Abstract
Since the initial marketing in 2005, the use of e-cigarettes has increased exponentially. Nonetheless, accumulating evidence has demonstrated the ineffectiveness of e-cigarettes in leading to smoking cessation, and decreasing the adverse health impacts of cigarette smoking. The number of adolescents adapted to e-cigarettes has been increasing substantially each year, and this adaptation has promoted openness to tobacco smoking. The present review discusses controversies regarding the smoking cessation effects of e-cigarettes, recent governmental policies and regulations of e-cigarette use, toxic components and vaporization products of e-cigarettes, and the novel molecular mechanisms underlying the adverse health impacts of e-cigarettes leading to oxidative stress in target tissues, and consequent development of cardiopulmonary diseases (i.e. COPD), neurodegenerative disorders (i.e. Alzheimer's’ disease), and cancer. Health warning signs on the packaging and professional consultation to avoid adaptation in risk groups might be helpful solutions to control negative impacts of e-cigarettes. It is also recommended to further expand basic and clinical investigations to reveal more detailed oxidative stress mechanisms of e-cigarette induced damages, which would ultimately result in more effective protective strategies.
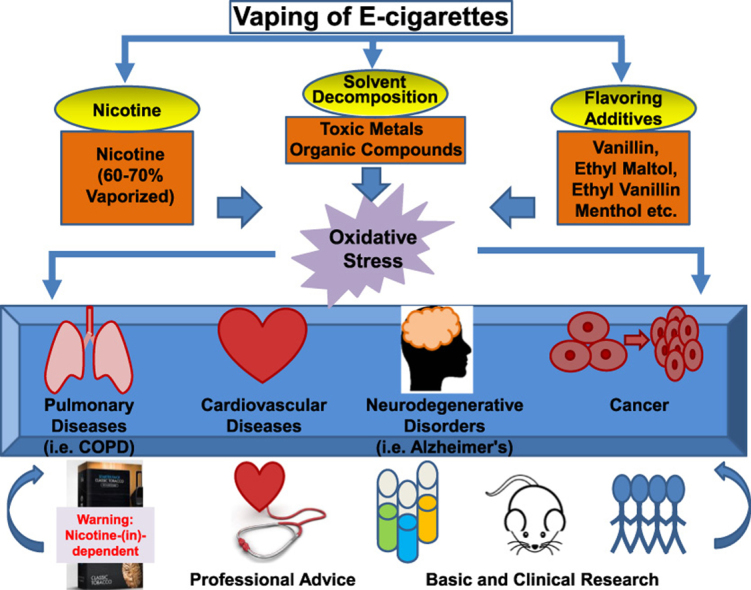
Graphical abstract
Highlights
-
•
E-cigarette use has increased exponentially but controversial in smoking cessation.
-
•
Governmental policies and regulations of e-cigarette use are being implemented.
-
•
E-cigarette components and vaporization products exert adverse health impacts.
-
•
The toxicity of e-cigarettes seems mediated by oxidative stress.
-
•
Health warning signs and professional consultation for control of negative impacts.
-
•
Recommend to expand basic and clinical mechanistic investigations of oxidative stress.
Since the initial marketing in 2005, the use of e-cigarettes has increased exponentially [1]. There are approximately 3 million e-cigarette users in the UK (United Kingdom) [2]. In the USA (United States of America), the total sales of e-cigarettes are anticipated to exceed tobacco products within a decade [3]. It has been shown that more than half of the current and ex-smokers in US [4], and 61% of the current smokers in UK [2], have tried e-cigarettes. China as the primary manufacturing center for e-cigarettes (~80%), has the largest smoking population in the world (3 out of 10 billion, 45% of adult men are smokers), and is rapidly developing into a nation with the largest population of e-cigarette users [1]. Despite being anticipated to help cease smoking, two recent meta-analyses of clinical trials have indicated that e-cigarettes are not effective in this regard [5]. Of those studies that showed a significant effect of e-cigarettes on smoking cessation, it is unclear whether the reduction in tobacco smoking was simply a consequence of partial replacement with e-cigarettes to make the smokers dual users. In addition, the Center for Disease Control (CDC) reports data showing a dramatic increase in e-cigarette use in high school students [6], and that this adaptation to e-cigarettes has been shown to promote openness to tobacco smoking in adolescents [7]. Among high school students, current e-cigarette use tripled in a single year – from 660,000 users in 2013 to 2,000,000 in 2014 [8]. It is important to note that the American Thoracic Society and the American Heart Association cited concerns regarding ineffectiveness in smoking cessation and potential adverse effects of e-cigarettes in the 2014 recommendations [9], [10], both urging restricted use of e-cigarettes until their health impacts are fully understood. It is stated in both that e-cigarette use may promote nicotine addiction and early adaptation to e-cigarettes and/or tobacco smoking in teenagers. Importantly, more recent studies have provided evidence that oxidative stress mediate many of the adverse effects of e-cigarettes.
