Abstract
Background
Sophisticated conventional medicine (CM) has brought significant advances to cancer prevention, detection, and treatment. However, many cancer patients still turn to complementary and alternative medicine (CAM) treatment. This study explored the prevalence, patterns, and perceived value of CAM among cancer patients.
Methods
This quantitative descriptive study was conducted between March 1, 2015, and July 31, 2015, among a cross-sectional, convenience sample of patients from the Oncology Department of San Fernando General Hospital in Trinidad and Tobago. Face-to-face interviews were conducted at the oncology clinic and treatment suite after obtaining informed consent. Data analysis included descriptive analysis, chi-square tests, and binary logistic regression analysis.
Results
The prevalence of CAM use among a sample of 350 cancer patients was 39.1% (39.6% for breast cancer, 44.4% for prostate cancer, 37% for ovarian cancer, and 38.7% for colon cancer patients). Herbs were the most common type of CAM used (93.4%), followed by spiritual therapy (73.7%). CAM use was more prevalent among females (68.6%), Indo-Trinidadians (63.5%), and patients aged 41–50 years (37.2%). The majority (70%–80%) rated CAM efficacy on perceived value. CAM was used mainly because of a desire to try anything that might help (67.6%), followed by it being congruent with the patients’ beliefs (59.1%). Patients knew about CAM mainly through friends (69.3%) and family (69.3%). Most patients were generally satisfied (93.6%) and considered CAM helpful (89.8%), but the majority never informed their health care provider of CAM use (78.8%). Patients reported the simultaneous use of more than one type of CAM, without considering or knowing of possible side-effects. The perceived value of CAM included empowerment, control, cure, and improved quality of life. CAM use was associated with age, but no predictors of CAM use could be identified.
Conclusion
Medicinal herbs and spiritual therapy are commonly used among cancer patients because of perceived benefits and satisfaction. CAM use is more prevalent among females, Indo-Trinidadians, and patients aged 41–50 years old. There are no useful predictors of CAM use. More than one type of CAM is commonly used simultaneously without disclosure to health care providers.
Keywords: Cancer treatment, Complementary and alternative medicine, Non-disclosure, Patient satisfaction, Side-effect
Background
Complementary and alternative medicine (CAM) is defined as “a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine” [1]. It includes herbs, spiritual therapies (prayers, faith healing, divinations, meditation, psychic therapy, folk magic/sorcery [obeah], and mind–body techniques), dietary supplements, biofeedback, hypnosis, acupuncture, Ayurveda, homeopathy, naturopathy, Chinese medicine, chiropractic, massage, tai chi, yoga, electromagnetic therapy, kinesiology, reiki, and qigong. According to the WHO, “the term complementary and alternative medicine is used in some countries to refer to a broad set of health care practices that are not part of the country's own tradition and are not integrated into the dominant health care system” [2]. The overall prevalence of CAM was reported as being 36% in the US (2007), 26% in the UK (2005), and 52% in Australia (2004) [3]. The global prevalence is reported to be 9.8%–76.0% [3].
In Trinidad and Tobago, cancer is one of the top 10 causes of death [4]. Treatment with conventional medicine (CM) has caused significant advances in the prevention, detection, and treatment of cancer [5]. Nonetheless, many patients choose CAM over CM in the hope of maintaining wellness and curing the disease [6–9]. The decision to use CAM is typically influenced by factors such as poor doctor–patient communication, the emotional effect of a cancer diagnosis, perceived severity of conventional treatment side-effects, the individual’s need for decision-making control, and strong beliefs in holistic healing and the mind–body–spirit connection [10]. CM focuses on curative aspects without focusing on the social, psychological, and spiritual needs of the patient [11]. CAM therefore fills this void. Despite the perceived benefits and influences, only a small number of patients refuse CM and prefer CAM alone [9]. These numbers are increasing steadily, with patients reporting continued perceived efficacy since the early 1980s [9, 12–20].
With respect to cancer patients, a European study comprising 956 patients, conducted in 14 countries, revealed that the prevalence of CAM varies markedly among patients with different types of cancer: colon cancer (32.7%), breast cancer (44.7%), lung cancer (23.6%), pancreatic cancer (56.3%), brain cancer (50%), head and neck cancer (22.7%) [21]. A Canadian study revealed CAM usage to be 29.8% among men diagnosed with prostate cancer [22]. The types of CAM used also vary according to the types of cancer: vitamin E, saw palmetto, and selenium are used for prostatic cancer [22, 23]; vitamin A, selenium, phytoestrogens, and traditional Chinese medicine (coumarin, flavonoids) for breast cancer; psychological and spiritual therapies for colorectal cancer [24]; vitamins and minerals for ovarian cancer [25]; herbal plants for lung cancer; and cyclopamine (a steroidal alkaloid extracted from Veratrum californicum) for pancreatic cancer. Spiritual therapy is also widely implemented by cancer patients [26].
