Abstract
Objective
To review the effectiveness of interventions to increase HIV serostatus disclosure in low- and middle-income countries.
Design
Systematic review of peer-reviewed articles providing pre-post or multi-arm evaluations of disclosure interventions, defined broadly as any intervention with the goal of increasing rates of voluntary disclosure of HIV serostatus through self-disclosure or partner notification.
Methods
Articles were included if they reported post-intervention evaluation results and were published between January 1, 1990 and August 1, 2014. Searching was conducted through 5 electronic databases, secondary searching of four journals, and hand searching reference lists of included articles. Systematic methods were used for screening and data abstraction, which was conducted in duplicate. Study quality (rigor) was assessed with eight items.
Results
Fourteen articles evaluating 13 interventions met the inclusion criteria, all from sub-Saharan Africa. Most interventions focused on people living with HIV and used cognitive-behavioral group sessions or peer/community health worker support to encourage disclosure to sexual partners, family members, or friends. One focused on maternal disclosure to HIV-uninfected children, while two examined voluntary partner notification interventions. Several studies had limitations due to weak designs, small sample sizes, or high attrition. Findings on disclosure were mixed, with most effect sizes being relatively small and some, more rigorous studies showing no effect. Partner notification interventions had the strongest evidence of impact.
Conclusion
The existing evidence base for interventions to increase disclosure is limited and shows variable results. Further research is needed to determine whether current approaches to increasing disclosure are effective or whether new approaches should be considered.
Keywords: HIV, Disclosure, Partner notification, Review, systematic, Intervention studies
Introduction
An effective global response to HIV requires people to be aware of their risk of HIV, get tested, engage in appropriate HIV care and treatment services if positive, and take appropriate steps to prevent HIV transmission or acquisition. There are many approaches to achieving these goals, but supporting individuals to disclose their HIV serostatus to others may facilitate these diverse outcomes in multiple ways. A recent meta-analysis showed that disclosure is associated with increased social support and reduced stigma [1], which may facilitate other interventions, such as engagement in care and treatment [2]. Disclosure to sexual partners could also increase rates of HIV testing and reduce transmission risk behaviors, although the evidence for this is limited and affected by multiple confounding factors [3]. However, there have also been concerns about potential negative aspects of disclosure, including stigma, violence, abandonment, and relationship dissolution [4]. Fear of HIV serostatus disclosure to partners has been among the most consistent barriers to prevention of mother-to-child transmission (PMTCT) program uptake among women in sub-Saharan Africa [5].Indeed, many benefits of disclosure require access to services and commodities that may not be guaranteed in many low and middle-income settings. Program planners must thus consider how best to support people to disclose their HIV status to others in order to achieve positive outcomes while avoiding potential negative consequences of disclosure.
Obermeyer et al. [6] have defined HIV serostatus disclosure as “the process of revealing a person's HIV status, whether positive or negative”. Disclosure is a complex process, not a single event, and involves different ways of communicating about HIV status with different people at different points in time. Self-disclosure – when a person directly shares information about his or her HIV status with another person – is the most commonly considered form of disclosure. Partner notification, also known as partner services or contact tracing, is a related concept that involves identifying the sexual or injection partners of people who test HIV-positive and informing them that they may have been exposed to HIV. Partner notification has been described as taking multiple forms, from self-disclosure by the index patient (“patient referral”) to notification by a provider with the index patient remaining anonymous (“provider referral”) [7]. Historically, partner notification was done with partners of index patients with sexually transmitted infections (STIs); partner notification for HIV has only more recently been considered.
Previous reviews on HIV serostatus disclosure have covered descriptive studies measuring rates, barriers, and outcomes of disclosure by different groups [4, 8-12]; theoretical models of disclosure [13]; and broad overviews of multiple aspects of disclosure, including laws, policies, and gender norms [6, 14]. Other reviews have examined the related, but distinct, concept of informing someone, often a child, that he or she is HIV-infected [15, 16]. Despite the large body of literature on HIV serostatus disclosure, there has been little focus on interventions designed to support people as they disclose their HIV status and increase rates of disclosure. We conducted the first systematic review of the effectiveness of interventions to increase HIV serostatus disclosure with a focus on low- and middle-income countries.
Methods
This review was conducted as part of the Evidence Project, a series of systematic reviews of behavioral interventions and aspects of HIV prevention in low- and middle-income countries. We follow standard systematic review methods and reporting guidelines [17] for all reviews.
Inclusion criteria
Disclosure interventions were defined broadly as any intervention with the goal of increasing rates of voluntary disclosure of HIV serostatus. Studies were included in the review if they met the following criteria:
Presents an evaluation of an intervention that has the explicit goal of increasing rates of voluntary disclosure of HIV serostatus (either positive or negative) by participants to others. Disclosure could be to anyone – e.g., sexual partners (current or past), children, family members, friends, or healthcare providers. Disclosure had to be voluntary (i.e., not involuntary or coercive) and could be either self-disclosure, facilitated disclosure (e.g., facilitated by a counselor), or disclosure by a third party without the presence of the individual, but with the individual's permission. Within this definition, we included interventions offering voluntary partner notification. We excluded studies that focused on informing a person about his or her own HIV diagnosis; this alternative definition of disclosure to someone, rather than by someone, has been examined elsewhere, particularly among children.
Measures one of the following primary outcomes (1) HIV serostatus disclosure or (2) partner/contact knowledge of potential HIV exposure (as assessed through measures such as return for HIV testing).
Compares one of the primary outcomes listed above among those who received the disclosure intervention to those who did not, or compares pre/post outcomes among intervention participants.
Conducted in a developing country according to the World Bank classifications of low-income, lower-middle income, or upper-middle income [18].
Published in a peer-reviewed journal from January 1, 1990 to August 1, 2014.
Although disclosure is generally discussed and encouraged in counseling accompanying HIV testing, we excluded studies that evaluated only HIV testing and counseling (HTC) programs as these have been reviewed elsewhere [19-21]. The Evidence Project uses the cut-off date of 1990 across all reviews to reduce temporal bias due to the dramatic changes in HIV treatment, behaviors, and social norms that have occurred in low- and middle-income countries over the past 25 years.
Studies not meeting all inclusion criteria but presenting relevant data from qualitative studies, cost studies, modeling studies, or reviews were included as background articles.
Search terms
We searched five electronic databases (PubMed, PsycINFO, Sociological Abstracts, the Cumulative Index to Nursing and Allied Health literature (CINAHL), and EMBASE) for relevant articles using the search terms (HIV OR AIDS) and (disclos* OR notif*). We also conducted hand-searching of the table of contents of four key journals in the field of HIV: AIDS, AIDS and Behavior, AIDS Care, and AIDS Education and Prevention. Finally, we searched the reference lists of included articles to further identify potential new citations and continued this process iteratively until no new citations were found.
