Abstract
Thermally influenced freshwater systems provide suitable conditions for non-native species of tropical and subtropical origin to survive and form proliferating populations beyond their native ranges. In Germany, non-native convict cichlids (Amatitlania nigrofasciata) and tilapia (Oreochromis sp.) have established populations in the Gillbach, a small stream that receives warm water discharge from a local power plant. Here, we report on the discovery of spotted tilapia (Pelmatolapia mariae) in the Gillbach, the first record of a reproducing population of this species in Europe. It has been hypothesized that Oreochromis sp. in the Gillbach are descendants of aquaculture escapees and our mtDNA analysis found both O. mossambicus and O. niloticus maternal lineages, which are commonly used for hybrids in aquaculture. Convict cichlids and spotted tilapia were most probably introduced into the Gillbach by aquarium hobbyists. Despite their high invasiveness worldwide, we argue that all three cichlid species are unlikely to spread and persist permanently beyond the thermally influenced range of the Gillbach river system. However, convict cichlids from the Gillbach are known to host both native and non-native fish parasites and thus, non-native cichlids may constitute threats to the native fish fauna. We therefore strongly recommend continuous monitoring of the Gillbach and similar systems.
Keywords: biological invasion, non-native species, thermally influenced freshwater systems, thermally polluted, invasion biology, tilapia
1. Introduction
In colder regions of the world, many freshwater systems are thermally influenced, i.e. show water temperatures above the normal range [1]. In addition to sites that receive warm water by geothermal sources (e.g. [2–8]), thermal conditions are often altered by anthropogenic activities such as discharges of heated waters from power plants or the dewatering of mines (e.g. [9–18]). These thermally influenced freshwater (TIF) systems are hotspots for non-native organisms, as they provide suitable habitats for species of tropical and subtropical origin (e.g. [2–4,7–15,17,19–25]). While biological invasions are known to be a major driver of species extinctions and biodiversity loss [26–30], the importance and influence of TIFs remain mostly understudied.
The release of unwanted pets by aquarium hobbyists has been assumed as the main introduction pathway for non-native species into TIFs [17,31–37]. For example, aggressive behaviour, rapid reproduction, large size and illness are factors that increase the likelihood of aquarium fish to be released into the wild [31,38,39]. However, also introductions by aquaculture escapees are documented (e.g. [15,40–42]). The resulting artificial communities in TIFs [43,44] often comprise native as well as non-native species of both invertebrates (e.g. crustaceans; [13,21,45]) and vertebrates (e.g. fish; [4,8,15]).
One of the TIFs in central Europe is the Gillbach near the city of Cologne in Germany. This stream receives warm water effluents year round from a lignite power plant. Near the influx water temperatures rarely drop below 19°C, whereas 2 km downstream a minimum of 13°C was reported (February 2012; [15]). These conditions allowed several non-native tropical and subtropical fish species like Ancistrus sp., Poecilia reticulata and Pseudorasbora parva as well as some invertebrates (Neocaridina davidi and Macrobrachium dayanum) and tropical plants (Vallisneria spiralis) to establish self-sustaining populations [13,15,46]. Most of these are popular ornamental species, making an introduction via aquarium release the most probable invasion pathway and plausible scenario for the Gillbach.
Our current paper focuses on members of another (sub)tropical fish family inhabiting the Gillbach: Cichlidae. All members of this taxonomic family stem from the tropics or subtropics and many of them have been dispersed worldwide over the past century as a result of intentional introductions. Larger predatory cichlids (e.g. Cichla ocellaris, Cichlasoma managuense, Serranochromis robustus; [47]) are selected for stock enhancement, whereas certain omnivorous and herbivorous species are used as agents in the control of aquatic weeds (e.g. O. aureus [48], Coptodon zillii [49]), disease vector insects (e.g. O. mossambicus [50]) or nuisance molluscs (Astatoreochromis alluaudi [51], Coptodon rendalli [52]).
The most famous representatives of this family are commonly known and collectively referred to as ‘tilapia’ (genera Sarotherodon, Oreochromis and Tilapia; sensu Trewavas [53]) (e.g. [54–56]). According to Canonico et al. [55] most introductions of these genera have occurred due to aquaculture activities. In fact, the farming of tilapia (Oreochromis spp.) is currently the most widespread type of aquaculture in the world and only second to carp by volume of production [57]. In 1998, first specimens of a tilapia hybrid have been reported for the Gillbach and were identified as O. niloticus × mossambicus based on live coloration [41]. As most of today's tilapia culture is based on hybrids (most often between O. niloticus, O. aureus and O. mossambicus; [58]), we used DNA analysis, alongside classical morphological analysis, for species identification in our current study.
Besides their use in aquaculture, cichlids are also very popular with aquarists as they show a rich array of coloration and behavioural displays. Some species (e.g. Aequidens pulcher, Amatitlania nigrofasciata, Astronotus ocellatus, Cichlasoma spp., Geophagus brasiliensis, Hemichromis letourneauxi, Sarotherodon melanotheron; [59]) have been transported widely around the world via the aquarium trade and many introductions have been the result of occasional releases from home aquaria or stock disposal from dedicated breeding facilities of the aquarium trade. One of the most popular species within the ornamental trade is the convict cichlid (Amatitlania nigrofasciata), originally stemming from Central America. So far, the only stable population of A. nigrofasciata in Germany seems to be established in the Gillbach and was first described in 1998 [17,41].
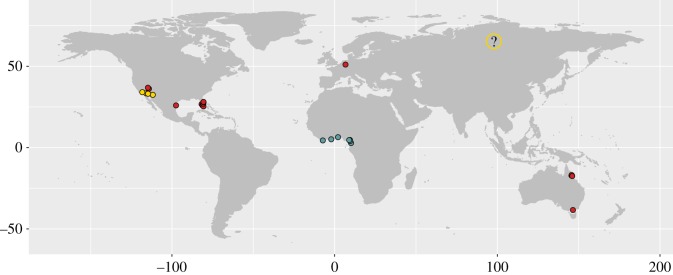
In the current paper, we first report on the occurrence of another reproducing cichlid species in the Gillbach, which we identified as the spotted tilapia, Pelmatolapia mariae. With the new record of P. mariae (figure 1), the Gillbach now seems to harbour stable populations of at least two large African cichlids (Oreochromis sp. and P. mariae) and one Central American cichlid (A. nigrofasciata)—all of which have a long invasive history all over the world [55].
Figure 1.
Distribution of Pelmatolapia mariae. P. mariae has been introduced beyond its natural range in West Africa (blue), with established populations in Australia, USA and Germany (red). Note that some records of P. mariae are location unspecific (indicated by question mark) or are suspected of having been subject to misidentification (yellow). For more detailed information on specific introduction sites of P. mariae refer to Bradford et al. [60] and Nico & Neilson [61].
