Abstract
Nonalcoholic fatty liver diseases (NAFLD) is one of the most common chronic liver disease in Western countries. Oxygen is a central component of the cellular microenvironment, which participate in the regulation of cell survival, differentiation, functions and energy metabolism. Accordingly, sufficient oxygen supply is an important factor for tissue durability, mainly in highly metabolic tissues, such as the liver. Accumulating evidence from the past few decades provides strong support for the existence of interruptions in oxygen availability in fatty livers. This outcome may be the consequence of both, impaired systemic microcirculation and cellular membrane modifications which occur under steatotic conditions. This review summarizes current knowledge regarding the main factors which can affect oxygen supply in fatty liver.
Abbreviations: ALA, α-lipoic acid; AMPK, AMP-activated protein kinase; eNOS, endothelial NO synthase; ECM, extracellular matrix; ET-1, endothelin-1; EVs, extracellular vesicles; HCS, Hepatic stellate cells; HI, intermittent hypoxia; NO, nitric oxide; NAFLD, nonalcoholic liver diseases; NASH, nonalcoholic steatohepatitis; OSA, obstructive sleep apnea; PC, phosphatidylcholine; PE, phosphatidylethanolamine; RBS, red blood cells; ROS, reactive oxygen species; TXA2, thromboxane A2; T2D, type 2 diabetes
Keywords: Oxygen, Fatty liver, Nitric oxide, Inflammation
Graphical abstract
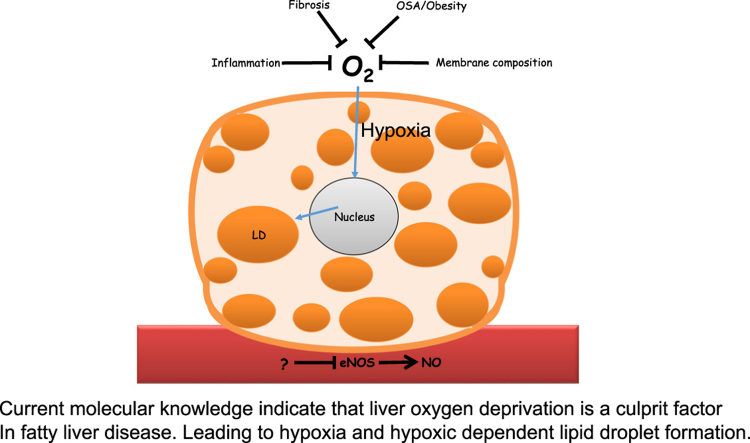
Current molecular knowledge indicate that liver oxygen deprivation is a culprit factor In fatty liver disease. Leading to hypoxia and hypoxic dependent lipid droplet formation.
1. Introduction
Aerobic organisms cannot survive without oxygen [1]. Oxygen is an important component of the cellular microenvironment, which regulates cell survival, differentiation and function. The liver is highly metabolic tissue, where oxygen is essential as an electron acceptor in energy metabolism. As such, adequate oxygen supply to the liver is extremely critical for this tissue's function [2], [3]. Due to the liver structure and metabolism, the blood composition significantly alters during the passage through the sinusoids, leading to the formation of periportal-to-perivenous concentration gradients of substrates, products and hormones [3]. Oxygen partial pressure in the periportal blood (zone 1) is about 60–65 mm Hg (84–91 µmol/L, 9–11% oxygen) and falls as blood percolates throw the liver lobules towards the perivenuous (zone 3), where the oxygen partial pressure is about 30–35 mm Hg (42–49 µmol/L, 5–7% oxygen) [3], [4]. Oxygen regulates metabolic zonation in normal liver and under pathological condition serves as a modulator of liver diseases [3]. Oxygen delivery also plays an important role in other hepatic process, such as hepatic redox state. It was also observed that reduced intrahepatic oxygen levels in zone 3 are associated with enhanced susceptibility of these cells to anoxia-induced damage [4]. These findings indicate that hepatocytes oxygen availability prior to the occurrence of stress can dramatically affect the outcome.
Non alcoholic liver diseases (NAFLD) encompass a wide spectrum of liver pathologies, ranging from simple steatosis through steatohepatitis (NASH) to cirrhosis. NAFLD is currently considered the most common chronic liver disease in Western countries and is steadily increasing along with the worldwide epidemic of obesity and type 2 diabetes (T2D) [5]. Substantial line of evidence suggests hepatic perfusion abnormalities exist in the presence of NAFLD. Moreover, it was further suggested that steatosis-induced reduced oxygen delivery to the liver may play a critical role in liver diseases related to steatosis [6]. In the present article we review several of the main causes for impaired liver oxygen availability under NAFLD. The consequences of such impairment are beyond the scope of this mini review.
2. Hepatic vascular network
The hepatic vascular network consists of dual distinct blood inflow systems: the hepatic artery, which delivers oxygen-rich blood and the portal vein which drains the gastrointestinal tract and delivers nutrient-rich blood [7]. The hepatic artery, contributes ~ 30% of the total hepatic blood flow and the portal vein, contributes ~ 70% of the total hepatic blood flow [8]. The intrahepatic branches of the portal vein, hepatic artery, along with the bile duct run together within the portal tract system, branching out through ~ 17–20 orders of branches in order to deliver the entire corpus of the liver [9]. Finally, blood returned to the systemic circulation through at least three distinct hepatic veins (right, middle and left) which drains into the inferior vena cava.
The hepatic sinusoids comprise one of the largest-caliber vascular beds in the body [9]. Hepatic sinusoids are the location of pressure equalization between systemic and portal venous flow [10]. Impairment of blood flow through this vascular bed constitutes a major loss of physiologic function, with profound influence on homeostasis for the entire human organism [9].
Substantial changes in blood flow were demonstrated in human and animal models of fatty liver [11], [12], [13], [14], [15]. Recently, it was suggested that NAFLD may serve as a risk factor for the development of cardiovascular diseases primarily due to dysfunction in endothelial dependent vasodilation [16], [17]. In relation to the hepatic vascular network, decreased portal vein flow was found in patients with fatty liver [18], [19]. By using Doppler flowmetry, Seifalian et al. observed reduced sinusoidal perfusion in steatotic human liver grafts compared to healthy livers [11]. Decreased parenchymal perfusion was also found by using positron emission tomography in individuals with T2D and liver steatosis [20]. Likewise, Guiu and colleagues also found decreased perfusion-related diffusion in patients with T2D and liver steatosis, which was attributed to decreased parenchymal perfusion [19]. In accordance with human studies, several animal models of liver steatosis (rats, mice and rabbits) also demonstrate the presence of microvascular abnormalities in models of fatty liver characterized by the presence of reduced sinusoidal perfusion and dysfunctional sinusoids [11], [12], [15], [21], [22], [23], [24].
3. Morphology changes
Abnormal microvascular function and reduced oxygen consumption under steatotic condition can be explained by several mechanisms/factors. Previous studies have suggested that reduction in sinusoidal perfusion arises initially from the influence of enlarged hepatocytes. Increased accumulation of lipids within the cytosol causes the hepatocytes to swell (enlargement of cell size). Subsequently, this enlargement widens the paranchymal cell plates, narrow and distorts the lumens sinusoids and reduces the intrasinusoidal volume as well as the architecture of the sinusoidal network, leading to reduced oxygen supply to hepatocytes. Morphological changes of reduced sinusoidal perfusion area as a result of enlarge hepatocytes were corroborate by using microscopic methods in genetic animal models obese zucker rats (fa/fa), ob/ob and foz/foz mice and dietry-induced liver steatosis [11], [25].
