Abstract
The inhibition of inflammation and vascular smooth muscle cell (VSMC) proliferation is an ideal strategy to suppress intimal hyperplasia after percutaneous transluminal angioplasty (PTA). Evidence has indicated that overexpression of A20 suppresses neointima formation, but its low transfection efficiency limits its application. Hence, we upregulated A20 expression via transfection of rAd.ATF (recombinant adenovirus vector of artificial transcription factor) and rAd.A20 in rat carotid arteries after balloon dilatation (in vivo) and isolated VSMCs (in vitro). In vivo, we found that after rAd.ATF and rAd.A20 transfection, A20 expression was markedly increased, whereas proliferating cell nuclear antigen (PCNA) and nuclear factor κB p65 (NF-κBp65) protein levels were significantly decreased, and intimal hyperplasia and secretion of proinflammatory factors were significantly reduced when compared with empty vector and saline control groups. Most importantly, the rAd.ATF-treated group showed more significant inhibition on intimal hyperplasia and expression of PCNA than the rAd.A20-treated group. In vitro, compared with the control group, transfection of rAd.ATF and rAd.A20 significantly increased A20 expression, which upregulated the proliferator-activated receptor (PPAR) level for both mRNA and protein, and reduced migration and proliferation of VSMCs and lipopolysaccharide (LPS)-induced inflammation. Furthermore, the PPARα agonist GW6471 could partially restore the effect of A20 on VSMCs. Our findings indicate that the ATF of A20 inhibits neointimal hyperplasia and, therefore, constitutes a novel potential alternative to prevent restenosis.
Keywords: A20, stroke, PPARα, restenosis, transcription factor
Introduction
Balloon dilation and stent implantation are widely applied in cases of vascular stenosis, which have a higher recurrence rate after 1 year (19.7%) and 5 years (60%) post-operation, hence seriously affecting the long-term curative effect.1, 2, 3 Restenosis after interventional therapy is largely due to injured local blood vessel walls caused by percutaneous transluminal angioplasty (PTA), which gives rise to excessive inflammation and repair responses and have always been a problem in interventional therapy.
Vascular endothelial cell injury initiates a series of pathological processes that promote excessive vascular smooth muscle cell (VSMC) proliferation, resulting in intravascular restenosis.2 Recently, it was shown that inflammatory responses play an important role in the genesis and development of restenosis.4 Histological and biochemical investigations have shown that platelets and white blood cells are very active following stent surgery in a series of inflammatory responses.5, 6 Hence, inhibition of the VSMC proliferation and inflammation induced by PTA may be essential in the suppression and treatment of restenosis.
Tumor necrosis factor α inducible protein 3 (TNFAIP3) or A20 is a plasmosin that is effective in decreasing inflammation, atherosclerosis, SMC proliferation, and cell apoptosis, as well as providing protection to endothelial cells.7, 8, 9 Evidence has shown that local transfection of the A20 gene effectively counteracts endomembrane hyperplasia after carotid artery injury.10, 11 However, the exogenous genes showed little incorporation into the transcriptionally active areas of the genome. Moreover, the number of endogenous cellular transcription factors is very limited; thus, the exogenous gene promoter may not recruit enough transcription factors. However, a small amount of artificial transcription factor (ATF) can effectively activate endogenous proteins.12 Presently, researchers have designed a zinc-finger protein (ZFP, which is the structural basis of ATFs) for A20, and the results have shown that the ZFP can activate the expression of A20 protein in vitro.13 Therefore, we hypothesized that overexpression of endogenous A20 protein through the ATF-based recombinant adenovirus vector (named rAd.ATF) may suppress PTA-induced restenosis.
Proliferator-activated receptor alpha (PPARα), a ligand-activated nuclear receptor, has been shown to play a protective role in inflammatory diseases, such as atherosclerosis, multiple sclerosis (MS), and trauma.14, 15, 16 Both A20 and PPARα could inhibit NF-κB activation, and this anti-inflammatory effect is related to their zinc-finger domain. Research has shown that zinc supplementation could inhibit the secretion of inflammatory cytokines in vitro and in vivo through the upregulation of the anti-inflammatory proteins A20 and PPARα. It was shown that A20 could protect mice from lethal liver ischemia reperfusion injury by increasing PPARα expression.17 In addition, studies have reported that PPARα may inhibit the proliferation and migration of VSMCs.18, 19 Thus, we hypothesized that A20 may suppress the occurrence and development of restenosis via the increased expression of PPARα in a rat carotid artery restenosis model.
Considering this important phenomenon, we constructed a recombinant adenovirus vector of ATF (rAd.ATF) and ascertained whether it could trigger endogenous A20 protein expression. We observed the inhibiting effect of rAd.ATF on balloon dilatation-induced restenosis in rat carotid arteries and elucidated the potential mechanism of A20 that is involved in the retardation of restenosis.
Results
A20 Expression Significantly Increased after Rat Carotid Angioplasty, Mainly in Proliferous VSMCs
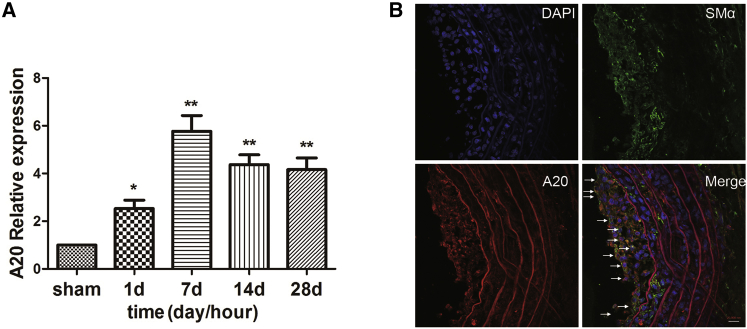
We detected A20 expression in rat carotid arteries after balloon dilation using real-time qPCR. The results showed that compared with the control group, A20 expression was significantly increased in rat carotid arteries at 7, 14, and 28 days after balloon dilation and reached its peak on day 7 (Figure 1A). The direct cause of restenosis is pathological VSMC proliferation. Hence, the expression of A20 in VSMCs was detected by immunofluorescence staining. The result demonstrated that A20 (red) is mainly expressed in VSMCs (green) in the intimal hyperplasia 7 days after balloon valvuloplasty (Figure 1B). These results suggest that A20 may play an important role in the PTA-induced proliferation of VSMCs.