2. Material and methods
2.1. Study system
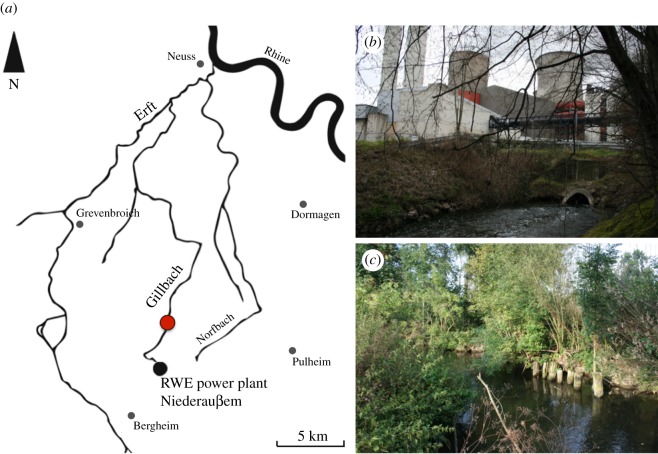
The Gillbach is a 28 km long stream within the Erft drainage, part of the Rhine basin of central Europe (figure 2a). The river flows through the North Rhine Lignite field in Germany, a hub for opencast mining and electrical energy industries. Its original headwaters being destroyed, it is now fed solely by the warm water discharge of the coal-fired power plant ‘Niederaußem’ (50°59'46.82′′ N, 6°39'50.56′′ E, RWE Power Inc.; figure 2b) located west of Cologne. At the site near Hüchelhoven/Rheidt (51°00'39.5′′ N, 6°41'02.1′′ E), the stream has been straightened to accommodate agriculture and developmental needs. The streambed of the Gillbach (approx. 3 m wide and 30–80 cm deep) consists almost entirely of artificially placed rocks as well as sand and mud. Owing to the coverage by bushes and trees, submerged vegetation is mostly absent (figure 2c).
Figure 2.
(a) Map of the Gillbach and its position within the Rhine catchment. Both the locations of the temperature logger placed by the inlet of the RWE power plant Niederaußem (black; b) and the sampling site near Rheidt (red; c) are indicated.
2.2. Sampling
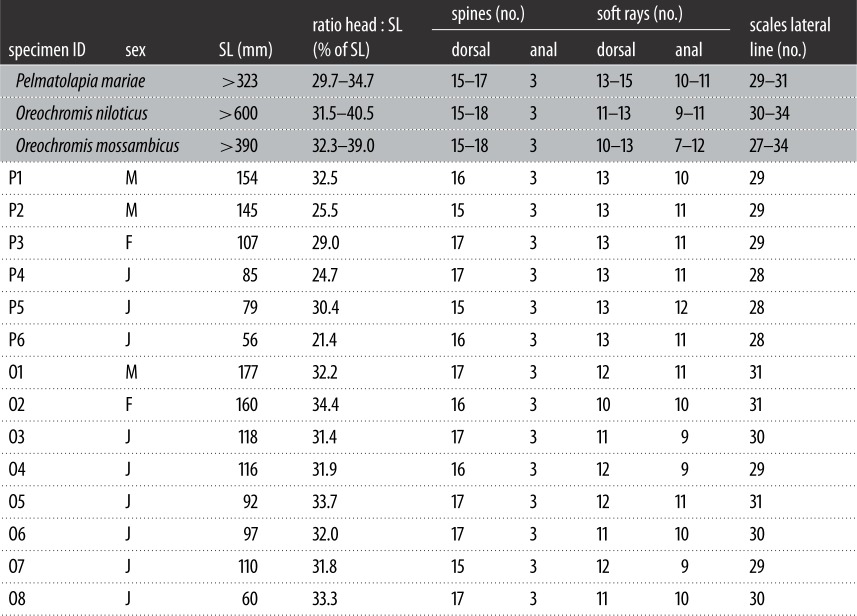
The water temperature at the stream's inlet was recorded every 4 h using an Onset HOBO data logger for the period of 19 March 2016 until 4 May 2017. Two kilometres downstream of this site, voucher specimens were caught using seine-netting (mesh size 6 mm) in September 2016. All specimens were immediately euthanized with an overdose of clove oil and preserved in 99% ethanol for further genetic analysis. Morphological species determination of all African cichlids was performed using standard keys (table 1; [53,62–64]). The identification of the Central American cichlid A. nigrofasciata followed Schmitter-Soto [65,66]. All native fish species were identified according to Kottelat & Freyhof [67]. Afterwards, all specimens were integrated into the ichthyologic collection at the Zoologisches Forschungsmuseum Alexander Koenig (ZFMK) in Bonn, Germany under the project numbers Lukas_Gillbach2016_01 to Lukas_Gillbach2016_15.
Table 1.
Basic data on specimens of African cichlid species collected in the Gillbach stream, including numbers of individuals captured, sex, size (standard length, SL) and meristic information. Reference values follow Teugels & Thys van den Audenaerde [62] for P. mariae and Trewavas [53] for Oreochromis spp. Note that fin ray counts can differ between localities [60].
 |
2.3. DNA extraction and mitochondrial DNA analysis
We extracted DNA from fin-clips of 14 fish specimens using the QIAGEN Blood and Tissue Kit (QIAGEN GmbH, Germany) as recommended by manufacturer's instructions. All extracts were measured with the NanoDrop™ Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and normalized to a concentration of approximately 10 ng µl−1 for further analysis. We analysed partial sequences of two mitochondrial DNA genes using the following primer pairs (for/rev): 1. Cytochrome b (Cyt b) L14734 (5′-AACCACCGTTGTTATTCAACT-3′) and H15557 (5′-GGCAAATAGGAARTATCAYTC-3′); 2. Cytochrome c oxidase subunit 1 (COI) L6199 (5′-GCCTTCCCWCGAATAAATAA-3′) and H6855 (5′-AGTCAGCTGAAKACTTTTAC -3′) [68]. PCR reactions were carried out in a total reaction volume of 15 µl, including 3 µl template, 3 mM MgCl2, 1X standard Taq (Mg-free) reaction buffer, 0.2 mM of each dNTP, 0.3 µM of each primer and 0.66 units Taq polymerase (New England BioLabs GmbH, Germany). We used the following thermal cycling parameters: 3 min initial denaturation at 95°C followed by 35 cycles (30 s at 94°C, 30 s at 54°C and 60 s at 72°C) plus a final extension step of 30 min at 72°C. PCR products were purified with a mixture of five units Exonuclease I (Thermo Scientific, Waltham, MA, USA) and 16 units of FastAP™ Thermosensitive Alkaline Phosphatase (Thermo Scientific). Sequencing was performed in both directions with PCR primers using the BigDye Terminator 3.1 sequencing kit (Life Technologies GmbH, part of Thermo Fisher Scientific) with an initial denaturation step of 60 s at 95°C, followed by 30 cycles of 10 s at 96°C, 10 s at 50°C and 120 s at 60°C. Products were purified with ABI-XTerminator beads (Life Technologies GmbH, part of Thermo Fisher Scientific) and separated on an ABI 3730 DNA Analyzer (Life Technologies GmbH, part of Thermo Fisher Scientific). The obtained sequence data were analysed in GENEIOUS 7.1.9 (Biomatters) and blasted for species determination using default settings in the National Centre for Biotechnology Information (NCBI) GenBank. The resulting sequences were submitted as Blast queries to Genbank. COI sequences were deposited in GenBank under accession numbers KY565238–KY565240 and Cyt b sequences can be accessed under KY582461–KY582463 (table 2).
Table 2.
