Abstract
Recent evidence for feathers in theropods has led to speculations that the largest tyrannosaurids, including Tyrannosaurus rex, were extensively feathered. We describe fossil integument from Tyrannosaurus and other tyrannosaurids (Albertosaurus, Daspletosaurus, Gorgosaurus and Tarbosaurus), confirming that these large-bodied forms possessed scaly, reptilian-like skin. Body size evolution in tyrannosauroids reveals two independent occurrences of gigantism; specifically, the large sizes in Yutyrannus and tyrannosaurids were independently derived. These new findings demonstrate that extensive feather coverings observed in some early tyrannosauroids were lost by the Albian, basal to Tyrannosauridae. This loss is unrelated to palaeoclimate but possibly tied to the evolution of gigantism, although other mechanisms exist.
Keywords: Tyrannosauridae, skin, feathers, gigantism
1. Introduction
Filamentous feathers on some large tyrannosauroids from China have raised the intriguing possibility that similar integumentary structures were widespread throughout the group, even among the largest, Late Cretaceous tyrannosaurids [1]. This hypothesis has yet to be tested although it has serious implications for feather evolution, in which the developmental sequence of modern feathers is generally assumed to have been recapitulated in the phylogeny of coelurosaurian theropods [2–6]. The earliest feathers were simple hollow shafts and gradual innovations, including the development of the rachis, barbs and barbules, towards the fully formed flight feather were added in maniraptoran theropods and eventually birds [3,5]. Although filamentous feathers likely represent the plesiomorphic condition for coelurosaurians [7], the majority of dinosaurian integumentary fossils—including the giant sauropodomorphs and most ornithischians—indicate scaly, reptilian-like skin [7]. Filamentous feathers have been described in two tyrannosauroids, both from the Early Cretaceous of China: Dilong paradoxus [8] and Yutyrannus huali, which, at around 9 m in length, approached the size of some Late Cretaceous tyrannosaurids. Extensive plumage on Yutyrannus led those authors to reason that large body size did not lead inevitably to a reduction of feathers and that their presence was a necessary adaptation to the cold palaeoclimate in western Liaoning [1]. Here, we provide the first detailed descriptions of Tyrannosaurus skin, which, augmented by other integumentary fossils from Late Cretaceous Laurasian tyrannosaurids, provide new context for the evolution of integument and body size in Tyrannosauroidea.
2. Methods
We examined skin impressions associated with a partial skull and skeleton of Tyrannosaurus rex (‘Wyrex'; HMNS (Houston Museum of Natural Science, Houston) 2006.1743.01 (formerly BHI 6230); Hell Creek Formation, upper Maastrichtian), collected near the town of Baker, Montana. Combined with new observations of skin from other Late Cretaceous tyrannosaurids, including Daspletosaurus, Tarbosaurus and, for the first time, Albertosaurus and Gorgosaurus, we assembled a new dataset of integument and body size in tyrannosauroids (see electronic supplementary material, table S1). We adopted a similar approach to VanBuren et al. [9] to explore the relative timing of changes in both integument and size within the tyrannosauroid tree [10] and performed ancestral state estimation of integument (discrete character; Ninteg = 7) and size (based on femoral length; continuous character; NFL = 15). All analyses were carried out in R v. 3.2.3 [11].