Figure 1.
A20 Expression Was Significantly Increased after Balloon Injury in Rat Carotid Artery
(A) A20 mRNA expression was examined at the indicated time points after balloon dilation injury in rat carotid arteries. Data are expressed as means ± SD (n = 3). *p < 0.05; **p < 0.01 (compared with sham). (B). Immunofluorescence staining showed that A20 was mainly expressed in smooth muscle cells at 7 days after balloon dilation injury in rat carotid arteries (anti-alpha smooth muscle actin [SMα]: green; A20: red). The arrows show co-localization of A20 with vascular smooth muscle cells (VSMCs) post balloon dilation injury. Scale bar, 20,000 nm.
Transfection of rAd.ATF in Rat Carotid Arteries Activated the Expression of A20
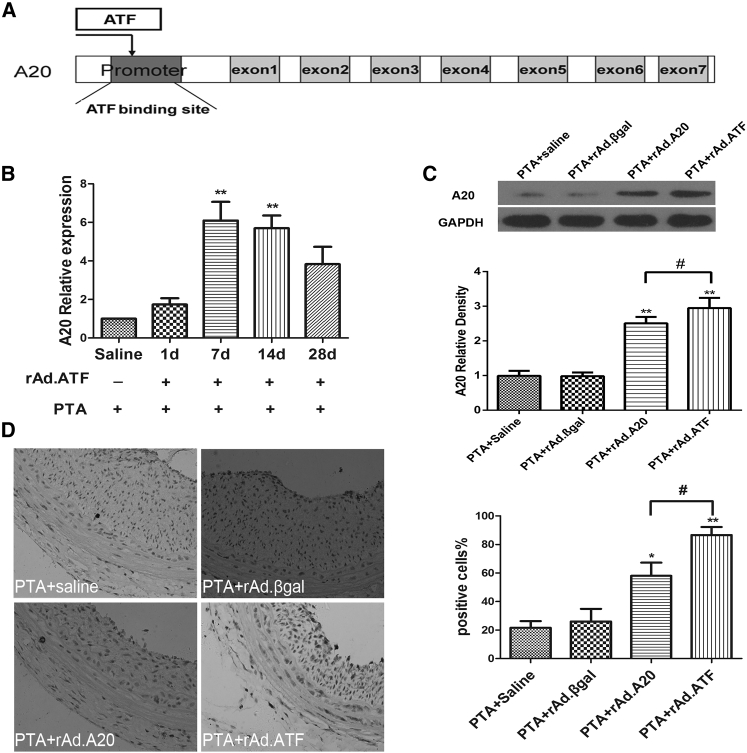
We constructed the ATF (targeting A20) adenovirus vector named rAd.ATF; its functional mechanism is shown in Figure 2A. rAd.ATF was transfected into rat carotid arteries during balloon dilation surgery; subsequently, the expression of A20 was evaluated. Blood vessels at the operative site were collected at days 1, 7, 14, and 28. Expression of A20 was detected by real-time qPCR. The results indicated that the level of A20 was significantly increased after rAd.ATF transfection compared with the sham group, and that the expression of A20 remained high at 28 days after rAd.ATF transfection (Figure 2B). Then the expression of A20 protein among the PTA + saline, PTA + rAd.βgal, PTA + rAd.A20, and PTA + rAd.ATF transfection groups was evaluated using western blot at 7 days after transfection. The result showed that the expression of A20 was markedly increased in the rAd.A20 and rAd.ATF groups compared with the non-transfected group (Figure 2C). Immunohistochemistry further validated that the expression of A20 was significantly increased in the rAd.A20 and rAd.ATF groups compared with the PTA + saline and PTA + rAd.βgal groups (Figure 2D). Interestingly, the increase of A20 in the rAd.ATF group was significantly higher than in the rAd.A20 group. These results confirmed that rAd.ATF transfection could effectively initiate A20 expression in vivo.
Figure 2.
rAd.ATF Transfection Activated A20 Expression in Rat Carotid Arteries in This Restenosis Model
(A) Schematic of the mechanism of A20 gene targeting by ATF. (B) Two microliters of rAd.ATF virus solution (1 × 108 TU/mL) was administered at the injured site immediately after balloon dilation of rat carotid arteries; then A20 mRNA was detected at the indicated time points after rAd.ATF transfection by real-time qPCR. **p < 0.01 (compared with saline and 1 day). (C) A20 protein expression was assayed by western blotting at the operative site of the carotid artery from the different groups at 7 days after balloon dilation injury. *p < 0.05 (compared with PTA + saline and PTA + rAd.βgal); **p < 0.01 (compared with PTA + saline and PTA + rAd.βgal); #p < 0.05. (D) A20 expression was assessed by immunohistochemical analysis at the surgical sites of the carotid arteries from the indicated groups at 7 days after balloon dilation injury (original magnification ×400). All data are expressed as the means ± SD (n = 3–4).
