Abstract
The association between air pollution and childhood respiratory disease is inconsistent. In the present study, we investigated a short-term effect of ambient air pollutants and daily childhood lower respiratory diseases (CLRD). Daily air pollutants, weather data, and CLRD data were collected from January 2014 to April 2015 (452 days) in Nanjing, China. Time-series regression and generalized additive models were used to assess the effects of air pollutants (PM10, PM2.5, NO2, SO2, O3, and CO) on CLRD. We observed that an interquartile range (IQR) increase in concentrations of PM10, NO2, and SO2 significantly increased the daily CLRD with 6 days cumulative effects (difference of estimates: 2.8%, 95% CI: 0.6–5.0%; 4.1%, 1.2–7.0%; 5.6%, 2.6–8.6%, respectively). However, no significant association was found in IQR concentrations of PM2.5, O3, and CO. Specifically, elevated PM10, PM2.5, NO2, and SO2 significantly increased the numbers of CLRD in cool season (3.6%, 1.5–5.7%; 2.4%, 0.3–4.5%; 4.9%, 2.9–7.0%; 6.3%, 3.7–9.0%, respectively). Additionally, the effect estimates of PM10, NO2, and SO2 in female and age >27 months were more pronounced than in male and age ≤27 months. This study suggested that short-term exposure to ambient PM10, NO2, and SO2 were associated with the increased CLRD numbers.
Introduction
Air pollution is the important environment risk factor. Extensive data have indicated that air pollution is associated with a negative effect on human health1. WHO had proposed outdoor air pollution as the 14th mortality risk factor in 2004. The ischemic heart disease and stroke were the main cause of deaths associated with air pollution. Additionally, pulmonary disorder also contributed to the disease mortality, among which acute lower respiratory diseases (LRD) was the common cause of children deaths under five years old2.
It is well known that the response of individual air pollutant exposure may be determined by age, sex, genetics, lifestyle and disease. The children are vulnerable to the detrimental effects of outdoor air pollutants on health. Several previous studies demonstrated significantly harmful effects of outdoor air pollutants on childhood lung function, e.g. exposure to particulate matter less than 2.5 µm in aerodynamic diameter (PM2.5), particulate matter less than 10 µm in aerodynamic diameter (PM10) and nitrogen dioxide (NO2) associated with reduced forced expiratory flows3. Recently, a few studies have found that acute and short-term exposure to outdoor air pollutants may increase the number of childhood allergic rhinitis, pneumonia, asthma symptoms4, 5. A recent meta-analysis including several cohort and cross-sectional studies suggested that exposure to traffic related PM10, PM2.5, NOx, sulfur dioxide (SO2), carbon monoxide (CO), ozone (O3) had increased frequency of childhood asthma and wheeze6. Potential mechanisms may be air pollution inducing allergic sensitization and oxidative stress, increasing the individual susceptibility to respiratory infection7.
At present, few association studies between air pollutants and Childhood LRD (CLRD) have been conducted in China8, 9. A recent study in Changsha (China) reported that early-life exposure to air pollutants can induce the higher childhood asthma10. Liu et al. collected the daily concentrations of PM10, NO2, and SO2 during the children life time (2006–2012) where they lived, and found that exposure to NO2 was associated with respiratory diseases in children11. Although the association studies of air pollutants and CLRD have been widely studied, the results remain inconsistent. Molter et al. found that outdoor air pollutants were not related with the multi-center childhood asthma in Europe12. Studies demonstrated that different NO2 exposure years of life were not associated with childhood asthma and asthma-related symptoms in the United States13.
It was worth to note that these studies had provided the effects of air pollutants on CLRD in location for public health assessments, however, the results might be affected by the local air pollutants levels, components and population susceptibility. It is important to study the effects of city air pollutants on CLRD numbers for the local city policy development14. Nanjing is a city located in the east of China, which is the provincial capital of Jiangsu province. With the development of economics, the concentrations of Nanjing’s air pollutants in central monitoring sites are above the nation’s levels. In the present study, we aim to conduct a short-term effect of ambient air pollutants, including PM10, PM2.5, NO2, CO, and O3, and daily CLRD in Nanjing, China.