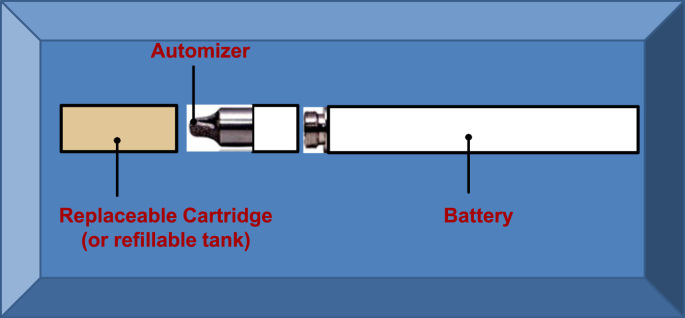
The e-cigarettes are comprised of three components; a battery powered heating element, a cartridge (replaceable) or tank (refillable) containing a solution made of propylene glycol, glycerine, nicotine, water and flavorings, and an atomizer that vaporizes the solution when heated (Fig. 1). More than 1000 brands of e-cigarettes and 7764 flavors of the solution have been produced, with production innovation evolving rapidly [1], [8], [11]. The market is primarily dominated by small companies although the large tobacco firms also have product lines. The regulatory policies on e-cigarettes have been lacking, at least in part due to the incompletely understood health impacts of the e-cigarettes. With encouragement of the government to promote self-regulation, the first National Association of Electronic Cigarettes (NAEC) was established in 2015 in China, which is a professional association with 1800 industrial participants [1]. Back in 2006, The State Tobacco Monopoly Administration (STMA) classified e-cigarettes as potentially harmful chemical products, recommending regulation under State Administration of Work Safety [1]. Appropriate regulation is an urgent need given the potential health hazard particularly considering the e-cigarette marketing to youth and non-smokers. In the UK, e-cigarettes that contain nicotine are regulated either as tobacco-related products or as licensed medicines [2]. In the US, the Food and Drug Administration (FDA) established new rules on e-cigarette regulations in 2016, with a series of timelines starting in August 2016 (http://fdaregs.info/fda-deeming-regulations/timeline/).
Fig. 1.
The making of the e-cigarettes. The e-cigarettes are comprised of three components; a battery powered heating element, a cartridge (replaceable) or tank (refillable) containing a solution made of propylene glycol, glycerine, nicotine, water and flavorings, and an atomizer that vaporizes the solution when heated.
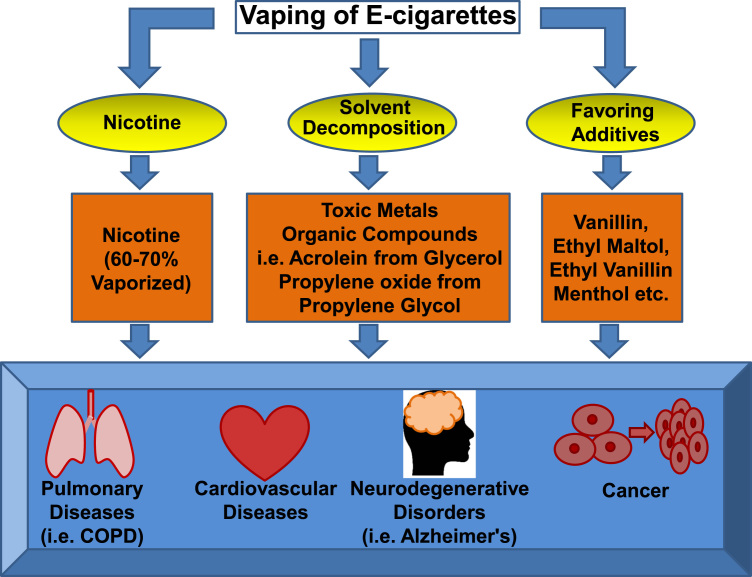
For habitual e-cigarette users or people who use e-cigarettes as a smoking cessation aid, the chronic use of e-cigarettes, and the use of e-cigarettes during and after attempted smoking cessation, is associated with worrisome adverse effects. Most of the e-cigarettes tested for smoking cessation contain nicotine [9], [10], which is known to contribute to cardiopulmonary diseases, neurodegenerative disorders and cancer [12]. Although consumed at lower levels in e-cigarette users compared to tobacco cigarette users, this lower level of nicotine has already passed the threshold of being potentially toxic [13]. For teenagers, nicotine exposure will affect brain development and lead to nicotine addiction that has a lifelong impact. Thermal decomposition of the solvents in e-cigarettes generates an array of organic compounds including carbonyls, one of which is acrolein (from glycerol/glycerine). Of note, acrolein has been shown to be capable of inducing COPD [14], the third leading cause of death worldwide [9]. The levels of acrolein are elevated in lung fluids of COPD patients [14]. Many of the identified components released from e-cigarette vaporization are potential carcinogens, including toxic metals (cadmium, chromium, lead, manganese and nickel), acrolein, and other organic compounds such as propylene oxide formed from propylene glycol [10], [15]. The latter, propylene glycol, is not present in traditional tobacco cigarettes. Direct exposure of mice to e-cigarette vapors