Abstract
Activation-induced deaminase (AID) is essential for class switch recombination and somatic hypermutation, and it has the ability to deaminate single-stranded DNA at cytidines. Mammalian class switch regions form R-loops upon transcription in the physiological orientation. The displaced DNA strand of an R-loop is forced to wrap around the RNA-DNA hybrid; hence, it may not have complete exposure to proteins. A fundamental question concerns the extent to which AID is accessible to the displaced strand of a transcription-generated R-loop. We used a minimal R-loop to carry out high-resolution analysis of the precise locations of AID action. We found that AID deaminates on the displaced DNA strand across the entire length of the R-loop. Displaced strand locations with a WRC (where W is A or T and R is A or G) sequence are preferred targets, but there are clear exceptions. These WRC deviations may be due to steric constraints on the accessibility of AID to these sites as the displaced strand twists around the RNA-DNA duplex. This phenomenon may explain the lack of WRC site preference at the mutations surrounding class switch recombination junctions.
Class switch recombination (CSR) is a DNA recombinational event that occurs in germinal-center B cells (6, 14, 39). By undergoing class switch recombination, mature B cells can switch their immunoglobulin heavy-chain constant regions from μ (which encodes the IgM subtype) to other heavy-chain isotypes (γ for IgG, α for IgA, and ε for IgE, respectively). Class switch recombination is mediated by large (2- to 12-kb) repetitive regions, called switch regions, upstream of each coding constant-region exon (14). Recombination is thought to occur through a DNA double-strand break (DSB) intermediate (25). CSR generates DSBs that can be detected by ligation-mediated PCR (4, 30, 36); moreover, the deleted intervening DNA between the two breaks forms a circular molecule (17, 18, 22, 35), which is also detectable in class-switching B cells. The joining of the two chromosomal ends of the DSB is thought to be mediated by the nonhomologous DNA end-joining pathway (3, 6, 20, 21), although dependence on some components is not certain (1).
A key step in committing a certain switch region to recombination is transcription through that switch region from an upstream promoter (called an I exon promoter) (39). These transcripts are called germ line transcripts [to be distinguished from the transcript containing the V(D)J exon] or sterile transcripts, because they do not encode any protein (14, 39). The germ line transcripts are highly G rich, due to an asymmetry between a G-rich nontemplate and a C-rich template DNA strand of the switch region. The two switch regions involved in CSR do not share any extensive homology, and switch junctions do not reveal any consensus sequence motif. This type of DNA recombination event can be best described as regionally specific recombination (19, 39).
When switch regions are transcribed in vitro, the RNA transcripts stay bound to the DNA template, forming an RNA-DNA hybrid (9, 29, 34, 37). Biochemical probing has provided evidence that the RNA-DNA hybrid forms in vivo in switch regions and is most consistent with an R-loop structure (37). R-loop formation depends on transcription through switch regions in the correct orientation. Recent mouse models with inverted Sγ1 switch regions show markedly reduced CSR efficiency (32). In addition, an artificial DNA fragment can partially replace Sγ1 in CSR but only in the orientation capable of R-loop formation and not in the other orientation (32). All of these features are consistent with R-loop formation at the switch regions upon transcription.
The only lymphoid-specific factor required for CSR was identified by a subtractive hybridization from a B-cell line stimulated to undergo switching (23, 24). The identified gene product was termed activation-induced cytidine deaminase (AID) because it bears sequence homology to an RNA-editing enzyme, APOBEC1, which deaminates a cytidine residue in the ApoB100 RNA. How AID functions in CSR is currently under debate. One model proposes that AID, like APOBEC1, edits an mRNA which then encodes the active recombinase (15). The other model proposes that AID directly deaminates cytidine in the DNA (11, 26) and the resulting uracil is then processed by the base excision repair components, including uracil DNA glycosylase (UDG) or uracil nucleotide glycosylase (UNG) and the apurinic-apyrimidinic (AP) endonuclease (APE), to generate a nick on the DNA strand. UNG null mice (28) and human patients bearing UNG mutations (16) are impaired for CSR, supporting the DNA deaminase model. A number of biochemical studies have shown that purified recombinant AID protein is capable of deamination of cytidine residues on single-stranded DNA in vitro (2, 8, 10, 33, 38). The elucidation of the R-loop structure in vitro and in vivo immediately implied a possible mechanism for generating DNA breaks in the switch region. The displaced template in an R-loop is single stranded, and that template might be the target for AID (6, 13, 39).