Screening
Initial citations retrieved were screened by one trained study team member to exclude articles that were clearly not relevant to the review's goals based on title and abstract alone. The remaining citations were double-screened by two senior study team members working independently; results were compared and differences resolved through consensus. A final decision on inclusion of articles was based on a full-text review and, often, discussion among all coauthors. Relevant articles not meeting the inclusion criteria were retained and included as background material; background studies underwent data abstraction using a greatly simplified form.
Data abstraction
Each article meeting the inclusion criteria underwent data abstraction using a structured form by two trained study team members working independently; results were compared and differences resolved through consensus. Authors were contacted as needed to provide clarification or additional information on their studies.
The following information was gathered from each included study: location, setting and target group; years (period of study); description of the intervention; study design; sample size; age range, gender; random or non-random allocation of participants; length of follow-up; outcome measures; comparison groups; effect sizes; confidence intervals; significance levels; and limitations identified by authors and reviewers. Study quality (rigor) was assessed using an 8-item assessment tool developed by The Evidence Project for other systematic reviews of HIV behavioral interventions [19-28].
Data analysis
Data were synthesized across studies and presented narratively. We did not meta-analyze due to substantial heterogeneity in study populations, settings, specific intervention approaches, and ways of measuring disclosure as an outcome.
Results
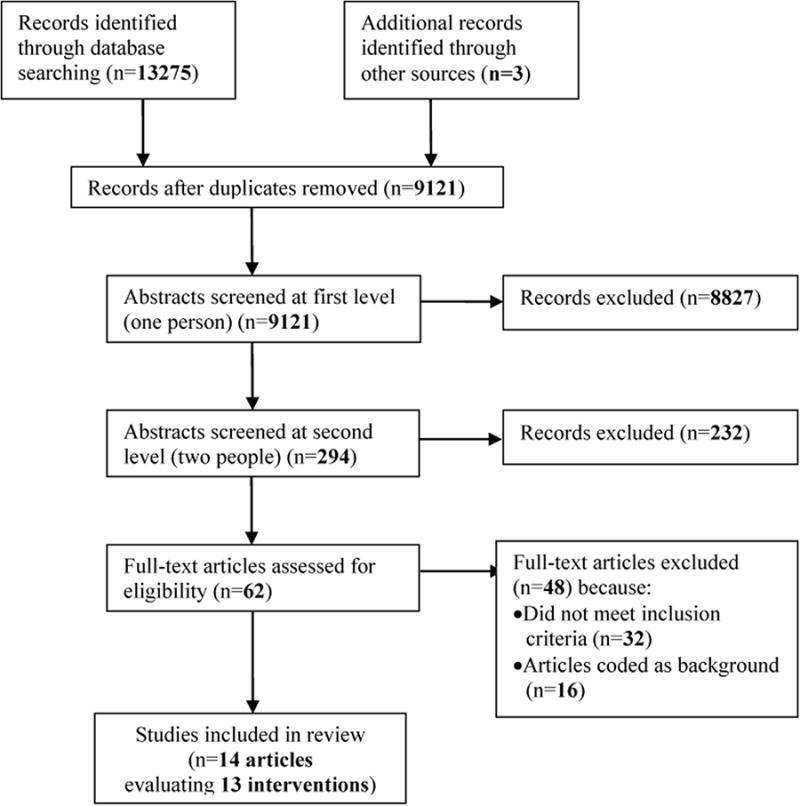
Our initial database search yielded 13,275 published citations; 3 additional citations were identified through other means, such as searching reference lists of relevant articles (Figure 1). After removing duplicates, 9,121 citations remained; 8,827 of these were excluded in the initial screening. The remaining 294 were screened in duplicate, and 232 were excluded at this level. The full text of 62 articles was retrieved for additional review. Of these, 32 did not meet inclusion criteria and 16 were coded as background. One intervention was evaluated through both a pilot study [29] and a full evaluation [30]; both studies were included in the review as they present unique data, but we discuss them together as the same intervention. Thus, fourteen studies evaluating thirteen interventions ultimately met the criteria for inclusion in the review.
Figure 1. PRISMA flowchart showing disposition of citations through the search and screening process.
Study descriptions
Table 1 presents descriptions of the 13 included interventions [29-42]. All 13 were conducted in sub-Saharan Africa: 7 in South Africa, 3 in Tanzania, and 1 each in Botswana, Cameroon, and Malawi. One study from Tanzania was published in 1999; the remaining studies were published between 2007 and 2014. All but one intervention either targeted only women or had a majority of female participants. Five interventions targeted pregnant women [32, 34, 35, 37, 41]; of these, four focused on HIV-positive pregnant women [32, 35, 37, 41], while one included both positive and negative women and their partners [34]. Six interventions targeted HIV-infected adults, including newly diagnosed people living with HIV (PLHIV) [31, 33], HIV-infected women [29, 40], people initiating ART [39], and adults living with HIV generally [36, 38]. One intervention focused on HIV-infected youth [42].
Table 1. Descriptions of included studies.
