T-follicular helper (TFH) cells are a specialised subset of effector CD4+ T cells, endowed with the function of facilitating B-cell activation and differentiation. As such they are crucial to the generation of robust humoral immune responses during natural infection or following vaccination. Interactions between a TFH cell and a cognate B cell occur in the B-cell follicle within secondary lymphoid tissues. Herein lies the problem of studying human TFH cells; in contrast to mouse models, where lymphoid tissues can be conveniently imaged and collected, immune responses in secondary lymphoid organs of humans cannot be easily accessed. In a bid to circumvent this issue, numerous groups have focused on identifying the circulating counterparts of bona fide TFH cells in peripheral blood. In secondary lymphoid structures, TFH cells upregulate expression of the B-cell zone-homing chemokine receptor CXCR5 and downregulate the T-cell zone-homing chemokine receptor CCR7, allowing for the localisation of these cells to the follicle where they are positioned to help B cells. A CXCR5+ subset of CD4+ T cells can also be identified in peripheral blood, subsequently referred to as circulating TFH (cTFH) cells. How these cells relate to tissue TFH cells remains unclear. For example, these cTFH cells express much lower levels of ICOS and PD-1 than tissue TFH cells, and do not express Bcl-6.1, 2, 3 On the other hand they produce increased amounts of interleukin 21 (IL-21) and, importantly, are superior B-cell helpers when compared to their CXCR5− counterparts.1, 4
Further work sought to identify a subset of cells within the cTFH population that more closely resembled tissue TFH cells and correlated with antibody responses. This lead to the idea that the majority of CXCR5+ cells in the blood represented memory TFH cells.5 Thus, tetanus, cytomegalovirus (CMV) and flu-specific cells can be identified within the CD4+CXCR5+ population in healthy adults.1, 5 In contrast, recently activated TFH cells could be identified by the expression of activation markers such as ICOS and CD38, as well as high expression of PD-1. Thus, following administration of the influenza vaccine, an increase in proliferating cells expressing PD-1, ICOS and/or CD38 has been observed.6, 7 However, exactly how these two populations are related to one another, and from which population of antigen (Ag)-specific cells they arise following challenge remains unclear. In a recent article in Science Immunology, Herati et al.8 sought to address these questions by longitudinal tracking of the influenza-specific response in different populations of CD4+ T cells. In particular, they compare CXCR5+PD1+ cTFH cells that lack ICOS and CD38 (similar to the previously described ‘memory’ TFH cell) with those that co-express ICOS and CD38 that are expanded post influenza vaccination (the previously described ‘activated’ TFH cell). Akin to lymphoid TFH cells, and consistent with the idea that these are activated cells, ICOS+CD38+ cTFH cells express high levels of the activation markers CD27, CD28, Ki67, and PD-1 and have downregulated CD127. Furthermore, consistent with published work, ICOS+CD38+ cTFH cells express the transcription factor c-Maf; however, one key difference is that, similar to lymphoid TFH cells, ICOS+CD38+ cTFH cells also express the TFH lineage transcription factor Bcl-6, whereas others have failed to detect Bcl-6 expression in cTFH populations.1, 4, 9 Interestingly ICOS+CD38+ cTFH cells also expressed higher levels of Tbet, Foxo1 and GATA3 compared to naive, CXCR5− and CXCR5+ ICOS−CD38− populations. In regards to cytokine expression, ICOS+CD38+ cTFH cells were observed to express higher frequencies of IL-21 as well as IL-10 and IL-17 than ICOS−CD38− cTFH cells.