3. Results
(a). Description
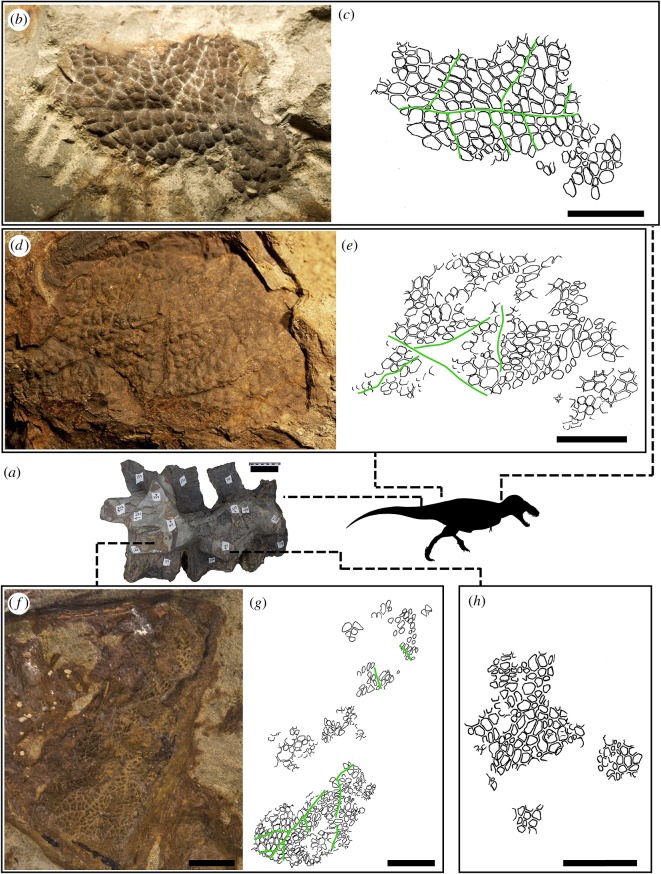
HMNS 2006.1743.01 preserves numerous patches of skin on the neck, pelvis and tail. The integument generally comprises a basement of smooth, unornamented scales variably preserved in bold or negative relief, but devoid of feature scales (sensu [12]). Individual scales are highly variable in shape, ranging through elongate, elliptical, subrectangular and irregular three- to six-sided polygons. Despite this variation, there is no evidence to suggest that scales were polarized (i.e. having distinct anterior–posterior axes, as in avian scutate scales). On the tail, up to ten patches of integument, ranging from 1 to 30 cm2, are preserved on the right lateral side between caudal vertebrae 6–8. Skin over the neck, ilium and tail is composed of minute scales (≤1 mm in diameter) arranged into somewhat trapezoidal or triangular clusters (approx. 50 mm2) separated by deeply inscribed bands of interstitial skin (figure 1a–h). These bands are slightly wider than the hinge areas between individual scales. Where the patches of integument are sufficiently large, the bands are arranged in a branching pattern reminiscent of the midrib and lateral veins on a plant leaf (figure 1b,c,f,g). The ‘midrib' thus gives rise to numerous alternating lateral ‘veins' that extend obliquely from the midrib and lie subparallel to one another.
Figure 1.
Integument of Tyrannosaurus rex (HMNS 2006.1743.01). (a) Proximal caudal vertebrae 6–8 in right lateral view. Integument from the neck (b,c), the ilium (d,e) and caudal vertebrae (f–h). Green lines indicate ‘vein and midrib' patterns. Scale in a = 10 cm; b–e = 5 mm and f–h = 10 mm. (Online version in colour.)
Integument is preserved in several other tyrannosaurids (see descriptions in the electronic supplementary material), including the first definitive evidence of scales in Albertosaurus and Gorgosaurus. Collectively, these reveal that much of the abdomen, tail and thoracic regions were scaly. Scale architecture is similar in most respects to that in HMNS 2006.1743.01, although none preserves the ‘midrib and vein' arrangement. The basement scales of Gorgosaurus (CMN 11593), which are preserved along the ventrolateral surface of a haemal spine, are polygonal to circular, with an average diameter of 3.6 mm (range = 2.5–4.9 mm). Most notable, however, is a specimen of Albertosaurus sarcophagus (TMP 1994.186.0001) that preserves evidence of feature scales on the abdomen. The feature scales are conical (7 mm in diameter, 2.5 mm high) with radial corrugations and are embedded in a patch of pebbly, circular to hexagonal basement scales (diameter range = 1.4–2.5 mm; electronic supplementary material, figure S1a–d).