Importantly, reduction in oxygen availability can impair fatty acid oxidation, leading to further fat accumulation and exacerbation of fat-induced disturbances in sinusoidal perfusion, which generates a vicious circle of disease progression [25].
Extracellular vesicles (EVs) are lipid coated particles with a diameter of up to 1000 nm, which are released from different cell types. EVs and have been shown to have pathophysiological roles in a many disease states. NAFLD is consider the hepatic manifestation of metabolic syndrome. During a metabolic diseases organ crosstalk and lipid delivery from the adipose tissue to the liver or from liver non-parenchymal cells to hepatocytes could be a major factor for liver hypoxic/steatotic response. Indeed Evs released from adipose tissue may modulate liver steatosis [26]. A cross talk relationship was recently demonstrated between hypoxia inducible factor activation, nitric oxide production and EVs modulation. This has major response in endothelial cells EVs production [27], and could be an early marker for NAFLD [28].
4. Nitric oxide (NO)
NO is an essential vasodilator molecule. Beside this effect, intensive evidence further illustrate that NO involves in various processes that are beneficial to vascular homeostasis, including reduction of vascular smooth muscle migration and growth, platelet aggregation and thrombosis, monocyte and macrophage adhesion and inflammation [29].
Deficiency in endothelial NO synthase (eNOS)-derived NO may be important in the etiology of NAFLD. Alterations in NO metabolism and bioavailability can contribute to tissue injury under NAFLD as well as other NAFLD-related diseases, such as obesity and diabetes [30]. Decreased NO bioavailability was found in steatotic livers following high fat diet (HFD) or high cholesterol diet (diets induced steatosis) in rodents [24], [30]. Impaired NO metabolism was associated with significant lower portal blood flow and reduced hepatic microcirculation. Conversely, in steatotic livers, l-Arginine administration improved hepatic arterial and portal blood flows as well as microcirculation and increased hepatic tissue oxyhemoglobin, whereas L-NAME significantly worsened these parameters [24]. Decreased NO bioavailability in diet-induced steatosis models was couples with changes in two key enzymes involved in the control of NO metabolism in liver, eNOS and/or arginase 1. Although total eNOS levels were not found to be profoundly altered, the extent of Ser1177 phosphorylation of eNOS was significantly decreased by a diet-induced liver steatosis [24], [30].
Insulin is one of the main factors possibly involved in microvascular abnormalities and specifically in impaired eNOS activation observed in NAFLD. Beneficial vascular effects of insulin, particularly on the endothelium was demonstrated. Insulin promotes NO production through the activation of the PI3K/Akt/eNOS signaling pathway, leading to vasodilation and vascular protection [31]. NAFLD is often associated with insulin resistance [25]. Therefore, the development of insulin resistance under diet-induced liver steatosis might subsequently impairs insulin-induced vasodilation and cause the development of endothelial dysfunction, which further contribute to vascular damage [31]. Pasarin and colleagues [23] have demonstrated that vascular responses to insulin are indeed impaired in a model of early NAFLD and are associated with insulin resistance in the liver sinusoidal endothelium. By using a model of diet-induced obesity as well as isolated and perfused livers, they observed an increased hepatic vascular resistance with impaired vasodilatory response of the liver vascular bed to acetyl-choline. These abnormalities were associated with a decreased Akt-dependent eNOS phosphorylation and NOS activity which may impair endothelial dependent vasodilation. Importantly, in their studies, liver endothelial dysfunction occurred before the development of fibrosis or inflammation [23].
AMP-activated protein kinase (AMPK) has also been shown to phosphorylate and activate eNOS at Ser1177 in cultured endothelial cells [32]. It was suggested that treatments which induce liver/hepatic fat accumulation are also associated with abnormalities in AMPK activation [33], [34], [35], [36], [37]. However, the direct association between AMPK and eNOS phosphorylation and its effects on the microvascular system and endothelial function has yet to be shown. Nevertheless, Lee et al., have also demonstrated endothelial dysfunction in obese rats was associated with lower AMPK activating in aortic endothelium [38]. In their study, reduced AMPK activation was connected with impaired endothelium-dependent vascular relaxation and decreased NO synthesis. Administration of α-lipoic acid (ALA) normalized AMPK activation and improved endothelial function. However, eNOS phosphorylation was not assessed in that study. The postulated mechanism underlying reduced NO bioavailability was inactivation of NO by oxygen-derived free radicals. It was proposed that reduced AMPK activity leads to a decrease in fatty acid oxidation and an increase in intracellular malonyl coenzyme A levels. Thus, increased flux of free fatty acids (FFA) from the circulation concomitantly with decreased FFA oxidation in aortic endothelial cells would eventually lead to excessive accumulation of triglyceride and long chain acyl coenzyme A. This was suggested to be an initial event which leads to a cascade of increased reactive oxygen species (ROS) production, increased apoptosis, and decreased NO bioavailability in endothelial cells and vascular dysfunction.
Arginase 1 levels were found to be significantly increased by HFD [30]. Arginase 1 catalyzes the hydrolysis of l-arginine into ornithine and urea. Consequently, it is speculated that increased arginase 1 also contributes to the decrease in NO metabolites observed in fatty livers, by depleting eNOS substrate, l-arginine.
eNOS regulation is further complex as its regulation also occurs at the post-translational level. Post translation modification of eNOS can also be conducted by caveolin-1 (cav-1). Caveolae are flask-shaped vesicular invaginations in the plasma membrane and are considered to be a subset of lipid rafts [39]. Caveolae have also been implicated in chronic inflammatory conditions and other pathologies, including atherosclerosis and generalized dyslipidaemia [40]. Evidence indicates caveolae are also important to vascular function and homoestasis [40]. Cav-1 represents the main structural protein of caveolae [41]. Although little information exist regarding the pathophysiological role of cav-1 in NAFLD, increased cav-1 levels were found in the livers of rodents models of this disease [39], [41], [42].
Cave-1 is important regulator of vascular tone and vascular reactivity, among others, by regulating endothelial NO production, microvascular permeability, cellular Ca2+ entry, vascular remodeling and angiogenesis. Direct protein–protein interaction between cav-1 and eNOS was demonstrated in vivo. This interaction significantly inhibits eNOS activity, resulting in sequestering of eNOS in caveolae and reduced NO production [40]. Accordingly, increased binding of cav-1 to eNOS in injured liver is associated with reduced eNOS activity [43], while loss of cav-1 leads to persistent eNOS activation and high levels of NO in cells [40].
These findings highlight the importance of cav-1 in the regulation of eNOS function. More research is needed to elucidate the importance of cav-1 in vascular alternations observed in NAFLD.
5. Inflammation and fibrosis
NASH is characterized by hepatic inflammation and varying degrees of fibrosis [44]. Both of which can affect sinusoidal function as well as other factors thus influence hepatic perfusion.
5.1. Inflammation
The concept that hypoxia can promote the development of inflammation is well accepted. However, as hypoxia can induce inflammation, inflamed lesions often become severely hypoxic. Indeed, hypoxia or anoxia, hypoglycaemia and acidosis are characteristic features of inflamed tissues [45]. Hypoxia and inflammation are intertwined at the molecular, cellular, and clinical levels. Although the contribution of hypoxia to the inflammatory response is beyond the scope of this review, it seems that oxygen-sensing mechanisms and hypoxia signaling are potential therapeutic targets for the treatment of inflammatory diseases [46].