A20 Expression Initiated by ATF Significantly Reduces Balloon Dilatation-Induced Neointima Formation
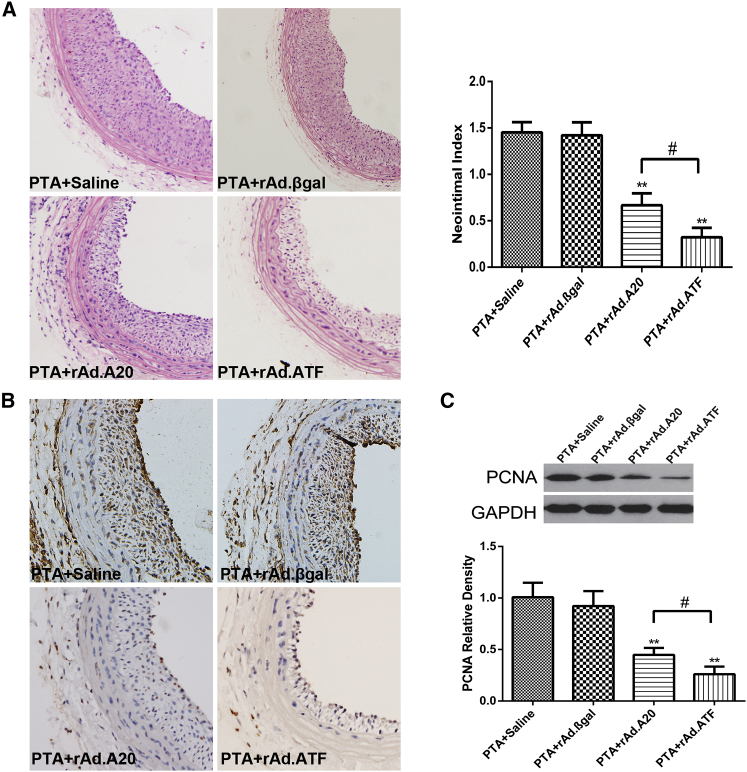
The inhibitive effect of A20 protein on PTA-induced neointima formation in rats was previously reported using rat carotid artery restenosis models.11 To explore whether ATF can prevent neointima formation more effectively, all animals underwent PTA surgery, and the balloon-injured vessels were locally treated with saline, rAd.βgal, rAd.A20, or rAd.ATF. Histomorphological analyses of the balloon-injured carotid artery sections revealed significant focal neointimal hyperplasia in the saline and rAd.βgal groups, but obvious neointimal hyperplasia was not observed in the rAd.ATF- and rAd.A20-treated groups. The neointimal hyperplasia in the PTA + saline and PTA + rAd.βgal groups was 3.9-fold larger than that in the rAd.ATF group. At the same time, compared with the rAd.A20 group, the intimal hyperplasia in the rAd.ATF group was markedly decreased (Figure 3A). Moreover, to clarify the inhibitory effect of ATF transfection on intimal hyperplasia, we detected the expression of proliferating cell nuclear antigen (PCNA) using immunochemistry and western blot, which showed that PCNA was clearly reduced in the rAd.ATF group (Figures 3B and 3C). All results confirmed that ATF transfection could strongly inhibit neointimal formation following vascular injury.
Figure 3.
rAd.ATF Transfection Significantly Reduces Neointima Formation in This Rat Carotid Artery Restenosis Model
(A) Neointima formation was assessed by H&E staining (original magnification ×400). (B) Proliferating cell nuclear antigen (PCNA) expression was assessed by immunohistochemical analysis at the operative site of carotid artery from each group at 7 days after PTA (original magnification ×400). (C) PCNA protein expression was assessed by western blotting at the operative sites of the blood vessels from each group at 7 days after PTA. Data are expressed as the means ± SD (n = 3–6). **p < 0.01 (compared with PTA + saline and PTA + rAd.βgal); #p < 0.05.
Transfection of rAd.ATF Inhibited Inflammation in This Rat Carotid Artery Restenosis Model
Evidence has shown that the inflammatory response plays a central role in the development and progression of restenosis and that it can promote neointimal proliferation after PTA. NF-κB mediates normal biological phenomena and different states of pathology, such as cell growth, cell death, atherosclerosis, and inflammation. NF- κB activation caused by PTA may induce the expression of cytokines, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α).5, 6 In the present study, the role of ATF in PTA-induced inflammation was also studied by examining certain inflammatory molecules, such as NF-κBp65, IL-6, and TNF-α. Western blot for NF-κBp65 protein detection showed a substantial reduction in the neointima of the rAd.A20 and rAd.ATF groups 7 days after PTA (Figure 4A). Furthermore, IL-6, TNF-α, and matrix metallopeptidase (MMP)-9 expression in rat carotid arteries 7 days after PTA was downregulated in the rAd.A20 and rAd.ATF groups (Figures 4B–4D). These data demonstrated that rAd.ATF transfection attenuated the inflammatory responses induced by PTA.
Figure 4.
rAd.ATF Transfection Significantly Reduces the Inflammatory Response Induced by PTA
(A) NF-κBp65 protein expression was assessed by western blotting at the operative site of the carotid artery from each group at 7 days after PTA. (B–D) Proinflammatory factor (B, IL-6; C, TNF-α; D, MMP-9) expression was assessed by ELISA at the operative sites of the blood vessels at 7 days after PTA. All data are expressed as the means ± SD (n = 3–4). *p < 0.05 (compared with PTA + saline and PTA + rAd.βgal); **p < 0.01 (compared with PTA + saline and PTA + rAd.βgal); #p < 0.05.
A20 Inhibited Proliferation, Migration, and Lipopolysaccharide-Induced Inflammation of SMCs via Increasing PPARα Expression
To further confirm whether ATF induces A20 expression in rat VSMCs, we incubated cells with rAd.ATF, rAd.A20, rAd.βgal, or saline for 7 d; then A20 expression was evaluated with real-time qPCR and western blot. The mRNA and protein levels of A20 in the rAd.A20 and rAd.ATF groups were significantly increased compared with those in the rAd.βgal and saline groups (Figures 5A and 5B). The mRNA and protein expression of A20 in the rAd.ATF-transfected group were significantly higher than that in the rAd.A20 group. These results indicate that ATF more efficiently induced A20 protein expression.