Material and Methods
Data collection
Nanjing is a large city of Jiangsu province with a moderate subtropical climate (6597 km2). It had a population of 8.22 million in 2015. Daily CLRD data from 28 January 2014 to 24 April 2015 were obtained from Children’s Hospital of Nanjing Medical University, The Second Affiliated Hospital of Nanjing Medical University, Jiangsu Women and Clinical Health Hospital, Nanjing Jiangning Hospital, Nanjing First Hospital, Nanjing Gaochun People’s Hospital, Nanjing Maternity and Child Health Care Hospital. In this study, CLRD were comprised of doctor-diagnosed pneumonia (J18), bronchitis (J20), capillary bronchitis (J21), and acute asthma (J45), which were diagnosed based on the International Classification of Diseases 10th revision15.
Air pollution data including PM10, PM2.5, NO2, SO2, O3, and CO were obtained from China Environmental Monitoring Centre. These monitoring stations are not in direct vicinity to traffic or industrial sources, housing emissions such as oil, coal, or waste burning. Thus, the daily air pollution data of monitoring stations can represent the environmental air pollutants’ levels. To control the confounding effects of meteorological conditions on CLRD, we also obtained the data of daily weather data (relative humidity and mean temperature) from the Meteorological Bureau.
Statistical analysis
Spearman’s correlation coefficient was applied to investigate the association between air pollutants and weather variables. We used the time-series regression model to explore the effects of each air pollutant (PM10, PM2.5, NO2, SO2, O3, and CO) on CLRD. We also used generalized additive model (GAM) to calculate the data16. Herein, the data of the respiratory symptoms followed the over-dispersed Poisson distribution, thus, we applied the quasi-Poisson regression in the GAM17. In order to control the potential confounding effect, firstly, we included a natural cubic smooth function of calendar time with 7 degrees of freedom (df) per year to remove unmeasured long-term and seasonal trends18; secondly, we included the natural smooth functions of the current-day relative humidity (3 df) and mean temperature (3 df) to exclude the weather confounding effects19; thirdly, we also included the indicator variable for “day of the week (DOW)” in the model. In this study, we also calculated the data in the cool period (November through April, 4 df) and warm period (May to October, 4 df) to separately analyze the effect of air pollutants on CLRD.
Further, we used single-pollutant model to explore the air pollution’s effect on CLRD with single lag days (lag0, 1, 2, 3, 4, 5, 6). To remove the potential misalignment of single day lag exposure, we considered the moving average exposure of multiple days (lag 0–1, 0–2, 0–3, 0–4, 0–5, 0–6) in additional analyses. We conducted the concentration-response relationship curves for the air pollutants concentrations with daily CLRD using a 3 df for the smoothing function in single-pollutant model. Herein, we performed the two-pollutants model to exam the stability of results.
The effect estimates were shown as the percentage change and 95% confidence intervals (CIs) in daily CLRD per interquartile range (IQR) increased of air pollutants’ levels. In this study, all models were conducted in R software (version 3.2.1) with mgcv package. All tests were two-sided, and P < 0.05 were considered as statistically significant.
Results
Data description
Table 1 presents the data of descriptive variables. There were 26,423 CLRD cases (male: 16,078, female: 10,344, sex unknown: 1; mean age: 26.6 months, Supplementary Table 1) during 28 January 2014 to 24 April 2015 (452 days), among which there were 5,551 bronchitis, 19,098 pneumonia, 1,560 capillary bronchitis, and 214 asthma cases. The daily average CLRD numbers were 58.5, and the maximum was 105.0. The daily average concentrations were 117.4 µg/m3 for PM10, 69.9 µg/m3 for PM2.5, 50.1 µg/m3 for NO2, 21.5 µg/m3 for SO2, 0.94 mg/m3 for CO, and 95.8 µg/m3 for O3. The daily average relative humidity and temperature were 72.5% and 15.1 °C, respectively.
Table 1.