Though it is clear that the displaced strand in an R-loop is accessible to the bisulfite anion, it remains to be tested whether the same single strand is accessible to agents of protein size, specifically the 24-kDa AID, which, even if a globular monomer, would have a theoretical radius of approximately 25 Å. The displaced strand must still wrap around the RNA-DNA duplex, so it is not entirely like a segment of free single-stranded DNA (Fig. 1). The distance between the displaced strand and the RNA-DNA duplex (which itself is likely to be A form) can vary from less than 4 to more than 21 Å. Hence, AID may experience steric hindrance in acting on cytidines within this displaced strand.
FIG. 1.
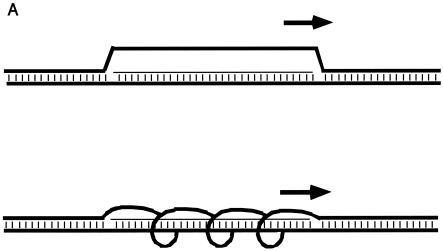
Two- and three-dimensional representations of R-loops. (A) Two-dimensional diagrams of R-loops. The thick horizontal lines represent each of the two DNA strands. The thin horizontal line represents the RNA transcript. Vertical dashes indicate base pairing. The short thick arrow above the R-loop indicates the position and direction of the RNA polymerase that is generating the RNA. The upper diagram shows the displaced strand with maximal displacement from the RNA-DNA duplex but with zero twist relative to the RNA-DNA duplex. The lower diagram illustrates the displaced DNA strand with shorter distance of displacement from the RNA-DNA duplex and with some twist relative to the RNA-DNA duplex. (B) Three-dimensional model of an R-loop. One switch repeat is shown configured as an R-loop. On the left, the structure is viewed from one end, with the RNA-DNA duplex (A-form) in the center and the displaced nontemplate strand (green) on the perimeter. On the right, a longitudinal view is shown. The template strand loses two helical turns (720°) as it unwinds and extends away from the RNA-DNA duplex, with a maximum displacement of 21 Å from the duplex. For comparison, a globular 24-kDa protein (the size of an AID monomer) would have a radius of approximately 50 Å. Green, nontemplate strand (displaced R-loop); red, template strand; blue, RNA; yellow, cytosine bases in the R-loop.
In the context of an important study of AID function in concert with replication protein A, low-resolution agarose gel data have been reported suggesting that AID can act on displaced G-rich strands when transcription is directed off the edge of a linearized template (7, 8). This particular aspect of that study was complicated by the possibility that the transcription might generate a Y-structure rather than an R-loop as the RNA polymerase runs off the template at the downstream end of the switch region.
Here we explore R-loop-AID interactions at sequencing gel resolution. This resolution can be achieved only by limiting the region of R-loop formation to a much shorter length than that of a full switch region. We find that AID does have access to the displaced strand of the R-loop and a much more limited access to the template DNA strand. However, the preferred action of AID at WRC (where W is A or T and R is A or G) sites on single-stranded DNA is altered on the displaced strand of the R-loop, presumably reflecting the steric constraints to AID access.
MATERIALS AND METHODS
Chemicals and purified recombinant protein.
T7 and T3 RNA polymerases, ribonucleotides (rATP, rUTP, rGTP, rCTP), and RNase H were purchased from Promega (Madison, Wis.). Thermostable DNA polymerase Vent exo− was purchased from New England BioLabs (Beverly, Mass.). Uracil glycosylase was purchased from Invitrogen (Carlsbad, Calif.). The UNG1-deficient bacterial strain NR8052 was kindly provided by Eric Radany. Recombinant mouse AID was purified from baculovirus-infected insect cells as described previously (38) with a minor modification. RNase A (100 μg/ml) was added to the insect cell lysate while it was being mixed with the glutathione agarose (overnight at 4°C). This step relieved the dependence of AID activity on RNase A during the deamination reaction.
In vitro transcription.
One microgram of supercoiled plasmid prepared from a CsCl gradient was transcribed in vitro in a 20-μl reaction mixture with either T7 or T3 RNA polymerase according to a Promega protocol. The reaction mixture was incubated at 37°C for 1 h, and the RNA polymerase was heat inactivated by incubation at 70°C for 20 min. Free RNA was removed by adding 1 μg of RNase A and incubating the mixture for 30 min at 37°C. Nucleic acid was then purified by phenol and chloroform extraction followed by ethanol precipitation.
Detection of AID-mediated cytidine deamination on an R-loop.