Figure 5.
A20 inhibited Proliferation, Migration, and LPS-Induced Inflammation of VSMCs via Increasing PPARα Expression
(A and B) A20 mRNA and protein expression were assessed by real-time qPCR (A) and western blotting (B) in VSMCs at 7 days after transfection. **p < 0.01 (compared with control and Ad.βgal); #p < 0.05. (C and D) PPARα mRNA and protein expression were detected by real-time qPCR (C) and western blotting (D) in VSMCs from each group that had been treated with platelet-derived growth factor (PDGF; 50 ng/mL). **p < 0.01 (compared with PDGF and PDGF + Ad.βgal); #p < 0.05. (E and F) The proliferation (E) and migration (F) of PDGF-induced VSMCs were detected by MTT and Transwell assays, respectively. *p < 0.05 (compared with control and Ad.βgal); **p < 0.01 (compared with control and Ad.βgal); #p < 0.05. (G and H) The secretion of IL-6 (G) and TNF-α (H) in supernatants of VSMCs from each group that had been stimulated with LPS (200 ng/mL) for 48 hr was detected by ELISA. *p < 0.05 (compared with control and Ad.βgal); **p < 0.01 (compared with control and Ad.βgal); #p < 0.05. These experiments were repeated at least three times; all data are expressed as the means ± SD (n = 3–4).
Interestingly, expression of A20 could also increase the expression of PPARα in hepatocytes. PPARα plays a key role in smooth muscle cell (SMC) proliferation and migration.18 To investigate the possible mechanism underlying the A20-meditied inhibition of PTA-induced restenosis, we treated VSMCs from all groups with platelet-derived growth factor (PDGF), which plays a direct role in lesion formation after vascular injury.20 We also used the PPARα antagonist GW6471 (1 mg/mL) to treat VSMCs in the rAd.ATF group. The results showed that PPARα expression was increased in the rAd.A20 and rAd.ATF groups, whereas, as expected, GW6471 partially attenuated PPARα expression in the rAd.ATF + GW group (Figures 5C and 5D). These results indicate that PPARα may be involved in the A20-mediated prevention of VSMC proliferation.
To confirm the involvement of A20 in the prevention of SMC proliferation and migration, we observed the MTT value and mobility of VSMCs in a simulated in vitro proliferation model. The MTT (methyl-thiazolyl-tetrazolium) test was conducted to evaluate VSMC proliferation following PDGF treatment. The MTT values were significantly decreased in the rAd.ATF and rAd.A20 groups compared with the control and rAd.βgal groups (Figure 5E). Representative images showed the mobility of VSMCs following PDGF treatment, indicating that fewer VSMCs migrated in the rAd.ATF and rAd.A20 groups compared with the control, rAd.βgal, and GW groups. In the absence of rAd.ATF and rAdA20, the ability to migrate to the lower compartment was enhanced in the VSMCs treated with PDGF. However, rAd.ATF or rAd.A20 significantly inhibited cell migration under these conditions (Figure 5F). In addition, the suppressive effect of ATF on VSMC proliferation and migration was weakened by GW6471 (Figures 5E and 5F). Moreover, A20 decreased the inflammatory factor expression induced by LPS (lipopolysaccharide) stimulation (200 ng/mL), and this effect was partially reversed by the PPARα antagonist GW6471 (Figures 5G and 5H). These results established that A20, a PPARα agonist, potently inhibits SMC proliferation, migration, and inflammation in vitro, partially by inducing the expression of PPARα.
Discussion
Based on these results, it is clear that ATF-based recombinant adenoviruses can induce the expression of endogenous A20. The expression of A20 protein was markedly increased following the transfection of ATF rather than the transfection of A20 itself. Furthermore, ATF transfection can effectively inhibit carotid artery intimal hyperplasia in the rat model of restenosis. This anti-restenosis role of A20 may be mediated by the increased expression of PPARα and the inhibition of inflammation caused by the NF-κB pathway. We first initiated the expression of A20 using an ATF in restenosis model, and we determined that A20 may suppress restenosis partially through enhancing the expression of PPARα.
Eukaryotic nuclear genes are tightly regulated at both the transcriptional and the translational levels. Transcription factor (TF) proteins are the master regulators of transcriptional activity and gene expression, and hence received much attention in scientific research for drug development and the treatment of diseases.21, 22 Therefore, we developed an ATF, which was delivered using adenoviruses, with the aim to specifically bind to a region upstream of A20 and mimic the structure and function of TFs. In the present study, the ATF size was only 552 bp, whereas A20 cDNA is 4,426 bp, and thus was easily transfected into the cells. We demonstrated that transfection of the ATF could more effectively upregulate endogenous gene expression than transfecting A20 itself in vitro and in vivo, making ATF transfection more desirable. Notably, we did not observe neoplasia during the long-term follow-up (up to 6 months) of mice treated with rAd.ATF, and these results clearly indicated a great potential for ATF in clinical applications.
The main drawbacks of stent implantation are in-stent restenosis (ISR) and stent thrombosis. Inflammation plays a central role in the formation and development of restenosis. NF-κB activation can promote the secretion of various inflammatory mediators, such as IL-6, monocyte chemotactic protein 1 (MCP 1), and TNF-α, and contribute to plaque formation as well as narrowing of the vessel injury site.23, 24, 25 Meanwhile, NF-κB activation enhances the proliferation of SMCs, contributing to the intimal hyperplasia.26, 27 Therefore, inhibiting inflammatory activity is a crucial step for the prevention and treatment of restenosis after angioplasty. A20 is an important negative regulator of the NF-κB pathway and could inhibit neointimal hyperplasia by controlling inflammatory conditions after carotid artery balloon injury.11 Previously, we showed that A20-modified cerebrovascular stent implantation led to mild neointimal hyperplasia compared with the control group.28 However, the A20-modified stent could not effectively control vascular stenosis after implantation, because the stent carried few genes and poor operational stability. Hence, we increased the expression of A20 via the transfection of its ATF, which successfully inhibited local PTA-induced inflammation.