Decreased tissue oxygenation during inflammation is most likely a consequence of altered blood flow, which is secondary to microvascular injury, thrombosis or increased interstitial pressure coupled with increased metabolic demands of cells and activities of multiplication of intracellular pathogens that can deprive infected cells of oxygen [45], [46].
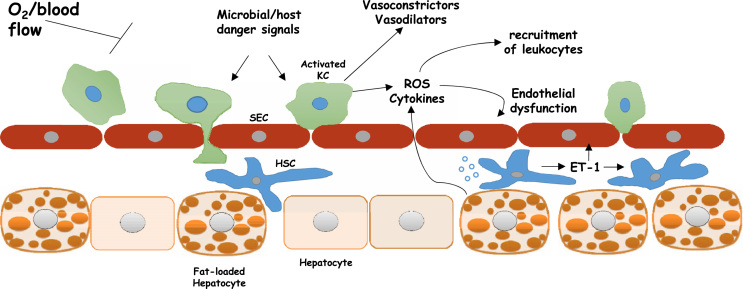
Kupffer cells have been suspected to be critically involved in the progression of non alcoholic steatosis to steatohepatitis and fibrosis. Although several factors/causes can induce kupffer cells activation, the space-occupying effect of fat-laden hepatocytes may not only lead to impaired sinusoidal perfusion but may also interfere with sinusoid microcirculation and hepatocellular clearance of microbial and host-derived danger signals, thus, enhancing responsiveness of Kupffer cells [47]. In turn, activation of kupffer cells can also contribute to the reduction of hepatic perfusion. Indeed, Kupffer cells were found to contribute directly to hepatic microcirculatory dysfunction as well as to liver damage following inflammatory stress. Conversely, depletion of Kupffer cells significantly ameliorates the microcirculatory perturbations of trauma and sepsis [48]. In accord with these findings, Kupffer's cell depletion led to a strong effect on portal and arterial blood flow, with an > 50% increase in total liver blood flow following liver transplantation in pigs [49]. Kupffer cells are known to express a variety of vasoregulatory enzyme systems with the release of vasoactive mediators, including endothelins, ROS, NO and prostanoids, which can cause changes in endothelial cells and activation and contraction of hepatic stellate cells (HSC) [22]. Kupffer cells are also likely the primary source of increased Thromboxane A2 (TXA2) release under LPS injection. TXA2-induced receptor activation was observed to mediate hyperresponsiveness of hepatic portal circulation to endothelin-1 (ET-1), significantly potentiate its contractile effect and maybe also to contribute to endotoxin-induced hepatic microcirculatory failure [50]. However, it should be mentioned that Kupffer cells activation may not always results in reduction of hepatic perfusion. One should keep in mind that the finely tuned balance between vasoconstrictors and vasodilators is essential for the local regulation of the hepatic microcirculation. As such, it was proposed that during regeneration Kupffer cells may be involved in the local regulation of hepatic microcirculation, being mandatory for mediating intrahepatic hyperperfusion [51] (Fig. 1).
Fig. 1.
Sinusoidal cellular interactions that attenuates oxygen availability within the liver. Such interaction regulates oxygen availability During the different stages of the non-alcoholic fatty liver disease. Fat infiltration, Inflammation, and fibrosis. HSC, hepatic stellate cells; ET-1, endothelin-1; Kupffercells; ROS, reactive oxygen species; SEC, sinusoidal endothelial cells.
In the presence of liver steatosis, hepatocytes, Kupffer cells, and possibly other resident liver cells, can be stimulated to produce cytokines and chemokines. The production of these factors can be enhanced through intracellular or extracellular pathways, by the activation of IκB kinase and c-Jun N-terminal kinase or Toll-like receptors, respectively. Thereafter, these compounds can act in an autocrine or paracrine manner to promote cell death, ROS production and the recruitment of leukocytes from the circulation [52]. Indeed, one of the characteristic morphological features of steatohepatitis is, among others, inflammation that is predominantly lobular inflammation and includes the presence of leukocytes [53]. As abovementioned, the existence of liver steatosis can reduce sinusoidal blood flow as well as the diameters of sinusoids. At locations with narrowed lumen, leukocytes can transiently occlude the vessel and thus obstruct the blood flow [54]. Importantly, although stagnant leukocytes probably unable to completely occlude the sinusoidal lumen, they can further add to the impeded flow of the hepatic microcirculation [55]. Transient leukocyte plugging appears to more common at the periportal sinusoids given that they are narrower and more tortuous compared to those at the centrilobular region [54].
Growing body of experimental studies also link inflammation to endothelial dysfunction and loss of NO bioactivity. In vitro studies illustrated proinflammatory factors such as tumor necrosis factor alpha, C-reactive protein, and ox-LDL lead to lower eNOS expression and consequently decrease NO production. Decreased eNOS messenger RNA stability, at least in part, appears to mediate this effect. Cytokines can further decrease the bioavailability of NO by increasing the production of ROS. Enhanced ROS generation and levels in endothelial cells can directly react and decrease the activity of NO as well as to ameliorate the production or effects of NO through oxidative modification of eNOS and/or guanylyl cyclase. In agreement with these findings, studies also showed impairment in endothelial function in normal arteries which were exposed to chronic immune response. This finding strongly implies proinflammatory states lead to a loss of the bioactivity of endothelium-derived NO also in humans. Consist with this notion, correlation between circulating markers of inflammation and endothelial dysfunction was found in observational studies. Finally, anti-inflammatory therapies, including nonselective and selective COX inhibitors, have been reported to improve endothelial function and endothelium-dependent dilation [56]. However, given that NASH state involves several factors that have impact on endothelial cells the specific importance of inflammation to the abnormalities observed in NASH is difficult to reveal.
5.2. Fibrosis
Hepatic fibrogenesis is the liver's wound healing response to injury and can lead to cirrhosis [57]. Recently, NASH has been recognized as a major cause of liver fibrosis [58] with the degree of fibrosis being an important factor for monitoring disease progression. Fibrosis due to NASH is usually characterized in Perisinusoidal/pericellular collagen deposition, with primary accumulation of extracellular matrix (ECM) in the space of Disse. Collagen is initially deposited in acinar zone 3. As the disease progress, portal fibrosis can develop and ultimately bridging fibrosis and finally cirrhosis are noted [59]. Collagen deposition in the space of Disse may also restrict sinusoidal perfusion. McCuskey et al. [22] have demonstrated that collagen deposition in the space of Disse, along with hepatocytes swelling, render sinusoids tortuous and increase the proportion of sinusoids that completely fail to allow passage of erythrocytes. Consequently, sinusoids become inefficient conduit of blood, which lead to impaired tissue perfusion. Under fibrotic conditions, deposition of collagen as well as other extracellular proteins in the space of Disse covers the sinusoids with a connective tissue. These changes impede oxygen diffusion and impair nutrients/waste products exchange among intravascular compartment and the hepatocytes [22]. HSCs play a fundamental role in fibrogenesis. Following chronic injury, perisinusoidal HSCs become activated and this activation increases their contractility (Fig. 1) [40].
Beside their ability to modulate ECM, activation of HSCs is also thought to play a role in the regulation of sinusoidal caliber and blood flow [40], [60]. Indeed, accumulating evidence point at the perisinusoidal stellate cell as the cell responsible for controlling sinusoidal diameter. Tonic contraction of HSCs, an important feature of activated HSCs, causes increased resistance to blood flow in the hepatic sinusoid and plays a pivotal role in portal hypertension [58], [61], [62]. Accordingly, contractility of stellate cells may be a major determinant of early and late increases in portal resistance during liver fibrosis [63].