Molecular species identification. Processed samples of ‘tilapia’ species included in this study and top matches from GenBank database. Maximum identity percentage of the sequences refers to pair-wise alignments with the closest match (n.a. identifies no match greater than 90%).
| GenBank accession number |
best BLAST hit with GenBank accession number and maximum identity percentage |
||||
|---|---|---|---|---|---|
| specimen ID | COI | Cyt b | COI | Cyt b | identified species |
| P1 | KY565240 | KY582463 | KJ669646.1 (100%) | n.a. | P. mariae |
| P2 | KY565240 | KY582463 | KJ669646.1 (100%) | n.a. | P. mariae |
| P3 | KY565240 | KY582463 | KJ669646.1 (100%) | n.a. | P. mariae |
| P4 | KY565240 | KY582463 | KJ669646.1 (100%) | n.a. | P. mariae |
| P5 | KY565240 | KY582463 | KJ669646.1 (100%) | n.a. | P. mariae |
| P6 | KY565240 | KY582463 | KJ669646.1 (100%) | n.a. | P. mariae |
| O1 | KY565238 | KY582461 | AY597335.1 (99%) | AY597335.1 (99%) | O. mossambicus |
| O2 | KY565239 | KY582462 | GU370126.1 (98%) | GU477628.1 (97%) | O. niloticus |
| O3 | KY565238 | KY582461 | AY597335.1 (99%) | AY597335.1 (99%) | O. mossambicus |
| O4 | KY565239 | KY582462 | GU370126.1 (98%) | GU477628.1 (97%) | O. niloticus |
| O5 | KY565238 | KY582461 | AY597335.1 (99%) | AY597335.1 (99%) | O. mossambicus |
| O6 | KY565238 | KY582461 | AY597335.1 (99%) | AY597335.1 (99%) | O. mossambicus |
| O7 | KY565238 | KY582461 | AY597335.1 (99%) | AY597335.1 (99%) | O. mossambicus |
| O8 | KY565238 | KY582461 | AY597335.1 (99%) | AY597335.1 (99%) | O. mossambicus |
3. Results
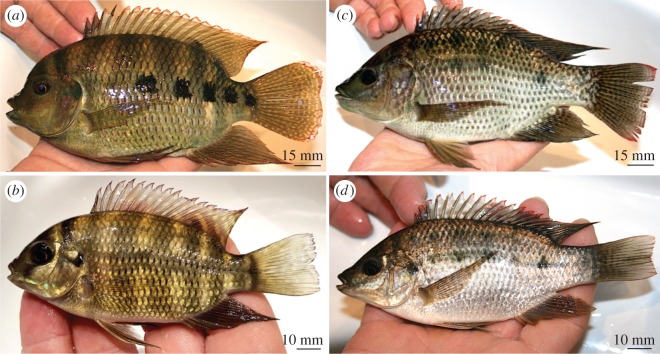
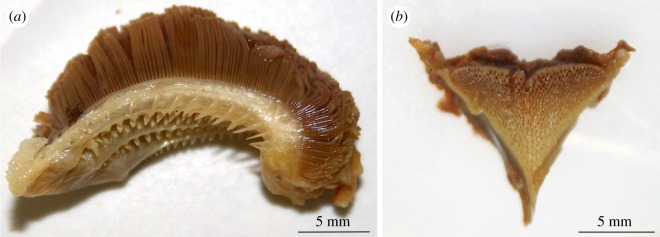
We first observed individuals of Pelmatolapia mariae near Rheidt in August 2016 (video observations, see the electronic supplementary material). In September 2016, we captured six specimens of P. mariae including two juveniles (figure 3b) and four adults (figure 3a). These ranged in size from 56 to 154 mm standard length (SL; table 1). All showed typical morphological features (table 1) and the distinctive coloration of P. mariae (e.g. ventral bars in juveniles (figure 3b) and dark caudal spots in adult specimens (figure 3a)). Furthermore, we counted a maximum of 15 gill rakers on the lower limb of the first branchial arch (figure 4a). Analysis of the lower pharyngeal jaw (LPJ; figure 4b) showed that its ventral keel was shorter than the toothed section. Both features are in accordance with the species' description by Teugels & Thys van den Audenaerde [62,63]. Molecular analysis of the mitochondrial COI gene confirmed the identity of P. mariae (100% matching with accession number KJ669646.1).
Figure 3.
Live coloration of caught African cichlids. (a) Pelmatolapia mariae, adult male SL 154 mm; (b) Pelmatolapia mariae, juvenile SL 56 mm; (c) Oreochromis sp., adult female SL 160 mm; (d) Oreochromis sp., juvenile SL 92 mm.
Figure 4.
Anatomical features of Pelmatolapia mariae. A maximum of 15 gill rakers on lower limb of isolated first gill arch (a). The LPJ (b) is triangular with blade shorter than toothed section.
We caught eight specimens of the genus Oreochromis. Our morphological analysis found all individuals to show overlapping morphological characteristics of both O. mossambicus and O. niloticus (table 1) and a more O. mossambicus-like coloration and body shape (figure 3c,d; reddish fins, elongated snout, no bars at caudal fin typical for O. niloticus). However, our molecular analysis found both mitochondrial lineages of O. mossambicus (species O1, O3, O5–O8, matching with 99% identity in all cases; table 2) as well as O. niloticus (species O2 and O4, matching with greater than or equal to 96% in all cases; table 2), indicating that both maternal lineages were once introduced (O. mossambicus and O. niloticus and/or their hybrids). The wide range of sizes indicates that the Oreochromis population is breeding in the Gillbach. In fact, an adult female was mouthbrooding newly hatched fry at the point of capture.
A third cichlid species, the convict cichlid (Amatitlania nigrofasciata) was also caught and we observed many breeding pairs in shallow areas along the stream. In addition, two native European chub (Squalius cephalus), one native barbel (Barbus barbus), as well as one specimen of the tropical Ancistrus sp. were caught. Assignment of armoured catfish specimens further than the genus Ancistrus remains tentative until systematics are further resolved [69].
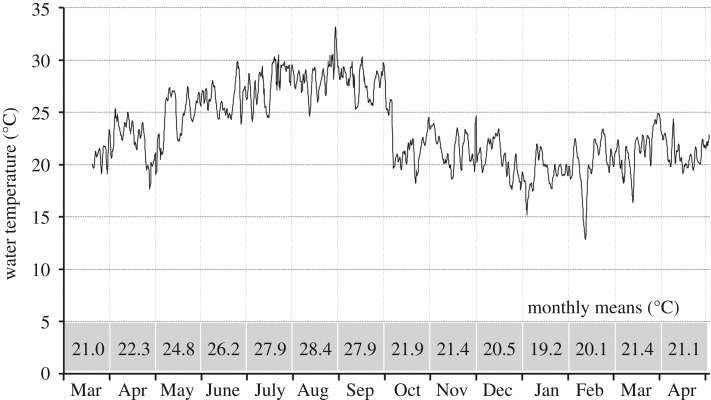
Our temperature measures largely confirmed previous measures [15] and water temperatures never dropped below 8.38°C (only three readings were below 12.5°C). Monthly means at the Gillbach (figure 5, bottom panel) ranged from 19.2°C (January 2017) to 28.4°C (August 2016). Reference measurements taken at the sampling site in Hüchelhoven revealed an average temperature difference of ±2 K compared with the stream's source.
Figure 5.