PPARα is widely expressed in many organs and tissues (such as the liver, heart, brain, kidney, and lung).29 Studies have shown that PPARα can inhibit the inflammatory response and is considered to be a new target for the treatment of inflammatory diseases.30, 31 Studies have shown that PPARα agonists can improve endothelial function by inhibiting the production of adhesion molecules, such as vascular cell adhesion protein 1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1), thus altering the recruitment of lymphocytes and monocytes to the vascular wall, inhibiting the smooth muscle cell proliferation induced by IL-1 and IL-6, and repressing the progression of atherosclerosis.18, 32, 33, 34 It is believed that the sustained overactivation of NF-κB plays an important role in the pathogenesis of hypertrophic cardiomyopathy. Studies have shown that PPARα agonists can inhibit the myocardial remodeling induced by pressure overload and improve myocardial systolic function in rats.35, 36 PPARα also plays a significant role in the regulation of neuroinflammation in numerous CNS diseases. Studies have demonstrated that the PPARα agonists inhibit the secretion of inflammatory cytokines IL-1β, IL-6, and TNF-α by LPS-induced astrocytes. PPARα agonists can also reduce neurological defects, brain edema, and cell adhesion factor 1 (ICAM-1) expression in traumatic brain injury (TBI) models by activating PPARα.37 Another study showed that fenofibrate may inhibit nerve inflammation by inhibiting IL-12 family cytokines and MyD88-dependent TLR signaling in experimental autoimmune encephalomyelitis models. In addition, PPARα can also control diseases such as airway disease, inflammatory bowel disease, and liver inflammation by repressing inflammation. These results suggest that PPARα may protect against inflammation and inflammation-related diseases.
The pathological proliferation of SMCs is the direct cause of restenosis and can be promoted by the inflammatory environment of local vascular walls after PTA. Meanwhile, constitutive NF-κB activation is essential for the SMC proliferation response to vascular injury.38, 39 Therefore, anti-inflammatory methods and the inhibition of SMC proliferation are ideal strategies for the prevention and treatment of ISR. PPARα is a member of the PPAR family and is expressed in SMCs; once activated, it may inhibit the proliferation and migration of SMCs treated with PDGF through inhibiting Cdk2 phosphorylation, PCNA expression, and MMP-9 production.16 Thus, increased PPARα has anti-inflammatory effects and inhibits VSMC proliferation. The present study showed that A20 overexpression may raise endogenous PPARα expression and decrease inflammatory cytokine secretion. Interestingly, as shown in the rAd.ATF + GW group (Figures 5E–5H), the PPARα antagonist GW6471 only partly diminished the effects of A20 on SMC proliferation and migration and LPS-induced inflammation, which indicates that A20 may inhibit neointimal hyperplasia partly through increasing PPARα expression. To date, the mechanism underlying A20-induced PPARα expression remains unclear. Available evidence demonstrated that A20 can potently downregulate TLR-mediated inflammation.40 On the other hand, PPARα can negatively regulate TLR4 activity, and therefore exert anti-inflammatory actions.41 Hence, whether A20 upsets the balance between inflammation and PPARα or whether there is a crosstalk between A20 and PPARα is still unclear; additional studies are required to resolve these points.
We sought to demonstrate the ability of an ATF-based recombinant adenovirus to initiate endogenous A20 protein expression and effectively inhibit neointimal hyperplasia. The effects of A20 may be partially mediated by the attenuation of PPARα-dependent inflammation, which could be a potential alternative treatment for post-operative restenosis.
Materials and Methods
Animal Study
Male Sprague Dawley rats, 8–10 weeks old (180–220 g), were obtained and maintained in the Animal Care and Use Center of The Third Military Medical University in Chongqing, China. All procedures involving animal treatments adhered to the NIH Guide for the Care and Use of Laboratory Animals published by the NIH (8th edition, 2011) and were approved by the Institutional Committee of Animal Care and Use. Rats were randomly divided into different groups. All rats in this study were sacrificed by an intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg) followed by cervical dislocation.
Construction of the Balloon Dilatation Restenosis Model in Sprague Dawley Rats
Rats were anesthetized by an intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg). Heart rate and respiration were monitored during the surgical procedure. A quarter of the original dose of anesthesia was supplemented if the rats moved restlessly. The rat restenosis model (the PTA model) was generated as previously described.11 In brief, the right common carotid artery, right external carotid artery, and right internal carotid artery were isolated via a midline incision. The right external carotid artery was ligated with a 6-0 nylon suture, whereas the right internal and common carotid arteries were clamped with vascular clamps. A small opening was cut in the external carotid artery with eye scissors, and the balloon was inserted and inflated at three atmospheres for 20 s. The control, rAd.βgal, rAd.A20, and rAd.ATF groups were given 2 μL of saline, rAd.βgal (1 × 108 transducing units [TU]/mL), rAd.A20 (1 × 108 TU/mL), or rAd.ATF (1 × 108 TU/mL) and maintained for 20 min following the ligation of the opening in the right external carotid artery. The blood vessels affected by this surgery were collected for follow-up tests after 14 d.