As mentioned earlier, ET-1 represents the most potent vasoconstrictor [64]. Under normal conditions, the primary source of ET-1 is the sinusoidal endothelial cells (along with endothelial cells in the peripheral vasculature). However, during liver injury, stellate cells become the major source as well as a target of this cytokine while its production by sinusoidal endothelial cells is actually decreased. Endothelin receptors can be identified on the surface of all hepatic cell types, but are mostly located on stellate cells. Moreover, experimental models of fibrogenesis demonstrated upregulation of endothelin receptors levels/density specifically on stellate cells after injury. ET-1 has a prominent contractile effect on stellate cells and myofibroblasts. Indeed, ET-1, was shown to cause contraction of isolated stellate cells. Exposure of isolated cultured stellate cells to ET-1 also led to the stimulation of smooth muscle actin, proliferation, and extracellular matrix protein synthesis. Therefore, increased ET-1 levels and activity during liver injury not only promote fibrogenesis but also results in augmented resistance to sinusoidal blood flow. The later appears to be primarily due to the net effect of enhanced stellate cell contractility and sinusoidal constriction by ET-1. Stellate cells also produce NO and its production is attributable to the activity of the inducible form of NO synthase (iNOS) [43], [60], [62]. Currently the role of iNOS-derived NO in hepatic fibrogenesis is controversial. Nevertheless, eNOS-dependent NO production is reduced after chronic injury and fibrogenesis [65].
Beside ET-1 and NO, many other vasoactive compounds have effects on stellate cells and therefore can modulate intrahepatic resistance. Yet, available data suggest ET-1 and NO have the most prominent effects on isolated stellate cells and also that they have the most profound effects on intrahepatic resistance and blood flow in the liver in vivo [43], [60], [62]. However, the fact that stellate cells can be affected by many vasoactive compounds reinforces and emphasizes their potential role in vasoregulation.
6. Obstructive sleep apnea (OSA)
Obstructive sleep apnea (OSA) was found to be prevalence in NAFLD patients [66]. This can cause chronic intermittent hypoxia (IH) and therefore to aggravate irregularities in liver perfusion as well as this disease development and progression [67], [68], [69], [70]. OSA is characterized by recurrent upper airway collapse during sleep resulting in fragmentation of sleep and recurrent oxyhemoglobin desaturations termed chronic IH [70]. OSA is accompanied by hypoxia in target tissues and with hemodynamic changes associated with dysregulation of the sympathetic nervous system [69]. Accumulating evidence connects chronic IH, caused by OSA, to the development and progression of NAFLD [67]. In a mouse model of IH, which mimics oxyhemoglobin desaturations as observed in patients with OSA, lean mice did not immediately develop liver injury and a mild increase in serum ALT was observed only after 12 weeks. Conversely, in obese mice, IH dramatically exacerbated liver injury as indicated by increased degree of hepatic steatosis, liver lipid peroxidation, elevated plasma liver enzymes levels and aggravated insulin resistance as early as after 4 weeks. In obese mice IH also enhanced the expression of pro-inflammatory cytokines indicating the progression of hepatic steatosis to steatohepatitis. These finding suggest the presence of obesity and/or liver steatosis potentiate the detrimental effects of IH. Consistency, Savransky et al., also found that prolonged IH (6 months) promoted the progression of diet-induced hepatic steatosis to NASH with liver fibrosis [70]. However, it should be noted that these experiments in obese mice illustrate that IH and obesity interact to exacerbate hepatic steatosis converting it to steatohepatitis. Although patients with NAFLD often also suffer from obesity, the exact contribution of IH to the transition of NAFLD to NASH in the absence of obesity cannot be concluded from these findings. In this regard, a recently meta-analysis conducted by Musso et al. [71] which pooled 2183 participants from 18 cross-sectional studies suggests that the presence of OSA confers an over twofold increased risk of having NAFLD, and about 2-fold increased risk of progressive NASH and fibrosis in patients with NAFLD independent of BMI and waist circumference (as wells as of age and gender). This meta-analysis also found a dose–response relationship between the severity of OSA and the severity of liver disease in NAFLD patients. Currently, obesity is associated with liver hypoxia which may further aggravate in the presence of IH or OSA [72]. However, obesity is also associated with other disorders which may affect oxygen availability such as obesity hypoventilation syndrome. Whether or not intrahepatic oxygen tension is lower in non-obese NAFLD individuals with OSA compared with subjects without NAFLD but with OSA is yet to be determined.
Nevertheless, the presence of liver hypoxia may enhance tumor malignancy, including cellular carcinoma [73], [74], [75], [76]. Importantly, hypoxia may also promote radioresistance of carcinoma cells potentially by upregulating nonhomologous end-joining pathway, which plays a central role in DNA repair of irradiated cells [75]. In agreement with these findings, recent evidence suggests sleep apnea and, specially, IH may worsen the prognosis of cancer [77]. Thus, the coincidence of OSA and NAFLD under the background of HCC may suggest enhanced vulnerability and possibly poor outcome.
7. Membrane composition
Along with systemic factors, another parameter which may influence intracellular oxygen concentration is the oxygen permeability coefficient across the membrane.
One of the most essential properties of biological membranes is that they serve as barriers which inhibit to the penetration of polar molecules [78].
According to the Fluid Mosaic Model, a biological membrane is a two dimensional fluid of oriented proteins and lipids. Proper functions of membranes require a fluid plasticity which is achieved by the modification in lipid composition [79]. Oxygen is classified as a fat-soluble, non polar, molecule and can rapidly dissolve in the hydrocarbon core of the cell membrane [80]. Results obtained by several works implied that the membrane bilayer is a resistance factor to oxygen diffusive passage or to the diffusion of small soluble lipid molecules [79]. Contrary to these studies, recent data indicates that membranes are not barriers to oxygen transport into the cell as well as to the mitochondrion and that the created oxygen concentration differences across these membranes under physiological conditions are insignificant [78]. However, this conclusion is not unambiguous. In membranes with high cholesterol concentrations the solubility and diffusion of oxygen are impaired [78], [79], [81], [82], [83], [84], [85], [86].
Cholesterol is the most prominent sterol of mammalian cells. Cholesterol predominantly located in the plasma membrane, where it serves as a non-polar hydrophobic lipid component. One of the most important functions of cholesterol is its ability to modulate the physicochemical properties of cellular membranes and maintaining its fluidity and architecture [87], [88]. In the membrane, cholesterol also prevents the leaking of polar molecules across the membrane [82].
Cholesterol and oxygen seem to be intricately entangled and the relationship between the two seems to be reciprocal. While cholesterol synthesis is an oxygen-intensive process, which requires 11 oxygen molecules, it has been reported that cholesterol restricts membrane oxygen diffusion and plays as a determinant of oxygen gradient in cellular systems [82], [83], [88].
The ability of cholesterol accumulation in the plasma membrane to ameliorate the diffusion of oxygen across membranes and to limit intracellular oxygen availability was demonstrated in several cells as well as in model membranes. Studies conducted in Chinese hamster ovary (CHO) cells suggest plasma membrane cholesterol serves as a barrier to oxygen and can be an important determinant for the extent of oxygen gradient observed across the plasma membrane [81].
Likewise, in fiber-cells, which comprise the mammalian lens, cholesterol levels in membranes are extremely high, exhibiting cholesterol-to-phospholipid mole ratios from one to two in the cortex of the lens to as high as three to four in the lens nucleus. The lens fiber membrane provides resistance to oxygen transport. It was elucidate that plasma cholesterol concentrations are major factor affecting oxygen transport within and across lens lipid membrane and responsible for the unique properties of this membrane [84], [85], [86], [89], [90].