resulted in inflammation and reduced clearance of bacteria and virus, which are key features of COPD [3]. A recent study has demonstrated that e-cigarette exposure induced DNA strand break and apoptosis regardless of nicotine contents [16]. Moreover, some of the flavoring additives have been shown to be pathogenic [8], [17]. These flavorings contain cytotoxic aldehydes [8], [17], which is a compound class recognized as “primary irritants” of mucosal tissue of the respiratory tract [18]. Using gas chromatography/mass spectrometry (GC/MS) to analyze 28 e-cigarette liquids from seven manufacturers, Hutzler and colleagues found that vanillin, ethyl maltol, ethyl vanillin and menthol were the four most frequently found flavor chemicals, which were present in 79%, 57%, 50% and 43% of the 28 samples, respectively [19]. Of note, Bahl et al. examined 41 e-cigarette refill fluids for cytotoxicity to human pulmonary fibroblasts, human embryonic stem cells and mouse neural stem cells, and noted that when present, the cytotoxicity was related to the flavor chemicals, especially for cinnamon-flavored refill fluids [20], [21]. The potential pathological effects of these e-cigarette vaporization products and flavoring additives are summarized in Fig. 2.
Fig. 2.
Pathological effects induced by e-cigarette components and vaporization products. Most of the e-cigarettes used for smoking cessation contain nicotine, which can be vaporized by 60–70%. Thermal decomposition of e-cigarette solvents results in release of toxic metals, and formation of an array of organic compounds such as acrolein from glycerol, and propylene oxide from propylene glycol. Frequently used flavoring additives include vanillin, ethyl matol, ethyl vanillin, and methol. All of these components and vaporization products have been shown to be either directly carcinogenic, or toxic in inducing cardiopulmonary diseases and neurodegenerative disorders.
Importantly, the latest studies have demonstrated that the use of e-cigarettes is associated with increased oxidative stress, which seems to mediate the adverse effects of e-cigarettes. Oxidative stress develops in e-cigarette-exposed human bronchial and lung epithelial cells [22], [23], human lung vascular endothelial cells and human umbilical vein endothelial cells [24], [25], resulting in inflammation, cytotoxicity and increased endothelial cell permeability [22], [23], [24], [25], [26]. In these studies, intracellular production of reactive oxygen species (ROS) was determined using fluorescent probes such as DCF-DA for intracellular hydrogen peroxide [23], [25], [27]. Exposure to nicotine that was specifically generated by the use of e-cigarettes, was shown to promote oxidative stress and impairment of autophagy, which in turn serves as a potential mechanism leading to development of COPD [27]. Learner and colleagues have recently shown that e-cigarette aerosols and copper nanoparticles induce mitochondrial ROS production, mitochondrial stress (reduced stability of OxPhos electron transport chain (ETC) complex IV subunit) and DNA fragmentation in lung fibroblasts [28]. Acrolein has been shown to provoke oxidative stress and inflammation to result in loss of endothelial cell barrier integrity in the lung [24]. Propylene glycol, glycerine, and methanol have all been shown to increase production of hydrogen peroxide [28]. The flavoring additive of cinnamon roll stimulated dramatic increase in the production of inflammatory cytokine IL-8 in human lung fibroblasts [28].