R-loop DNA (T7-transcribed plasmid) was incubated with purified recombinant mouse AID and bacterial UNG (Invitrogen) for 1 h at 37°C in a buffer containing 25 mM Tris-HCl (pH 8.0), 50 mM NaCl, and 5 mM EDTA. Typically, 50 ng of purified recombinant AID protein (0.8 pmol) was used to treat 100 ng of R-loop plasmid (0.15 pmol, corresponding to approximately 4 pmol of potential C residues on the displaced G-rich strand of the R-loop) in a 10-μl total reaction volume. Nucleic acid was purified by phenol and chloroform extraction followed by ethanol precipitation. Purified nucleic acid was resuspended in Tris-EDTA buffer (pH 8.0). In the primer extension reaction, 10 ng of purified nucleic acid from the AID-treated sample was added to a 10-μl reaction mixture containing a 1 μM concentration of 32P-labeled T3 primer, a 200 μM concentration of each deoxynucleoside triphosphate, 0.5 U of Vent exo−, and 1× Thermopol buffer supplied by New England BioLabs. Primer extension was done by incubation of the mixture for 2 min at 95°C, 5 min at 48°C, and 15 min at 72°C with a Robocycler 96 (Stratagene, La Jolla, Calif.). The primer extension mixture was then resolved on a 6% denaturing polyacrylamide gel containing 1× Tris-borate-EDTA and 7 M urea. Primer extension products were visualized by exposing the dried gel to a phosphorimager screen and scanned with a molecular imager FX (Bio-Rad, Hercules, Calif.). Gel quantitation was done with Quantity One v4.2.3 (Bio-Rad).
Three-dimensional model of an R-loop.
R-loop structures were built with an in-house nucleic acid-building algorithm, NASDAC (5). Initial base positions were generated through a series of rotational and translational input parameters. A-DNA helical parameters were used for duplex generation. The “unwound” structures were built by allowing the terminal bases of the coding strand to remain as a part of the hybrid while unwinding the remainder of the strand. The nontemplate strand gradually loses two helical turns (720° of twist over 39 bases), which allows it to unwind and extend away from the A-form duplex. A maximum displacement of 21 Å from the duplex axis was achieved; further displacement causes too great a distance between glycosidic N atoms of successive bases in the displaced strand. NASDAC-generated structures were energy minimized in the AMBER force field. Na+ cations were added for electroneutrality. Energy minimization of the structures was carried out in two steps. Initially, all the base atoms were constrained to their initial positions, allowing only the backbone atoms to relax. This constraint was followed by relaxation of all atoms. Three thousand cycles of minimization were performed in each step: 500 cycles of steepest-descent minimization followed by 2,500 cycles of conjugate gradient minimization. Conditions included a distance-dependent dielectric constant and a nonbonded cutoff of 12 Å. Structures were visualized with the Weblab viewer visualization program.
RESULTS
Length of mouse Sγ3 sequence required for generation of a stable R-loop on a plasmid.
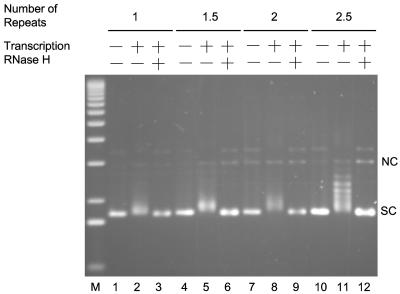
In order to access AID sites of deamination upon sequencing gel resolution, it was necessary to use short lengths of switch sequences that were still sufficient to efficiently form R-loops. We constructed a series of plasmids that contained 1, 1.5, 2, and 2.5 repeats of mouse Sγ3 (Fig. 2). When transcribed in a physiological orientation by T7 phage RNA polymerase, only plasmids containing 2.5 repeats (pTW-EL54) showed a significant electrophoretic mobility shift (Fig. 2, lane 11), whereas the smaller switch regions were equivalent to plasmids lacking switch region DNA (Fig. 2, lanes 2, 5, and 8). The resulting shift was sensitive to RNase H treatment (Fig. 2, lane 12), indicating the presence of an RNA-DNA hybrid. Bisulfite sequencing of these transcribed 2.5-repeat molecules confirmed that the top strand was single stranded and the bottom strand was base paired, consistent with the R-loop structure that we have demonstrated previously (data not shown) (37). Hence, 2.5 repeats appeared to be sufficient for stable R-loop formation.
FIG. 2.
R-loop formation on short segments of Sγ3 switch region DNA. Plasmids harboring 1, 1.5, 2, and 2.5 Sγ3 repeats were transcribed in vitro with T7 RNA polymerase. The transcription products were resolved on an agarose gel stained with ethidium bromide after electrophoresis. The components of each reaction are indicated at the top of the gel. NC, nicked circular; SC, supercoiled.
Extension of R-loops upstream of the switch region.