Daily water temperatures in the Gillbach in 2016/2017. Fluctuations in the daily means (six data points per day) were recorded for the source of the Gillbach from March 2016 to May 2017 and monthly means were calculated (bottom panel).
4. Discussion
The Gillbach near Cologne represents a thermal refuge, which provides suitable conditions for a variety of introduced non-native species. In fact, the cichlids O. niloticus × O. mossambicus, A. nigrofasciata and Maylandia (Pseudotropheus) aurora have been reported for the Gillbach/Erft system during previous samplings [15,17,41]. The occurrence of M. aurora could not be confirmed after 1998, but our sampling now adds another species to that list: the West African spotted tilapia P. mariae.
Our mtDNA analysis found both O. mossambicus and O. niloticus maternal lineages. Sequencing of the Cyt b and COI gene, however, does not enable us to clearly detect hybrids. Nevertheless, found specimens from the Gillbach largely share the same morphological phenotype corroborating the presence of a hybrid population. Oreochromis species hybridize well when occurring syntopically [58] and the use of hybrids is common practice in aquaculture. The Gillbach's Oreochromis sp. are assumed to be (descendants of) escapees from a closed aquaculture facility that employed the power plant's warm water discharge for its production [41]. We do not know whether both species (O. niloticus and O. mossambicus) were initially introduced and hybridized afterwards in the Gillbach or whether hybrids were initially released. Samplings in 2012 [15] and 2016 (present study) found both adults and juveniles, thus, Oreochromis sp. can be considered to have established a reproducing population within the Gillbach.
Amatitlania nigrofasciata have successfully persisted in the Gillbach for more than 18 years now (first record by Höfer & Staas [41]). Individuals of different size classes were plentiful during samplings in 1998 [41], 2012 [15], 2014 [17] and 2016 (present study). Established wild populations are known for Asia, the Middle East, North and Central America and Australia [59,70–73]. So far the only other introduction sites within Europe are two thermal refugia in Italy [4] and Austria [3], both of which are very similar to the Gillbach system in their habitat characteristics and species assemblage.
To this day, P. mariae has been introduced to at least three continents (figure 1; [59–61]). Records from Australia show an established population in the cooling waters of a power station [74] very similar to the Gillbach system. The Gillbach, with its sandy streambed and shallow littoral zones, allows for conditions that correspond to P. mariae's natural habitats in Nigeria [75]. Submerged vegetation is mostly absent in this section of the Gillbach; however, artificially placed rocks may be suitable as spawning substrate [76] and provide shelter during the larval and juvenile stages, which are most prone to predation [77]. Mature individuals have virtually no predators in the Gillbach, but eggs and larvae are most probably cannibalized or preyed on by bigger Oreochromis sp., as well as native species, such as European chub and Common barbel. The dental and gill morphology of the spotted tilapia allows for foraging behaviour that includes both plankton-filtering and grazing [78,79] and thus P. mariae finds suitable conditions in the Gillbach for a diet dominated by plant material [17]. Moreover, the species' documented tolerance to a wide range of temperatures, salinity and dissolved oxygen concentrations [60], not only fosters its dispersal, but also makes it a potential candidate for aquaculture [80,81]. However, the spotted tilapia is an available aquarium fish and its first occurrence in the Gillbach almost two decades later than the closure of the aquaculture facility renders a release by aquarists the most likely reason for the introduction of the species. Similar introduction pathways are known from certain locations in Australia [82–84] and North America ([85,86]; figure 1).
As all cichlid species currently present in the Gillbach stem from the tropics and subtropics, they cannot cope with water temperatures commonly encountered during harsh German winters. Several studies report on the temperature ranges in their natural habitats (17°C–35°C for O. mossambicus and 13.5°C–33°C for O. niloticus [87]; 20°C–36°C for A. nigrofasciata [88]; 20°C–25°C for P. mariae [89]). However, extended temperature ranges have been shown for introduced populations of Oreochromis spp. [87] and P. mariae [90,91] with lethal limits of all three species being reported to be below 11.5°C [92–94]. While average monthly temperatures in the Gillbach never dropped below 19°C (figure 5), we did record temperature spikes (less than 4 h) down to 9.5°C (26 April 2016) and even 8.4°C (30 April 2016, 2 January 2017). However, fish originating from the ornamental trade are often more cold-tolerant than their wild-type counterparts (e.g. [15] for Poecilia reticulata; [14] for Xiphophorus variatus). In fact, several studies showed certain tilapias (Oreochromis spp.) are capable of surviving in rapid temperature fluctuations down to 10°C with seemingly no detrimental effect [9,95,96].
The Gillbach drains into the Erft river, which is equally influenced by the effluents of nearby power plants (e.g. RWE power plant ‘Frimmersdorf’; [97,98]) and mine dewatering (Lignite mining area ‘Garzweiler’). Temperatures in 2016 never dropped below 10°C (e.g. February near Glesch; [99]) and tropical non-natives such as Poecilia reticulata and even piranhas have been found here regularly (Udo Rose 2007, personal communication). The Erft drains into the Rhine, which is currently the most thermally polluted river in the world [1] with a high richness and abundance of non-native species [100,101]. Nevertheless, temperatures in the Rhine sometimes drop to below 4°C (e.g. January 2017 near Düsseldorf-Flehe; [99]) and thus most non-natives with tropical or subtropical origin would not survive outside the areas affected by warm water influx. In fact, both Deacon et al. [102] and Jourdan et al. [15] suggested that an expansion of the tropical guppy into adjacent, not artificially heated streams is unlikely.
One often neglected risk emanating from non-native species is their ability to distribute non-native pathogens [103,104]. Emde et al. [17] demonstrated that TIFs may function as reservoirs for non-native pathogens and parasites. The authors found convict cichlids from the Gillbach to serve as both intermediate and final host for one native (A. anguillae) and three introduced fish parasite species (A. crassus, B. acheilognathi, C. cotti), thereby increasing the risk of spread of these parasites beyond their current distribution. First samples of Oreochromis sp. indicate that this species plays no significant role in the spread of parasites within the Gillbach system due to its mainly plant-based diet (Sebastian Emde 2016, personal communication). Whether P. mariae constitutes a greater threat in this regard should be in the focus of future investigations as its diet differs from that of Oreochromis sp. In its native range, P. mariae carries heavy parasite loads with a large proportion of the population being infected (greater than 50%; [105–107]).
The Gillbach exemplifies that TIFs can accumulate more and more non-native species over time [13,15,41,46]. Observations from the Warmbach near Villach (Austria) provide similar results: each consecutive sampling found new non-native species (2001: Hemichromis letourneauxi; 2002: Hemichromis fasciatus; 2005: Procambarus clarkia, Lepomis gibbosus, Poecilia reticulata, Xiphophorus maculatus, Xiphophorus hellerii; 2007: Amatitlania nigrofasciata, Oreochromis mossambicus, Ancistrus dolichopterus, Maylandia aurora, Hyphessobrycon erythrostigma [3,108]). Overall, greater effort in prevention of the release of non-native species is required to stop the spread outside their native range through a raising of awareness in fish keepers and society alike.
5. Conclusion
(i) The Gillbach—a TIF that has accumulated non-native species over time—is now harbouring stable populations of three cichlid species. We confirm the occurrence of A. nigrofasciata and Oreochromis sp., both of which have been previously described for this system. In fact, molecular analyses of Oreochromis specimens identified the existence of mitochondrial lineages of O. mossambicus and O. niloticus. We further report on the occurrence of P. mariae, which is the first record of this species in Europe.