However, the CAM use and practices among cancer patients in Trinidad and Tobago are unknown. This study therefore explored the prevalence, patterns, and perceived value of CAM among adult cancer patients in Trinidad and Tobago.
Public health relevance
Wahner-Roedler et al. [27] reported 67% of physicians agreed that some CAM therapies hold promise for the treatment of symptoms, conditions, and diseases. However, the majority (70%) of physicians in the US feel that the current practice of CAM represents a threat to public health [27]. CAM usage is based largely on perceived benefits, which have given hope to many patients to accomplish wellness and improved quality of life, but little thought is spent on the multitude of interactions that may result. Furthermore, many CAM therapies lack a scientific basis and are of questionable safety and efficacy, which may lead to major health consequences: delayed treatment, disease complications, and even death. In addition, there is the possibility of herbal toxicity and herb–herb and herb–drug interactions. Oncolytic drugs have a narrow therapeutic window, and CAM use increases the risk of clinically relevant herb–anticancer drug interactions. Such a relevant interaction is that of St. John’s wort with the anticancer drugs irinotecan and imatinib. It is therefore estimated that CAM–anticancer drug interactions are responsible for substantially more unexpected toxicities of chemotherapeutic drugs and possible under-treatment of cancer patients [28]. CAM use may therefore pose a major public health problem. These concerns were raised by the WHO Traditional Medicine Strategy of 2002–2005, which emphasised four public health areas of CAM: policy; safety, efficacy, and quality; access; and rational use [2].
Methods
Study design and population
This cross-sectional study was conducted among all cancer patients undergoing treatment at the South West Regional Health Authority (SWRHA) of Trinidad and Tobago between March 1, 2015, and July 31, 2015. The sample size required for adequate power was 384, based on a 5% margin of error. Inclusion criteria were age > 18 years, the absence of confusion (i.e., no cognitive or behavioural problems) and communication problems, and informed consent to participate in the study. A convenience sample, comprising every sixth consecutive patient of oncology clinic attendees, and all patient attendees of the oncology treatment suite, were identified for interview. A premedical research student interviewed the consenting individuals.
Data collection
The data collection instrument was a 37-item questionnaire covering patient demographics (age, sex, marital status, ethnicity, educational level, employment status, residence, religion, and religiosity) (8 items), oncology-related variables (5 items), and various aspects of CAM usage (types, experiences, reasons, benefits, influences, effects and consequences, source, and access to CAM) (24 items). CAM types were detailed with each type being further categorised into the many areas of practice as follows: Medicinal herbs/Biological-based medicine (Aloe Vera, Evening Primrose, Calcium, Ginger, Vitamins, etc.), Spiritual therapy/Mind-body systems (Faith healing, Divinations, Meditation, Hypnotherapy, etc.), Alternative systems (Chinese medicine, Indian/Ayurveda medicine, Acupuncture, Homeopathy), Physical therapy/Body manipulations (Chiropractic, Osteopathy, Massage, Manual healing), Energy therapies (bio-electro magnetics, Oxygen/Ozone treatment), Local/Folk remedies (Bloodletting cupping, Local surgery/Sacrification, Ritual sacrifice, Urine therapy, etc.). This questionnaire was tested and used in a previous study of cardiac patients in Trinidad [15]. Face-to-face interviews were conducted on site at clinic locations and the oncology suite. Data were collected and entered on a computer with secured access to the researcher, statistician, and research assistant.
Statistical analysis
Statistical analysis using descriptive and inferential methods was performed using SPSS version 20 software [29]. Graphs were produced using EXCEL software after obtaining the relevant information from the SPSS output. Descriptive methods were used to obtain frequency tables and graphs. Inferential methods included tests of equality of proportions, chi-squared tests of association (e.g., Fisher’s exact test and McNemar’s test of paired proportions, as applicable) between selected socio-demographic or other attribute variables and CAM use. Binary logistic regression was used to identify predictors of CAM use. All hypotheses were tested at the 5% level of significance.
Results
Prevalence and patterns of CAM usage
Of the 384 eligible patients in the study, 350 (91.1%) completed the interview. The other 8.9% did not wish to take part in the study. The reliability of the questionnaire (Cronbach’s alpha) was 0.922. Patients were predominantly female (n = 249, 71.1%), Indo-Trinidadian (n = 210, 60.0%), secondary school educated (n = 152, 43.4%), and Christian (n = 206, 59.8%) (Table 1). CAM users and non-users had similar socio-demographic characteristics, except for the percentages of Afro-Trinidadians and of patients aged 51–60 years, which were lower among CAM users than non-users (25.5% vs. 31.9%; p = 0.035 and 8.0% vs. 17.8%; p = 0.011, respectively) (Table 1). The most common type of cancer was breast cancer (n = 169, 48.3%; or 67.9% of all female patients), followed by prostate cancer (n = 81, 23.1%; or 80.2% of all male patients) (Fig. 1). Only 2 (0.6%) patients had lung cancer. Twenty-three (6.6%) cases had other types of cancer, namely Hodgkin’s lymphoma, or bone, cervix, lung, stomach, throat, brain, ovary, liver, or throat cancer. The total number of cases did not add up to 350 because some patients had multiple cancers (e.g., bone and prostate cancer [1 patient], bone and lung cancer [1 patient], and lung, bone, and ovarian cancer [1 patient]).