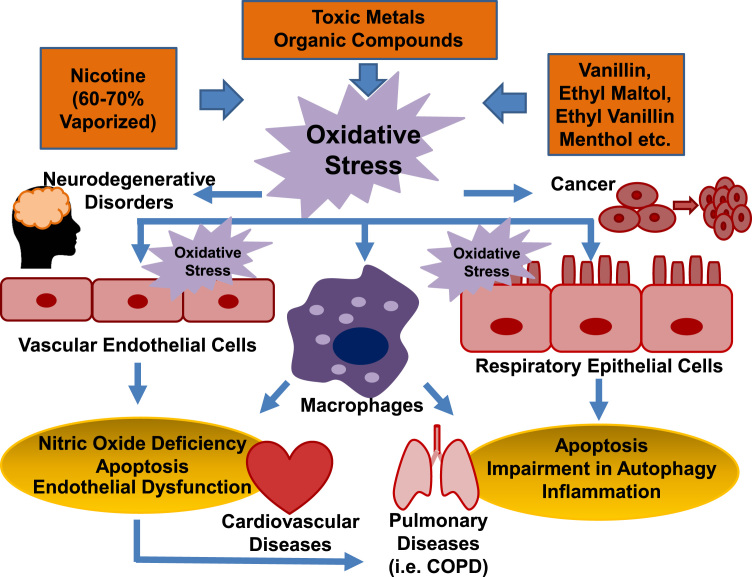
Furthermore, recent reports have confirmed oxidative stress inducing effects of e-cigarettes in animal models in vivo [3], [28], and in human e-cigarette users [10], [15]. Electron paramagnetic/spin resonance (EPR/ESR) determination of free radical production indicated increased ROS release from e-cigarette vaporization in both cell-free and cell systems, which is associated with increased lipid peroxidation in the lung homogenates (assessed by thiobarbituric acid reactive substances (TBARS)) of e-cigarette exposed mice [3]. The authors also observed a 58% increase in macrophage counts in the bronchoalveolar lavage (BAL) 24 h after the final exposure (twice per day for two weeks), which was accompanied by an elevated production of IL-6 [3]. These responses have resulted in reduced bacterial and viral clearance [3]. In healthy human subjects, the use of e-cigarettes stimulated oxidative stress, nitric oxide deficiency, and endothelial/vascular dysfunction that translated into impaired flow-mediated dilatation [29]. In addition, Moheimani et al. recently reported occurrence of oxidative stress and increased cardiac sympathetic activity in habitual e-cigarette users, which are known risk factors for cardiovascular diseases [30]. The role of oxidative stress in mediating adverse effects of e-cigarettes to result in cardiopulmonary pathogenesis, neurodegenerative disorders and cancer, is illustrated in Fig. 3.
Fig. 3.
A mediator role of oxidative stress in e-cigarette induced pathogenesis. The toxic components and vaporization products of e-cigarettes have been shown by recent studies to induce oxidative stress in human lung and branchial epithelial cells, and human vascular endothelial cells, resulting in inflammation, cytotoxicity and increased endothelial permeability. It also induces tissue infiltration of activated macrophages. Impairment in branchial epithelial cell autophagy induced by e-cigarette contained nicotine, and reduced bacterial and viral clearance in e-cigarette exposed mouse lung, represent potential mechanisms leading to development of COPD. Healthy human subjects responded to e-cigarette exposure with increased oxidative stress, reduced nitric oxide bioavailability, and impaired endothelial/vascular dysfunction that precede to cardiovascular diseases.
One may propose that e-cigarette users could take some anti-oxidants to offset the toxicity of oxidative stress. Given the complexities of how ROS function in vivo for both physiological (low levels of ROS production are required for growth signaling) and pathological processes, and that the detailed molecular mechanisms underlying e-cigarette induction of oxidative stress have remained unclear, it is not yet possible to deliver precise anti-oxidative treatments effectively to control oxidative stress damage. The oxidase systems selectively responsible for the development of different human diseases are still under intensive investigations to enable targeted therapies. Nonetheless, recent studies have demonstrated whereas NADPH oxidase (NOX) isoform 1 (NOX1) is responsible for diabetic vascular complications [31], NOX4 activation mediates ischemia reperfusion injury in the heart [32]. Cigarette smoking is known to induce oxidative stress to result in endothelial dysfunction and vascular diseases [33], [34], [35]. A role of endogenous NOX and extracellular superoxide dismutase (ecSOD) in smoking induced oxidative stress has been implicated [36]. Of note, recent studies indicate that airway smooth muscle NOX4 can be upregulated to produce ROS in COPD [37].