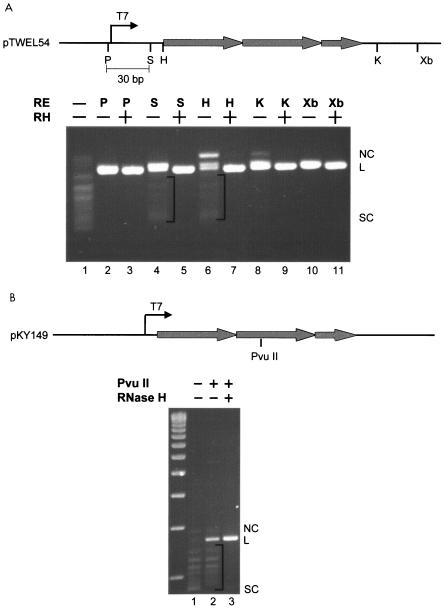
The in vitro R-loop formed on 2.5 Sγ3 repeats was digested with restriction enzymes to determine whether the structure extends outside of the switch region (Fig. 3A). We found that unique enzyme sites (SacI and HindIII) located upstream of the switch sequence but downstream of the T7 promoter could not be digested to completion (Fig. 3A, lanes 4 and 6) unless RNase H was added to the digestion mixture (Fig. 3A, lanes 5 and 7). This finding indicated that a fraction of the R-loop extends upstream of the switch region. However, unique sites upstream of the T7 promoter (PstI) could be digested to completion even in the absence of RNase H, indicating that the R-loop does not extend beyond the promoter. When we digested the R-loop with enzymes (KpnI and XbaI) that have unique sites downstream of the switch region, the digestion always went to completion, even in the absence of RNase H. This finding suggests that R-loop formation terminates promptly by the time that the RNA polymerase reaches the end of the 2.5-repeat switch region.
FIG. 3.
Extent of R-loop formation on a 2.5-Sγ3 repeat segment. (A) The top panel is a diagram representing a part of pTW-EL54. Shaded thick arrows represent Sγ3 repeats. The lower panel shows the digestion products of T7 RNA polymerase-transcribed pTW-EL54 (R-loop). Irrelevant lanes between lanes 7 and 8 have been removed. The location of the R-loop was probed by testing for the cleavability of restriction sites upstream and downstream of the switch region. Bands below the linear position represent uncut plasmid due to the R-loop formation. RNase H removes the RNA of the R-loop, thereby permitting the two DNA strands to anneal and become cleavable by the restriction enzymes. The brackets indicate the undigested plasmid due to R-loop formation in the region of the restriction enzyme sites. (B) R-loop status of the middle of the 125-bp switch region. A PvuII restriction site within the middle of the 2.5-repeat switch region was tested for cleavability. NC, nicked circular; L, linear; SC, supercoiled; T7, T7 promoter; P, PstI; S, SacI; H, HindIII; K, KpnI; X, XbaI.
We constructed a plasmid (pKY149) with a point mutation in the middle of the switch region to create a unique PvuII site. After R-loop formation (Fig. 3B, lane 1), we digested the plasmid with PvuII and found that a significant amount of the R-loop could not be digested unless RNase H was also added (Fig. 3B, compare lanes 2 and 3), consistent with the idea that the R-loop region centers on the switch sequences.
AID can deaminate cytidine on the displaced G-rich strand of an R-loop.
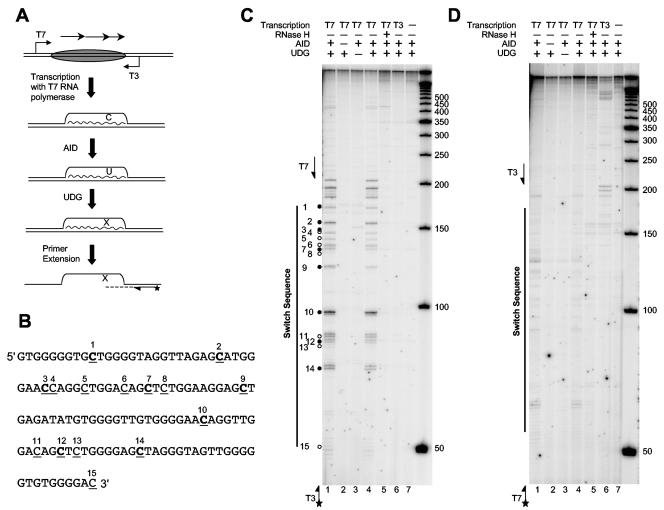
To detect AID-mediated deamination on an R-loop, we treated a minimal R-loop (Fig. 2, lane 11) with recombinant mouse AID purified from baculovirus-infected insect cells (Fig. 4A). AID-treated R-loops were then incubated with Escherichia coli uracil glycosylase, which removes the U to generate an abasic site. The resulting AP site could be detected by primer extension because the DNA polymerase used here (Vent exo−) could not bypass this AP site.