Table 1.
Socio-demographic characteristics of CAM users and non-users (n = 350)
| Characteristic | CAM users (%) (n = 137) | CAM non-users (%) (n = 213) | P | Total (n) | % |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 43 (31.4) | 58 (27.2) | 0.401 | 101 | 28.9 |
| Female | 94 (68.6) | 155 (72.8) | 0.401 | 249 | 71.1 |
| Age | |||||
| < 20 | 0 (0.0) | 2 (0.9) | 0.522 | 2 | 0.6 |
| 21–30 | 4 (2.9) | 4 (1.9) | 0.716 | 8 | 2.3 |
| 31–40 | 22 (16.1) | 26 (12.2) | 0.341 | 48 | 13.7 |
| 41–50 | 51 (37.2) | 58 (27.2) | 0.058 | 109 | 31.1 |
| 51–60 | 11 (8.0) | 38 (17.8) | 0.011 | 49 | 14.0 |
| > 60 | 49 (35.8) | 85 (39.9) | 0.499 | 134 | 38.3 |
| Religion | |||||
| Christianity | 71 (51.8) | 135 (63.4) | 0.081 | 206 | 59.8 |
| Hinduism | 49 (35.8) | 58 (27.2) | 0.880 | 107 | 29.7 |
| Islam | 11 (8.0) | 15 (7.0) | 0.139 | 26 | 7.4 |
| Other | 6 (4.4) | 5 (2.3) | 0.823 | 11 | 3.1 |
| Ethnicity | |||||
| Afro-Trinidadian | 35 (25.5) | 68 (31.9) | 0.035 | 103 | 29.4 |
| Indo-Trinidadian | 87 (63.5) | 123 (57.7) | 0.097 | 210 | 60.0 |
| Mixed | 10 (7.3) | 19 (8. 9) | 0.835 | 29 | 8.3 |
| Other | 5 (3.6) | 3 (1.4) | 0.351 | 8 | 2.3 |
| Employment status | |||||
| Unemployed | 91 (66.4) | 153 (71.8) | 0.287 | 244 | 69.7 |
| Employed | 46 (33.6) | 60 (28.2) | 0.287 | 106 | 30.3 |
| Education level | |||||
| Less than primary school | 2 (1.5) | 10 (4.7) | 0.137 | 12 | 3.4 |
| Primary school | 44 (32.1) | 83 (39.0) | 0.211 | 127 | 36.3 |
| Secondary school | 63 (46.0) | 89 (41.8) | 0.442 | 152 | 43.4 |
| Tertiary | 28 (20.4) | 31 (14.6) | 0.188 | 59 | 16.9 |
Data are the number (percentage)
Fig. 1.
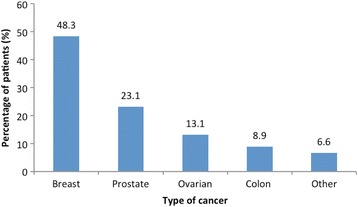
Distribution of cancer types among the study patients (n = 350)
One hundred and thirty-seven (39.1%) patients used at least one type of CAM. This figure comprised 67/169 (39.6%) breast cancer patients, 38/81 (44.4%) prostate cancer patients, 17/46 (37.0%) ovarian cancer patients, 12/31 (38.7%) colon cancer patients, and 5/23 (21.7%) patients with other types of cancer. Medicinal herbs (n = 129, 94.2%) and spiritual therapy (n = 101, 73.7%) were the most common types of CAM used (Fig. 2), regardless of cancer type (Table 2). Alternative systems, physical therapies/body manipulations, and energy therapies were not common among cancer patients.
Fig. 2.
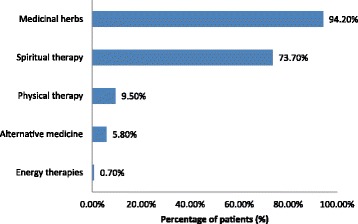
Types of complementary and alternative medicine (CAM) therapy used by cancer patients (n = 137)
Table 2.