(ii) Cichlids in TIFs may play a role in disease and parasite transmission. It has been shown that convict cichlids from the Gillbach serve as hosts for both non-native and native parasites. Thus, we strongly recommend further investigations on the potential transmitter role of Oreochromis sp. and P. mariae in the Gillbach system.
(iii) We urgently call for an inclusion of TIFs into continuous monitoring programmes. The Gillbach provides a fruitful system to study invasion processes in detail and improve our understanding of potential impacts on native species and ecosystems. We further prompt that raising public awareness is much needed. While there are several scientific publications on the Gillbach's non-native fish fauna, alien species databases either show outdated records [109,110] or no records of any introduced cichlids in Germany [111,112] as of May 2017. Furthermore, we urge fish keepers to refrain from releasing their pets into ‘suitable’ habitats (which is already prohibited by the German Animal Welfare Act [113]; §3 Abs. 3, 4 TierSchG).
Acknowledgements
The authors thank Dr Udo Rose—biologist at the Erftverband and member of the Erftfischereigenossenschaft (Bergheim, Germany)—for the permission to do research at the Gillbach. Further, we would like to express our gratitude to the Gesellschaft für Ichthyology e.V. (GfI) for financially supporting the project. We were further supported by several fish enthusiasts: Geoffrey P. F. Mazué, Vivek Hari Sridhar, Bernd Neu, Roman De Giorgi, Judith Kochmann, as well as Petr Zajicek and Marcus Ebert from IGB Berlin. We want to express our sincere appreciation for their lively participation during numerous samplings. Lastly, we would like to thank the anonymous reviewers for their valuable comments and suggestions during the revision process.
Ethics
Permission to collect specimens of the family Cichlidae from the Gillbach was granted to the authors by the Erftfischereigenossenschaft (Bergheim, Germany) through Dr Udo Rose. No further collecting permits or approvals were needed. No animal care protocol was required for our research.
Data accessibility
Our data are deposited at Dryad (http://dx.doi.org/10.5061/dryad.sd7vh; [114]). mtDNA sequences are available at the NCBI GenBank under the following accession codes: KY565238–KY565240 and KY582461–KY582463. All specimens were integrated into the ichthyologic collection at the ZFMK in Bonn, Germany under the project numbers Lukas_Gillbach2016_01 to Lukas_Gillbach2016_15.
Authors' contributions
J.A.Y.L., J.J., G.K., S.E., F.W.M. and D.B. jointly collected the fish. J.A.Y.L. performed the morphological identification and prepared DNA samples, which were further analysed by J.J., B.C. and H.J. J.A.Y.L., J.J. and D.B. interpreted the results. J.A.Y.L. wrote the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Financial support was given by the Gesellschaft für Ichthyologie e.V. (German Ichthyological Society).
References
- 1.Raptis CE, van Vliet MT, Pfister S. 2016. Global thermal pollution of rivers from thermoelectric power plants. Environ. Res. Lett. 11, 104011 (doi:10.1088/1748-9326/11/10/104011) [Google Scholar]
- 2.Specziár A. 2004. Life history pattern and feeding ecology of the introduced eastern mosquitofish, Gambusia holbrooki, in a thermal spa under temperate climate, of Lake Hévíz, Hungary. Hydrobiologia 522, 249–260. (doi:10.1023/B:HYDR.0000029978.46013.d1) [Google Scholar]
- 3.Petutschnig J, Honsig-Erlenburg W, Pekny R. 2008. Zum aktuellen Flusskrebs- und Fischvorkommen des Warmbaches in Villach. Carinthia II 198, 95–102 [In German]. [Google Scholar]
- 4.Piazzini S, Lori E, Favilli L, Cianfanelli S, Vanni S, Manganelli G. 2010. A tropical fish community in thermal waters of southern Tuscany. Biol. Invasions 12, 2959 (doi:10.1007/s10530-010-9695-x) [Google Scholar]
- 5.O'Gorman EJ, et al. 2012. Impacts of warming on the structure and functioning of aquatic communities: individual- to ecosystem-level responses. Adv. Ecol. Res. 47, 81–176. (doi:10.1016/B978-0-12-398315-2.00002-8) [Google Scholar]
- 6.O'Gorman EJ, Benstead JP, Cross WF, Friberg N, Hood JM, Johnson PW, Sigurdsson BD, Woodward G. 2014. Climate change and geothermal ecosystems: natural laboratories, sentinel systems, and future refugia. Glob. Change Biol. 20, 3291–3299. (doi:10.1111/gcb.12602) [DOI] [PubMed] [Google Scholar]
- 7.Milenković M, Žikić V, Stanković SS, Marić S. 2013. First study of the guppy fish (Poecilia reticulata Peters, 1859) occurring in natural thermal waters of Serbia. J. Appl. Ichthyol. 30, 160–163. (doi:10.1111/jai.12218) [Google Scholar]
- 8.Sas-Kovács I, Telcean IC, Covaciu-Marcov SD. 2015. A non-native fish assemblage in geothermal waters of Romania. J. Appl. Ichthyol. 31, 211–213. (doi:10.1111/jai.12652) [Google Scholar]
- 9.Langford TE, Aston RJ. 1972. The ecology of some British rivers in relation to warm water discharges from power stations. Proc. R. Soc. Lond. B 180, 407–419. (doi:10.1098/rspb.1972.0027) [DOI] [PubMed] [Google Scholar]
- 10.Langford T. 1990. Ecological effects of thermal discharges. Heidelberg, Germany: Springer Science & Business Media. [Google Scholar]
- 11.Fuller PL, Nico LG, Williams JD. 1999. Nonindigenous fishes introduced into inland waters of the United States. Am. Fish. Soc. 27, 613. [Google Scholar]
- 12.Simard MA, Paquet A, Jutras C, Robitaille Y, Blier PU, Courtois R, Martel AL. 2012. North American range extension of the invasive Asian clam in a St. Lawrence River power station thermal plume. Aquat. Invasions 7, 81–89. (doi:10.3391/ai.2012.7.1.009) The path is (http://www.aquaticinvasions.net/2012/AI_2012_1_Simard_etal.pdf) [Google Scholar]
- 13.Klotz W, Miesen FW, Hüllen S, Herder F. 2013. Two Asian fresh water shrimp species found in a thermally polluted stream system in North Rhine-Westphalia, Germany. Aquat. Invasions 8, 333–339. (doi:10.3391/ai.2013.8.3.09) The path is (http://www.aquaticinvasions.net/2013/AI_2013_3_Klotz_etal.pdf) [Google Scholar]
