. Characterization of autoantibodies against components of the nuclear pore complexes: high frequency of anti-p62 nucleoporin antibodies. Ann NY Acad Sci 2007; 1109: 519–530. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Courvalin JC. Antinuclear antibodies specific for primary biliary cirrhosis. Autoimmun Rev 2003; 2: 211–217. [DOI] [PubMed] [Google Scholar]
- Sternsdorf T, Guldner HH, Szostecki C, Grotzinger T, Will H. Two nuclear dot-associated proteins, PML and Sp100, are often co-autoimmunogenic in patients with primary biliary cirrhosis. Scand J Immunol 1995; 42: 257–268. [DOI] [PubMed] [Google Scholar]
- Bloch DB, Nakajima A, Gulick T, Chiche JD, Orth D, de La Monte SM et al. Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator. Mol Cell Biol 2000; 20: 6138–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Rey C, Bogdanos D, Yang CY, Roberts K, Leung PS, Anaya JM et al. Primary biliary cirrhosis and the nuclear pore complex. Autoimmun Rev 2012; 11: 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratori P, Muratori L, Ferrari R, Cassani F, Bianchi G, Lenzi M et al. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol 2003; 98: 431–437. [DOI] [PubMed] [Google Scholar]
- Wesierska-Gadek J, Hohenauer H, Hitchman E, Penner E. Autoantibodies from patients with primary biliary cirrhosis preferentially react with the amino-terminal domain of nuclear pore complex glycoprotein gp210. J Exp Med 1995; 182: 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Ichida T, Yoshida T, Hayakawa A, Uchida M, Tashiro-Itoh T et al. Autoantibodies against a 210 kDa glycoprotein of the nuclear pore complex as a prognostic marker in patients with primary biliary cirrhosis. J Gastroenterol Hepatol 1998; 13: 257–265. [DOI] [PubMed] [Google Scholar]
- Makinen D, Fritzler M, Davis P, Sherlock S. Anticentromere antibodies in primary biliary cirrhosis. Arthritis Rheum 1983; 26: 914–917. [DOI] [PubMed] [Google Scholar]
- Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010; 51: 660–678. [DOI] [PubMed] [Google Scholar]
- Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol 2008; 14: 3781–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis 2006; 26: 31–41. [DOI] [PubMed] [Google Scholar]
- Seibold F, Slametschka D, Gregor M, Weber P. Neutrophil autoantibodies: a genetic marker in primary sclerosing cholangitis and ulcerative colitis. Gastroenterology 1994; 107: 532–536. [DOI] [PubMed] [Google Scholar]
- Chapman RW. The enigma of anti-neutrophil antibodies in ulcerative colitis primary sclerosing cholangitis: important genetic marker or epiphenomenon? Hepatology 1995; 21: 1473–1474. [DOI] [PubMed] [Google Scholar]
- Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther 2012; 36: 517–533. [DOI] [PubMed] [Google Scholar]
- Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol 2011; 54: 374–385. [DOI] [PubMed] [Google Scholar]
- Roll J, Boyer JL, Barry D, Klatskin G. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N Engl J Med 1983; 308: 1–7. [DOI] [PubMed] [Google Scholar]
- Mitchison HC, Bassendine MF, Hendrick A, Bennett MK, Bird G, Watson AJ et al. Positive antimitochondrial antibody but normal alkaline phosphatase: is this primary biliary cirrhosis? Hepatology 1986; 6: 1279–1284. [DOI] [PubMed] [Google Scholar]
- Toh BH, Taylor R, Pollock W. F-actin reactive smooth muscle antibody in subjects with normal liver function. Pathology 2009; 41: 572–575. [DOI] [PubMed] [Google Scholar]
- Healey R, Corless L, Gordins P, Holding S. Do anti-smooth muscle antibodies predict development of autoimmune hepatitis in patients with normal liver function?—a retrospective cohort review. Autoimmun Rev 2016; 15: 668–672. [DOI] [PubMed] [Google Scholar]