FIG. 4.
Enzyme and strand specificity for AID action on an R-loop. (A) Schematic diagram illustrating the assay for AID action on an R-loop (top strand). R-loops were generated by transcribing supercoiled plasmid with T7 RNA polymerase. Purified R-loops were then treated with AID and UDG, followed by primer extension with a radioactively labeled primer to detect the AP site that results from AID and UDG action. Diagrammed DNA strands are represented by straight lines, and the RNA transcript is represented by a wavy line. The oval represents the switch region. Arrows above the oval indicate switch repeats. The T7 and T3 promoters are indicated by arrows flanking the switch region. A star indicates the radioactive label. (B) Sequence of the G-rich displaced DNA strand of the 2.5-Sγ3 repeat switch region. All C residues are underlined. Each bold C corresponds to a WRC sequence. (C) AID action on the displaced G-rich strand of an R-loop. Primer extension was carried out with a labeled T3 primer, shown at the bottom left of the gel. Each dot indicates the position of a cytidine residue on the G-rich strand (each closed circle conforms to a WRC sequence, and open circles do not). The components of the reaction are shown at the top of the gel. Single-stranded markers (in nucleotides) are shown on the right. (D) AID action on the C-rich strand of an R-loop, which is base paired with the RNA transcript. Primer extension was carried out with a labeled T7 primer, shown at the bottom left of the gel. All other symbols are the same as described for panel C. The components of the reaction are shown at the top of the gel. Single-stranded markers are shown on the right.
We used an amount of AID enzyme that previously gave only one cytidine deamination per single-stranded DNA molecule (38). Doublet bands were observed for each site, because Vent exo− DNA polymerase adds an additional nucleotide at the 3′ end (with an efficiency of about 50% for filling in an EcoRI-generated end) (Fig. 4C, lanes 1 and 4). When either AID or UDG was omitted from the reaction, no primer extension pause site product was detected (Fig. 4C, lanes 2 and 3), indicating that the pause sites were indeed due to the C deamination by AID and uracil removal by UDG.
When the assay was performed with DNA that does not have an R-loop structure, such as an untranscribed supercoiled plasmid (Fig. 4C, lane 7), a plasmid transcribed with T3 RNA polymerase (nonphysiological orientation) (Fig. 4C, lane 6), or an R-loop that was treated with RNase H (Fig. 4C, lane 5), we did not detect any stalled primer extension product. This finding indicates that AID action is dependent on the R-loop structure. In addition, the primer extension products align exactly with each C residue of the G-rich strand within the switch region (Fig. 4C, lanes 1 and 4; sequencing ladder not shown). When we used another primer (KY439) further downstream for the primer extension, a corresponding shift in size for every band was observed (data not shown), confirming that the primer extension results are attributable to the C deamination sites inferred.
When we analyzed the C-rich strand by using labeled T7 primer as for the extension reaction, we also observed some weak bands (Fig. 4D, lanes 1 and 4), although the intensity of the bands was only slightly higher than the background intensity. From our previous bisulfite sequencing analysis, we knew that the C-rich strand was paired with the RNA transcript. We suspected that this minimal amount of activity on the C-rich strands results from deamination of unpaired C's at the border of the R-loop. Similar to that of the G-rich strand, the primer extension product observed for C-rich strands was also dependent on AID and UDG (Fig. 4D, lanes 2 and 3) and dependent on the R-loop structure (Fig. 4D, lanes 5 to 7). When AID- but not UDG-treated R-loops were transformed into an ung null bacterial strain (NR8052), C-to-T mutations were detected (data not shown), consistent with the action of AID as a cytidine deaminase. However, clones containing these conversions were rare among all cloned molecules.
The deamination intensities conformed largely to the WRCr (where W is A or T, R is A or G, and r indicates a small preference for purine) preferences defined previously for single-stranded DNA. However, there were clear deviations. For example, the third labeled cytidine (Fig. 4D, lanes 1 and 4) was a strong WRC site on purely single-stranded DNA but was barely detectable as a site of AID action. Similarly, the first labeled cytidine should have been deaminated as well as the second labeled cytidine (Fig. 4D, lanes 1 and 4), yet it was not. These deviations are most readily explained by steric constraints within the R-loop conformation that restrict access by AID at these particular sites.
DISCUSSION
AID can deaminate at the displaced strand of a transcription-induced R-loop.