- 14.Cohen AE, Dugan LE, Hendrickson DA, Martin FD, Huynh J, Labay BJ, Casarez MJ. 2014. Population of variable platyfish (Xiphophorus variatus) established in Waller Creek, Travis County, Texas. Southwest. Nat. 59, 413–419. (doi:10.1894/MP-10.1) [Google Scholar]
- 15.Jourdan J, Miesen FW, Zimmer C, Gasch K, Herder F, Schleucher E, Plath M, Bierbach D. 2014. On the natural history of an introduced population of guppies (Poecilia reticulata Peters, 1859) in Germany. BioInvasions Rec. 3, 175–184. (doi:10.3391/bir.2014.3.3.07) The path is (http://www.reabic.net/journals/bir/2014/3/BIR_2014_Jourdan_etal.pdf) [Google Scholar]
- 16.Worthington TA, Shaw PJ, Daffern JR, Langford TEL. 2015. The effects of a thermal discharge on the macroinvertebrate community of a large British river: implications for climate change. Hydrobiologia 753, 81–95. (doi:10.1007/s10750-015-2197-1) [Google Scholar]
- 17.Emde S, Kochmann J, Kuhn T, Dörge DD, Plath M, Miesen FW, Klimpel S. 2016. Cooling water of power plant creates ‘hot spots’ for tropical fishes and parasites. Parasitol. Res. 115, 85–98. (doi:10.1007/s00436-015-4724-4) [DOI] [PubMed] [Google Scholar]
- 18.Mulhollem JJ, Colombo RE, Wahl DH. 2016. Effects of heated effluent on Midwestern US lakes: implications for future climate change. Aquat. Sci. 78, 743–753. (doi:10.1007/s00027-016-0466-3) [Google Scholar]
- 19.Koschel RH, Gonsiorczyk T, Krienitz L, Padisák J, Scheffler W. 2002. Primary production of phytoplankton and nutrient metabolism during and after thermal pollution in a deep, oligotrophic lowland lake (Lake Stechlin, Germany). Proc. Int. Assoc. Theor. Appl. Limnol. 28, 569–575. [Google Scholar]
- 20.Bianco PG, Turin P. 2009. Record of two established populations of Nile tilapia, Oreochromis niloticus, in freshwaters of northern Italy. J. Appl. Ichthyol. 26, 140–2. (doi:10.1111/j.1439-0426.2009.01315.x) [Google Scholar]
- 21.Jaklič M, Vrezec A. 2011. The first tropical alien crayfish species in European waters: the redclaw Cherax quadricarinatus (Von Martens, 1868)(Decapoda, Parastacidae). Crustaceana 84, 651–665. (doi:10.1163/001121611X577936) [Google Scholar]
- 22.Burchardt L, et al. 2014. Spring phytoplankton and periphyton composition: case study from a thermally abnormal lakes in Western Poland. Biodiv. Res. Conserv. 36, 17–24. (doi:10.2478/biorc-2014-0010) [Google Scholar]
- 23.Pilecka-Rapacz M, Piasecki W, Czerniawski R, Sługocki Ł, Krepski T, Domagała J. 2015. The effect of warm discharge waters of a power plant on the occurrence of parasitic Metazoa in freshwater bream, Abramis brama (L.). Bull. Eur. Ass. Fish Pathol. 35, 94–103. [Google Scholar]
- 24.Yanygina LV. 2015. Spatial distribution of Gmelinoides fasciatus Steb. in thermally polluted water (Belovo Reservoir, Southwest Siberia). Int. J. Environ. Res. 9, 877–884. [Google Scholar]
- 25.Lipták B, Mrugała A, Pekárik L, Mutkovič A, Gruľa D, Petrusek A, Kouba A. 2016. Expansion of the marbled crayfish in Slovakia: beginning of an invasion in the Danube catchment? J. Limnol. 75, 305–312. (doi:10.4081/jlimnol.2016.1313) [Google Scholar]
- 26.Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl. 10, 689–710. (doi:10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2) [Google Scholar]
- 27.Clavero M, García-Berthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110 (doi:10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 28.Shvidenko A. 2005. Ecosystems and human well-being: synthesis. Millennium Ecosystem Assessment 2005. Washington, DC: Island Press. [Google Scholar]
- 29.Butchart SH, et al. 2010. Global biodiversity: indicators of recent declines. Science 328, 1164–1168. (doi:10.1126/science.1187512) [DOI] [PubMed] [Google Scholar]
- 30.Dudgeon D. 2014. Threats to freshwater biodiversity in a changing world. In Global environmental change (ed. Freddman B.), pp. 243–253. The Netherlands: Springer. [Google Scholar]
- 31.Padilla DK, Williams SL. 2004. Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front. Ecol. Environ. 2, 131–138. (doi:10.1890/1540-9295(2004)002[0131:BBWAAO]2.0.CO;2) [Google Scholar]
- 32.Copp GH, et al. 2005. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 21, 242–262. (doi:10.1111/j.1439-0426.2005.00690.x) [Google Scholar]
- 33.Gozlan RE, Britton JR, Cowx I, Copp GH. 2010. Current knowledge on non-native freshwater fish introductions. J. Fish. Biol. 76, 751–786. (doi:10.1111/j.1095-8649.2010.02566.x) [Google Scholar]
- 34.Strecker AL, Campbell PM, Olden JD. 2011. The aquarium trade as an invasion pathway in the Pacific Northwest. Fisheries 36, 74–85. (doi:10.1577/03632415.2011.10389070) [Google Scholar]
- 35.Chucholl C. 2013. Invaders for sale: trade and determinants of introduction of ornamental freshwater crayfish. Biol. Invasions 15, 125–141. (doi:10.1007/s10530-012-0273-2) [Google Scholar]
- 36.Maceda-Veiga A, Escribano-Alacid J, de Sostoa A, García-Berthou E. 2013. The aquarium trade as a potential source of fish introductions in southwestern Europe. Biol. Invasions 15, 2707–2716. (doi:10.1007/s10530-013-0485-0) [Google Scholar]
- 37.Rabitsch W, Milasowszky N, Nehring S, Wiesner C, Wolter C, Essl F. 2013. The times are changing: temporal shifts in patterns of fish invasions in central European fresh waters. J. Fish. Biol. 82, 17–33. (doi:10.1111/j.1095-8649.2012.03457.x) [DOI] [PubMed] [Google Scholar]
- 38.Duggan IC, Rixon CA, MacIsaac HJ. 2006. Popularity and propagule pressure: determinants of introduction and establishment of aquarium fish. Biol. Invasions 8, 377–382. (doi:10.1007/s10530-004-2310-2) [Google Scholar]
- 39.Gertzen E, Familiar O, Leung B. 2008. Quantifying invasion pathways: fish introductions from the aquarium trade. Can. J. Fish. Aquat. Sci. 65, 1265–1273. (doi:10.1139/F08-056) [Google Scholar]
- 40.Stauffer JR Jr, Boltz SE, Boltz JM. 1988. Cold shock susceptibility of blue tilapia from Susquehanna River, Pennsylvania. N. Am. J. Fish. Manage. 8, 329–332. (doi:10.1577/1548-8675(1988)008<0329:CSSOBT>2.3.CO;2) [Google Scholar]
- 41.Höfer S, Staas S.1998. Bericht zur fischereibiologischen Untersuchung des Gillbaches im Bereich Bergheim-Auenheim Zoologisches Institut der Universität zu Köln, Abt. Allgemeine Ökologie und Limnologie, Köln [In German].