Cell Culture and Transfection
Rat VSMCs were prepared from thoracic aortas of male Sprague-Dawley rats (180–220 g) using the collagenase digestion method and cultured as previously described.42 The cells were grown in DMEM (HyClone) supplemented with 10% FBS (Invitrogen) at 37°C with 5% CO2. Approximately 1 × 108 cells were collected and washed three times with PBS. The cells were fixed with 10% neutralized formaldehyde (WAKO) for 24 hr at 4°C. After three washes and incubation at 37°C for 2 hr to remove residual formaldehyde, the cells were washed again and re-suspended in PBS for use as immunogens. At 85% cell confluence in six-well plates, the medium (DMEM + 10% FBS) was changed to serum-free medium for an additional 24 hr to starve the cells prior to the experiments. A ZFP plasmid targeting the A20 gene was kindly provided by Dr. Yong Wei (Department of Anatomy, The Third Military Medical University). The A20 plasmids were from our laboratory, and the recombinant adenoviral vectors were generated by Invitrogen. VSMCs in the rAd.βgal, rAd.A20, and rAd.ATF groups were transduced at an MOI of 100 plaque-forming units (PFUs) per cell, resulting in the expression of the transgene in 95%–100% of cells. The medium was replaced by normal culture medium, and the cells were cultured for 7 days after 8 hr of rAd.βgal, rAd.A20, or rAd.ATF transfection. Control groups were treated with saline.
Assays of Cell Proliferation, Migration, and Inflammation
The cell proliferation was measured using the MTT assay as previously described.43 In brief, cells from different groups were washed with PBS and treated with PDGF (10 ng/mL) for an additional 72 hr; then each well of the plate was treated with 100 μL of MTT solution (0.5 mg/mL) and incubated at 37°C with 5% CO2 for an additional 2 hr. The resultant crystals were solubilized in 500 μL of DMSO, and the optical density (OD) at 450 nm was evaluated and used to calculate the cell proliferation.
The migration of VSMCs in each group through polycarbonate filters with 8-μm pores was measured with a Boyden chamber (24-well unit; Corning) as previously described.22 VSMCs were seeded (1 × 105 cells) into the upper chamber of the plate, whereas serum-free DMEM supplemented with 50 ng/mL PDGF was placed in the lower compartment. After 72 hr of incubation at 5% CO2, the cells on the underside of the membrane were visualized with a Giemsa stain and quantified. VSMCs of the rAd.ATF + GW group were treated with 25 μM GW6471 (GW, a specific PPARα antagonist dissolved in DMSO; Sigma), and the GW was added to the upper compartment. Research has demonstrated that LPS stimulation concurrent with balloon angioplasty in rabbit iliac arteries facilitated neointimal formation. Therefore, for the evaluation of cell inflammation, LPS was used to simulate the inflammatory responses induced by PTA.44 VSMCs from each group were treated with LPS (200 ng/mL) for 48 hr; then the cell culture supernatant was collected, and the concentration of IL-6 or TNF-α was assayed using ELISA.
Real-Time qPCR
Total RNA was isolated with a TRIzol reagent kit (Invitrogen), and cDNA was synthesized using the iScript cDNA synthesis reagent (Fermentas) according to the manufacturer’s instructions. Real-time qPCR was performed in the Bio-Rad iQ PCR machine (Bio-Rad) with the iQ SYBR Green reagent. The primers were purchased from Shanghai Sangon Biotech. The primer sequences used in the study are TNFAIP3, F (forward): 5′-CTCTTCTCCTTTCTGTCCTCAGGTG-3′, R (reverse): 5′CGTGTGTCTGTTTCCTTGAGCGTGGTG-3′; and PPARα, F: 5′-CGGGTCATACTCGCAGGAAAGR-3′, R: 5′-TGGCAGCAGTGGAAGAATCG-3′. Relative gene expression was calculated using the comparative cycle threshold (Ct) method (2-ΔΔCT) as described by the manufacturer. The results were expressed relative to controls.
Western Blot
A20, PCNA, PPARα, and NF-κBp65 proteins from carotid artery tissues or cultured VSMCs were resolved by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes by electroblotting. A monoclonal rabbit anti-TNFAIP3 antibody (Novus), a monoclonal mouse anti-PCNA antibody (Abcam), a polyclonal rabbit anti-PPARα antibody (Santa Cruz), and an NF-κBp65 antibody (Abcam) were used as primary antibodies, and β-actin (Santa Cruz) served as the internal control.
Histology and Immunochemistry
Rats were perfused with saline and 4% paraformaldehyde (PFA); then carotid artery samples were recovered and fixed in 4% buffered PFA. Sections (5.0 μM) were stained with H&E for morphological evaluation. We examined neointima formation with the intima/media (I/M) ratio, which was determined using NIH Imaging software in a blinded fashion as previously described.42 Six rats were used per time point per group, and 6–10 serial sections, 50 μm apart, were examined for each vessel. Immunohistochemical analyses were performed using anti-TNFAIP3 antibody (Novus) and anti-p65 antibody. Immunostaining was evaluated by counting the number of positive cells per high-power field (HPF). We used the appropriate secondary antibodies and isotype-specific negative controls for each antibody. A minimum of three HPFs per slide were analyzed in a blind fashion by inverted microscope.
ELISA
The blood vessels at the operative site of each group were collected 3 days following balloon injury. Cytokine and MMP-9 levels in plasma were measured using the mouse MMP-9, IL-6, and TNF-α ELISA kits (R&D Systems) according to the manufacturer’s protocol.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism. All data are expressed as means ± SD or SE. The results were analyzed by one-way ANOVA followed by a Bonferroni post hoc test or Kruskal-Wallis test for multiple comparisons. All p values <0.05 were considered statistically significant.
Author Contributions
Z.M., P.G., L.C., J.P., and M.W. conducted experiments and analyzed data; J.H. performed statistical analyses; Z.M., K.C., and Z.Z. designed experiments; Z.M. and Z.Z. wrote the paper; and all co-authors contributed to data interpretation, review, and editing of the final paper. The final version of the manuscript was approved by all authors.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Chuhong Zhu and Weng Zeng for their excellent technical assistance. This work was supported in part by the National Natural Science Foundation of China (grants 81000671 and 81471194).