In the blood, plasma cholesterol concentration, which is in equilibrium with red blood cells (RBS) membrane cholesterol levels, affects oxygen exchange in and out of these cells. Indeed, plasma cholesterol and RBS membrane cholesterol levels were shown to be negatively correlated with in the percentage changes in oxygen saturation. Treatment with statin decreases blood and RBS cholesterol levels and was associated with increased blood oxygen diffusion capacity [82]. Thus, given the association between high RBC membrane cholesterol contents and reduced oxygen transport, plasma cholesterol levels in NAFLD individuals is an additional factor which can affect liver perfusion. Indeed, blood cholesterol levels as well as glycemic control are the two metabolic aspects that must be aggressively addressed in patients with NAFLD, and especially in NASH [91].
In regard to hepatocytes specifically, published and unpublished results from our lab indicate that cholesterol overload to AML12 hepatocytes can significantly affect oxygen availability in these cells [92]. These results demonstrate the same suppressive effect of cholesterol on oxygen permeability into cells in hepatocytes and propose a new parameter which may affect the progression of NAFLD.
The direct effect of membrane cholesterol on oxygen diffusion was investigated by Subczynski et al. [79], [84] using saturated and unsaturated phosphatidylcholine membranes. Regarding oxygen transport parameter, they showed that at baseline (in the absence of cholesterol) both membranes have a bell-like shape with a gradual increase toward the center of the membrane. However, interestingly, oxygen transport parameter was slightly greater in saturated membrane. The addition of 50% cholesterol differently affected oxygen transport in different regions of the membrane. At the polar head group region and the hydrocarbon region near the bilayer surface, cholesterol significantly decreased oxygen transport. Conversely, little or no effect was observed in the center part of the bilayer. An abrupt increase in oxygen transport occurred between the C9 and C10 positions of the lipid alkyl chain. These alternations in oxygen transport were in association with changes in membrane hydrophobicity profile. Indeed, in the absence of cholesterol saturated and unsaturated membranes showed gradual increase in hydrophobicity toward the center of the bilayer. The addition of cholesterol significantly enhanced the polarity (reduced hydrophobicity) in the headgroup and hydrocarbon regions while decreased the polarity in the central region of the bilayer. A sharp increase in hydrophobicity was observed between the ninth and tenth carbon atoms, which means within the carbon-carbon bound along the alkyl chain.
On the basis of overall results, and mainly the similarities observed between oxygen transport parameter and the hydrophobicity profiles, the authors suggested the possibility of lateral transport of molecular oxygen and other small non-polar molecules along the inner core region of the membrane (parallel to the membrane surface), which is referred to as “hydrophobic channeling”. It was further proposed that high membrane cholesterol contents are responsible for creating a barrier to oxygen transport across the membrane that is located in the polar head group and near-surface regions of the membrane, to the depth of the ninth carbon, where the major barriers for oxygen permeability are located.
Taken together, a high membrane cholesterol concentration seems to cause a significant barrier, impeding oxygen transport across membrane into intracellular compartment.
Beside cholesterol, plasma membrane also contains phospholipids. It was shown that membrane alkyl chain saturation can affect oxygen transport, which is slightly greater in saturated membrane [80], [84]. Phosphatidylcholine (PC) and Phosphatidylethanolamine (PE) are two major phospholipids in the plasma membrane. Recently several studies have implicated that decreased PC to PE ratio promotes hepatocytes cell damage and the transition to NASH [93]. Although this change was found to be associated with the loss of membrane integrity, its effect on oxygen availability is yet known. In eukaryotic membranes, PE and phosphatidylserine are more unsaturated than other phospholipids [87]. Since phospholipids composition may influence oxygen permeability across the membrane and can also interact with plasma membrane cholesterol [87] it is of great interest to evaluate whether this change in PC to PE ratio can further manipulate oxygen transport.
8. Summary and conclusion
Reduced oxygen supply appears to exist in the presence of NAFLD. This review tried to provide an overview of the main factors affecting oxygen availability to the liver which are relevant under steatotic conditions. Morphological changes of reduced intrasinusoidal volume due to the enlargement of hepatocytes cell size along with sinusoidal endothelial dysfunction are among the main causes for microcirculation abnormalities observed in steatotic livers. Additional systemic alterations, including inflammation, collagen deposition in the space of Disse, OSA and obesity, causing further narrowing of sinusoid lumens and/or reduction of blood oxygen saturation and thus aggravating the decrease in liver perfusion. Finally, cellular membrane composition modifications may also lead to diminished availability of oxygen to hepatocytes.
Given the importance of oxygen to the liver, the development of therapeutic interventions aimed to improve liver perfusion is highly essential.
Acknowledgment
This review was supported by an Israel Science Foundation Grant 371/12 to OT and ZM.
References
- 1.Kulkarni A.C., Kuppusamy P., Parinandi N. Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid. Redox Signal. 2007;9(10):1717–1730. doi: 10.1089/ars.2007.1724. (Epub 2007/09/08) [DOI] [PubMed] [Google Scholar]
- 2.Nahmias Y., Kramvis Y., Barbe L., Casali M., Berthiaume F., Yarmush M.L. A novel formulation of oxygen-carrying matrix enhances liver-specific function of cultured hepatocytes. FASEB J. 2006;20(14):2531–2533. doi: 10.1096/fj.06-6192fje. (Epub 2006/11/02) [DOI] [PubMed] [Google Scholar]
- 3.Jungermann K., Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31(2):255–260. doi: 10.1002/hep.510310201. (Epub 2000/02/03) [DOI] [PubMed] [Google Scholar]
- 4.Broughan T.A., Naukam R., Tan C., Van De Wiele C.J., Refai H., Teague T.K. Effects of hepatic zonal oxygen levels on hepatocyte stress responses. J. Surg. Res. 2008;145(1):150–160. doi: 10.1016/j.jss.2007.04.014. (Epub 2008/01/01) [DOI] [PubMed] [Google Scholar]
- 5.Vanni E., Mezzabotta L., Bugianesi E. NAFLD and hepatocellular Carcinoma: how big a problem is this really? Curr. Hepatol. Rep. 2014;13(2):113–118. [Google Scholar]