| Study | Setting | Population Characteristics | Intervention Description | Study Design |
|---|---|---|---|---|
| Cognitive-behavioral support groups | ||||
|
| ||||
| Futterman et al., 2010 [32] | South Africa Western Cape Province, Cape Town, Gugulethu and Vanguard Townships |
HIV-positive pregnant women Gender:100% female Age: Mean: 26.4 years Range: 16-42 years |
Participants in the Mamekhaya intervention were linked to mentor mothers through the Mother2mothers program in addition to standard PMTCT services. Mentor mothers provided support to participants during pregnancy and in the weeks following delivery. Participants also attended an 8-session small group cognitive-behavioral intervention focusing on healthy living, feeling happy and strong, partnering and preventing transmission, and parenting. | Non-randomized trial – Group. Assessments took place at baseline (N=160) and at 6 months after delivery (N=71) post-intervention. Participants were non-randomly selected. |
|
| ||||
| Jones et al., 2013 [34] | South Africa Mpumalanga Province; GertSibande and Nkangala districts |
Pregnant women who completed HIV testing and counseling and their male partners (both HIV-positive and HIV-negative) Gender: 50% male 50% female Age: Mean: 28.2 years |
The intervention consisted of 4 weekly 90 -120 minute sessions emphasizing cognitive-behavioral skill building to improve communication, sexual negotiation, conflict resolution, STI/HIV prevention, PMTCT, use of male and female condoms, and gender-relevant issues, including disclosure. Intervention sessions were closed, structured, gender- concordant group sessions limited to 10 participants per group; male and female groups were conducted concurrently. Participants in the control condition received the standard of care (PMTCT) and four time-matched sessions (health-related videos). All participants were provided with male and female condoms at the close of each session. | Randomized controlled trial – Group. Assessments took place at baseline (N=239 couples, N= 478 individuals) and at 6-8 weeks (N=239 couples, N= 478 individuals) follow-up. Participants were non-randomly selected. |
|
| ||||
| Kaaya et al., 2013 [35] | Tanzania Dar es Salaam |
HIV-positive pregnant women Gender:100% female Age: Mean: 26 years |
Participants in the intervention group received a 6-week support group intervention in groups of 6-8 women. Content of group sessions included: whom to disclose to, initial exploration about MTCT of HIV and possible ways to prevent it, available support systems as home/or in the community, living with uncertainty and making plans for the future, safer sex practices and health implications of HIV, accessing health care and social services, ways of reducing the risk of MTCT of HIV, disclosure to partners, family, and friends, ongoing psychosocial support to facilitate discussion and sharing of possible support systems, relaxation breathing exercises trainings, and sharing of successful partner disclosure. | Randomized controlled trial – Individual. Assessments took place at baseline (N=331) and 8-10 weeks (N=188) follow-up. Participants were non-randomly selected. |
|
| ||||
| Mundell et al., 2011 [37] | South Africa Tshwane township, Pretoria |
Pregnant women recently diagnosed as HIV-positive Gender:100% female Age: Intervention mean: 27.35 years Comparison mean: 26.89 years |
The intervention consisted of a structured 10-session weekly psychosocial support group. The support group sessions focused on HIV-related knowledge; the emotional impact of HIV infection; disclosure of serostatus; how to cope more effectively with HIV infection personally, in the home, the family, and within intimate relationships; stress management; coping with stigma, discrimination; and education on basic human rights, life planning and goal setting, so as to leave participants with a sense of hope and a feeling of empowerment. | Non-randomized trial – individual. Assessments took place at baseline (N=361) and at 2 months (N= 279) and 8 months (N=240) follow-up. Participants were non-randomly selected. |
|
| ||||
| Sarnquist et al., 2014 [41] | Zimbabwe Chitungwiza |
HIV-infected women seeking antenatal care Gender:100% female Age: Range: 18-40 years |
This intervention sought to integrate enhanced family planning and PMTCT services through nurse training. The “Peers Undertaking Reproductive and Sexual Health Education” (PURSE) intervention took place at one of four clinics over the course of three, 90 minute sessions. The intervention used Social Cognitive Theory and the Theory of Gender and Power to improve communication skills and condom use. Sessions included information on HIV, PMTCT and family planning, sexual negotiation skills and empowerment, and communication skills related to family planning and sex. Discussions, behavior modelling, songs, dramatizations and role-playing were all used as learning techniques. | Non-randomized trial – individual. Assessments took place at baseline (N=98) and at 6 weeks (N=83) and 3 months (N=94) post-partum. Participants were non-randomly selected. |
|
| ||||
| Snyder et al., 2014 [42] | South Africa Cape Town, Nyanga and Khayelitsha sub-districts |
HIV-infected youth in Cape Town, South Africa Gender:95% female 5% male Age: Median: 22 years |
The Hlanganani intervention took place over a three week period with one, two hour session per week in the form of a cognitive behavioral structured support group devised to cover three domains: coping and support, HIV health, and positive prevention. Role play, relaxation exercises, interactive discussions, goal setting and a set of cognitive behavioral tools were all used to facilitate linkage to care, healthy behavior change, social support, coping and disclosure. | Before-after study. Assessments took place at baseline (N=109) and 3 weeks (N=74) post-intervention. Participants were non-randomly selected. |
|
| ||||
| Wouters et al., 2009 [39] | South Africa Free State Province |
Adults living with HIV enrolled in a public sector ART program Gender: 33.2% male 66.8% female Age: Mean: 37.9 years(SD=8.6) |
The intervention consisted of two community support components of the South African ART program—Community Health Workers (CHWs) and HIV support groups. CHWs are multiskilled and multipurpose HIV/tuberculosis workers, involved in counseling, social support, and home-based care. HIV peer-support groups facilitate the discussion of factors that may enhance or impede adherence, disclosure, and other psycho-social issues, and also act as forums for health promotion and education. CHWs predominantly provided lifestyle counseling and encouraged ART adherence. Patients attending an HIV support group reported receiving encouragement, motivation, and emotional support by sharing their mutual experiences and ideas. | Cohort study. Assessments took place at baseline (6 months after ART initiation, N=268), at 12 months (N= 234) and 18 months (N= 183) after ART initiation. Participants were randomly selected. |
|
| ||||
| Home-based or peer/community health workers | ||||
| MacNeil et al., 1999 [36] | Tanzania Specific location not reported |
HIV-positive sexually active adults Gender: 33.77% male 66.23% female Age: Mean: 31.93 years |
Enhanced care and support intervention consisted of providing ongoing counselling on prevention and problem-solving for the HIV-infected persons, education to other family members, provision of condoms and, when necessary, referral for treatment. Subjects received enhanced care and support at least once a month either in the counselling center or at the home of the subject. Counseling was defined as the ongoing dialogue and relationship between the counsellor and the client with the aims of preventing the transmission of HIV and providing psychological support to those already infected. | Randomized control trial – Individual. Assessments took place at baseline (N=155) and at 3 month (N=NR) and 6 month (N= NR) follow-up. Participants were non-randomly selected. |
|
| ||||
| Ncama, 2007 [38] | South Africa Port Shepstone and Zululand areas |
Adults living with HIV Gender: 26% male 74% female Age: NR |
Participants received integrated community/home-based care provided by community caregivers, including counseling, HIV education, symptom control, support, and welfare interventions. | Cross-sectional study design. N=363. Intervention participants were randomly selected and comparison group participants were non-randomly selected. |
|
| ||||
| Rochat et al., 2013 [29] | South Africa KwaZulu-Natal province |