NOX family oxidases are known to activate other oxidase systems to result in a vicious cycle of prolonging ROS production, resulting in sustained oxidative stress and initiation of pathological processes. It would be important to investigate the oxidase networks to reveal detailed mechanisms of oxidative stress development in response to e-cigarette exposure. At the present, however, the best solution to prevent damages caused by e-cigarette-derived oxidative stress might be to avoid adaptation to e-cigarettes, particularly when their smoking cessation effects are questionable.
Therefore, it is important to give deeper consideration to the adaptation and regulation of e-cigarettes as an alternative to tobacco smoking since they introduce new toxic agents, toxicity of which is poorly understood. With the availability of objective data from human trials and basic research, appropriate health warnings could be included on the packaging alerting nicotine-dependent and independent adverse effects. Recent data indicate that only 22% of e-cigarette liquid bottles used a warning statement that indicated the product "contained nicotine" [38]. None of the statements included the information that nicotine was "addictive". Only about half of the websites for e-cigarette marketing have a minimum age requirement barrier that prevented under-aged persons from entering [38].
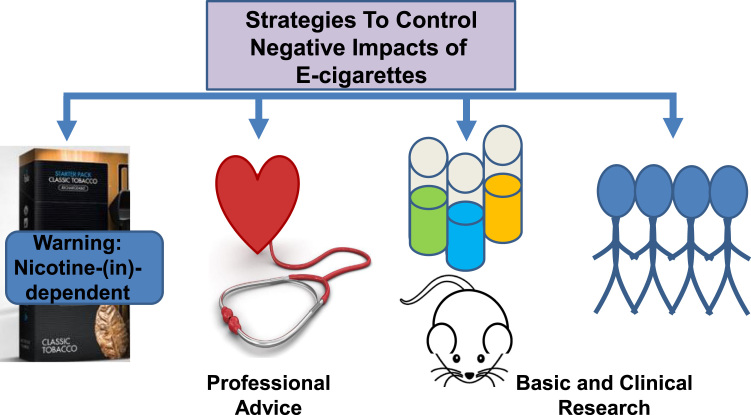
Furthermore, it is probably not too early to start advising the tobacco users in the community not to rush into the harmful adaptation of e-cigarettes, and for physicians to guide populations at risk of relevant diseases, including cardiopulmonary diseases, neurodegenerative disorders and cancer, to avoid them. These recommended strategies to better control negative impacts of e-cigarettes are summarized in Fig. 4. Increasing the awareness of the oxidative stress aspects of the e-cigarette use may prove beneficial in promoting basic and clinical investigations to further our understandings of the adverse effects of e-cigarettes to better protect against these detrimental consequences.
Fig. 4.
Strategies to better control negative impacts of e-cigarettes. Health warning signs on the packaging and professional consultation to avoid adaptation in risk groups might be helpful solutions to control negative impacts of e-cigarettes. It is also recommended to further expand basic and clinical investigations to reveal more detailed oxidative stress mechanisms of e-cigarette induced damages, which would ultimately result in more effective protective strategies.
Acknowledgements
This work was supported by The National Key Research and Development Program of China Grants 2016YFC1303900 (CW), 2016YFC0901102 (CW), National Institute of Health National Heart, Lung and Blood Institute (NHLBI) Grants HL077440 (HC), HL088975 (HC), HL108701 (HC, DGH), HL119968 (HC), and an American Heart Association Established Investigator Award (EIA) 12EIA8990025 (HC).
References
- 1.Xu X., Wang X., Zhang X., Liu Y., He H., Mackay J. The debate on regulation of e-cigarettes in China. Lancet Respir. Med. 2016;4:856–858. doi: 10.1016/S2213-2600(16)30313-7. (Epub2016 Oct 12) [DOI] [PubMed] [Google Scholar]
- 2.House of Parliament Postnote Number 533 on Electronic Cigarettes, Parlimentary Office of Science and Technology.