- 6.Mantena S.K., Vaughn D.P., Andringa K.K., Eccleston H.B., King A.L., Abrams G.A. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417(1):183–193. doi: 10.1042/BJ20080868. (Epub 2008/08/30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wambaugh J., Shah I. Simulating microdosimetry in a virtual hepatic lobule. PLoS Comput. Biol. 2010;6(4):e1000756. doi: 10.1371/journal.pcbi.1000756. (Epub 2010/04/28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. (Epub 2009/12/31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford J.M. Vascular disorders of the liver. Clin. Liver Dis. 2010;14(4):635–650. doi: 10.1016/j.cld.2010.08.002. (Epub 2010/11/09) [DOI] [PubMed] [Google Scholar]
- 10.Brunt E.M., Gouw A.S., Hubscher S.G., Tiniakos D.G., Bedossa P., Burt A.D. Pathology of the liver sinusoids. Histopathology. 2014;64(7):907–920. doi: 10.1111/his.12364. (Epub 2014/01/08) [DOI] [PubMed] [Google Scholar]
- 11.Farrell G.C., Teoh N.C., McCuskey R.S. Hepatic microcirculation in fatty liver disease. Anat. Rec. 2008;291(6):684–692. doi: 10.1002/ar.20715. (Epub 2008/05/20) [DOI] [PubMed] [Google Scholar]
- 12.Shigefuku R., Takahashi H., Kobayashi M., Ikeda H., Matsunaga K., Okuse C. Pathophysiological analysis of nonalcoholic fatty liver disease by evaluation of fatty liver changes and blood flow using xenon computed tomography: can early-stage nonalcoholic steatohepatitis be distinguished from simple steatosis? J. Gastroenterol. 2012;47(11):1238–1247. doi: 10.1007/s00535-012-0581-4. (Epub 2012/05/12) [DOI] [PubMed] [Google Scholar]
- 13.Shigefuku R., Takahashi H., Kato M., Yoshida Y., Suetani K., Noguchi Y. Evaluation of hepatic tissue blood flow using xenon computed tomography with fibrosis progression in nonalcoholic fatty liver disease: comparison with chronic hepatitis C. Int. J. Mol. Sci. 2014;15(1):1026–1039. doi: 10.3390/ijms15011026. (Epub 2014/01/16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocciolillo S., Parruti G., Marzio L. CEUS and Fibroscan in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. World J. Hepatol. 2014;6(7):496–503. doi: 10.4254/wjh.v6.i7.496. (Epub 2014/07/30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Beers B.E. Science to Practice: can we diagnose nonalcoholic Steatohepatitis with intravoxel incoherent motion diffusion-weighted MR imaging? Radiology. 2014;270(1):1–2. doi: 10.1148/radiol.13132294. [DOI] [PubMed] [Google Scholar]
- 16.Villanova N., Moscatiello S., Ramilli S., Bugianesi E., Magalotti D., Vanni E. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42(2):473–480. doi: 10.1002/hep.20781. (Epub 2005/06/28) [DOI] [PubMed] [Google Scholar]
- 17.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. (Epub 2010/10/01) [DOI] [PubMed] [Google Scholar]
- 18.Erdogmus B., Tamer A., Buyukkaya R., Yazici B., Buyukkaya A., Korkut E. Portal vein hemodynamics in patients with non-alcoholic fatty liver disease. Tohoku J. Exp. Med. 2008;215(1):89–93. doi: 10.1620/tjem.215.89. (Epub 2008/05/30) [DOI] [PubMed] [Google Scholar]
- 19.Guiu B., Petit J.M., Capitan V., Aho S., Masson D., Lefevre P.H. Intravoxel incoherent motion diffusion-weighted imaging in nonalcoholic fatty liver disease: a 3.0-T MR study. Radiology. 2012;265(1):96–103. doi: 10.1148/radiol.12112478. (Epub 2012/07/31) [DOI] [PubMed] [Google Scholar]
- 20.Rijzewijk L.J., van der Meer R.W., Lubberink M., Lamb H.J., Romijn J.A., de Roos A. Liver fat content in type 2 diabetes: relationship with hepatic perfusion and substrate metabolism. Diabetes. 2010;59(11):2747–2754. doi: 10.2337/db09-1201. (Epub 2010/08/10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenstengel S., Stoeppeler S., Bahde R., Spiegel H.U., Palmes D. Type of steatosis influences microcirculation and fibrogenesis in different rat strains. J. Investig. Surg. 2011;24(6):273–282. doi: 10.3109/08941939.2011.586094. (Epub 2011/11/04) [DOI] [PubMed] [Google Scholar]
- 22.McCuskey R.S., Ito Y., Robertson G.R., McCuskey M.K., Perry M., Farrell G.C. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40(2):386–393. doi: 10.1002/hep.20302. (Epub 2004/09/16) [DOI] [PubMed] [Google Scholar]
- 23.Pasarin M., La Mura V., Gracia-Sancho J., Garcia-Caldero H., Rodriguez-Vilarrupla A., Garcia-Pagan J.C. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7(4):e32785. doi: 10.1371/journal.pone.0032785. (Epub 2012/04/18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ijaz S., Yang W., Winslet M.C., Seifalian A.M. The role of nitric oxide in the modulation of hepatic microcirculation and tissue oxygenation in an experimental model of hepatic steatosis. Microvasc. Res. 2005;70(3):129–136. doi: 10.1016/j.mvr.2005.08.001. (Epub 2005/10/06) [DOI] [PubMed] [Google Scholar]
- 25.Schleicher J., Guthke R., Dahmen U., Dirsch O., Holzhuetter H.G., Schuster S. A theoretical study of lipid accumulation in the liver-implications for nonalcoholic fatty liver disease. Biochim. Biophys. Acta. 2014;1841(1):62–69. doi: 10.1016/j.bbalip.2013.08.016. (Epub 2013/09/04) [DOI] [PubMed] [Google Scholar]
- 26.Huang-Doran I., Zhang C.Y., Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol. Metab.: TEM. 2017;28(1):3–18. doi: 10.1016/j.tem.2016.10.003. (Epub 2016/11/05) [DOI] [PubMed] [Google Scholar]
- 27.Burnley-Hall N., Willis G., Davis J., Rees D.A., James P.E. Nitrite-derived nitric oxide reduces hypoxia-inducible factor 1alpha-mediated extracellular vesicle production by endothelial cells. Nitric Oxide: Biol. Chem. 2017;63:1–12. doi: 10.1016/j.niox.2016.12.005. (Epub 2016/12/27) [DOI] [PubMed] [Google Scholar]
- 28.Ban L.A., Shackel N.A., McLennan S.V. Extracellular vesicles: a new frontier in biomarker discovery for non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016;17(3):376. doi: 10.3390/ijms17030376. (Epub 2016/03/18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwakiri Y., Groszmann R.J. Vascular endothelial dysfunction in cirrhosis. J. Hepatol. 2007;46(5):927–934. doi: 10.1016/j.jhep.2007.02.006. (Epub 2007/03/30) [DOI] [PubMed] [Google Scholar]
- 30.Eccleston H.B., Andringa K.K., Betancourt A.M., King A.L., Mantena S.K., Swain T.M. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid. Redox Signal. 2011;15(2):447–459. doi: 10.1089/ars.2010.3395. (Epub 2010/10/06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasarin M., Abraldes J.G., Rodriguez-Vilarrupla A., La Mura V., Garcia-Pagan J.C., Bosch J. Insulin resistance and liver microcirculation in a rat model of early NAFLD. J. Hepatol. 2011;55(5):1095–1102. doi: 10.1016/j.jhep.2011.01.053. (Epub 2011/03/02) [DOI] [PubMed] [Google Scholar]
- 32.Reihill J.A., Ewart M.A., Hardie D.G., Salt I.P. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys. Res Commun. 2007;354(4):1084–1088. doi: 10.1016/j.bbrc.2007.01.110. (Epub 2007/02/06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anavi S., Ilan E., Tirosh O., Madar Z. Infusion of a lipid emulsion modulates AMPK and related proteins in rat liver, muscle, and adipose tissues. Obesity. 2010;18(6):1108–1115. doi: 10.1038/oby.2009.489. (Epub 2010/01/09) [DOI] [PubMed] [Google Scholar]