CAM use by type of cancer
| CAM used | Type of cancer: Number of users (%) | |||||
|---|---|---|---|---|---|---|
| Breast (n = 67) | Prostate (n = 36) | Ovarian (n = 17) | Colon (n = 12) | Other (n = 5) | p-value | |
| Medicinal herbs | 65 (97.0) | 34 (94.4) | 15 (88.2) | 10 (83.36) | 5 (100.0) | 0.292 |
| Spiritual therapy | 46 (68.7) | 27 (75.0) | 13 (76.5) | 10 (83.3) | 5 (100.0) | 0.503 |
| Alternative medicine | 4 (6.0) | 5 (13.9) | 1 (5.9) | 3 (25.0) | 0 (0.0) | 0.205 |
| Physical therapy | 3 (4.5) | 2 (5.6) | 0 (0.0) | 3 (25.0) | 0 (0.0) | 0.048 |
| Energy therapy | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.902 |
CAM complementary and alternative medicine
Data are the number (percentage)
The responses to the initiation and substitution of CAM, as well as the abandonment of CM, varied among CAM users. With respect to initiation, most patients (n = 113, 82.5%) began using CAM while they were being treated with CM, while the remaining patients (n = 24, 17.5%) had begun CAM prior to CM. Most patients, 131 (95.6%) stated that they never resorted to CAM as a substitute for CM on a permanent basis. Abandonment of CM was not so common among patients since the vast majority (93.4%) were not prepared to give up CM. Only nine (6.6%) patients reported having abandoned CM for CAM at some time.
Age was the only socio-demographic variable associated with CAM use (χ2 = 11.365; df = 5; p = 0.045) with increasing age associated with increased use of CAM. However, neither age nor any of the other socio-demographic variables (age, sex, marital status, ethnicity, highest level of education and employment status, income, and religion) were useful predictors of CAM use.
The majority of patients (73.1% of breast cancer patients, 88.9% of prostate cancer patients, 82.4% of ovary cancer patients, 83.3% of colon cancer patients, 80.0% of patients with other types of cancer) claimed to have received some particular benefit from CAM use. The main benefits were that it would treat the condition directly (n = 77, 56.2%), that it would improve their psychological/emotional well-being (n = 68, 49.6%), that it would allow them to relax/sleep (n = 61, 44.5%), and that it would relieve the side-effects of conventional medicine (n = 36, 26.5%). Less than 10 patients failed to mention any particular item from a long, open-ended list of benefits. There was no association between the type of cancer and whether or not users claimed to have obtained particular benefits from CAM use (p = 0.432).
The most commonly reported reason for deciding to use CAM was the desire to try anything that could help (n = 96, 67.6%), followed by being congruent with their beliefs and their inner self (n = 81, 59.1%) (Table 3). The least common reason was that CM was too mechanistic and lacked the human touch (n = 12, 8.8%). Fifty-four (39.4%) patients said that they decided to use CAM because CM was too expensive.
Table 3.
Reasons for deciding to use CAM
| Reason | Type of cancer: Number of patients (%) | |||||
|---|---|---|---|---|---|---|
| Breast (n = 67) | Prostate (n = 36) | Ovarian (n = 17) | Colon (n = 12) | Other (n = 5) | Total (n = 137) | |
| The patient was disappointed with CM | 11 (16.4) | 5 (13.9) | 5 (29.4) | 3 (25.0) | 2 (40.0) | 26 (19.0) |
| CM was too toxic or damaging | 4 (6.2) | 4 (11.1) | 4 (11.8) | 2 (16.7) | 1 (20.0) | 13 (9.6) |
| CAM was more in keeping with personal beliefs and the inner self | 38 (56.7) | 22 (61.1) | 10 (58.8) | 9 (75.0) | 2 (40.0) | 81 (59.1) |
| The patient felt the desire to take control of treatment | 20 (29.9) | 14 (38.9) | 6 (35.3) | 1 (8.3) | 1 (20.0) | 42 (30.7) |
| CM was too mechanistic and lacked the human touch | 4 (6.0) | 4 (11.1) | 2 (11.8) | 2 (16.7) | 0 (0.0) | 12 (8.8) |
| The patient felt the desire to try everything that could help | 45 (67.2) | 26 (72.2) | 11 (64.7) | 9 (75.0) | 1 (25.0) | 92 (67.6) |
| CM was too expensive | 32 (34.3) | 18 (50.0) | 9 (52.9) | 4 (33.3) | 0 (0.0) | 54 (39.4) |
CAM complementary and alternative medicine, CM conventional medicine. Data are the number (percentage)
Outcome of CAM treatments
Overall, 60.0% of patients were “Satisfied”, 33.6% were “Very Satisfied”, and only 4.4% were “Dissatisfied”. The percentage of patients very satisfied, satisfied, and not satisfied with CAM for each type of cancer is shown in Fig. 3. Patients with prostate and breast cancer were the two groups with the highest percentages of “Satisfied-to-Very satisfied” patients. However, the level of satisfaction was found to be independent of cancer type. The majority of CAM users (n = 128, 89.8%) considered CAM helpful, and 14 (10.2%) said that it was not helpful. Of those who found CAM helpful, 94 (76.4%) had been using it once per day; 16 (13.0%), 4–6 times per week; and 12 (9.8%), 1–3 times per week. The majority of CAM users (n = 131, 95.6%) described the outcome of CAM treatments as good; and only 1 (0.7%) patient reported complications related to the use of CAM.
Fig. 3.