(b). Character evolution
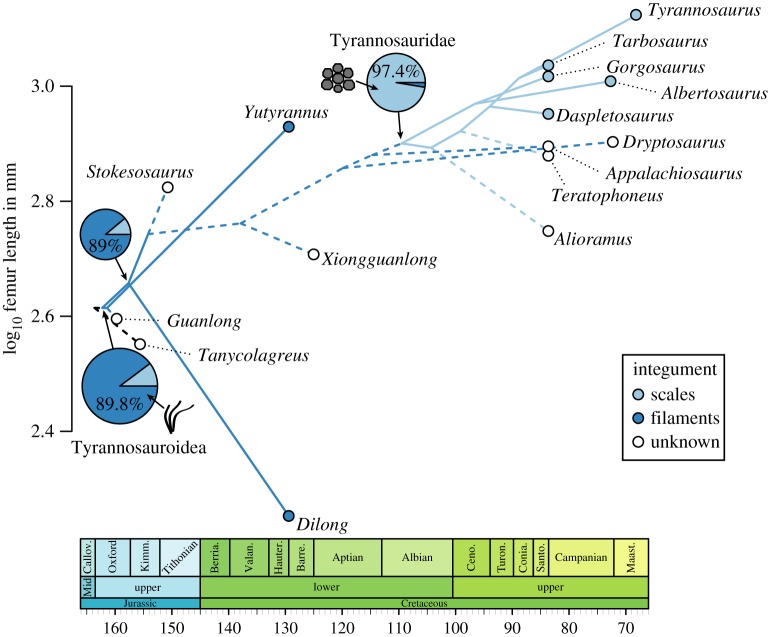
Body size evolution in tyrannosauroids reveals two instances of evolution towards large body size (figure 2): (1) a drastic shift in the feathered Yutyrannus (from 2.615 to 2.929 log10 mm) and (2) a seemingly gradual trend towards the larger sizes of tyrannosaurids, starting with the common ancestor of Stokesosaurus, Xiongguanlong, Dryptosaurus, Appalachiosaurus and Tyrannosauridae. The ancestral tyrannosauroid integument likely comprised filamentous feathers (88.9–89.8% likelihood) with a loss of feathers occurring no earlier than the tyrannosaurid ancestor. Results are insensitive to differences in topology and branch scaling method (see the electronic supplementary material).
Figure 2.
Body size and integumentary evolution in Tyrannosauroidea. The phenogram traces body size evolution, measured as log10 femur length (in mm), upon which the integument character is optimized using maximum-likelihood to estimate ancestral states (pie charts). Dashed lines represent areas of ambiguity resulting from missing data. (Online version in colour.)
4. Discussion
Combined with evidence from other tyrannosaurids, the integument of HMNS 2006.1743.01 provides compelling evidence of an entirely squamous covering in Tyrannosaurus. Integument in Albertosaurus, Daspletosaurus, Gorgosaurus, Tarbosaurus and Tyrannosaurus collectively covers parts of the neck, abdomen, hips and tail, suggesting that most (if not all) large-bodied tyrannosaurids were scaly and, if partly feathered, these were limited to the dorsum. Tyrannosaurids do not, therefore, exhibit the widely distributed filamentous feathers present in Dilong and Yutyrannus, where scales are unknown [1,8]. In fact, within Coelurosauria, where feathers are present, they cover virtually the entire body [13,14]. On the other hand, the co-occurrence of epidermal scales and filamentous structures has only been reported in some neornithischians [15], although the homology of these filamentous structures with theropod feathers has been questioned [7]. Finally, the presence of epidermal scales in a large adult individual does not rule out the possibility that younger individuals possessed feathers—a developmental switchover that, to our knowledge, would be unprecedented at any rate.
The increase in feather complexity predicted by developmental models of feather evolution is generally considered congruent with the fossil record of non-avian theropods [3,6]. This evolutionary succession is also compatible with phylogenetic reconstructions of Coelurosauria, in which increasingly complex feather morphotypes appear in progressively more derived clades [2,4,5]. However, widespread epidermal scales among Tyrannosauridae indicate that feather evolution was more complex than previously assumed and did not evolve in a purely progressive fashion across the whole of Coelurosauria; feathers had appeared in Dilong and Yutyrannus by the Hauterivian [1,8] but were apparently secondarily lost by the Albian. If, as some have posited [16], the integumentary structures such as those in Yutyrannus and Dilong are not feathers, then our findings still reveal notable changes within the integument of tyrannosauroids that require explanation.