- 34.Barnea M., Shamay A., Stark A.H., Madar Z. A high-fat diet has a tissue-specific effect on adiponectin and related enzyme expression. Obesity. 2006;14(12):2145–2153. doi: 10.1038/oby.2006.251. (Epub 2006/12/26) [DOI] [PubMed] [Google Scholar]
- 35.Ix J.H., Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J. Am. Soc. Nephrol. 2010;21(3):406–412. doi: 10.1681/ASN.2009080820. (Epub 2010/02/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman S.M., Qadri I., Janssen R.C., Friedman J.E. Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J. Lipid Res. 2009;50(11):2193–2202. doi: 10.1194/jlr.M800633-JLR200. (Epub 2009/06/09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraegen E.W., Saha A.K., Preston E., Wilks D., Hoy A.J., Cooney G.J. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am. J. Physiol. Endocrinol. Metab. 2006;290(3):E471–E479. doi: 10.1152/ajpendo.00316.2005. (Epub 2005/10/20) [DOI] [PubMed] [Google Scholar]
- 38.Lee W.J., Lee I.K., Kim H.S., Kim Y.M., Koh E.H., Won J.C. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005;25(12):2488–2494. doi: 10.1161/01.ATV.0000190667.33224.4c. (Epub 2005/10/15) [DOI] [PubMed] [Google Scholar]
- 39.Hahn-Obercyger M., Graeve L., Madar Z. A high-cholesterol diet increases the association between caveolae and insulin receptors in rat liver. J. Lipid Res. 2009;50(1):98–107. doi: 10.1194/jlr.M800441-JLR200. (Epub 2008/09/02) [DOI] [PubMed] [Google Scholar]
- 40.Chidlow J.H., Jr, Sessa W.C. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86(2):219–225. doi: 10.1093/cvr/cvq075. (Epub 2010/03/06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mastrodonato M., Calamita G., Rossi R., Mentino D., Bonfrate L., Portincasa P. Altered distribution of caveolin-1 in early liver steatosis. Eur. J. Clin. Investig. 2011;41(6):642–651. doi: 10.1111/j.1365-2362.2010.02459.x. (Epub 2011/01/22) [DOI] [PubMed] [Google Scholar]
- 42.Qiu Y., Liu S., Chen H.T., Yu C.H., Teng X.D., Yao H.T. Upregulation of caveolin-1 and SR-B1 in mice with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2013;12(6):630–636. doi: 10.1016/s1499-3872(13)60099-5. (Epub 2013/12/11) [DOI] [PubMed] [Google Scholar]
- 43.Rockey D.C. Vascular mediators in the injured liver. Hepatology. 2003;37(1):4–12. doi: 10.1053/jhep.2003.50044. (Epub 2002/12/25) [DOI] [PubMed] [Google Scholar]
- 44.Savard C., Tartaglione E.V., Kuver R., Haigh W.G., Farrell G.C., Subramanian S. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57(1):81–92. doi: 10.1002/hep.25789. (Epub 2012/04/18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nizet V., Johnson R.S. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 2009;9(9):609–617. doi: 10.1038/nri2607. (Epub 2009/08/26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. (Epub 2011/02/18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J. Hepatol. 2009;51(1):212–223. doi: 10.1016/j.jhep.2009.03.008. (Epub 2009/05/19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keller S.A., Paxian M., Ashburn J.H., Clemens M.G., Huynh T. Kupffer cell ablation improves hepatic microcirculation after trauma and sepsis. J. Trauma. 2005;58(4):740–749. doi: 10.1097/01.ta.0000158246.74816.18. (discussion 9-51. Epub 2005/04/13) [DOI] [PubMed] [Google Scholar]
- 49.von Frankenberg M., Golling M., Mehrabi A., Nentwich H., Thies J., Schaeffer F. Destruction of Kupffer's cells increases total liver blood flow and decreases ischemia reperfusion injury in pigs. Transplant. Proc. 1999;31(8):3253–3254. doi: 10.1016/s0041-1345(99)00714-9. (Epub 2000/01/05) [DOI] [PubMed] [Google Scholar]
- 50.Xu H., Korneszczuk K., Karaa A., Lin T., Clemens M.G., Zhang J.X. Thromboxane A2 from Kupffer cells contributes to the hyperresponsiveness of hepatic portal circulation to endothelin-1 in endotoxemic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288(2):G277–G283. doi: 10.1152/ajpgi.00256.2004. (Epub 2005/01/14) [DOI] [PubMed] [Google Scholar]
- 51.Abshagen K., Eipel C., Kalff J.C., Menger M.D., Vollmar B. Kupffer cells are mandatory for adequate liver regeneration by mediating hyperperfusion via modulation of vasoactive proteins. Microcirculation. 2008;15(1):37–47. doi: 10.1080/10739680701412989. (Epub 2007/10/24) [DOI] [PubMed] [Google Scholar]
- 52.Maher J.J., Leon P., Ryan J.C. Beyond insulin resistance: innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48(2):670–678. doi: 10.1002/hep.22399. (Epub 2008/07/31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. (Epub 1999/09/14) [DOI] [PubMed] [Google Scholar]
- 54.McCuskey R.S. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20(1):3–7. doi: 10.1034/j.1600-0676.2000.020001003.x. (Epub 2000/03/22) [DOI] [PubMed] [Google Scholar]
- 55.Serracino-Inglott F., Habib N.A., Mathie R.T. Hepatic ischemia-reperfusion injury. Am. J. Surg. 2001;181(2):160–166. doi: 10.1016/s0002-9610(00)00573-0. (Epub 2001/06/27) [DOI] [PubMed] [Google Scholar]
- 56.Huang A.L., Vita J.A. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc. Med. 2006;16(1):15–20. doi: 10.1016/j.tcm.2005.10.002. (Epub 2006/01/03) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svegliati-Baroni G., De Minicis S., Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28(8):1052–1064. doi: 10.1111/j.1478-3231.2008.01825.x. (Epub 2008/09/12) [DOI] [PubMed] [Google Scholar]
- 58.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Investig. 2005;115(2):209–218. doi: 10.1172/JCI24282. (Epub 2005/02/04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sumida Y., Nakajima A., Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014;20(2):475–485. doi: 10.3748/wjg.v20.i2.475. (Epub 2014/02/28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynaert H., Thompson M.G., Thomas T., Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50(4):571–581. doi: 10.1136/gut.50.4.571. (Epub 2002/03/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adrian J.E., Poelstra K., Scherphof G.L., Meijer D.K., van Loenen-Weemaes A.M., Reker-Smit C. Effects of a new bioactive lipid-based drug carrier on cultured hepatic stellate cells and liver fibrosis in bile duct-ligated rats. J. Pharmacol. Exp. Ther. 2007;321(2):536–543. doi: 10.1124/jpet.106.117945. (Epub 2007/02/23) [DOI] [PubMed] [Google Scholar]
- 62.Friedman S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. (Epub 2008/01/16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar M., Sarin S.K. Is cirrhosis of the liver reversible? Indian J. Pediatr. 2007;74(4):393–399. doi: 10.1007/s12098-007-0067-1. (Epub 2007/05/04) [DOI] [PubMed] [Google Scholar]
- 64.Abraham D., Dashwood M. Endothelin--role in vascular disease. Rheumatology. 2008;47(Suppl 5):v23–v24. doi: 10.1093/rheumatology/ken282. (Epub 2008/09/17) [DOI] [PubMed] [Google Scholar]
- 65.Leung T.M., Tipoe G.L., Liong E.C., Lau T.Y., Fung M.L., Nanji A.A. Endothelial nitric oxide synthase is a critical factor in experimental liver fibrosis. Int J. Exp. Pathol. 2008;89(4):241–250. doi: 10.1111/j.1365-2613.2008.00590.x. (Epub 2008/04/24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh H., Pollock R., Uhanova J., Kryger M., Hawkins K., Minuk G.Y. Symptoms of obstructive sleep apnea in patients with nonalcoholic fatty liver disease. Dig. Dis. Sci. 2005;50(12):2338–2343. doi: 10.1007/s10620-005-3058-y. (Epub 2006/01/18) [DOI] [PubMed] [Google Scholar]