References
- 1.Stettler C., Wandel S., Allemann S., Kastrati A., Morice M.C., Schömig A., Pfisterer M.E., Stone G.W., Leon M.B., de Lezo J.S. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 2.Losordo D.W., Isner J.M., Diaz-Sandoval L.J. Endothelial recovery: the next target in restenosis prevention. Circulation. 2003;107:2635–2637. doi: 10.1161/01.CIR.0000071083.31270.C3. [DOI] [PubMed] [Google Scholar]
- 3.Di Gioia G., Campanale C.M., Mega S., Ragni L., Creta A., Di Sciascio G. Percutaneous treatment of recurrent in-stent restenosis of carotid artery stenting: a case report and state-of-the-art review. Am. J. Case Rep. 2015;16:558–562. doi: 10.12659/AJCR.894198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okura H., Takagi T., Yoshida K. Therapies targeting inflammation after stent implantation. Curr. Vasc. Pharmacol. 2013;11:399–406. doi: 10.2174/1570161111311040004. [DOI] [PubMed] [Google Scholar]
- 5.Bonta P.I., Pols T.W., van Tiel C.M., Vos M., Arkenbout E.K., Rohlena J., Koch K.T., de Maat M.P., Tanck M.W., de Winter R.J. Nuclear receptor Nurr1 is expressed in and is associated with human restenosis and inhibits vascular lesion formation in mice involving inhibition of smooth muscle cell proliferation and inflammation. Circulation. 2010;121:2023–2032. doi: 10.1161/CIRCULATIONAHA.109.885673. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M. Genetic susceptibility to restenosis: role of bone marrow cells and inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2009;29:1407–1408. doi: 10.1161/ATVBAHA.109.194928. [DOI] [PubMed] [Google Scholar]
- 7.McGillicuddy F.C., Moll H.P., Farouk S., Damrauer S.M., Ferran C., Reilly M.P. Translational studies of A20 in atherosclerosis and cardiovascular disease. Adv. Exp. Med. Biol. 2014;809:83–101. doi: 10.1007/978-1-4939-0398-6_6. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar I., Paul J. The deubiquitinase A20 in immunopathology of autoimmune diseases. Autoimmunity. 2014;47:307–319. doi: 10.3109/08916934.2014.900756. [DOI] [PubMed] [Google Scholar]
- 9.Coornaert B., Carpentier I., Beyaert R. A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damrauer S.M., Fisher M.D., Wada H., Siracuse J.J., da Silva C.G., Moon K., Csizmadia E., Maccariello E.R., Patel V.I., Studer P. A20 inhibits post-angioplasty restenosis by blocking macrophage trafficking and decreasing adventitial neovascularization. Atherosclerosis. 2010;211:404–408. doi: 10.1016/j.atherosclerosis.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel V.I., Daniel S., Longo C.R., Shrikhande G.V., Scali S.T., Czismadia E., Groft C.M., Shukri T., Motley-Dore C., Ramsey H.E. A20, a modulator of smooth muscle cell proliferation and apoptosis, prevents and induces regression of neointimal hyperplasia. FASEB J. 2006;20:1418–1430. doi: 10.1096/fj.05-4981com. [DOI] [PubMed] [Google Scholar]
- 12.Mattei E., Corbi N., Di Certo M.G., Strimpakos G., Severini C., Onori A., Desantis A., Libri V., Buontempo S., Floridi A. Utrophin up-regulation by an artificial transcription factor in transgenic mice. PLoS ONE. 2007;2:e774. doi: 10.1371/journal.pone.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y., Ying D., Hou C., Cui X., Zhu C. Design of a zinc finger protein binding a sequence upstream of the A20 gene. BMC Biotechnol. 2008;8:28. doi: 10.1186/1472-6750-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derosa G., Sahebkar A., Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J. Cell. Physiol. 2017 doi: 10.1002/jcp.25804. Published online January 18, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Esmaeili M.A., Yadav S., Gupta R.K., Waggoner G.R., Deloach A., Calingasan N.Y., Beal M.F., Kiaei M. Preferential PPAR-α activation reduces neuroinflammation, and blocks neurodegeneration in vivo. Hum. Mol. Genet. 2016;25:317–327. doi: 10.1093/hmg/ddv477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito E., Rinaldi B., Mazzon E., Donniacuo M., Impellizzeri D., Paterniti I., Capuano A., Bramanti P., Cuzzocrea S. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: involvement of PPAR-α. J. Neuroinflammation. 2012;9:81. doi: 10.1186/1742-2094-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey H.E., Da Silva C.G., Longo C.R., Csizmadia E., Studer P., Patel V.I., Damrauer S.M., Siracuse J.J., Daniel S., Ferran C. A20 protects mice from lethal liver ischemia/reperfusion injury by increasing peroxisome proliferator-activated receptor-alpha expression. Liver Transpl. 2009;15:1613–1621. doi: 10.1002/lt.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gizard F., Amant C., Barbier O., Bellosta S., Robillard R., Percevault F., Sevestre H., Krimpenfort P., Corsini A., Rochette J. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J. Clin. Invest. 2005;115:3228–3238. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahradka P., Wright B., Fuerst M., Yurkova N., Molnar K., Taylor C.G. Peroxisome proliferator-activated receptor alpha and gamma ligands differentially affect smooth muscle cell proliferation and migration. J. Pharmacol. Exp. Ther. 2006;317:651–659. doi: 10.1124/jpet.105.096271. [DOI] [PubMed] [Google Scholar]
- 20.Ferns G.A., Raines E.W., Sprugel K.H., Motani A.S., Reidy M.A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Blue R., Zeitler B., Strange T.L., Pearl J.R., Huizinga D.H., Evans S., Gregory P.D., Urnov F.D., Petolino J.F. Activation domains for controlling plant gene expression using designed transcription factors. Plant Biotechnol. J. 2013;11:671–680. doi: 10.1111/pbi.12057. [DOI] [PubMed] [Google Scholar]