Within the context of integumentary evolution in dinosaurs ([7]; figure 2), the secondary appearance of expansive scalation observed in Tyrannosauridae raises the novel question: why did a coelurosaurian lineage lose the extensive filamentous coverings common to its close relatives? Interestingly, the scales on the legs of modern birds may not be homologous with reptile scales but are secondarily derived from feathers, likely acquired in response to behaviour (e.g. perching, wading) [17–19]. The corollary, therefore, is that tyrannosaur scales may also be derived from primitive feathers such as those in Yutyrannus. However, morphological evidence alone is insufficient and only future developmental studies will shed light on this hypothesis. It is possible that reversals to scales occurred within ornithischian dinosaurs (e.g. Ceratopsia and Ornithopoda); however, such scenarios remain ambiguous as it is also possible that integumentary structures in Kulindadromeus and Tianyulong represent the derived condition [7]. Accordingly, the unambiguous loss of an expansive filamentous cover in tyrannosaurids merits further discussion.
While patches of prominent/brightly coloured feathers could serve as effective display structures, the extensive feather coverings of most coelurosaurs are generally accepted as thermal insulation [1,20]. The adaptive loss of plumage, therefore, suggests a reduction in the importance of heat retention. Four hypotheses related to feather loss are discussed, none of which is mutually exclusive.
One hypothesis is that tyrannosaurids inhabited substantially warmer climates than their earlier counterparts [1]. However, this can be rejected based on (1) contemporaneous coelurosaurs did not undergo similar feather loss [21] and (2) estimates of the mean annual air temperature (MAAT) for the North American and Mongolian Late Cretaceous localities are comparable to that of the Yixian Formation (electronic supplementary material, table S2). Tyrannosaurids are also known from diverse palaeoenvironments [22], whereas the Yixian Formation is characterized by extensive forests [23], potentially imparting differential selective pressures on their respective physiologies. For example, modern Javan rhinos and Asian elephants are hairier than their African counterparts, not simply because they are smaller, but also because they inhabit dense forests where direct sunlight is less intense [24].
Increased activity level and higher athletic performance are also potential drivers of hair loss. For their size, tyrannosaurids possessed proportionately longer legs that, along with the primitive arctometatarsalian foot condition, suggest increased cursoriality [25]. More frequent, intensive or prolonged cursorial pursuits could have demanded greater heat-loss ability.
Finally, gigantism (i.e. increased body mass) affords greater heat retention: a thermodynamic by-product of the square-cube law and linked to reductions in hair in large modern terrestrial mammals [26]. Gigantism has been offered as an explanation for the absence of feathers in large herbivorous dinosaurs [27]. Therefore, the greater ability of larger tyrannosaurids to maintain a constant body temperature may have outweighed the advantages of insulation by a more pressing need to shed heat. In the Horseshoe Canyon Formation, the juxtaposition of feathered ornithomimids [21] and non-feathered Albertosaurus supports this hypothesis. Such relatively modest-sized theropods would have proportionately less thermal inertia. However, although the mass estimate for the feathered Yutyrannus (1414 kg [1]) is considerably lower than most tyrannosaurids with integumentary data (Gorgosaurus, 2145–3577 kg [28]; Tarbosaurus, 1744–2945 kg [29]; Tyrannosaurus, 5014–8361 kg [28]), it is similar in size to Albertosaurus (1325–2210 kg [28]) and the size at which our model predicts tyrannosauroid feather loss might have first evolved (figure 2). Our results, therefore, reveal an intriguing counterintuitive pattern between size and integumentary evolution within Tyrannosauroidea that can only be tested by future fossil discoveries.