- 67.Musso G., Cassader M., Olivetti C., Rosina F., Carbone G., Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes. Rev. 2013;14(5):417–431. doi: 10.1111/obr.12020. (Epub 2013/02/08) [DOI] [PubMed] [Google Scholar]
- 68.Ahmed M.H., Byrne C.D. Obstructive sleep apnea syndrome and fatty liver: association or causal link? World J. Gastroenterol. 2010;16(34):4243–4252. doi: 10.3748/wjg.v16.i34.4243. (Epub 2010/09/08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sookoian S., Pirola C.J. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes. Surg. 2013;23(11):1815–1825. doi: 10.1007/s11695-013-0981-4. (Epub 2013/06/07) [DOI] [PubMed] [Google Scholar]
- 70.Mirrakhimov A.E., Polotsky V.Y. Obstructive sleep apnea and non-alcoholic Fatty liver disease: is the liver another target? Front. Neurol. 2012;3:149. doi: 10.3389/fneur.2012.00149. (Epub 2012/10/23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armstrong M.J., Adams L.A., Canbay A., Syn W.K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174–1197. doi: 10.1002/hep.26717. (Epub 2013/09/05) [DOI] [PubMed] [Google Scholar]
- 72.Reinke C., Bevans-Fonti S., Drager L.F., Shin M.K., Polotsky V.Y. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J. Appl. Physiol. 2011;111(3):881–890. doi: 10.1152/japplphysiol.00492.2011. (Epub 2011/07/09) (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu X.Z., Xie G.R., Chen D. Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2007;22(8):1178–1182. doi: 10.1111/j.1440-1746.2007.04997.x. (Epub 2007/06/15) [DOI] [PubMed] [Google Scholar]
- 74.Bogaerts E., Heindryckx F., Devisscher L., Paridaens A., Vandewynckel Y.P., Van den Bussche A. Time-dependent effect of hypoxia on tumor progression and liver progenitor cell markers in primary liver tumors. PLoS One. 2015;10(3):e0119555. doi: 10.1371/journal.pone.0119555. (Epub 2015/03/21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren Y., Hao P., Dutta B., Cheow E.S.H., Sim K.H., Gan C.S. Hypoxia modulates A431 cellular pathways association to tumor radioresistance and enhanced migration revealed by comprehensive proteomic and functional studies. Mol. Cell. Proteom. 2013;12(2):485–498. doi: 10.1074/mcp.M112.018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park J.E., Tan H.S., Datta A., Lai R.C., Zhang H., Meng W. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteom. 2010;9(6):1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campos-Rodriguez F., Martinez-Garcia M.A., Martinez M., Duran-Cantolla J. Peña Mdl, Masdeu MJ, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am. J. Respir. Crit. Care Med. 2013;187(1):99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 78.Widomska J., Raguz M., Subczynski W.K. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta. 2007;1768(10):2635–2645. doi: 10.1016/j.bbamem.2007.06.018. (Epub 2007/07/31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Catala A. lipid peroxidation modifies the picture of membranes from the "fluid mosaic model" to the "lipid whisker model". Biochimie. 2012;94(1):101–109. doi: 10.1016/j.biochi.2011.09.025. (Epub 2011/10/11) [DOI] [PubMed] [Google Scholar]
- 80.Sidell B.D. Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J. Exp. Biol. 1998;201(Pt 8):1119–1128. doi: 10.1242/jeb.201.8.1119. (Epub 1998/05/29) [DOI] [PubMed] [Google Scholar]
- 81.Khan N., Shen J., Chang T.Y., Chang C.C., Fung P.C., Grinberg O. Plasma membrane cholesterol: a possible barrier to intracellular oxygen in normal and mutant CHO cells defective in cholesterol metabolism. Biochemistry. 2003;42(1):23–29. doi: 10.1021/bi026039t. (Epub 2003/01/08) [DOI] [PubMed] [Google Scholar]
- 82.Galea A.M., Brown A.J. Special relationship between sterols and oxygen: were sterols an adaptation to aerobic life? Free Radic. Biol. Med. 2009;47(6):880–889. doi: 10.1016/j.freeradbiomed.2009.06.027. (Epub 2009/06/30) [DOI] [PubMed] [Google Scholar]
- 83.Brown A.J., Galea A.M. Cholesterol as an evolutionary response to living with oxygen. Evolution. 2010;64(7):2179–2183. doi: 10.1111/j.1558-5646.2010.01011.x. (Epub 2010/04/17) [DOI] [PubMed] [Google Scholar]
- 84.Subczynski W.K., Widomska J., Feix J.B. Physical properties of lipid bilayers from EPR spin labeling and their influence on chemical reactions in a membrane environment. Free Radic. Biol. Med. 2009;46(6):707–718. doi: 10.1016/j.freeradbiomed.2008.11.024. (Epub 2008/12/30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Subczynski W.K., Raguz M., Widomska J., Mainali L., Konovalov A. Functions of cholesterol and the cholesterol bilayer domain specific to the fiber-cell plasma membrane of the eye lens. J. Membr. Biol. 2012;245(1):51–68. doi: 10.1007/s00232-011-9412-4. (Epub 2011/12/31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mainali L., Raguz M., O'Brien W.J., Subczynski W.K. Properties of membranes derived from the total lipids extracted from the human lens cortex and nucleus. Biochim. Biophys. Acta. 2013;1828(6):1432–1440. doi: 10.1016/j.bbamem.2013.02.006. (Epub 2013/02/27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohvo-Rekila H., Ramstedt B., Leppimaki P., Slotte J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002;41(1):66–97. doi: 10.1016/s0163-7827(01)00020-0. (Epub 2001/11/06) [DOI] [PubMed] [Google Scholar]
- 88.Miersch S., Espey M.G., Chaube R., Akarca A., Tweten R., Ananvoranich S. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J. Biol. Chem. 2008;283(27):18513–18521. doi: 10.1074/jbc.M800440200. (Epub 2008/05/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Widomska J., Raguz M., Dillon J., Gaillard E.R., Subczynski W.K. Physical properties of the lipid bilayer membrane made of calf lens lipids: epr spin labeling studies. Biochim. Biophys. Acta. 2007;1768(6):1454–1465. doi: 10.1016/j.bbamem.2007.03.007. (Epub 2007/04/25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thao M.T., Perez D., Dillon J., Gaillard E.R. Measuring the viscosity of whole bovine lens using a fiber optic oxygen sensing system. Mol. Vis. 2014;20:125–131. (Epub 2014/02/08) [PMC free article] [PubMed] [Google Scholar]
- 91.McPherson D.D. Circulatory dysfunction in NAFLD--which is first, which is last, and what do we do in between? Hepatology. 2005;42(2):270–272. doi: 10.1002/hep.20834. (Epub 2005/07/19) [DOI] [PubMed] [Google Scholar]
- 92.Anavi S., Hahn-Obercyger M., Madar Z., Tirosh O. Mechanism for HIF-1 activation by cholesterol under normoxia: a redox signaling pathway for liver damage. Free Radic. Biol. Med. 2014;71:61–69. doi: 10.1016/j.freeradbiomed.2014.03.007. (Epub 2014/03/19) [DOI] [PubMed] [Google Scholar]
- 93.Li Z., Agellon L.B., Allen T.M., Umeda M., Jewell L., Mason A. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3(5):321–331. doi: 10.1016/j.cmet.2006.03.007. (Epub 2006/05/09) [DOI] [PubMed] [Google Scholar]