The summary of descriptive statistics in this study.
| Variables | Mean ± SD | Minimum | 25% quartile | Median | 75% quartile | Maximum | Inter-quartile range |
|---|---|---|---|---|---|---|---|
| Daily CLRD numbers | 58.5 ± 13.9 | 23.0 | 48.0 | 58.5 | 68.3 | 105.0 | 20.3 |
| Air pollution (µg/m3) | |||||||
| PM10 | 117.4 ± 56.5 | 17.8 | 76.0 | 108.9 | 147.8 | 389.2 | 71.8 |
| PM2.5 | 69.9 ± 36.7 | 13.7 | 45.4 | 63.0 | 87.6 | 247.3 | 42.2 |
| NO2 | 50.1 ± 18.8 | 14.2 | 36.2 | 47.5 | 62.3 | 118.1 | 26.1 |
| SO2 | 21.5 ± 11.5 | 3.21 | 12.4 | 19.8 | 28.8 | 71.7 | 16.4 |
| O3 | 95.8 ± 52.7 | 7.00 | 53.0 | 84.5 | 129.0 | 273.0 | 76.0 |
| CO (mg/m3) | 0.94 ± 0.89 | 0.35 | 0.70 | 0.89 | 1.11 | 2.45 | 0.41 |
| Weather conditions | |||||||
| Mean temperature (°C) | 15.1 ± 8.2 | −2.23 | 7.93 | 15.3 | 22.4 | 31.8 | |
| Relative humidity (%) | 72.5 ± 15.5 | 28.0 | 62.2 | 72.9 | 85.4 | 97.2 | |
CLRD, Childhood lower respiratory diseases.
As shown in Table 2, there were positively correlated among 6 air pollutants, except CO and O3 (r = −0.17, P = 0.001). In addition, air pollutants were also correlated with temperature and humidity.
Table 2.
Spearman correlation between air pollutants and weather variables in Nanjing.
| Variables | PM10 | PM2.5 | NO2 | SO2 | CO | O3 | Mean temperature (°C) | Relative humidity (%) |
|---|---|---|---|---|---|---|---|---|
| PM10 | — | 0.87** | 0.67** | 0.75** | 0.61** | 0.24** | −0.07 | −0.49** |
| PM2.5 | 0.87** | — | 0.53** | 0.56** | 0.68** | 0.22** | 0.03 | −0.21** |
| NO2 | 0.67** | 0.53** | — | 0.75** | 0.53** | 0.05 | −0.22** | −0.46** |
| SO2 | 0.75** | 0.56** | 0.75** | — | 0.47** | 0.14** | 0.28** | 0.71** |
| CO | 0.61** | 0.68** | 0.53** | 0.47** | — | −0.17** | −0.22** | −0.13** |
| O3 | 0.24** | 0.22** | 0.05 | 0.14** | −0.17** | — | 0.58** | −0.26** |
| Mean temperature (°C) | −0.07 | 0.03 | −0.22** | −0.28** | −0.22** | 0.58** | — | 0.24** |
| Relative humidity (%) | −0.50** | −0.21** | −0.46** | −0.71** | −0.13** | −0.26** | 0.24** | — |
**P < 0.001.
Main results
Single-pollutant models were applied to obtain the percentage change of CLRD associated with an IQR increase in air pollutants concentrations for single day lag (lag0, lag1, lag2, lag3, lag4, lag5, lag6) and multi-day lag (lag0–1, lag0–2, lag0–3, lag0–4, lag0–5, lag0–6) (Figure 1). An IQR increase in concentrations of PM10, NO2, SO2 at multi-day exposure were more significant associated with estimated increase in the numbers of CLRD than single day exposure. For lag0–5, we observed the highest significant association between PM10, NO2, SO2 and CLRD numbers (difference of estimates: 2.8%, 95% CI: 0.6–5.0%; 4.1%, 1.2–7.0%; 5.6%, 2.6–8.6%, respectively). However, no significant association was found in IQR concentrations of PM2.5, O3, and CO.
Figure 1.
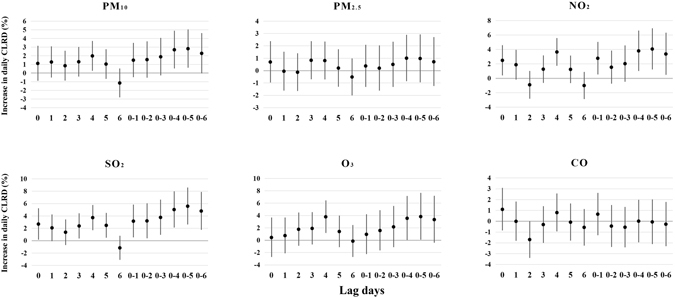
Estimated changes with 95% confidence intervals in daily CLRD (Childhood lower respiratory diseases) percentage deviations (%) associated with an interquartile range increase in PM10, PM2.5, NO2, SO2, O3 or CO concentrations with different lag days in single-pollutant model.