- 42.Peterson MS, Slack WT, Woodley CM. 2005. The occurrence of non-indigenous Nile tilapia, Oreochromis niloticus (Linnaeus) in coastal Mississippi, USA: ties to aquaculture and thermal effluent. Wetlands 25, 112–121. (doi:10.1672/0277-5212(2005)025[0112:TOONNT]2.0.CO;2) [Google Scholar]
- 43.Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Envrion. 5, 475–482. (doi:10.1890/070037) [Google Scholar]
- 44.Lurgi M, López BC, Montoya JM. 2012. Novel communities from climate change. Proc. R. Soc. B 367, 2913–2922. (doi:10.1098/rstb.2012.0238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lőkkös A, Müller T, Kovács K, Várkonyi L, Specziár A, Martin P. 2016. The alien, parthenogenetic marbled crayfish (Decapoda: Cambaridae) is entering Kis-Balaton (Hungary), one of Europe's most important wetland biotopes. Knowl. Manag. Aquat. Ecosyst. 417, 1–9. (doi:10.1051/kmae/2016003) [Google Scholar]
- 46.Kempkes M, Budesheim F, Rose U. 2009. Etho-ecological observations of a guppy population (Poecilia reticulata Peters, 1859) in a thermally polluted stream in Germany. BrehmSpace: Westarp Wissenschaften; [In German]. [Google Scholar]
- 47.Silva SS, Amarasinghe US. 1989. Stunting in Oreochromis mossambicus (Peters)(Pisces, Cichlidae): an evaluation of past and recent data from Sri Lankan reservoir populations. J. Appl. Ichthyol. 5, 203–210. (doi:10.1111/j.1439-0426.1989.tb00493.x) [Google Scholar]
- 48.Heaton W. 2015. Data from: Evaluation of blue tilapia (Oreochromis aureus) for duckweed (Lemna minor) control in South Carolina's private waters. Doctoral dissertation, Clemson University TigerPrints. [Google Scholar]
- 49.Rickel BW. 1975. Data from: The effectiveness of Tilapia zillii in controlling aquatic vegetation in a southwestern pond. MS thesis, Universsity of Arizona Campus Repository; See http://hdl.handle.net/10150/566546 (accessed on 1 February 2017). [Google Scholar]
- 50.Hauser WJ, Legner EF, Medved RA, Platt S. 1976. Tilapia—a management tool for biological control of aquatic weeds and insects. Bull. Am. Fish. Soc. 1, 15–16. [Google Scholar]
- 51.Slootweg R, Malek EA, McCullough FS. 1994. The biological control of snail intermediate hosts of schistosomiasis by fish. Rev. Fish. Biol. Fish. 4, 67–90. (doi:10.1007/BF00043261) [Google Scholar]
- 52.Graber M, Gevrey JP, Euzeby JA. 1981. Biological control of the mollusc vector of Katayama disease: predatory action of Tilapia rendalli and Sarotherodon mossambicus with regard to Biomphalaria glabrata. Hydrobiologia 78, 253–257. (doi:10.1007/BF00008521) [Google Scholar]
- 53.Trewavas E. 1983. Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia. London, UK: British Museum (Natural History). [Google Scholar]
- 54.Bhassu S, Yusoff K, Panandam JM, Embong WK, Oyyan S, Tan SG. 2004. The genetic structure of Oreochromis spp. (Tilapia) populations in Malaysia as revealed by microsatellite DNA analysis. Biochem. Genet. 42, 217–229. (doi:10.1023/B:BIGI.0000034426.31105.da) [DOI] [PubMed] [Google Scholar]
- 55.Canonico GC, Arthington A, McCrary JK, Thieme ML. 2005. The effects of introduced tilapias on native biodiversity. Aquat. Conserv. 15, 463–483. (doi:10.1002/aqc.699) [Google Scholar]
- 56.Dunz AR, Schliewen UK. 2013. Molecular phylogeny and revised classification of the haplotilapiine cichlid fishes formerly referred to as ‘Tilapia’. Mol. Phylogenet. Evol. 68, 64–80. (doi:10.1016/j.ympev.2013.03.015) [DOI] [PubMed] [Google Scholar]
- 57.FAO (United Nations Food and Agriculture Organization). 2014. The state of world fisheries and aquaculture. Rome: FAO. [Google Scholar]
- 58.D'Amato ME, Esterhuyse MM, Van Der Waal BC, Brink D, Volckaert FA. 2007. Hybridization and phylogeography of the Mozambique tilapia Oreochromis mossambicus in southern Africa evidenced by mitochondrial and microsatellite DNA genotyping. Conserv. Genet. 8, 475–488. (doi:10.1007/s10592-006-9186-x) [Google Scholar]
- 59.Welcomme RL. 1988. International introductions of inland aquatic species. FAO Fisheries Technical Paper No. 294, p. 318 Rome, Italy: FAO. [Google Scholar]
- 60.Bradford M, Kroon FJ, Russell DJ. 2011. The biology and management of Tilapia mariae (Pisces: Cichlidae) as a native and invasive species: a review. Mar. Freshw. Res. 62, 902–917. (doi:10.1071/MF10289) [Google Scholar]
- 61.Nico L, Neilson M. 2017. Tilapia mariae. Gainsville, FL: Data from: USGS Nonindigenous Aquatic Species Database; See https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=482 (Revision Date: 4/19/2013). [Google Scholar]
- 62.Teugels GG, Thys van den Audenaerde DFE. 2003. Cichlidae. In Faune des poissons d'eaux douces et saumâtres d'Afrique de l'Ouest. Volume 40. Coll. Faune Tropicale n° 28 (eds Paugy DC, Lévêque A, Teugels GG), pp. 521–600. Paris: Musée Royal de l'Afrique Centrale, Tervuren, Belgique and ORSTOM. [Google Scholar]
- 63.Teugels GG, Thys van den Audenaerde DFE. 1992. Cichlidae. In Faune des poissons d'eaux douces et saumâtres d'Afrique de l'Ouest. Volume 2. Coll. Faune Tropicale n° 28 (eds Lévêque C, Paugy D, Teugel GG), pp. 714–779. Paris: Musée Royal de l'Afrique Centrale, Tervuren, Belgique and ORSTOM. [Google Scholar]
- 64.Stiassny ML, Lamboj A, De Weirdt D, Teugels GG. 2007. Cichlidae. In Poissons d'eaux douces et saumâtres de basse Guinée, ouest de l'Afrique central, vol. 2 (eds Stiassny ML, Teugels GG, Hopkins CD), pp. 269–403. Paris, France: IRD Éditions. [Google Scholar]
- 65.Schmitter-Soto JJ. 2007. A systematic revision of the genus Archocentrus (Perciformes: Cichlidae), with the description of two new genera and six new species. Zootaxa 1603, 1–78. [Google Scholar]
- 66.Schmitter-Soto JJ. 2007. Phylogeny of species formerly assigned to the genus Archocentrus (Perciformes: Cichlidae). Zootaxa 1618, 1–50. [Google Scholar]
- 67.Kottelat M, Freyhof J. 2007. Handbook of European freshwater fishes, 646p Berlin, Germany: Publications Kottelat. [Google Scholar]
- 68.Inoue JG, Miya M, Tsukamoto K, Nishida M. 2000. Complete mitochondrial DNA sequence of the Japanese sardine Sardinops melanostictus. Fisheries Sci. 66, 924–932. (doi:10.1046/j.1444-2906.2000.00148.x) [Google Scholar]