HIV-infected women with HIV-uninfected school-aged children Gender:100% female Age: Median: 32 years Mean: 33.5 years Range: 24-46 years |
The Amaguguintervention was designed to help HIV-infected women disclose their own HIV status to their HIV-uninfected children. The intervention incorporated printed materials, therapeutic tools and child-friendly games and activities to support the mother's disclosure. Six sessions were to be completed with a trained counselor in the home over the course of six to eight weeks, in order to facilitate developmentally appropriate disclosure. Additional goals were to benefit family relationships with disclosure, to increase the child's knowledge about HIV and health and to improve custody planning for children of HIV-positive mothers. | Time-series study. Assessments took place at baseline (N=24), at the 5th (N=24) and 7th (N=24) study visits (within 8 weeks). Participants were non-randomly selected. |
|
| ||||
| Rochat et al., 2014 [30] | South Africa KwaZulu-Natal province |
HIV-infected women with HIV-uninfected school-aged children Gender:100% female Age: Range: 23-54 years |
The Amaguguintervention was designed to help HIV-infected women disclose their own HIV status to their HIV-uninfected children. It consisted of two stages: a pre-disclosure stage when the counsellor worked with the mother to prepare and train her towards disclosure, and a post-disclosure stage when the mother was counseled on health promotion and custody planning. Mothers received six counselor-led sessions at home as well as printed materials and child-friendly activities and games to support age-appropriate disclosure. | Before-after study. Assessments took place at baseline (N=291) and at follow-up (within 8 weeks) (N=281) post-intervention. Participants were non-randomly selected. |
|
| ||||
| Wouters et al., 2009 [39] | See above | |||
|
| ||||
| Zuyderduin et al., 2008 [40] | Botswana Location not reported |
Women living with HIV Gender:100% female Age: Mean: 31.16 years Range: 20-52 years |
The intervention consisted of training a group of HIV-positive women to serve as “buddies” to their peers. Buddies received information on HIV/AIDS knowledge, compliance with TB treatment, CD4 cell counts, ARV adherence, and support skills in order to provide other women living with HIV with support and guidance 4-6 times a week for the duration of 6 months. | Non-randomized trial – individual. Assessments took place at baseline (N=116) and 6 months (N=111) after the intervention's initial implementation. Assessments included buddies as well as buddy intervention and control participants. Participants were non-randomly selected. |
|
| ||||
| Partner notification | ||||
|
| ||||
| Brown et al., 2011 [31] | Malawi Lilongwe |
Persons with newly diagnosed HIV infection attending outpatient STI clinics Gender:41.7% male 58.3% female Age: Median: 28 years |
Participants were assigned to one of three groups: passive referral, contract referral, or provider referral. Index patients provided informed consent, were given referral cards to give to their partners, were counseled on the importance of safe sex behavior, and had blood drawn for CD4 counts. The passive referral group was expected to notify their partners on their own. The contact referral group was given 7 days to notify their partners, after which a health care provider contacted partners who had not reported for counseling and testing and counseled them to visit the clinic. Notification in the provider referral group occurred by providers within 48 hours. | Other study design: index clients were randomized to one of three partner notification strategies; assessment was conducted through rates of partner return for HTC. Assessments took place at baseline (N=240) for index patients and follow-up was conducted with named sexual partners (N=302) Participants were non-randomly selected. |
|
| ||||
| Henley et al., 2013 [33] | Cameroon Northwest and Southwest regions |
Persons testing positive for HIV Gender (index cases):72.9% female 27.1% male Age (index cases): Median: 31 years Range: 15-70 years |
The intervention was available to clients who tested HIV positive during HCT. After collecting information on the index partner's clients, health advisors asked cases how they wanted to notify each partner, offering them the options of provider, contract, or patient referral options. If cases chose provider referral, health advisors attempted to directly notify partners. If cases chose contract or patient referral, the health advisor counseled the case on how to notify their partner(s) and gave them a letter to give to each of their partners; the letter explained the importance of testing and included information on where to test and that the test would be free. For contract referral, the health advisor and the case established a period of time during which the case would attempt to notify their partner(s) themselves. If an identified partner did not come to a project site by the end of that period, the health advisor attempted to notify the partner directly. | Other study design: index clients self-selected one of three partner notification strategies; assessment was conducted through rates of partner return for HTC. Assessments took place at baseline (N=1462) for index patients and follow-up was conducted with named sexual partners (N=1607) Participants were non-randomly selected. |
Study rigor
Table 2 presents an assessment of study design and rigor for each included study. There were three randomized controlled trials (RCTs) [34-36] and five additional studies that included control groups but did not randomize participants to the intervention [32, 37, 39-41]. One study used a cross-sectional design to compare individuals who received the intervention to those who did not [38], while three studies used a before/after design just among intervention participants [29, 30, 42]. Two studies evaluating partner notification interventions were considered “other” study designs: one randomized index patients to different partner notification strategies [31] while the other let index patients choose their preferred strategy [33]; both were evaluated by assessing rates of partner return for HTC.
Table 2. Study design and rigor assessment.
| Study | Cohort | Control or comparison group | Pre/post intervention data | Random assignment of participants to the intervention | Random selection of participants for assessment | Follow-up rate of 80% or more | Comparison groups equivalent on socio-demographics | Comparison groups equivalent at baseline on disclosure |
|---|---|---|---|---|---|---|---|---|
| Cognitive-behavioral support groups | ||||||||
| Futterman et al., 2010 [33] | Yes | Yes | Yes | No | No | No | No | Yes1 |
| Jones et al., 2013 [34] | Yes | Yes | Yes | Yes | No | Yes | No | No1 |
| Kaaya et al., 2013 [35] | Yes | Yes | Yes | Yes | No | No | Yes | NR |
| Mundell et al., 2011 [37] | Yes | Yes | Yes | No | No | No | No | No |
| Sarnquist et al., 2014 [41] | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Snyder et al., 2014 [42] | Yes | No | Yes | NA | No | No | NA | NA |
| Wouters et al., 2009 [39] | Yes | Yes | No | No | Yes | Yes | NR | NR |
| Home-based or peer/community health workers | ||||||||
| MacNeil et al., 1999 [36] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Ncama, 2007 [38] | No | Yes | No | No | Partial2 | NA | No | NA |
| Rochat et al., 2013 [29] | Yes | No | Yes | NA | No | Yes | NA | NA |
| Rochat et al., 2014 [30] | Yes | No | Yes | NA | No | Yes | NA | NA |
| Wouters et al., 2009 [39] | See above | |||||||
| Zuyderduin et al., 2008 [40] | Yes | Yes | Yes | No | No | Yes | Yes | NR3 |
| Partner notification | ||||||||
| Brown et al., 2011 [31] | No | Yes | No | Yes | No | NA | NR | Yes4 |
| Henley et al., 2013 [33] | No | Yes | No | No | No | NA | NR | NA |
NR: Not reported; NA: Not applicable
Calculated from additional data provided by authors
Intervention group randomly selected, control group non-randomly selected
Not calculable based on data provided in the article
All participants were newly diagnosed, so presumably none had disclosed prior to the intervention.
Several studies had limitations that should be considered when interpreting findings. Four studies were described as pilot studies or had relatively small sample sizes [29, 40-42], although one later published an additional full evaluation of the intervention strategy. Three studies with pregnant women reported high attrition rates [32, 35, 37].