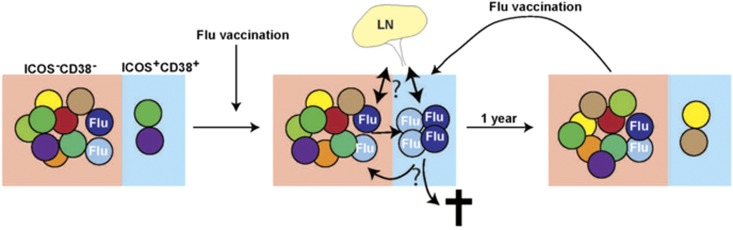
A key question is whether the cTFH cells induced following influenza vaccination are Ag specific. Herati et al. investigated this, first, by using MHC class II tetramers and by stimulating the cells with influenza virus-derived peptides, which resulted in upregulation of CD69 and CD200 (OX2)—activation-induced markers that have previously been used to identify Ag-specific CD4+ T cells. These studies revealed that while influenza-specific cells could be identified in all three CD4+ T-cell populations (CXCR5−, CXCR5+ICOS−CD38− and CXCR5+ICOS+CD38+), these cells were dramatically enriched in the ICOS+CD38+ population 7 days after influenza vaccination. Interestingly, sequencing of the T-cell receptor (TCR) Vβ chain of ICOS+CD38+ cTFH cells revealed that following influenza vaccination there was an increase in the clonality of the response; however this did not reflect the dominance of one clonotype, but rather an increase in multiple clonotypes. Furthermore, this increase in clonality within the ICOS+CD38+ cTFH cells positively correlated with the increase in cTFH cells, as well as the presence of circulating plasmablasts post influenza infection. The real novelty of this study was their ability to retrospectively analyse archived peripheral blood mononuclear cell (PBMC) samples from individuals receiving the annual influenza vaccine over a 3-year period. In each instance, the frequency of ICOS+CD38+ cTFH cells increased 7 days post influenza vaccination, declined back to baseline 1-year post vaccination, but was found to be increased again 7 days following a further vaccination (Figure 1). Examining TCR Vβ usage over time revealed a high turnover in ICOS+CD38+ cTFH in the absence of vaccination. For example, there was no overlap in TCR Vβ usage in the ICOS+CD38+ cTFH population 7 days after vaccination and 1-year post influenza vaccination, yet 7 days after revaccination many of the same TCRs were observed as those seen 7 days after the initial vaccination. In contrast, the TCR Vβ usage of CXCR5− and CXCR5+ICOS−CD38− populations was more stable over time. A similar pattern was seen when the authors focused only on TCRs associated with influenza-specific cells—namely, there was a constant low level of these clones in the CXCR5− and CXCR5+ICOS−CD38− populations, but there was a cyclical increase then decrease in these TCR clonotypes within the CXCR5+ICOS+CD38+ population following each round of vaccination. This helped answer another key question of whether the ICOS+CD38+ cTFH cells induced following vaccination form part of the memory cell repertoire. It appears ICOS+CD38+ cTFH cells quickly turned over post vaccination, which is consistent with the activated phenotype and high Ki67 expression in this population. Instead Ag-specific memory cells persist in the ICOS−CD38− subset, with some evidence of a low level of conversion between ICOS−CD38− and ICOS+CD38+ cTFH cells (Figure 1).
Figure 1.
cTFH cells can be divided into ICOS−CD38− and ICOS+CD38+ populations. The ICOS−CD38− population has a more stable TCR repertoire and likely to represent a memory population, while the ICOS+CD38+ cells are the activated cTFH cell population. The repertoire of the ICOS+CD38+ is less stable, likely reflecting current antigenic stimulus. Thus 7 days after administration of the flu vaccine there is an expansion of flu-specific clones in this population. These are then lost at later times post vaccination either through death, redistribution to lymphoid tissues or reversion to an ICOS−CD38− phenotype. However, these clones may still be retained in the ICOS−CD38− population. Following revaccination some of the same clones will be reactivated and appear in the ICOS+CD38+ population for a second or third time.
In summary, Herati et al. reveal ICOS+CD38+ cTFH cells contain Ag-specific cells post influenza vaccination, but stable memory cTFH cells persist within the ICOS−CD38− subset (Figure 1). This study highlights that ICOS+CD38+ and ICOS−CD38− cTFH cells may be new populations to monitor during natural infection or in response to vaccination to determine the quality of an immune response and the persistence of a memory response. Nevertheless, it still remains unknown how these cTFH populations relate to bona fide lymphoid TFH cells in regards to function, clonality and lineage. Identification of the true nature of ICOS+CD38+ cTFH cells will be required if they are to be an important biomarker for the development of improved vaccine strategies to stimulate robust antibody responses and the establishment of long-lived memory.
Footnotes
The authors declare no conflict of interest.
References
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34: 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2009; 62: 234–244. [DOI] [PubMed] [Google Scholar]
- He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013; 39: 770–781. [DOI] [PubMed] [Google Scholar]
- Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol 2015; 136: 993–1006.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013; 39: 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herati RS, Reuter MA, Dolfi DV, Mansfield KD, Aung H, Badwan OZ et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J Immunol 2014; 193: 3528–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel S-E, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013; 5: 176ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herati RS, Muselman A, Vella L, Bengsch B, Parkhouse K, Del Alcazar D et al. Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Sci Immunol 2017; 2: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol 2011; 186: 5556–5568. [DOI] [PubMed] [Google Scholar]