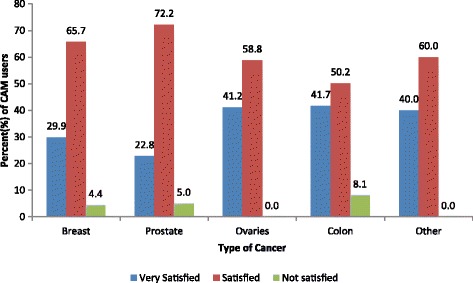
Level of satisfaction with complementary and alternative medicine (CAM) use by type of cancer
Awareness about CAM
Patient awareness/information about CAM usage was obtained from friends (n = 95, 69.3%), family members (n = 95, 69.3%), and other patients (n = 60, 43.8%) (Table 4). Health personnel outside of the hospital setting were the least influential factor (n = 2, 1.5%). All but 3 (2.2%) patients agreed that the greater the level of knowledge a person has regarding CAM, the greater the likelihood that he/she would use it. The majority of patients (n = 125, 91.2%) agreed that if they had had more knowledge about CAM, they would have encouraged others to use it. Reported sources of CAM information included friends (n = 28; 20.4%); relatives (n = 21; 15.3%); a CAM practitioner (n = 90; 6.6%); religious groups (n = 8; 5.8%); and other unspecified groups or individuals (n = 71; 51.8%). Only 13 (9.5%) of the patients had a trained healthcare provider (allopathic or CAM practitioner) supervise or guide their CAM treatment. Furthermore, only 29 (21.2%) patients informed their physician of their use of CAM.
Table 4.
Reported sources of CAM awareness/information
| Source of CAM awareness/information | Type of cancer: Number of patients (%) | |||||
|---|---|---|---|---|---|---|
| Breast (n = 67) | Prostate (n = 36) | Ovarian (n = 17) | Colon (n = 12) | Other (n = 5) | Total (n = 137) | |
| Health personnel outside the hospital | 1 (1.5) | 1 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0%) | 2 (1.5%) |
| In-hospital health personnel | 14 (20.9) | 5 (13.9) | 1 (5.9) | 3 (25.0) | 1 (20.0) | 24 (17.5) |
| Friends | 46 (68.7) | 30 (83.3) | 9 (52.9) | 8 (66.7) | 2 (40.0) | 95 (69.3) |
| Family members | 46 (68.7) | 29 (80.6) | 8 (47.1) | 9 (75.0) | 3 (60.0) | 95 (69.3) |
| CAM practitioner | 4 (6.0) | 5 (13.9) | 0 (0.0) | 3 (25.0) | 0 (0.0) | 12 (8.8) |
| Mass media | 9 (11.9) | 2 (5.6) | 8 (47.1) | 1 (8.3) | 1 (20.0) | 20 (14.6) |
| Religious groups | 15 (22.4) | 14 (38.9) | 5 (29.4) | 2 (16.73) | 0 (0.0) | 36 (26.3) |
| Other patients | 29 (43.3) | 17 (42.7) | 6 (35.3) | 8 (66.7) | 0 (0.0) | 60 (43.8) |
| Other persons (unspecified) | 1 (1.5) | 1 (2.8) | 2 (11.8) | 0 (0.0) | 0 (0.0) | 4 (2.9) |
CAM complementary and alternative medicine. Data are the number (percentage)
Discussion
In this study, the overall prevalence of CAM among cancer patients was 39.1%. In this study, the prevalence of CAM varied for different cancers, namely ovarian (37.0%), colon (38.7%), breast (39.6%), and prostate cancer (44.4%). This was in agreement with a previous study [21]. Of all socio-demographic variables tested, CAM usage was only associated with age. However, neither age nor any of the other socio-demographic variables were useful predictors for CAM usage.
Herbs were the most common type of CAM used (94.2%), followed by spiritual therapy (73.7%). Molassiotis et al. also reported herbs as the top CAM therapy among cancer patients in 9 out of 14 countries, including Turkey, Israel, Serbia, Czech Republic, Denmark, Italy, Switzerland, Spain, and Greece [21]. The majority of CAM users use at least 1 herb. Nonetheless, patients were not prepared to give up CM (93.4%). This may reflect patients’ lack of complete trust in CAM and fear of losing the benefits of CM. CAM was used mainly because of a desire to try anything (67.6%), followed by being congruent with their beliefs and inner self (59.1%).
CAM is used in nearly all types of cancer. There were no significant differences in the choice of medicinal herbs and spiritual CAM therapies, which were the most commonly used types of CAM among all types of cancer. However, while patients recognise the perceived value of CAM, 78.8% of patients from this study failed to communicate their use of CAM to their health care provider. In contrast, Saxe et al. [30] found that there was a high disclosure rate of CAM to physicians, with as much as 85% of breast cancer patients disclosing their use of naturopathy to their physicians [30]. This contrasts with the findings of Eisenberg et al. [31], who found that 63%–72% of patients practiced CAM without informing their health care providers. Non-disclosure may not be in the best interest in patient care, since vital information required for the management of patients is lost. Furthermore, few doctors are prepared to enquire from patients about CAM usage [32]. Patients also fear disclosing CAM information. This has led the US National Center for Complementary and Alternative Medicine (NCCAM) to launch the “Time to Talk” campaign encouraging both health care providers and patients to communicate about CAM use [33].