- 3.Sussan T.E., Gajghate S., Thimmulappa R.K., Ma J., Kim J.H., Sudini K., Consolini N., Cormier S.A., Lomnicki S., Hasan F., Pekosz A., Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10:e0116861. doi: 10.1371/journal.pone.0116861. (eCollection2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenborn C.A., Gindi R.M. Electronic Cigarette Use Among Adults: United States, 2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 5.Kalkhoran S., Glantz S.A. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir. Med. 2016;4:116–128. doi: 10.1016/S2213-2600(15)00521-4. (Epub 2016 Jan 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.R.A. Arrazola, T. Singh, C.G. Corey, C.G. Husten, L.J. Neff, B.J., Apelberg, R.E. Bunnell, C.J. Choiniere, B.A. King, S. Cox, T. McAfee, R.S. Caraballo, Tobacco use among middle and high school students – United States, MMWR Morb Mortal Wkly Rep. 2015, 64, 2011–2014, pp. 381–5. [PMC free article] [PubMed]
- 7.Coleman B.N., Apelberg B.J., Ambrose B.K., Green K.M., Choiniere C.J., Bunnell R., King B.A. Association between electronic cigarette use and openness to cigarette smoking among US young adults. Nicotine Tob. Res. 2015;17:212–218. doi: 10.1093/ntr/ntu211. (Epub 2014 Nov 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leone F.T., Ferkol T.W. Such are the impositions of quackery: e-cigarettes. Ann. Am. Thorac. Soc. 2015;12:787–788. doi: 10.1513/AnnalsATS.201504-252ED. [DOI] [PubMed] [Google Scholar]
- 9.Schraufnagel D.E., Blasi F., Drummond M.B., Lam D.C., Latif E., Rosen M.J., Sansores R., Van Zyl-Smit R. Electronic cigarettes. A position statement of the forum of international respiratory societies. Am. J. Respir. Crit. Care Med. 2014;190:611–618. doi: 10.1164/rccm.201407-1198PP. [DOI] [PubMed] [Google Scholar]
- 10.Bhatnagar A., Whitsel L.P., Ribisl K.M., Bullen C., Chaloupka F., Piano M.R., Robertson R.M., McAuley T., Goff D., Benowitz N. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130:1418–1436. doi: 10.1161/CIR.0000000000000107. (Epub2014 Aug 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu S.H., Sun J.Y., Bonnevie E., Cummins S.E., Gamst A., Yin L., Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob. Control. 2014:23. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durazzo T.C., Mattsson N., Weiner M.W. Smoking and increased Alzheimer's disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10:S122–S145. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Ruiz C.D., Graff D.W., Yan X.S. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health. 2015;15:991. doi: 10.1186/s12889-015-2349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moretto N., Volpi G., Pastore F., Facchinetti F. Acrolein effects in pulmonary cells: relevance to chronic obstructive pulmonary disease. Ann. N. Y. Acad. Sci. 2012;1259:39–46. doi: 10.1111/j.1749-6632.2012.06531.x. [DOI] [PubMed] [Google Scholar]
- 15.Hess C.A., Olmedo P., Navas-Acien A., Goessler W., Cohen J.E., Rule A.M. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res. 2017;152:221–225. doi: 10.1016/j.envres.2016.09.026. (Epub 2016 Oct 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu V., Rahimy M., Korrapati A., Xuan Y., Zou A.E., Krishnan A.R., Tsui T., Aguilera J.A., Advani S., Crotty Alexander L.E., Brumund K.T., Wang-Rodriguez J., Ongkeko W.M. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. (Epub 2015 Nov 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khlystov A., Samburova V. Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ. Sci. Technol. 2016;50:13080–13085. doi: 10.1021/acs.est.6b05145. (Epub2016 Nov 8) [DOI] [PubMed] [Google Scholar]
- 18.William P.L. Jr., S.M. Roberts, eds. Principles of Toxicology: Environmental and Industrial Applications, John Wiley & Sons, 346.