Further, we evaluated the effect estimates stratified by the season (warm and cool period). Figure 2 showed that the associations of PM10, PM2.5, NO2, and SO2 and CLRD numbers in cool period (3.6%, 1.5–5.7%; 2.4%, 0.3–4.5%; 4.9%, 2.9–7.0%; 6.3%, 3.7–9.0%, respectively) were more pronounced than in warm period. Additionally, we also explored the effect estimates of air pollutants on CLRD in the subgroup of sex, age and diseases (bronchitis, pneumonia, capillary bronchitis, and asthma cases) (Supplementary Figure 1–4). For PM10, we observed that the effect estimates were pronounced in female (4.0%, 0.6–7.6%), age >27 months (13.3%, 9.0–17.6%), and pneumonia (2.7%, 0.1–5.3%). For NO2 and SO2, results suggested that the significant effect was found both in male (3.9%, 0.4–7.5%; 4.4%, 0.7%, 8.2%) and female (5.2%, 0.8–9.8%; 7.8%, 3.1–12.7%). When stratified by age, besides PM10, an IQR increase in concentrations of PM2.5, NO2, SO2 and O3 exposure were significant associated with estimated increase in the numbers of CLRD (age >27 months) (12.2%, 8.5–16.0%; 13.2%, 7.8–19.0%; 9.9%, 4.5–15.6%; 12.9%, 6.2–19.9%, respectively). In the stratification of diseases, we found that PM2.5 was associated with positively increased risk of childhood asthma (28.2%, 3.2–59.3%), and NO2 was associated with increased risk of pneumonia (4.0%, 0.7–7.4%) and capillary bronchitis (19.7%, 8.1–32.6%), and SO2 was associated with increased risk of bronchitis (8.0%, 1.5–14.9%), pneumonia (4.3%, 0.9–7.8%) and capillary bronchitis (14.1%, 2.4–27.1%), and O3 was associated with increased risk of bronchitis (14.9%, 6.5–23.8%).
Figure 2.
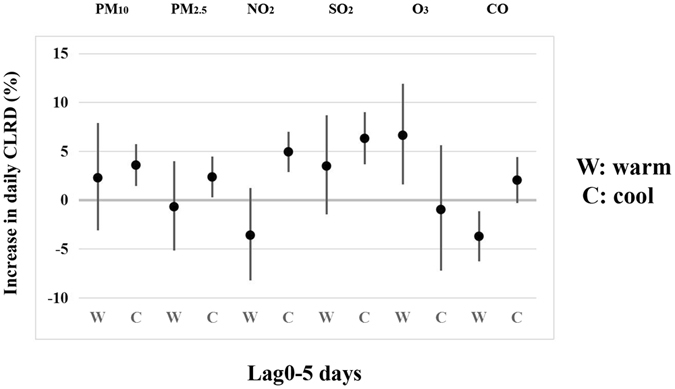
Estimated changes with 95% confidence intervals in daily CLRD percentage deviations (%) associated with an interquartile range increase in PM10, PM2.5, NO2, SO2, O3 or CO concentrations (lag0–5 days) by season using the single-pollutant model.
Concentration-response relationships of CLRD numbers with air pollutants also were performed (Figure 3). The curves for PM10, NO2 and O3 were similar and linearly positive and flat at higher concentrations. The effects of SO2 tend to be linearly positive with its concentrations, and appeared to be stable when concentration was beyond 40 µg/m3. However, the curves for PM2.5 and CO were linearly associated without any thresholds.
Figure 3.