Supplementary Material
Supplementary Material
Acknowledgements
TMP 1994.186.0001 was discovered by Tess Owen. We thank Tess Owen, Tom Owen and S. Kagan for assistance; B. Strilisky (Royal Tyrrell Museum of Palaeontology) for access to specimens; J. Mallon for photographs and information on CMN 11593; and M. Larson for assistance and information on HMNS 2006.1743.01. Silhouette in figure 1 by S. Hartman (phylopic.org). We thank T. Carr, two anonymous reviewers and the handling editor, R. Honeycutt, for insightful comments that improved the final version.
Ethics
All specimens are appropriately reposited in accordance with the ICZN, and all necessary permissions and permits were obtained to carry out this research.
Data accessibility
Supplemental descriptions, figures, palaeoclimate and body mass data and code are available as electronic supplementary material.
Authors' contributions
P.R.B. conceived and designed the study, acquired data and wrote the manuscript; N.E.C. conceived/performed statistical analyses, acquired/analysed data and wrote the manuscript; W.S.P. analysed the data and wrote the manuscript; P.J.C., P.L.L., D.H.T. and R.T.B. acquired data and critically revised the manuscript. All authors gave final approval for publication and agree to be accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
We received no funding for this study.
References
- 1.Xu X, Wang K, Zhang K, Ma Q, Xing L, Sullivan C, Hu D, Cheng S, Wang S. 2012. A gigantic feathered dinosaur from the Lower Cretaceous of China. Nature 484, 92–95. ( 10.1038/nature10906) [DOI] [PubMed] [Google Scholar]
- 2.Prum RO, Brush AH. 2002. The evolutionary origin and diversification of feathers. Q. Rev. Biol. 77, 261–295. ( 10.1086/341993) [DOI] [PubMed] [Google Scholar]
- 3.Prum RO. 1999. Development and evolutionary origin of feathers. J. Exp. Zool. 285, 291–306. () [DOI] [PubMed] [Google Scholar]
- 4.Prum RO, Brush AH. 2014. Which came first, the feather or the bird? Sci. Am. 23, 76–85. ( 10.1038/scientificamericandinosaurs0514-76) [DOI] [PubMed] [Google Scholar]
- 5.Norell MA, Xu X. 2005. Feathered dinosaurs. Annu. Rev. Earth Planet Sci. 33, 277–299. ( 10.1146/annurev.earth.33.092203.122511) [DOI] [Google Scholar]
- 6.Xu X, Zheng X, You H. 2009. A new feather type in a nonavian theropod and the early evolution of feathers. Proc. Natl Acad. Sci. USA 106, 832–834. ( 10.1073/pnas.0810055106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett PM, Evans DC, Campione NE. 2015. Evolution of dinosaur epidermal structures. Biol. Lett. 11, 20150229 ( 10.1098/rsbl.2015.0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Norell MA, Kuang X, Wang X, Zhao Q, Jia C. 2004. Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature 431, 680–684. ( 10.1038/nature02855) [DOI] [PubMed] [Google Scholar]
- 9.VanBuren CS, Campione NE, Evans DC. 2015. Head size, weaponry, and cervical adaptation: testing craniocervical evolutionary hypotheses in Ceratopsia. Evolution 69, 1728–1744. ( 10.1111/evo.12693) [DOI] [PubMed] [Google Scholar]
- 10.Brusatte SL, Carr TD. 2016. The phylogeny and evolutionary history of tyrannosauroid dinosaurs. Sci. Rep. 6, 20252 ( 10.1038/srep20252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R Development Core Team. 2015. R: a language and environment for statistical computing. v. 3.2.3. See http://www.R-project.org.