- 19.Hutzler C., Paschke M., Kruschinski S., Henkler F., Hahn J., Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol. 2014;88:1295–1308. doi: 10.1007/s00204-014-1294-7. (Epub2014 Jun 11) [DOI] [PubMed] [Google Scholar]
- 20.Bahl V., Lin S., Xu N., Davis B., Wang Y.H., Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. (Epub2012 Aug 20) [DOI] [PubMed] [Google Scholar]
- 21.Tierney P.A., Karpinski C.D., Brown J.E., Luo W., Pankow J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control. 2016;25:e10–e15. doi: 10.1136/tobaccocontrol-2014-052175. (Epub 2015 Apr 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheffler S., Dieken H., Krischenowski O., Forster C., Branscheid D., Aufderheide M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J. Environ. Res. Public Health. 2015;12:3915–3925. doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerner C.A., Sundar I.K., Yao H., Gerloff J., Ossip D.J., McIntosh S., Robinson R., Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732. doi: 10.1371/journal.pone.0116732. (eCollection2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweitzer K.S., Chen S.X., Law S., Van Demark M., Poirier C., Justice M.J., Hubbard W.C., Kim E.S., Lai X., Wang M., Kranz W.D., Carroll C.J., Ray B.D., Bittman R., Goodpaster J., Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L175–87. doi: 10.1152/ajplung.00411.2014. (Epub2015 May 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putzhammer R., Doppler C., Jakschitz T., Heinz K., Forste J., Danzl K., Messner B., Bernhard D. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One. 2016;11:e0157337. doi: 10.1371/journal.pone.0157337. (eCollection2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubenstein D.A., Hom S., Ghebrehiwet B., Yin W. Tobacco and e-cigarette products initiate Kupffer cell inflammatory responses. Mol. Immunol. 2015;67:652–660. doi: 10.1016/j.molimm.2015.05.020. (Epub2015 Jun 11) [DOI] [PubMed] [Google Scholar]
- 27.Bodas M., Van Westphal C., Carpenter-Thompson R., KM D., Vij N. Nicotine exposure induces bronchial epithelial cell apoptosis and senescence via ROS mediated autophagy-impairment. Free Radic. Biol. Med. 2016;97:441–453. doi: 10.1016/j.freeradbiomed.2016.06.017. (Epub2016 Jul 6) [DOI] [PubMed] [Google Scholar]
- 28.Lerner C.A., Rutagarama P., Ahmad T., Sundar I.K., Elder A., Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem. Biophys. Res. Commun. 2016;477:620–625. doi: 10.1016/j.bbrc.2016.06.109. (Epub2016 Jun 23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carnevale R., Sciarretta S., Violi F., Nocella C., Loffredo L., Perri L., Peruzzi M., Marullo A.G., De Falco E., Chimenti I., Valenti V., Biondi-Zoccai G., Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150:606–612. doi: 10.1016/j.chest.2016.04.012. (Epub2016 Apr 22) [DOI] [PubMed] [Google Scholar]
- 30.Moheimani R.S., Bhetraratana M., Yin F., Peters K.M., Gornbein J., Araujo J.A., Middlekauff H.R. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol. 2017;2:278–284. doi: 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youn J.Y., Gao L., Cai H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia. 2012;55:2069–2079. doi: 10.1007/s00125-012-2557-6. (Epub2012 May 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siu K.L., Lotz C., Ping P., Cai H. Netrin-1 abrogates ischemia/reperfusion-induced cardiac mitochondrial dysfunction via nitric oxide-dependent attenuation of NOX4 activation and recoupling of NOS. J. Mol. Cell Cardiol. 2014;78:174–185. doi: 10.1016/j.yjmcc.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai H. Hydrogen peroxide regulation of endothelial function: mechanisms, consequences and origins. Cardiovasc. Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ. Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 35.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 36.Tollefson A.K., Oberley-Deegan R.E., Butterfield K.T., Nicks M.E., Weaver M.R., Remigio L.K., Decsesznak J., Chu H.W., Bratton D.L., Riches D.W., Bowler R.P. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free Radic. Biol. Med. 2010;49:1937–1946. doi: 10.1016/j.freeradbiomed.2010.09.022. (Epub2010 Sep 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollins F., Sutcliffe A., Gomez E., Berair R., Russell R., Szyndralewiez C., Saunders R., Brightling C. Airway smooth muscle NOX4 is upregulated and modulates ROS generation in COPD. Respir. Res. 2016;17:84. doi: 10.1186/s12931-016-0403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagan P., Pokhrel P., Herzog T.A., Guy M.C., Sakuma K.K., Trinidad D.R., Cassel K., Jorgensen D., Lynch T., Felicitas-Perkins J.Q., Palafox S., Hamamura F., Maloney S., Degree K., Sterling K., Moolchan E., Clanton M.S., Eissenberg T. Warning statements and safety practices among manufacturers and distributors of electronic cigarette liquids in the United States. Nicotine Tob. Res. 2017:18. doi: 10.1093/ntr/ntx101. [DOI] [PMC free article] [PubMed] [Google Scholar]