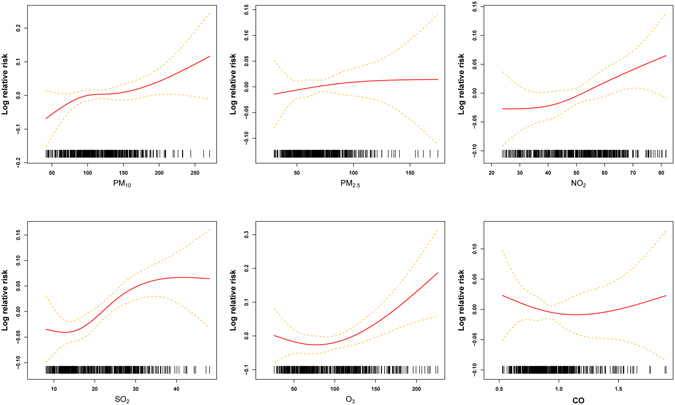
The concentration–response relationship curves for the air pollutants concentrations (lag0–5 day) with daily CLRD.
For lag 0–5, we also performed the two-pollutants model (Figure 4). Results suggested that the effect estimates of all six pollutants attenuated a little. However, when adjusted for PM2.5, two-pollutants model of PM10 produced the higher effect estimates for CLRD numbers than single-pollutant model (7.0%, 95% CI: 2.8–11.5%). Additionally, when adjusted for PM10, an IQR increase in PM2.5 pollutant concentration decreased CLRD numbers (−4.1%, 95% CI: −7.6–0.6%).
Figure 4.
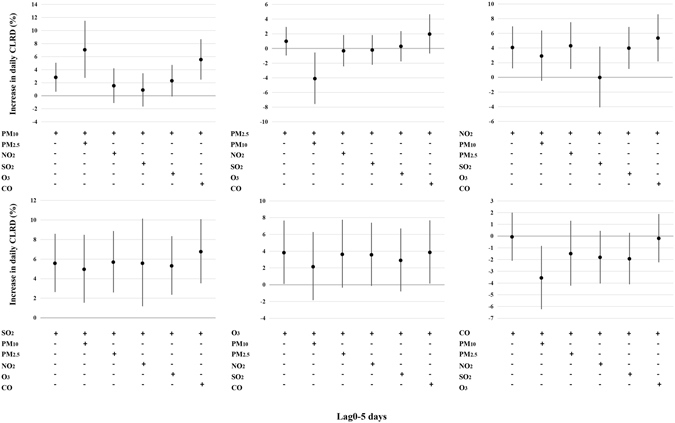
Estimated changes with 95% confidence intervals in daily CLRD percentage deviations (%) associated with an interquartile range increase in PM10, PM2.5, NO2, SO2, O3 or CO concentrations (lag0–5 days) in two-pollutants model.
Discussion
The source of air pollution is mainly manufacturing industries, traffic, agriculture, forest fires, etc., which result in increasing air pollutants level. Exposure to outdoor air pollutants such as particulate matter, NO2, SO2 had the adverse effects on the airway responsiveness and affected the immune competent cells20, 21. Epidemiological association studies between the air pollutants and childhood respiratory health have been studied widely; however, results are conflicting. For example, in a prospective birth cohort, Molter et al. did not find any evidence of the effect on the association between PM10 and NO2 and childhood asthma or wheeze22. Similar to their study, a study in Norway suggested that exposure to NO2 was not associated with childhood asthma at age 9–11 years old23. European BAMSE study also observed no significant association between PM10 exposure and childhood asthma24. Nevertheless, Clarket et al. found the significant association between NO2, PM10, SO2 exposure and Canadian childhood asthma25.
Children exposure to air pollutants is vulnerable to suffering from pulmonary diseases, owing to a larger surface area of lung space than adult26. Exposure to higher air pollutants levels may induce the pathologic changes in childhood airway mucous and make children prone to the prevalence of chronic inflammation in respiratory system27. Many studies have investigated the association between air pollutants and CLRD, of which the majority suggested the effects of short-term exposure to air pollutants up to 4 or 5 days28, 29. Similarly, we also found a cumulative effect of PM10, NO2, and SO2 (lag0–5) on the short term effects with CLRD. Besides, we also investigated the effect estimates of PM10, NO2, and SO2 on CLRD by stratification of diseases (bronchitis, pneumonia, capillary bronchitis, and asthma cases). Some differences observed between PM10, NO2, and SO2 and diseases.