Interventions and disclosure outcomes
We identified three broad intervention approaches: (1) cognitive behavioral, multi-session support groups, (2) home-based or peer/community health worker outreach and support, and (3) partner notification interventions (Table 1). Table 3 presents a summary of the disclosure measures and outcomes for each study.
Table 3. Disclosure interventions, measures and outcomes.
| Study and year of publication |
Year of data collection |
Time since HIV diagnosis |
Intervention type | Disclosure recipient (outcome measure) |
Baseline disclosure rates |
Follow-up time | Follow-up disclosure rates |
Statistical significance (p- value) |
|---|---|---|---|---|---|---|---|---|
| Cognitive-behavioral support groups | ||||||||
| Futterman et al., 2010 [32] | 2006-07 | NR | 8-session cognitive-behavioral group intervention plus peer support (mother mentors) | Sexual partners | Intervention: 41/59 (69.5%), Control: 41/77 (53.2%) | 6 months | Intervention: 34/40 (87.2%) Control: 25/31 (83.3%) | NS (p=0.055) |
| Jones et al., 2013 [34] | 2010-12 | NR | 4-session cognitive-behavioral group intervention | Sexual partners (accurate disclosure to partner) | Intervention – Men: 86%Intervention – Women: 80%Control – Men: 72%Control – Women: 70% | 6-8 weeks (immediately post-intervention) | Intervention – Men: 83%Intervention – Women: 81%Control – Men: 80%Control – Women: 65% | NR |
| Kaaya et al., 2013 [35] | 2001-04 | NR | 6-session support group intervention | Anyone(disclosure to specific people measured but not reported) | NR | 8-10 weeks follow-up | Intervention: 56%Control: 46%(RR: 1.2, 95% CI: 0.91-1.6) | NS (p=0.19) |
| Mundell et al., 2011 [37] | 2005-06 | Mean: 14 weeks | 10-session support group intervention | Anyone | Intervention: 71%Control: 60% | 8 months | Intervention: 97%Control: 82% | NR |
| Sarnquist et al., 2014 [41] | 2011 | NR | 3-session group intervention plus provider training | Sexual partners | Intervention: 84.1%Control: 78.8% | 6-weeks post-partum3-months post-partum | Intervention: 98.2%Control: 86.2%Intervention: 98.4%Control: 87.5% | Sig (p=0.048)Sig (p=0.04) |
| Snyder et al., 2014 [42] | 2010 | <12 months | 3-session cognitive behavioral support group | Anyone/number of people (Mean number of people disclosed to) | Mean: 2 | 3 weeks | Mean:4Disclosed to a friend or family member during the course of the intervention: 6/65 | |
| Wouters et al., 2009 [39] | 2004-07 | Mean: 31 months (range: 6 months – 15 years) | Home-based CHW visits and support group intervention (support group results presented here) | Family members (parents, relatives living in household, relatives living elsewhere) | Parents: 77.1%Relatives in household: 74.6%Relatives living elsewhere: 58.3% | 18 months after ART initiation (exact time after disclosure interventions unspecified) | Support group at time 0, disclosure from time 0 – time 1: Path coefficient: 0.103, t-value: 1.016Support group at time 1, disclosure from time 1 – time 2: Path coefficient: 0.191, t-value: 3.665 | Sig (p<0.05)Sig (p<0.001) |
| Home-based or peer/community health workers | ||||||||
| MacNeil et al., 1999 [36] | 1996-97 | Up to 4 weeks | Individual counseling and family education | Friend, parents, spouse, sexual partner, and anyone | Disclosed to friendI: 3 (3.8%) C: 6 (7.8%) Total: 9 (5.8%)Disclosed to parentsI: 15 (19.5%) C: 8 (10.4%) Total: 23 (14.9%)Disclosed to spouseI: 10 (13.0%) C: 9 (11.7%) Total: 19 (12.3%)Disclosed to sexual partnerI: 3 (3.9%) C: 6 (7.8%) Total: 9 (5.8%)Disclosed to someoneI: 16 (20.8%) C: 13 (16.9%) Total: 29 (18.8%) | 6 months | Disclosed to friendI: 11 (14.3%) C: 7 (9.1%) Total: 18 (11.7%)Disclosed to parentsI: 38 (49.4%) C: 23 (29.9%) Total: 61 (39.6%)Disclosed to spouseI: 20 (26.0%) C: 18 (23.4%) Total: 38 (24.7%)Disclosed to sexual partnerI: 12 (15.6%) C: 17 (22.1%) Total: 29 (18.8%)Disclosed to someoneI: 72 (93.5%) C: 58 (75.3%) Total: 130 (84.4%) | NSSig (p<0.05)NSNSSig (p<0.05) |
| Ncama, 2007 [38] | NR | NR | Home-based CHW visits | Anyone/number of people (more than 10 people) | Disclosed to anyone: 99% | NA (cross-sectional study design) | Disclosed to more than 10 people:Intervention: 555Control: 30%(r=26.442, d.f.=11) | Sig (p=0.006) |
| Rochat et al., 2013 [29] | 2010 | ∼6-8 years | 6-session family structured intervention by counselor | School-aged children (index child) | 0% | 8 weeks | No disclosure: 0/24Full disclosure: 11/24Partial disclosure: 13/24 | NR |
| Rochat et al., 2014 [30] | 2010-12 | 5-10 years | 6-session family structured intervention by counselor | School-aged children (index child) | No disclosure: 255/281Full disclosure: 19/281Partial disclosure: 7/281 | Within 8 weeks | No disclosure: 0/281Full disclosure: 171/281Partial disclosure: 110/281 | NR |
| Wouters et al., 2009 [39] | 2004-07 | Mean: 31 months (range: 6 months – 15 years) | Home-based CHW visits and support group intervention (CHW results presented here) | Family members | Disclosed to:Parents: 77.1%Relatives in household: 74.6%Relatives living elsewhere: 58.3% | 18 months after ART initiation (exact time after disclosure interventions unspecified) | CHW at time 0, disclosure from time 0 – time 1: Path coefficient: 0.086, t-value: 1.663CHW at time 1, disclosure from time 1 – time 2: Path coefficient: 0.120, t-value: 2.332 | NSSig (p<0.05) |
| Zuyderduin et al., 2008 [40] | 2002 | NR | Peer support (buddies) | Anyone/number of people (categorical: number of people disclosed to) | Disclosed to:1-5 people:I: 53.8% ;C: 68.4%6-10 people:I: 15.4%; C: 10.5%11-30 people:I: 21.3%; C: 5.3%31 or more:I:7.7%; C: 15.8% | 6 months | Moved to a higher category (disclosed to more people):Intervention: 48.7%Control: 39.5% | NR |
| Partner notification | ||||||||
| Brown et al., 2011 [31] | 2008-09 | 0 days | Anonymous partner notification | Sexual partners | 0%2 | 30 days | Partner returned for HTC:Passive referral: 20/82 (24%)Contract referral: 45/88 (51%)Provider referral: 42/82 (51%) | Sig (p<0.001for both contract referral vs. passive and provider referral vs. passive) |
| Henley et al., 2013 [33] | 2009-10 | 0 days | Anonymous partner notification | Sexual partners | NR | NR (variable, but generally within several days) | Partner returned for HTC: 1 Patient referral: 146/316 (46%)Contract referral: 139/228 (61%)Provider referral: 570/956 (60%) | Sig (p=0.049 for contract referral vs. patient and p=0.007 for provider referral vs. patient) |
Sig: Significant; NR: Not reported; NA: Not applicable; I: Intervention; C: Control
Additional data provided by authors
All participants were newly diagnosed, so presumably none had disclosed prior to the intervention
Cognitive-behavioral support groups
Seven studies examined support groups, often based on a multi-session, cognitive-behavioral model. Five of these studies targeted pregnant women.