The majority of patients seem to have no clear guidance or basis for appropriate CAM use, with only 13 (9.5%) patients having their CAM supervised or guided by a trained health care provider. Furthermore, only a small percentage of patients (6.6%) stopped using CM to use CAM. According to van Kleffens and van Leeuwen [11], patients refused CM treatment because of the desire to stay in control, fear of losing breasts, or not wanting to fight any more. In contrast, a 2013 prospective study in terminally ill cancer patients found that CAM did not provide any definite survival benefit or improved health-related quality of life [34]. More unfavourable findings regarding CAM usage was revealed in a 2003 study on cancer patients from Norway, which revealed higher death rates (79%) among CAM users than non-users (65%) [35]. CAM usage is attributed to the increasing demand and expectations for more holistic and comprehensive care [36]. It is perceived as being “natural” and “safe” [37], effective [38], and with fewer adverse effects [39]. CAM users experience a feeling of being in control, coping, and adjustment. The rationale behind CAM, whether it be problem-focused (strategies based on biological therapies) or emotion-focused (based on prayers and meditation techniques), is reported to be largely based on erroneous logic and science [40]. However, some studies find that the benefits of CAM should not be disregarded, and oncologists should familiarize themselves with commonly used CAM, in order to provide their patients with proper guidance in all aspects of their treatment [41].
Public health relevance
High satisfaction levels have overshadowed or downplayed the complications of herbal toxicity, herb–herb and herb–drug interactions; or treatment problems because of CAM usage or CM avoidance. Furthermore, its use continues because of the lack of regulations in Trinidad and Tobago [42], exposure and influences from a multitude of sources, and increasing availability of various types of CAM. The lack of professional CAM supervision, the growing number of CAM users, low disclosure rates, lack of meaningful advice from CM providers, and poor monitoring contribute to a major public health problem. The simultaneous practice of CAM and CM necessitates greater understanding, communication, and integration of these 2 forms of treatment.
Limitations
This study was conducted in a single public health institution. The sample was skewed towards a less economically privileged population. Because of the sample size, subpopulation analysis would be difficult since there would be insufficient power to draw meaningful conclusions. Patients may withhold information that they may be embarrassed about. The database is based entirely on the memory and truthfulness of patients’ responses, which may have involved bias. The results and conclusions are unique to our setting in Trinidad and generalisations would be difficult unless the populations are similar.
Conclusions
The prevalence of CAM use was relatively high (39.1%) among cancer patients. The frequent use of herbs (93.4%) and spiritual therapy (73.7%), the abandonment of CM for CAM (although only 6.6%), the failure by the vast majority (78.8%) of CAM users to inform their health care provider, and the high satisfaction (over 90%) with CAM use may have major health implications (delayed treatment, drug interactions, disease complications, and the possibility of death). Females, Indo-Trinidadians, and patients in the age range of 41–50 years are the main users. Patients’ use of CAM was mainly to try anything that might help, and to remain congruent with their beliefs and inner self. Other reasons included wellbeing, relaxation, counteracting the side-effects of CM, and cost. Patients were influenced to use, or introduced to CAM, by friends, followed by family, other patients, religious groups, mass media, in-hospital personnel, CAM practitioners, and health personnel outside of a hospital. Patients believe more education would encourage them to use more CAM.
Acknowledgements
I wish to acknowledge Dr. George Legall, statistician and lecturer at the University of the West Indies (Mt. Hope, Trinidad), who assisted with statistical analysis and methodology; Miss Kimberly Rampersad, premedical student who assisted with data collection; and all the patients and staff at the Oncology Department.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on request.
Funding
Not applicable.
Abbreviations
- CAM
Complementary and alternative medicine
- CM
Conventional medicine
- HCP
Health care provider
- NCCAM
National Center for Complementary and Alternative Medicine
- NHS
National Health Service
- SFGH
San Fernando General Hospital
- SWRHA
South West Regional Health Authority
- WHO
World Health Organization
Author’s contributions
MB conceptualised, designed, conducted, and reviewed the study; and wrote and revised the manuscript.
Author information
MB is a Specialist Medical Officer and Consultant Physician at the San Fernando Hospital (San Fernando, Trinidad and Tobago) and a lecturer at the School of Medicine and Arthur Lok Jack Graduate School of Business at the University of the West Indies (Mt. Hope, Trinidad).