Exposure to PM10 can induce the lung function disorder of children. In this study, we observed that an IQR increase in PM10 daily concentrations increased the daily numbers of CLRD with 5, 6, or 7 days cumulative effects (lag0–4, lag0–5, lag0–6, respectively). Recently, a retrospective study also showed a positive association between the cumulative effects of 7 days (lag0–6) for PM10 and CLRD15. Further, we found the significant association between PM10 and pneumonia. A Jinan study revealed that PM10 were associated with increased number of Childhood pneumonia30. In 2010, Weinmayr and his colleagues conducted a systematic literature review to assess the effect of PM10 on respiratory health in children, which had provided the clear evidence of PM10 as the risk factor on childhood asthma symptom episodes31. However, we did not observe the similar association. It is possible that genetic background can contribute to the differences.
NO2 exposure can damage the lung function, and induce the change of allergic airway inflammation, and responses to allergens32. Similar to PM10, we found that exposure to NO2 was significantly associated with CLRD. The estimated effect for NO2 was consistent with many other studies33, 34. In Italy, Bono et al. found the adverse health effect of NO2 on the risk of emergency room admissions for childhood respiratory diseases34. A meta-analysis included seven countries also confirmed that exposure to NO2 was significantly associated with the asthma and wheezing of children aged 0–1835. In the present study, the significant effect estimate of NO2 on childhood pneumonia and capillary bronchitis was more pronounced than other diseases (bronchitis, and asthma).
SO2 is a well-known gas with strong irritant, and can induce the responses of respiratory system. A study showed that children living near a petrochemical complex with high SO2 exposure had significantly increased incidence of bronchitis, asthma and allergic rhinitis than low exposure group36. In addition, Samoli et al. also observed that exposure to SO2 (10 µg/m3) significantly increased the number of childhood asthma hospital admissions37. In this study, we also found that exposure to SO2 had a positive association with the numbers of CLRD, especially with childhood bronchitis, pneumonia, and capillary bronchitis.
Season was an important factor in modifying the association between air pollutants and population health effects38. In this study, we found the effects of air pollutants on CLRD numbers varied by season. Specifically, the effect estimates of PM10, NO2, and SO2 were positive associated with CLRD numbers in cool season. Chen’s findings also suggested that exposure to PM10 and NO2 had the largest effects in childhood asthma in cool season39. A system review and meta-analysis also proposed that children exposure to PM10, NO2, and SO2 have the stronger association of asthma-related hospital admission in warm season40. The exact seasonal reasons were still not clear, and might be related with the difference in air pollution levels, surrounding environment, or races.
Several studies have found that age and sex can influence the effect estimates of air pollutants on population health. In the present study, we explored the effect estimates of air pollutants on CLRD in the subgroup of sex and age. Results showed that the effect estimates of PM10, NO2, and SO2 in female were more pronounced than in male, which were consistent with previous study41. Similar results were observed in children with age >27 months. It was possible that children with age >27 months spent more time outdoors than age ≤27 months.
Herein, we should mention several limitations. Firstly, we obtained the data only from one city. Exposure to air pollutants effects may be influenced by person’s living and other factors. Results were difficult to spread to other cities. Secondly, we did not obtain the exactly individual exposure levels of air pollutants, thus, we merely calculated the average air pollution levels of local region to evaluate the estimate effects of air pollutants. Thirdly, we collected the useful data based on one and a half years data from the seven hospitals, which may not represent the entire populations. Besides, our study is the retrospective study, we cannot obtain the biochemical indexes of CLRD cases. Further larger studies should be conducted to comprehensively clarify the effects of air pollutants on CLRD. This study also had several strengths. Firstly, it was the first time to investigate the effect estimate of air pollutants on CLRD in Nanjing, China. Secondly, we used the time-series regression and GAM analysis to evaluate the effects of air pollution. Thirdly, although we obtained the data in a city, the results were consisted with many other studies39, 40, 42.
In summary, we found that the short-term exposure to ambient PM10, NO2, and SO2 were associated with increased CLRD numbers. It was worth to note that our data provided the limited information; therefore, further studies should be conducted to validate our findings.