Four studies provided multi-session support groups to pregnant women living with HIV [32, 35, 37, 41]. Of these, the Mamekhaya intervention additionally provided “mother mentors” – HIV positive mothers trained as positive role models [32], while the PURSE study was part of a larger effort to integrate family planning into prevention of mother-to-child transmission (PTMCT) services [41]. Although all four studies included control groups, they shared some other limitations: three had significant attrition, only one randomized women to the intervention, and only one reported that intervention and control groups had comparable rates of disclosure at baseline (Table 2). Two of the four studies found that the intervention significantly increased disclosure: a study conducted in South Africa found that, compared to control participants, intervention participants had 8.3 times the odds of disclosure to anyone (95% CI: 2.1-32.9)[37], while the family planning/PMTCT integration study found that 98.4% of women intervention participants had disclosed to their partners by 3 months post-partum compared to 87.5% of control group women (p=0.04) [41]. The other two studies found no statistically significant effect on disclosure [32, 35].
A fifth study enrolled pregnant women and their partners in a 4-session psychosocial support group intervention in South Africa [34]. Both HIV-positive and HIV-negative women and their partners were enrolled if they had previously tested for HIV, although testing did not necessarily occur during the current pregnancy. HIV sero status disclosure to partners did not increase over time for men or women within the experimental or control groups; unpublished data (provided by the authors) showed that when analyses were limited to only HIV-infected participants, there was similarly no change in disclosure over time within either the experimental or control groups.
The final two support group interventions did not focus on pregnant women. The first examined the impact of support groups for people initiating ART in public-sector clinics in South Africa and measured disclosure to specific family members (parents, relatives living in the household, and relatives living elsewhere) [39]. Using regression models that controlled for other factors, PLHIV who participated in support groups were found to disclose their status to more family members at each time point. While the impact of the intervention was not different by gender, women were more likely to disclose their status than men overall. The second study, also from South Africa, was the Hlanganani study, which developed cognitive-behavioral support groups for HIV-infected youth [42]. This pilot study found that 6 of 65 participants disclosed to a friend or family member during the course of the intervention, and the mean number of people disclosed to increased from 2 to 4.
Home-based or peer/community health workers
The second common intervention approach was using peers or community health workers (CHWs) to provide individual support – including support related to disclosure – and other services to PLHIV, often through home-based services. Five interventions used these more individually-focused models.
The South African study which examined public-sector support groups also examined the impact of CHWs providing counseling, support, and home-based care [39], finding a significant impact of CHWs on disclosure to family members in the second study time period, but not the first. In Botswana, a small non-randomized trial examined the impact of a buddy system on various self-care behaviors of women living with HIV [40]. Women who chose to have a buddy were more likely to disclose to more people than those who did not (Intervention: 48.7%; Control: 39.5%). Another South African cross-sectional study examined an integrated community/home-based care program [38]. Essentially all participants had disclosed to someone, but the study authors reported that intervention participants were more likely to have disclosed to more than 10 people than control participants.
One intervention provided individual counseling and other support services to sexually active men and women newly diagnosed with HIV in Tanzania [36]. This RCT conducted in the 1990s was the earliest study included in the review; it was also one of the few to have intervention and control groups that were comparable at baseline on levels of disclosure. Disclosure rates at baseline were very low and increased dramatically over the course of the study in both the intervention and control groups, though slightly more in the intervention group. There were no major differences by gender.
Finally, one intervention focused on maternal disclosure of HIV-positive status to children. The Amagugu intervention, a six-session, family-centered intervention to assist women in disclosing their HIV status to their HIV-uninfected school-aged children, was evaluated through a pilot before/after study [29] and full before/after evaluation [30]. The pilot study with 24 women found that 11 disclosed fully using the word “HIV” [29], while the full evaluation with 281 women found that 171 disclosed fully [30]; in both studies, the remaining women did not tell their children they had HIV, but did initiate conversations with their children using words like “virus”.
Partner notification
While most interventions focused on voluntary disclosure through the interventions described above, two studies evaluated HIV partner notification interventions [31, 33]. In Malawi, consenting individuals from STI clinics who were newly diagnosed with HIV were randomized to 1 of 3 methods of partner notification: “passive referral” (participants disclosed to partners themselves), “contract referral” (participants had a set period of time to notify their partners, after which a health care provider contacted partners who had not reported for HTC), or “provider referral” (a health care provider notified partners directly) [31]. Both contract and provider referral were conducted anonymously, without revealing the name of the index client. Partners in the contract (51%) and provider (51%) referral arms were more likely to return for HTC than those in the passive referral arm (24%; p<0.001). There were two social harms reported: one partner abandonment and a police call when a provider came to a house. The second study, conducted in Cameroon, did not randomize participants, but otherwise evaluated similar options for people newly diagnosed with HIV [33]. Of those who agreed to participate in some form of partner services, over half (59.5%) chose provider referral, and additional data provided by the authors showed that 60% (570/956) of partners in the provider referral arm and 61% (139/228) in the contract referral arm returned for HIV testing compared with 46% (146/316) in the patient (passive) referral arm. Data were not collected for those who declined participation in any partner services. While women were significantly more likely than men to report that they were afraid of being hit or hurt if their partner found out about their HIV status (7.4% vs. 3.4%, p<0.001), no incidents of domestic violence resulting from partner services were reported. Neither partner notification intervention reported major differences in partner return rates by gender.