This study demonstrates that the displaced strand of a transcription-induced R-loop has sufficient space between it and the RNA-DNA duplex to permit AID to bind and deaminate the cytidines. This result is important for several reasons. First, it shows that AID is able to gain substantial access to the displaced strand of the R-loop. This information is relevant for understanding not only the biology of AID but also the structure of R-loops. Second, this study provides the first sequence-level gel resolution for the deamination by AID of specific cytidines in the displaced strand of an R-loop region, thereby correlating the R-loop precisely with AID action. Third, this study shows that AID does not require any additional proteins for catalytic action at an R-loop. This finding does not rule out that other proteins might stimulate this activity (7, 8), but it does show that these other proteins are not essential. Fourth, the sites of action conform well to the WRC preference spectrum (27, 38), but not uniformly so.
Constraints on AID accessibility to the displaced DNA strand of the R-loop.
AID has been demonstrated to have quantitative site preferences within single-stranded DNA (27, 38). The two nucleotides upstream and the one nucleotide downstream of the cytidine influence the AID specificity over a 10-fold range (38). If the displaced strand within the R-loop existed simply as single-stranded DNA, then we would expect the profile of relative deaminations to match that seen for single-stranded DNA. Several specific deviations illustrate that this is not entirely the case. This finding indicates that the R-loop conformation does constrain some sites from AID action.
Interestingly, analysis of the mutations around chromosomal switch junctions also does not conform uniformly to the WRCr rule at C residues (12, 31). Our findings here provide a potential explanation for these mutations that is based in the R-loop conformation.
Structure of transcription-induced R-loops.
R-loops generated by transcription are quite distinct from those generated by assembly of RNA and DNA oligonucleotides (34). During transcription, positive superhelical tension is generated ahead of the RNA polymerase and negative superhelical tension is generated behind the polymerase. Even more importantly, the displaced DNA strand will wrap around the RNA-DNA duplex with a transient periodicity defined by the size of the RNA polymerase. The greater the distance that the polymerase pushes the displaced strand away from the RNA-DNA duplex, the less frequently the displaced strand will wrap around the RNA-DNA duplex during transcription. At the extreme, the displaced strand would not wrap around the duplex at all, which is how we and others have typically drawn R-loops in two dimensions (Fig. 1A). However, at the other extreme, the displaced DNA strand could wrap within the major groove and have a periodicity that is the same as that of the RNA-DNA duplex. The diameter of the RNA polymerase protein determines the distance with which the displaced strand is transiently pushed away from the RNA-DNA duplex.
After the RNA polymerase has passed through the region, the displaced DNA strand might conceivably change position, given that the positive superhelical tension ahead and the negative superhelical tension behind the R-loop will dissipate by diffusion into the adjacent regions of normal duplex DNA. In addition, topoisomerases will compensate for positive and negative superhelical deviations.
We are confronted with a particularly refractory conformational problem when considering transcription-induced R-loops. These must be generated by transcription, which means that they are too heterogeneous (37) and too large for nuclear magnetic resonance or crystallography. Visual resolution of the single strands by transmission electron microscopy, cryoelectron microscopy, or atomic force microscopy is not possible. In light of the lack of structural approaches, we are limited to chemical and enzymatic probing of the R-loop. We have previously reported sequence-level chemical probing of the bottom and top DNA strands of 2-kb transcription-induced R-loops (37). The present study uses a natural enzyme, AID, to probe the R-loop structure.
The fact that even this 50-kDa protein (24-kDa AID plus the 26-kDa glutathione S-transferase moiety) can gain access to the displaced strand of the R-loop is consistent with the results of our earlier chemical probing methods. The bisulfite chemical probing would be inconsistent with the displaced strand lying in the major groove in a triplex conformation, because stacking of the bases in that strand would prevent a nucleophilic attack by bisulfite. Access by AID to this strand provides an independent confirmation that this is a single strand and is accessible.
Upstream boundary of transcription-induced R-loops and AID action.
Our data are from sequencing gels rather than low-resolution gels. This fact permits the first precise correlation of the boundaries of the R-loop and the boundary of the AID action on the top strand of transcription-induced R-loops. The boundary of the R-loop extends further upstream than simply the 2.5 switch repeats. This finding is based on restriction enzyme digestion upstream of the switch region on R-loop plasmids. Restriction enzymes fail to cut for about 20 to 30 bp upstream of the R-loop. Interestingly, AID also deaminates for a distance of about 20 to 30 nucleotides on the displaced strand upstream of the switch region. Why should the R-loop extend upstream of the switch region, even if only for 20 to 30 bp? We are not certain, but branch migration of the R-loop is the most likely explanation. In such migration, the free RNA may anneal with the template DNA strand, thereby displacing the top strand. Further extension of the branch migration may be limited by constraints of superhelical tension.