Electronic supplementary material
Acknowledgements
This study was partly supported by grants from the Natural Science Foundation of Jiangsu Province (15KJB330002), Undergraduates Training Programs of Innovation and Entrepreneurship of Jiangsu Province (201510312001Z), The National Key Research and Development Program of China (2017YFC0211600), Collaborative Innovation Center for Cancer Personalized Medicine, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Author Contributions
L.Z., X.G., Y.C., Z.Z., H.C., M.W. and conceived and designed the study. L.Z. and H.C wrote the main manuscript. Y.C. and X.Z., W.P., X.Z., Q.Y., J.X., W.S., S.B. analyzed the data. D.W., Z.H., Z.Z. and H.C. analyzed and interpreted the data. All authors have read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Liyang Zhu, Xuhua Ge and Yaoyao Chen contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04310-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhengdong Zhang, Email: drzdzhang@gmail.com.
Haiyan Chu, Email: chy_grape@njmu.edu.cn.
Meilin Wang, Email: mwang@njmu.edu.cn.
References
- 1.Beelen R, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 2.Jassal MS. Pediatric asthma and ambient pollutant levels in industrializing nations. International health. 2015;7:7–15. doi: 10.1093/inthealth/ihu081. [DOI] [PubMed] [Google Scholar]
- 3.Oftedal B, et al. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19:129–137. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- 4.Leitte AM, et al. Size-segregated particle number concentrations and respiratory emergency room visits in Beijing, China. Environmental health perspectives. 2011;119:508–513. doi: 10.1289/ehp.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarnat SE, et al. Air pollution and acute respiratory response in a panel of asthmatic children along the U.S.-Mexico border. Environmental health perspectives. 2012;120:437–444. doi: 10.1289/ehp.1003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasana J, et al. Motor vehicle air pollution and asthma in children: a meta-analysis. Environmental research. 2012;117:36–45. doi: 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 7.La Grutta S, et al. Environmental risk factors and lung diseases in children: from guidelines to health effects. Early human development. 2013;89(Suppl 3):S59–62. doi: 10.1016/j.earlhumdev.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JJ, et al. Children’s respiratory morbidity prevalence in relation to air pollution in four Chinese cities. Environmental health perspectives. 2002;110:961–967. doi: 10.1289/ehp.02110961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Z, et al. Asthmatic symptoms among pupils in relation to winter indoor and outdoor air pollution in schools in Taiyuan, China. Environmental health perspectives. 2008;116:90–97. doi: 10.1289/ehp.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Q, et al. Early life exposure to ambient air pollution and childhood asthma in China. Environmental research. 2015;143:83–92. doi: 10.1016/j.envres.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, et al. Associations of gestational and early life exposures to ambient air pollution with childhood respiratory diseases in Shanghai, China: A retrospective cohort study. Environment international. 2016;92–93:284–293. doi: 10.1016/j.envint.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Molter A, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. The European respiratory journal. 2015;45:610–624. doi: 10.1183/09031936.00083614. [DOI] [PubMed] [Google Scholar]
- 13.Parker JD, Akinbami LJ, Woodruff TJ. Air pollution and childhood respiratory allergies in the United States. Environmental health perspectives. 2009;117:140–147. doi: 10.1289/ehp.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu F, et al. Short-term effects of air pollution on daily mortality and years of life lost in Nanjing, China. The Science of the total environment. 2015;536:123–129. doi: 10.1016/j.scitotenv.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Schvartsman, C., Pereira, L.A., Braga, A.L., Farhat, S.C. Seven-day cumulative effects of air pollutants increase respiratory ER visits up to threefold. Pediatric pulmonology. (2016). [DOI] [PubMed]
- 16.Dominici F, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, et al. Associations between fine particle, coarse particle, black carbon and hospital visits in a Chinese city. The Science of the total environment. 2013;458–460:1–6. doi: 10.1016/j.scitotenv.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Peng RD, et al. Seasonal analyses of air pollution and mortality in 100 US cities. American journal of epidemiology. 2005;161:585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, et al. The association between ambient air pollution and daily mortality in Beijing after the 2008 olympics: a time series study. PloS one. 2013;8:e76759. doi: 10.1371/journal.pone.0076759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delfino RJ, et al. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2009;102:138–144. doi: 10.1016/S1081-1206(10)60244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerrett M, et al. A review and evaluation of intraurban air pollution exposure models. Journal of exposure analysis and environmental epidemiology. 2005;15:185–204. doi: 10.1038/sj.jea.7500388. [DOI] [PubMed] [Google Scholar]