- 22.Guan X., Stege J., Kim M., Dahmani Z., Fan N., Heifetz P., Barbas C.F., 3rd, Briggs S.P. Heritable endogenous gene regulation in plants with designed polydactyl zinc finger transcription factors. Proc. Natl. Acad. Sci. USA. 2002;99:13296–13301. doi: 10.1073/pnas.192412899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasser K., Schnaudigel S., Wohlfahrt J., Psychogios M.N., Knauth M., Gröschel K. Inflammation and in-stent restenosis: the role of serum markers and stent characteristics in carotid artery stenting. PLoS ONE. 2011;6:e22683. doi: 10.1371/journal.pone.0022683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niccoli G., Montone R.A., Ferrante G., Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J. Am. Coll. Cardiol. 2010;56:1783–1793. doi: 10.1016/j.jacc.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 25.Niccoli G., Montone R.A., Ferrante G., Minelli S., Crea F. [Clinical value of inflammatory biomarkers after stent implantation] G. Ital. Cardiol. (Rome) 2011;12:635–644. doi: 10.1714/945.10348. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T., Yamashita M., Horimai C., Hayashi M. Smooth muscle-selective inhibition of nuclear factor-κB attenuates smooth muscle phenotypic switching and neointima formation following vascular injury. J. Am. Heart Assoc. 2013;2:e000230. doi: 10.1161/JAHA.113.000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander M.R., Murgai M., Moehle C.W., Owens G.K. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol. Genomics. 2012;44:417–429. doi: 10.1152/physiolgenomics.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z., Shi S., Song M., Huang H., Chen K., Mi J., Li L., Chen G., Hou C., Huang G., Zhu C. Development of transgenic endothelial progenitor cell-seeded stents. J. Biomed. Mater. Res. A. 2009;91:623–628. doi: 10.1002/jbm.a.32300. [DOI] [PubMed] [Google Scholar]
- 29.Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 30.Montecucco F., Liberale L., Bonaventura A., Vecchiè A., Dallegri F., Carbone F. The role of inflammation in cardiovascular outcome. Curr. Atheroscler. Rep. 2017;19:11. doi: 10.1007/s11883-017-0646-1. [DOI] [PubMed] [Google Scholar]
- 31.Li B., Li W., Li X., Zhou H. Inflammation: a novel therapeutic target/direction in atherosclerosis. Curr. Pharm. Des. 2017;23:1216–1227. doi: 10.2174/1381612822666161230142931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delerive P., De Bosscher K., Besnard S., Vanden Berghe W., Peters J.M., Gonzalez F.J., Fruchart J.C., Tedgui A., Haegeman G., Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 33.Marx N., Sukhova G.K., Collins T., Libby P., Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X., Otsuki M., Saito H., Sumitani S., Yamamoto H., Asanuma N., Kouhara H., Kasayama S. PPARalpha and GR differentially down-regulate the expression of nuclear factor-kappaB-responsive genes in vascular endothelial cells. Endocrinology. 2001;142:3332–3339. doi: 10.1210/endo.142.8.8340. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q., Chen Y., Auger-Messier M., Molkentin J.D. Interaction between NFκB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ. Res. 2012;110:1077–1086. doi: 10.1161/CIRCRESAHA.111.260729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaspar-Pereira S., Fullard N., Townsend P.A., Banks P.S., Ellis E.L., Fox C., Maxwell A.G., Murphy L.B., Kirk A., Bauer R. The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am. J. Pathol. 2012;180:929–939. doi: 10.1016/j.ajpath.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X.R., Besson V.C., Palmier B., Garcia Y., Plotkine M., Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J. Neurotrauma. 2007;24:1119–1131. doi: 10.1089/neu.2006.0216. [DOI] [PubMed] [Google Scholar]
- 38.Bourcier T., Sukhova G., Libby P. The nuclear factor kappa-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J. Biol. Chem. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- 39.Bellas R.E., Lee J.S., Sonenshein G.E. Expression of a constitutive NF-kappa B-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J. Clin. Invest. 1995;96:2521–2527. doi: 10.1172/JCI118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shembade N., Ma A., Harhaj E.W. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji Y.Y., Liu J.T., Liu N., Wang Z.D., Liu C.H. PPARalpha activator fenofibrate modulates angiotensin II-induced inflammatory responses in vascular smooth muscle cells via the TLR4-dependent signaling pathway. Biochem. Pharmacol. 2009;78:1186–1197. doi: 10.1016/j.bcp.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 42.Schiller N.K., McNamara D.B. Balloon catheter vascular injury of the alloxan-induced diabetic rabbit: the role of insulin-like growth factor-1. Mol. Cell. Biochem. 1999;202:159–167. doi: 10.1023/a:1007005919319. [DOI] [PubMed] [Google Scholar]
- 43.Li Y.H., Chung H.C., Liu S.L., Chao T.H., Chen J.C. A novel inhibitory effect of Antrodia camphorata extract on vascular smooth muscle cell migration and neointima formation in mice. Int. Heart J. 2009;50:207–220. doi: 10.1536/ihj.50.207. [DOI] [PubMed] [Google Scholar]
- 44.Danenberg H.D., Welt F.G., Walker M., 3rd, Seifert P., Toegel G.S., Edelman E.R. Systemic inflammation induced by lipopolysaccharide increases neointimal formation after balloon and stent injury in rabbits. Circulation. 2002;105:2917–2922. doi: 10.1161/01.cir.0000018168.15904.bb. [DOI] [PubMed] [Google Scholar]