Acknowledgments
This work was supported by NIH grants (M.R.L.).
REFERENCES
- 1.Bosma, G. C., J. Kim, T. Urich, D. M. Fath, M. G. Cotticelli, N. R. Ruetsch, M. Z. Radic, and M. J. Bosma. 2002. DNA-dependent protein kinase activity is not required for immunoglobulin class switching. J. Exp. Med. 196:1483-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bransteitter, R., P. Pham, M. D. Scharff, and M. F. Goodman. 2003. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc. Natl. Acad. Sci. USA 100:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casellas, R., A. Nussenzweig, R. Wuerffel, R. Pelanda, A. Reichlin, H. Suh, X. F. Qin, E. Besmer, A. Kenter, K. Rajewsky, and M. C. Nussenzweig. 1998. Ku80 is required for immunoglobulin isotype switching. EMBO J. 17:2404-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalan, N., F. Selz, K. Imai, P. Revy, A. Fischer, and A. Durandy. 2003. The block in immunoglobulin class switch recombination caused by activation-induced cytidine deaminase deficiency occurs prior to the generation of DNA double strand breaks in switch μ region. J. Immunol. 171:2504-2509. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, E. J., E. A. Price, M. C. Bayramyan, and I. S. Haworth. 2003. Computation of DNA backbone conformations. J. Biomol. Struct. Dyn. 21:111-125. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri, J., and F. W. Alt. 2004. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 4:541-552. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri, J., C. Khuong, and F. W. Alt. 2004. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature 430:992-998. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri, J., M. Tian, C. Khuong, K. Chua, E. Pinaud, and F. W. Alt. 2003. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature 422:726-730. [DOI] [PubMed] [Google Scholar]
- 9.Daniels, G. A., and M. R. Lieber. 1995. RNA:DNA complex formation upon transcription of immunoglobulin switch regions: implications for the mechanism and regulation of class switch recombination. Nucleic Acids Res. 23:5006-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickerson, S. K., E. Market, E. Besmer, and F. N. Papavasiliou. 2003. AID mediates hypermutation by deaminating single stranded DNA. J. Exp. Med. 197:1291-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Noia, J., and M. S. Neuberger. 2002. Altering the pathway of immunoglobulin hypermutation by inhibiting uracil-DNA glycosylase. Nature 419:43-48. [DOI] [PubMed] [Google Scholar]
- 12.Dunnick, W., G. Z. Hertz, L. Scappino, and C. Gritzmacher. 1993. DNA sequences at immunoglobulin switch region recombination sites. Nucleic Acids Res. 21:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fugmann, S. D., and D. G. Schatz. 2003. RNA aids DNA. Nat. Immunol. 4:429-430. [DOI] [PubMed] [Google Scholar]
- 14.Gritzmacher, C. A. 1989. Molecular aspects of heavy-chain class switching. Crit. Rev. Immunol. 9:173-200. [PubMed] [Google Scholar]
- 15.Honjo, T., M. Muramatsu, and S. Fagarasan. 2004. AID: how does it aid antibody diversity? Immunity 20:659-668. [DOI] [PubMed] [Google Scholar]
- 16.Imai, K., G. Slupphaug, W. I. Lee, P. Revy, S. Nonoyama, N. Catalan, L. Yel, M. Forveille, B. Kavli, H. E. Krokan, H. D. Ochs, A. Fischer, and A. Durandy. 2003. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat. Immunol. 4:1023-1028. [DOI] [PubMed] [Google Scholar]
- 17.Iwasato, T., A. Shimizu, T. Honjo, and H. Yamagishi. 1990. Circular DNA is excised by immunoglobulin class switch recombination. Cell 62:143-149. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita, K., M. Harigai, S. Fagarasan, M. Muramatsu, and T. Honjo. 2001. A hallmark of active class switch recombination: transcripts directed by I promoters on looped-out circular DNAs. Proc. Natl. Acad. Sci. USA 98:12620-12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieber, M. R. 1991. Site-specific recombination in the immune system. FASEB J. 5:2934-2944. [DOI] [PubMed] [Google Scholar]
- 20.Manis, J. P., D. Dudley, L. Kaylor, and F. W. Alt. 2002. IgH class switch recombination to IgG1 in DNA-PKcs-deficient B cells. Immunity 16:607-617. [DOI] [PubMed] [Google Scholar]