Ethics approval and consent to participate
This study received ethical approval from the Ethics Committee of South West Regional Health Authority on 28th September 2014. All participants gave their consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The author declares that he/she has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.US Department of Health and Human Services, National Institutes of Health. The Use of Complementary and Alternative Medicine in the United States. In: National Centre for Complementary and Alternative Medicine (NCCAM). 2008. https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed 27 June 2017.
- 2.World Health Organization. General guidelines for methodologies on research and evaluation of traditional medicine. WHO; Geneva. 2000. http://apps.who.int/iris/bitstream/10665/66783/1/WHO_EDM_TRM_2000.1.pdf. Accessed 24 May 2017.
- 3.Harris PE, Cooper KL, Relton C, Thomas KJ. Prevalence of complementary and alternative medicine (CAM) use by the general population: a systematic review and update. Int J Clin Pract. 2012;66:924–939. doi: 10.1111/j.1742-1241.2012.02945.x. [DOI] [PubMed] [Google Scholar]
- 4.Pan American Health Organization . Health in the Americas: 2012 edition: country volume. Washington, DC: PAHO; 2012. [Google Scholar]
- 5.Sudhakar A. History of cancer, ancient and modern treatment methods. J Cancer Sci Ther. 2009;1:1–4. doi: 10.4172/1948-5956.100000e2. [DOI] [PubMed] [Google Scholar]
- 6.Ezeome ER, Anarado AN. Use of complementary and alternative medicine by cancer patients at the University of Nigeria Teaching Hospital, Enugu, Nigeria. BMC Complement Altern Med. 2007;7:28. doi: 10.1186/1472-6882-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur K, Belliard JC, Hardin SB, Knecht K, Chen CS, Montgomery S. Reasons to use and disclose use of complementary medicine use—an insight from cancer patients. Cancer Clin Oncol. 2013;2:81–92. doi: 10.5539/cco.v2n2p81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhoef MJ, Rose MS, White M, Balneaves LG. Declining conventional cancer treatment and using complementary and alternative medicine: a problem or a challenge? Curr Oncol. 2008;15:s10–s16. doi: 10.3747/co.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassileth BR, Lusk EJ, Strouse TB, Bodenheimer BJ. Contemporary unorthodox treatments in cancer medicine. Ann Intern Med. 1984;101:105–112. doi: 10.7326/0003-4819-101-1-105. [DOI] [PubMed] [Google Scholar]
- 10.Ernst E. The role of complementary and alternative medicine. BMJ. 2000;321:1133–1135. doi: 10.1136/bmj.321.7269.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kleffens T, van Leeuwen E. Physicians’ evaluations of patients’ decisions to refuse oncological treatment. J Med Ethics. 2005;31:131–136. doi: 10.1136/jme.2004.008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahall M, Edwards M. Perceptions of complementary and alternative medicine among cardiac patients in South Trinidad: a qualitative study. BMC Complement Altern Med. 2015;15:99. doi: 10.1186/s12906-015-0577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement Y. Limited clinical evidence to support the integration of Caribbean herbs into conventional medicine. Focus Altern Complement Ther. 2011;16:289–292. doi: 10.1111/j.2042-7166.2011.01116.x. [DOI] [Google Scholar]
- 14.Richardson J. What patients expect from complementary therapy: a qualitative study. Am J Public Health. 2004;94:1049–1053. doi: 10.2105/AJPH.94.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahall M. Complementary and alternative medicine usage among cardiac patients: a descriptive study. BMC Complement Altern Med. 2015;15:100. doi: 10.1186/s12906-015-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans M, Shaw A, Thompson EA, Falk S, Turton P, Thompson T, et al. Decisions to use complementary and alternative medicine (CAM) by male cancer patients: information-seeking roles and types of evidence used. BMC Complement Altern Med. 2007;7:25. doi: 10.1186/1472-6882-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst E. Complementary and alternative medicine (CAM) and cancer: the kind face of complementary medicine. Int J Surg. 2009;7:499–500. doi: 10.1016/j.ijsu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Helyer LK, Chin S, Chui BK, Fitzgerald B, Verma S, Rakovitch E, et al. The use of complementary and alternative medicines among patients with locally advanced breast cancer – a descriptive study. BMC Cancer. 2006;6:39. doi: 10.1186/1471-2407-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabish SA. Complementary and alternative healthcare: is it evidence-based? Int J Health Sci (Qassim) 2008;2:V–IX. [PMC free article] [PubMed] [Google Scholar]
- 20.Citrin DL, Bloom DL, Grutsch JF, Mortensen SJ, Lisa CG. Beliefs and perceptions of women with newly diagnosed breast cancer who refused conventional treatment in favor of alternative therapies. Oncologist. 2012;17:607–612. doi: 10.1634/theoncologist.2011-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16:655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 22.Boon H, Westlake K, Stewart M, Gray R, Fleshner N, Gavin A, et al. Use of complementary/alternative medicine by men diagnosed with prostate cancer: prevalence and characteristics. Urology. 2003;62:849–853. doi: 10.1016/S0090-4295(03)00668-X. [DOI] [PubMed] [Google Scholar]