Discussion
We conducted the first systematic review of the effectiveness of interventions to increase HIV serostatus disclosure in low- and middle-income countries and found a limited evidence base: just thirteen interventions, all from sub-Saharan Africa, met the inclusion criteria. Most interventions focused on PLHIV and used cognitive-behavioral group sessions or peer/CHW support to encourage disclosure to sexual partners, family members, or friends. However, several were pilot studies or had significant limitations in study design or retention, and follow-up times were highly variable. Results on disclosure were mixed. When statistically significant effects were found, effect sizes were generally small, and several studies showed no intervention effects. There was no clear evidence suggesting that the effectiveness of interventions to increase disclosure varied by target population or intervention approach. One randomized study examining the effect of anonymous, voluntary partner notification found that it doubled the number of partners coming in for testing compared to self-disclosure; the strong study design likely provides the best evidence for an effective approach to increasing disclosure, with a similar intervention evaluated through a non-randomized design also showing promising, though smaller, absolute differences across groups.
Although our review covered the period from 1990-2014, almost all of the included articles were published since 2007. This suggests an increasing appreciation of the importance of disclosure and interventions addressing it over time, and articles in this supplement are already adding further to this evidence base. In addition, disclosure rates generally seemed to be higher in more recently conducted studies included in the review, perhaps suggesting more general trends in changing HIV-related norms, reduced stigma, and increased treatment access that support increased disclosure even in the absence of specific interventions.
The different interventions covered in this review targeted different groups, including pregnant women, adolescents, PLHIV generally, and newly diagnosed PLHIV. Each of these groups likely has different motivations for disclosure, communication skills, and perception of the potential benefits and harms of disclosure. Further, different interventions focused on disclosure to different individuals, from partners to children to family members to anyone in the wider community. Several interventions reported adapting their content to these different contexts and pilot testing with further refinement [30, 42]; these are likely to be critical considerations for the development of future disclosure interventions.
All but one of the interventions either focused only on women or had a majority of female participants. This likely reflects higher rates of HIV testing among women across sub-Saharan Africa as well as the focus on pregnancy as an opportunity for HTC and follow-up services. However, most studies that examined results by gender did not report significant differences in intervention effects between men and women, though a few did find that women were generally more likely to disclose than men, and one study reported some indications that participants found it easier to disclose to women than to men [38]. These findings are supported by the broader literature which does not suggest a clear pattern in disclosure rates by gender [6]; however, there is some indication that women (particularly mothers and sisters) are more likely to be recipients of disclosure, while studies have also documented clear gender differences in the contexts of, barriers to, and outcomes of disclosure [6].
Conclusions from this review must be considered in light of limitations of the existing evidence base. First, all studies that met the inclusion criteria were from just five countries in sub-Saharan Africa, so results cannot be generalized to other settings. Second, all but one study focused on disclosure by people living with HIV, so we know little about increasing serostatus disclosure among those who test HIV-negative. To date, disclosure by people who are HIV-negative has likely been of less interest to public health and program planners, but increased attention to pre-exposure prophylaxis (PrEP) and serosorting may change that in the future. Third, there were only a few studies for any particular population (such as pregnant women, adult PLHIV, adolescents, or parents disclosing to their children), and only a few studies for any particular intervention approach, limiting the evidence base for each approach and population group. Fourth, many of the included studies had small sample sizes, weak study designs, or significant limitations. Further, variations in study populations, settings, specific intervention approaches, and ways of measuring disclosure as an outcome meant that we were unable to combine the results from different studies in meta-analysis, which could have potentially reduced concerns about small sample size.
Our review focused on a wide range of interventions all with a similar goal: to increase rates of HIV serostatus disclosure. Obermeyer and colleagues [6] have argued that structural changes, such as increasing availability of services, could facilitate disclosure as much as individual approaches and counseling. We did not include articles that examined the effect of structural changes on disclosure if they did not have the specific goal of increasing disclosure. The articles included in this review thus focus more on strategies that engage individuals, which are generally more under the control of researchers and evaluators, rather than structural changes. While we wanted to focus on interventions that program planners could consider for their own settings, we recognize that our definition of disclosure interventions shapes the evidence base we are presenting in our review.
In sum, the existing evidence base for interventions to increase disclosure in low- and middle-income countries is limited and shows variable results. Further research is needed to determine whether current approaches to increasing disclosure are effective or whether new approaches should be considered.
Acknowledgments
C.K., V.F., K.O., and M.S. developed the initial study design and protocol. V.F. oversaw the search and data abstraction process. C.K. analyzed the findings and wrote the first draft of the manuscript with feedback on statistical issues from K.A. All authors collaboratively discussed key decisions through the course of the review, provided critical feedback on preliminary drafts and interpretation of results, and approved the final manuscript.
We wish to thank Erica D'Aquila, Hayley Droppert, Eric Filemyr, Elise Grover, Salwan Hager, and AnnisaHarshafor their screening and coding work on this review. We also thank all the study authors who responded to requests for additional information and clarification about their research. This research was supported by the US National Institute of Mental Health, grant number 1R01MH090173.
References
- 1.Smith R, Rossetto K, Peterson BL. A meta-analysis of disclosure of one's HIV-positive status, stigma and social support. AIDS Care. 2008;20:1266–1275. doi: 10.1080/09540120801926977. [DOI] [PubMed] [Google Scholar]
- 2.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16:18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simoni JM, Pantalone DW. Secrets and safety in the age of AIDS: does HIV disclosure lead to safer sex? Top HIV Med. 2004;12:109–118. [PubMed] [Google Scholar]
- 4.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bulletin of the World Health Organization. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 5.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obermeyer CM, Baijal P, Pegurri E. Facilitating HIV disclosure across diverse settings: a review. American Journal of Public Health. 2011;101:1011–1023. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev. 2013;10:CD002843. doi: 10.1002/14651858.CD002843.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krauss BJ, Letteney S, De Baets AJ, Baggaley R, Okero FA. Caregiver's HIV disclosure to children 12 years and under: a review and analysis of the evidence. AIDS Care. 2013;25:415–429. doi: 10.1080/09540121.2012.712664. [DOI] [PubMed] [Google Scholar]
- 9.Murphy DA. HIV-positive mothers' disclosure of their serostatus to their young children: a review. Clin Child Psychol Psychiatry. 2008;13:105–122. doi: 10.1177/1359104507087464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao S, Li X, Stanton B. Disclosure of parental HIV infection to children: a systematic review of global literature. AIDS Behav. 2013;17:369–389. doi: 10.1007/s10461-011-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan KM. Male self-disclosure of HIV-positive serostatus to sex partners: a review of the literature. J Assoc Nurses AIDS Care. 2005;16:33–47. doi: 10.1016/j.jana.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Thoth CA, Tucker C, Leahy M, Stewart SM. Self-disclosure of serostatus by youth who are HIV-positive: a review. J Behav Med. 2014;37:276–288. doi: 10.1007/s10865-012-9485-2. [DOI] [PubMed] [Google Scholar]
- 13.Qiao S, Li X, Stanton B. Theoretical models of parental HIV disclosure: a critical review. AIDS Care. 2013;25:326–336. doi: 10.1080/09540121.2012.712658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bott S, Obermeyer CM. The social and gender context of HIV disclosure in sub-Saharan Africa: a review of policies and practices. SAHARA J. 2013;10(Suppl 1):S5–16. doi: 10.1080/02664763.2012.755319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinzon-Iregui MC, Beck-Sague CM, Malow RM. Disclosure of their HIV status to infected children: a review of the literature. J Trop Pediatr. 2013;59:84–89. doi: 10.1093/tropej/fms052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vreeman RC, Gramelspacher AM, Gisore PO, Scanlon ML, Nyandiko WM. Disclosure of HIV status to children in resource-limited settings: a systematic review. J Int AIDS Soc. 2013;16:18466. doi: 10.7448/IAS.16.1.18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.World Bank Country and lending groups. [Accessed on March 21, 2012]; from: http://data.worldbank.org/about/country-classifications/country-and-lending-groups.