- 21.Manis, J. P., Y. Gu, R. Lansford, E. Sonoda, R. Ferrini, L. Davidson, K. Rajewsky, and F. W. Alt. 1998. Ku70 is required for late B cell development and immunoglobulin heavy chain class switching. J. Exp. Med. 187:2081-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka, M., K. Yoshida, T. Maeda, S. Usuda, and H. Sakano. 1990. Switch circular DNA formed in cytokine-treated mouse splenocytes: evidence for intramolecular DNA deletion in immunoglobulin class switching. Cell 62:135-142. [DOI] [PubMed] [Google Scholar]
- 23.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553-563. [DOI] [PubMed] [Google Scholar]
- 24.Muramatsu, M., V. S. Sankaranand, S. Anant, M. Sugai, K. Kinoshita, N. O. Davidson, and T. Honjo. 1999. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol. Chem. 274:18470-18476. [DOI] [PubMed] [Google Scholar]
- 25.Petersen, S., R. Casellas, B. Reina-San-Martin, H. T. Chen, M. J. Difilippantonio, P. C. Wilson, L. Hanitsch, A. Celeste, M. Muramatsu, D. R. Pilch, C. Redon, T. Ried, W. M. Bonner, T. Honjo, M. C. Nussenzweig, and A. Nussenzweig. 2001. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. Nature 414:660-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen-Mahrt, S. K., R. S. Harris, and M. S. Neuberger. 2002. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 418:99-103. [DOI] [PubMed] [Google Scholar]
- 27.Pham, P., R. Bransteitter, J. Petruska, and M. F. Goodman. 2003. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature 424:103-107. [DOI] [PubMed] [Google Scholar]
- 28.Rada, C., G. T. Williams, H. Nilsen, D. E. Barnes, T. Lindahl, and M. S. Neuberger. 2002. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr. Biol. 12:1748-1755. [DOI] [PubMed] [Google Scholar]
- 29.Reaban, M. E., J. Lebowitz, and J. A. Griffin. 1994. Transcription induces the formation of a stable RNA. DNA hybrid in the immunoglobulin alpha switch region. J. Biol. Chem. 269:21850-21857. [PubMed] [Google Scholar]
- 30.Rush, J. S., S. D. Fugmann, and D. G. Schatz. 2004. Staggered AID-dependent DNA double strand breaks are the predominant DNA lesions targeted to Sμ in Ig class switch recombination. Int. Immunol. 16:549-557. [DOI] [PubMed] [Google Scholar]
- 31.Schrader, C. E., S. P. Bradley, J. Vardo, S. N. Mochegova, E. Flanagan, and J. Stavnezer. 2003. Mutations occur in the Ig Sμ region but rarely in Sγ regions prior to class switch recombination. EMBO J. 22:5893-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinkura, R., M. Tian, M. Smith, K. Chua, Y. Fujiwara, and F. W. Alt. 2003. The influence of transcriptional orientation on endogenous switch region function. Nat. Immunol. [DOI] [PubMed]
- 33.Sohail, A., J. Klapacz, M. Samaranayake, A. Ullah, and A. S. Bhagwat. 2003. Human activation-induced cytidine deaminase causes transcription-dependent, strand-biased C to U deaminations. Nucleic Acids Res. 31:2990-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian, M., and F. W. Alt. 2000. Transcription-induced cleavage of immunoglobulin switch regions by nucleotide excision repair nucleases in vitro. J. Biol. Chem. 275:24163-24172. [DOI] [PubMed] [Google Scholar]
- 35.von Schwedler, U., H. M. Jack, and M. Wabl. 1990. Circular DNA is a product of the immunoglobulin class switch rearrangement. Nature 345:452-456. [DOI] [PubMed] [Google Scholar]
- 36.Wuerffel, R. A., J. Du, R. J. Thompson, and A. L. Kenter. 1997. Ig Sγ3 DNA-specific double strand breaks are induced in mitogen-activated B cells and are implicated in switch recombination. J. Immunol. 159:4139-4144. [PubMed] [Google Scholar]
- 37.Yu, K., F. Chedin, C. L. Hsieh, T. E. Wilson, and M. R. Lieber. 2003. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat. Immunol. 4:442-451. [DOI] [PubMed] [Google Scholar]
- 38.Yu, K., F. T. Huang, and M. R. Lieber. 2004. DNA substrate length and surrounding sequence affect the activation-induced deaminase activity at cytidine. J. Biol. Chem. 279:6496-6500. [DOI] [PubMed] [Google Scholar]
- 39.Yu, K., and M. R. Lieber. 2003. Nucleic acid structures and enzymes in the immunoglobulin class switch recombination mechanism. DNA Repair 2:1163-1174. [DOI] [PubMed] [Google Scholar]