- 23.Ponholzer A, Struhal G, Madersbacher S. Frequent use of complementary medicine by prostate cancer patients. Eur Urol. 2003;43:604–608. doi: 10.1016/S0302-2838(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 24.Tough SC, Johnston DW, Verhoef MJ, Arthur K, Bryant H. Complementary and alternative medicine use among colorectal cancer patients in Alberta, Canada. Altern Ther Health Med. 2002;8:54–56. [PubMed] [Google Scholar]
- 25.Ebbert JA, Donovan KA, Lengacher CA, Fabri D, Reich R, Daley E, et al. Right place, right time: preferences of women with ovarian cancer for delivery of CAM education. Medicines. 2015;2:236–250. doi: 10.3390/medicines2030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puchalski CM. Spirituality in the cancer trajectory. Ann Oncol. 2012;23:49–55. doi: 10.1093/annonc/mds088. [DOI] [PubMed] [Google Scholar]
- 27.Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3:495–501. doi: 10.1093/ecam/nel036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijerman I, Beijnena JH, Schellensa JH. Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist. 2006;11:742–752. doi: 10.1634/theoncologist.11-7-742. [DOI] [PubMed] [Google Scholar]
- 29.Krejice RV, Morgan DW. Determining sample size for research activities. Educ Psychol Meas. 1970;30:607–610. doi: 10.1177/001316447003000308. [DOI] [Google Scholar]
- 30.Saxe GA, Madlensky L, Kealey S, Wu DP, Freeman KL, Pierce JP. Disclosure to physicians of CAM use by breast cancer patients: findings from the Women’s healthy eating and living study. Integr Cancer Ther. 2008;7:122–129. doi: 10.1177/1534735408323081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg DM, Kessler RC, Van Rompay MI, Kaptchuk TJ, Wilkey SA, Appel S, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 2001;135:344–351. doi: 10.7326/0003-4819-135-5-200109040-00011. [DOI] [PubMed] [Google Scholar]
- 32.Most doctors not knowledgeable about herbals Health Day News. Modern Medicine Network. 2010. http://www.modernmedicine.com/%5Bnode-source-domain-raw%5D/news/clinical/clinical-pharmacology/most-doctors-not-knowledgeable-about-he?page=full. Accessed 31 Nov 2016.
- 33.National Centre for Complementary and Alternative Medicine (nccam) Bethesda: NCCAM; Time to Talk About CAM: Health care providers and patients need to ask and tell. National Institutes of Health. 2008. https://www.nih.gov/news-events/news-releases/time-talk-about-cam. Accessed 31 Nov 2016.
- 34.Yun YH, Lee MK, Park SM, Kim YA, Lee WJ, Lee KS, et al. Effect of complementary and alternative medicine on the survival and health-related quality of life among terminally ill cancer patients: a prospective cohort study. Ann Oncol. 2013;24:489–494. doi: 10.1093/annonc/mds469. [DOI] [PubMed] [Google Scholar]
- 35.Risberg T, Vickers A, Bremnes RM, Wist EA, Kaasa S, Cassileth BR. Does use of alternative medicine predict survival from cancer? Eur J Cancer. 2003;39:372–377. doi: 10.1016/S0959-8049(02)00701-3. [DOI] [PubMed] [Google Scholar]
- 36.Singh P, Chaturvedi A. Complementary and alternative medicine in cancer pain management: a systematic review. Indian J Palliat Care. 2015;21:105–115. doi: 10.4103/0973-1075.150202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams M, Jewell AP. The use of complementary and alternative medicine by cancer patients. Int Semin Surg Oncol. 2007;4:10. doi: 10.1186/1477-7800-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White MA, Verhoef MJ, Davison BJ, Gunn H, Cooke K. Seeking mind, body and spirit healing—why some men with prostate cancer choose CAM (complementary and alternative medicine) over conventional cancer treatments. Integr Med Insights. 2008;3:1–11. doi: 10.4137/imi.s377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhoef MJ, Balneaves LG, Boon HS, Vroegindewey A. Reasons for and characteristics associated with complementary and alternative medicine use among adult cancer patients: a systematic review. Integr Cancer Ther. 2005;4:274–286. doi: 10.1177/1534735405282361. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine (US) Committee on the Use of Complementary and Alternative Medicine by the American Public. 7, Integration of CAM and Conventional Medicine . In: complementary and alternative medicine in the United States. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 41.Dhanoa A, Yong TL, Yeap SJL, Lee ISZ, Singh VA. Complementary and alternative medicine use amongst Malaysian orthopaedic oncology patients. BMC Complement Altern Med. 2014;14:404. doi: 10.1186/1472-6882-14-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon Z. Health minister: laws coming to regulate herbal business. In: Guardian. 2012. http://www.guardian.co.tt/news/2012-05-16/health-minister-laws-coming-regulate-herbal-business. Accessed 26 Feb 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.