- 19.Denison JA, O'Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990--2005. AIDS Behav. 2008;12:363–373. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 20.Fonner VA, Denison J, Kennedy CE, O'Reilly K, Sweat M. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev. 2012;9:CD001224. doi: 10.1002/14651858.CD001224.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy CE, Fonner VA, Sweat MD, Okero FA, Baggaley R, O'Reilly KR. Provider-initiated HIV testing and counseling in low- and middle-income countries: a systematic review. AIDS Behav. 2013;17:1571–1590. doi: 10.1007/s10461-012-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand JT, O'Reilly K, Denison J, Anhang R, Sweat M. Systematic review of the effectiveness of mass communication programs to change HIV/AIDS-related behaviors in developing countries. Health Educ Res. 2006;21:567–597. doi: 10.1093/her/cyl036. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy C, O'Reilly K, Medley A, Sweat M. The impact of HIV treatment on risk behaviour in developing countries: a systematic review. AIDS Care. 2007;19:707–720. doi: 10.1080/09540120701203261. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy CE, Medley AM, Sweat MD, O'Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bull World Health Organ. 2010;88:615–623. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweat M, O'Reilly K, Kennedy C, Medley A. Psychosocial support for HIV-infected populations in developing countries: a key yet understudied component of positive prevention. AIDS. 2007;21:1070–1071. doi: 10.1097/QAD.0b013e3280f774da. [DOI] [PubMed] [Google Scholar]
- 26.Sweat MD, Denison J, Kennedy CE, Fonner V, O'Reilly KR. Effects of condom social marketing on condom use in developing countries: A systematic review and meta-analysis: 1990-2010. Bull World Health Organ. 2012;90:613–622A. doi: 10.2471/BLT.11.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy CE, Fonner VA, Sweat MD, Okero FA, Baggaley R, O'Reilly KR. Provider-initiated HIV testing and counseling in low- and middle-income countries: a systematic review. AIDS and Behavior. 2013;17:1571–1590. doi: 10.1007/s10461-012-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonner VA, Armstrong KS, Kennedy CE, O'Reilly KR, Sweat MD. School based sex education and HIV prevention in low- and middle-income countries: a systematic review and meta-analysis. PLoS One. 2014;9:e89692. doi: 10.1371/journal.pone.0089692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochat TJ, Mkwanazi N, Bland R. Maternal HIV disclosure to HIV-uninfected children in rural South Africa: a pilot study of a family-based intervention. BMC Public Health. 2013;13:147. doi: 10.1186/1471-2458-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochat TJ, Arteche AX, Stein A, Mkwanazi N, Bland RM. Maternal HIV disclosure to young HIV-uninfected children: an evaluation of a family-centred intervention in South Africa. AIDS. 2014;28(Suppl 3):S331–341. doi: 10.1097/QAD.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 31.Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: Opportunities for HIV treatment and prevention. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;56:437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Futterman D, Shea J, Besser M, Stafford S, Desmond K, Comulada WS, et al. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22:1093–1100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis. 2013;40:909–914. doi: 10.1097/OLQ.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones DL, Peltzer K, Villar-Loubet O, Shikwane E, Cook R, Vamos S, et al. Reducing the risk of HIV infection during pregnancy among South African women: A randomized controlled trial. AIDS Care. 2013;25:702–709. doi: 10.1080/09540121.2013.772280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaaya SF, Blander J, Antelman G, Cyprian F, Emmons KM, Matsumoto K, et al. Randomized controlled trial evaluating the effect of an interactive group counseling intervention for HIV-positive women on prenatal depression and disclosure of HIV status. AIDS Care. 2013;25:854–856. doi: 10.1080/09540121.2013.763891. [DOI] [PubMed] [Google Scholar]
- 36.MacNeil JM, Mberesero F, Kilonzo G. Is care and support associated with preventive behaviour among people with HIV? AIDS Care. 1999;11:537–546. doi: 10.1080/09540129947695. [DOI] [PubMed] [Google Scholar]
- 37.Mundell JP, Visser MJ, Makin JD, Kershaw TS, Forsyth BW, Jeffery B, et al. The impact of structured support groups for pregnant South African women recently diagnosed HIV positive. Women Health. 2011;51:546–565. doi: 10.1080/03630242.2011.606356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ncama BP. Acceptance and disclosure of HIV status through an integrated community/home-based care program in South Africa. International Nursing Review. 2007;54:391–397. doi: 10.1111/j.1466-7657.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 39.Wouters E, van Loon F, van Rensburg D, Meulemans H. Community support and disclosure of HIV serostatus to family members by public-sector antiretroviral treatment patients in the Free State Province of South Africa. AIDS Patient Care STDS. 2009;23:357–364. doi: 10.1089/apc.2008.0201. [DOI] [PubMed] [Google Scholar]
- 40.Zuyderduin JR, Ehlers VJ, Dm The impact of a buddy system on the self-care behaviours of women living with HIV/AIDS in Botswana. Health SA Gesondheid. 2008;13:4–15. [Google Scholar]
- 41.Sarnquist CC, Moyo P, Stranix-Chibanda L, Chipato T, Kang JL, Maldonado YA. Integrating family planning and prevention of mother to child HIV transmission in Zimbabwe. Contraception. 2014;89:209–214. doi: 10.1016/j.contraception.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder K, Wallace M, Duby Z, Aquino LDH, Stafford S, Hosek S, et al. Preliminary results from Hlanganani (Coming Together): A structured support group for HIV-infected adolescents piloted in Cape Town, South Africa. Children and Youth Services Review. 2014;45:114–121. [Google Scholar]