- 22.Molter A, et al. Effects of long-term exposure to PM10 and NO2 on asthma and wheeze in a prospective birth cohort. Journal of epidemiology and community health. 2014;68:21–28. doi: 10.1136/jech-2013-202681. [DOI] [PubMed] [Google Scholar]
- 23.Oftedal B, Nystad W, Brunekreef B, Nafstad P. Long-term traffic-related exposures and asthma onset in schoolchildren in oslo, norway. Environmental health perspectives. 2009;117:839–844. doi: 10.1289/ehp.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruzieva O, et al. Exposure to air pollution from traffic and childhood asthma until 12 years of age. Epidemiology. 2013;24:54–61. doi: 10.1097/EDE.0b013e318276c1ea. [DOI] [PubMed] [Google Scholar]
- 25.Clark NA, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environmental health perspectives. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng’ang’a LW, et al. Prevalence of exercise induced bronchospasm in Kenyan school children: an urban-rural comparison. Thorax. 1998;53:919–926. doi: 10.1136/thx.53.11.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MD, et al. Differences between children and adults: implications for risk assessment at California EPA. International journal of toxicology. 2002;21:403–418. doi: 10.1080/10915810290096630. [DOI] [PubMed] [Google Scholar]
- 28.Lee SL, Wong WH, Lau YL. Association between air pollution and asthma admission among children in Hong Kong. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2006;36:1138–1146. doi: 10.1111/j.1365-2222.2006.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farhat SC, et al. Effect of air pollution on pediatric respiratory emergency room visits and hospital admissions. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica… [et al.]. 2005;38:227–235. doi: 10.1590/s0100-879x2005000200011. [DOI] [PubMed] [Google Scholar]
- 30.Lv, C. et al. The impact of airborne particulate matter on pediatric hospital admissions for pneumonia among Jinan children: a case-crossover study. J Air Waste Manag Assoc (2016). [DOI] [PubMed]
- 31.Weinmayr G, et al. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environmental health perspectives. 2010;118:449–457. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poynter ME, et al. Nitrogen dioxide enhances allergic airway inflammation and hyperresponsiveness in the mouse. American journal of physiology. Lung cellular and molecular physiology. 2006;290:L144–152. doi: 10.1152/ajplung.00131.2005. [DOI] [PubMed] [Google Scholar]
- 33.Hansel NN, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environmental health perspectives. 2008;116:1428–1432. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bono R, et al. Air pollution, aeroallergens and admissions to pediatric emergency room for respiratory reasons in Turin, northwestern Italy. BMC public health. 2016;16:722. doi: 10.1186/s12889-016-3376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takenoue Y, et al. Influence of outdoor NO2 exposure on asthma in childhood: meta-analysis. Pediatrics international: official journal of the Japan Pediatric. Society. 2012;54:762–769. doi: 10.1111/j.1442-200X.2012.03674.x. [DOI] [PubMed] [Google Scholar]
- 36.Chiang TY, et al. Increased incidence of allergic rhinitis, bronchitis and asthma, in children living near a petrochemical complex with SO2 pollution. Environment international. 2016;96:1–7. doi: 10.1016/j.envint.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Samoli E, et al. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environmental research. 2011;111:418–424. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, et al. Acute effect of ambient air pollution on heart failure in Guangzhou, China. International journal of cardiology. 2014;177:436–441. doi: 10.1016/j.ijcard.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Chen, K. et al. The Effects of Air Pollution on Asthma Hospital Admissions in Adelaide, South Australia, 2003–2013: Time-Series and Case-crossover Analyses. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. (2016). [DOI] [PubMed]
- 40.Zheng XY, et al. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PloS one. 2015;10:e0138146. doi: 10.1371/journal.pone.0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strak M, et al. Associations between lifestyle and air pollution exposure: Potential for confounding in large administrative data cohorts. Environmental research. 2017;156:364–373. doi: 10.1016/j.envres.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, et al. Air Pollutants, Climate, and the Prevalence of Pediatric Asthma in Urban Areas of China. BioMed research international. 2016;2016:2935163. doi: 10.1155/2016/2935163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