- 12.Bell PR. 2012. Standardized terminology and potential taxonomic utility for hadrosaurid skin impressions: a case study for Saurolophus from Canada and Mongolia. PLoS ONE 7, e31295 ( 10.1371/journal.pone.0031295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie PJ, Chen P-J. 2001. Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can. J. Earth Sci. 38, 1705–1727. ( 10.1139/e01-050) [DOI] [Google Scholar]
- 14.Ji S, Ji Q, Lu J, Yuan C. 2007. A new giant compsognathid dinosaur with long filamentous integuments from Lower Cretaceous of northeastern China. Acta Geol. Sin. 81, 8–15. [Google Scholar]
- 15.Godefroit P, Sinitsa SM, Dhouailly D, Bolotsky YL, Sizov AV, McNamara ME, Benton MJ, Spagna P. 2014. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 345, 451–455. ( 10.1126/science.1253351) [DOI] [PubMed] [Google Scholar]
- 16.Feduccia A, Lingham-Soliar T, Hinchliffe JR. 2005. Do feathered dinosaurs exist? Testing the hypothesis on neontological and paleontological evidence. J. Morphol. 266, 125–166. ( 10.1002/jmor.10382) [DOI] [PubMed] [Google Scholar]
- 17.Sawyer RH, Knapp LW. 2003. Avian skin development and the evolutionary origin of feathers. J. Exp. Zool. B Mol. Dev. Evol. 298B, 57–72. ( 10.1002/jez.b.26) [DOI] [PubMed] [Google Scholar]
- 18.Godefroit P, Cau A, Dong-Yu H, Escuillie F, Wenhao W, Dyke G. 2013. A Jurassic avialan dinosaur from China resolves the early phylogenetic history of birds. Nature 498, 359–362. ( 10.1038/nature12168) [DOI] [PubMed] [Google Scholar]
- 19.Hu D, Hou L, Zhang L, Xu X. 2009. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature 461, 640–643. ( 10.1038/nature08322) [DOI] [PubMed] [Google Scholar]
- 20.Chen P-J, Dong Z-M, Zhen S-N. 1998. An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature 391, 147–152. ( 10.1038/34356) [DOI] [Google Scholar]
- 21.van der Reest AJ, Wolfe AP, Currie PJ. 2016. A densely feathered ornithomimid (Dinosauria: Theropoda) from the Upper Cretaceous Dinosaur Park Formation, Alberta, Canada. Cretaceous Res. 58, 108–117. ( 10.1016/j.cretres.2015.10.004) [DOI] [Google Scholar]
- 22.Lyson TR, Longrich NR. 2011. Spatial niche partitioning in dinosaurs from the latest Cretaceous (Maastrichtian) of North America. Proc. R. Soc. B 278, 1158–1164. ( 10.1098/rspb.2010.1444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhonghe Z. 2006. Evolutionary radiation of the Jehol Biota: chronological and ecological perspectives. Geol. J. 41, 377–393. ( 10.1002/gj.1045) [DOI] [Google Scholar]
- 24.Hone D. 2016. The tyrannosaur chronicles: the biology of the tyrant dinosaurs. London, UK: Bloomsbury Publishing. [Google Scholar]
- 25.Persons WS, Currie PJ. 2016. An approach to scoring cursorial limb proportions in carnivorous dinosaurs and an attempt to account for allometry. Sci. Rep. 6, 19828 ( 10.1038/srep19828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cave A. 1969. Hairs and vibrissae in the Rhinocerotidae. J. Zool. 157, 247–257. ( 10.1111/j.1469-7998.1969.tb01700.x) [DOI] [Google Scholar]
- 27.Bakker RT. 1988. The dinosaur heresies. London, UK: Penguin. [Google Scholar]
- 28.Campione NE, Evans DC, Brown CM, Carrano MT. 2014. Body mass estimation in non-avian bipeds using a theoretical conversion to quadruped stylopodial proportions. Methods Ecol. Evol. 5, 913–923. ( 10.1111/2041-210X.12226) [DOI] [Google Scholar]
- 29.Benson RBJ, Campione NE, Carrano MT, Mannion PD, Sullivan C, Upchurch P, Evans DC. 2014. Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 12, e1001853 ( 10.1371/journal.pbio.1001853) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplemental descriptions, figures, palaeoclimate and body mass data and code are available as electronic supplementary material.