- 69.Fisch-Muller S. 2003. Loricariidae-Ancistrinae (Armored catfishes). In Checklist of the freshwater fishes of South and Central America (eds Reis RE, Kullander SO, Ferraris CJ), pp. 373–400. Brasil: Porto Alegre. [Google Scholar]
- 70.Tachihara K, Tokunaga K, Chimura Y. 2002. Alien fishes in Okinawa Island. In Handbook of alien species in Japan (ed. Ecological Society of Japan), pp. 248–249. Tokyo: Chijin-Shokan. [Google Scholar]
- 71.Roll U, Dayan T, Simberloff D, Goren M. 2007. Characteristics of the introduced fish fauna of Israel. Biol. Invasion 9, 813–824. (doi:10.1007/s10530-006-9083-8) [Google Scholar]
- 72.Hovey TE, Swift CC. 2012. First record of an established population of the convict cichlid (Archocentrus nigrofasciatus) in California. Calif. Fish Game 98, 125–128. [Google Scholar]
- 73.Esmaeili HR, Teimori A, Feridon OW, Abbasi K, Brian WC. 2015. Alien and invasive freshwater fish species in Iran: diversity, environmental impacts and management. Iran J. Ichthyol. 1, 61–72. [Google Scholar]
- 74.Cadwallader PL, Backhouse GN, Fallu R. 1980. Occurrence of exotic tropical fish in the cooling pondage of a power station in temperate South-Eastern Australia. Mar. Freshw. Res. 31, 541–546. (doi:10.1071/MF9800541) [Google Scholar]
- 75.Ikomi RB, Jessa HO. 2003. Studies on aspects of the biology of Tilapia mariae (Boulenger, 1899) (Osteichthyes Cichlidae) in Ethiope River, Niger Delta, Nigeria. Afr. Zool. 38, 255–264. (doi:10.1080/15627020.2003.11407279) [Google Scholar]
- 76.Annett CA, Pierotti R, Baylis JR. 1999. Male and female parental roles in the monogamous cichlid, Tilapia mariae, introduced in Florida. Environ. Biol. Fish. 54, 283–293. (doi:10.1023/A:1007567028017) [Google Scholar]
- 77.Schwanck EJ. 1989. Parental care of Tilapia mariae in the field and in aquaria. Env. Biol. Fish. 24, 251–265. (doi:10.1007/BF00001399) [Google Scholar]
- 78.Trewavas E. 1974. The freshwater fishes of rivers Mungo and Meme and lakes Kotto, Mboandong and Soden, pp. 329–419. West Cameroon: British Museum (Natural History). [Google Scholar]
- 79.Anibeze CI. 2001. Stomach length and food preference of three tilapia species (Osteichthyes: Cichlidae) in Agulu Lake Basin, Nigeria. J. Aquat. Sci. 16, 57–60. (doi:10.4314/jas.v16i1.20004) [Google Scholar]
- 80.Ajuzie CC. 1996. Tilapia mariae: a possible candidate for culture in Nigeria. Aquacult. Mag. 22, 46–53. [Google Scholar]
- 81.Vassallo P, Bastianoni S, Beiso I, Ridolfi R, Fabiano M. 2007. Emergy analysis for the environmental sustainability of an inshore fish farming system. Ecol. Indic. 7, 290–298. (doi:10.1016/j.ecolind.2006.02.003) [Google Scholar]
- 82.Webb AC. 2007. Status of non-native freshwater fishes in tropical northern Queensland, including establishment success, rates of spread, range and introduction pathways. J. Proc. R. Soc. New South Wales 140, 63–78. [Google Scholar]
- 83.Greiner R, Gregg D. 2008. Tilapia in north Queensland waterways: Risks and potential economic impacts. Townsville, Australia: River Consulting. [Google Scholar]
- 84.Russell DJ, Thuesen PA, Thomson FE. 2012. A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Rev. Fish. Biol. Fish. 22, 533–554. (doi:10.1111/j.1095-8649.2012.03267.x) [Google Scholar]
- 85.Courtenay WR Jr, Hensley DA. 1980. Special problems associated with monitoring exotic species. In Biological monitoring of fish (eds Hocutt CM, Stauffer JR Jr), pp. 281–307. Washington, DC: Lexington Books. [Google Scholar]
- 86.Brooks WR, Jordan RC. 2010. Enhanced interspecific territoriality and the invasion success of the spotted tilapia (Tilapia mariae) in South Florida. Biol. Invasions 12, 865–874. (doi:10.1007/s10530-009-9507-3) [Google Scholar]
- 87.Philippart JC, Ruwet JC. 1982. Ecology and distribution of tilapias. In The biology and culture of tilapias (eds Pullin RSV, Lowe RH, McConnell RH), pp. 15–60. Manila, Philippines: ICLARM. [Google Scholar]
- 88.Bussing WA. 1998. Cichlidae. In Freshwater fishes of Costa Rica. Volume 2, pp. 293–383. San José, Costa Rica: Editorial Universidad de Costa Rica. [Google Scholar]
- 89.Baensch HA, Riehl R. 1991. Fische. In Aquarien Atlas. Band 5. Melle: Mergus Verlag für Natur- und Heimtierkunde, Germany; [In German]. [Google Scholar]
- 90.Courtenay WR Jr, Deacon JE. 1983. Fish introductions in the American Southwest: a case history of Rogers Spring, Nevada. Southwest. Nat. 28, 221–224. (doi:10.2307/3671390) [Google Scholar]
- 91.Siemien MJ, Stauffer JR. 1989. Temperature preference and tolerance of the spotted tilapia and Rio Grande cichlid. Arch. Hydrobiol. 115, 287–303. [Google Scholar]
- 92.Shafland PL, Pestrak JM. 1982. Lower lethal temperatures for fourteen non-native fishes in Florida. Env. Biol. Fish. 7, 149–156. (doi:10.1007/BF00001785) [Google Scholar]
- 93.Smitherman RO. 1988. Cold tolerance and growth of three strains of Oreochromis niloticus. In 2nd Int. Symp. on Tilapia in Aquaculture 16–20 March 1987, vol. 15 (ed. Department of Fisheries), pp. 215–218. Bangkok, Thailand. [Google Scholar]
- 94.Charo-Karisa H, Mahmoud AR, Bovenhuis H, Komen H. 2005. Heritability of cold tolerance in Nile tilapia Oreochromis niloticus juveniles. Aquacult. 249, 115–123. (doi:10.1016/j.aquaculture.2005.04.029) [Google Scholar]
- 95.Ross FF. 1970. Proc. Inst. Wat. Pollut. Control.
- 96.Arthington AH. 1991. Ecological and genetic impacts of introduced and translocated freshwater fishes in Australia. Can. J. Fish. Aquat. Sci. 48, 33–43. (doi:10.1139/f91-302) [Google Scholar]
- 97.Friedrich G.2000. Die untere Erft—ein subtropischer Fluss. Gewässergütebericht: 101–105 [In German].
- 98.Hussner A, Lösch R. 2005. Alien aquatic plants in a thermally abnormal river and their assembly to neophyte-dominated macrophyte stands (River Erft, Northrhine-Westphalia). Limnologica 35, 18–30. (doi:10.1016/j.limno.2005.01.001) [Google Scholar]
- 99.2017. LANUV (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen) Hydrologische Rohdaten Online: Messstation Düsseldorf-Flehe. See http://luadb.it.nrw.de/LUA/hygon/ (accessed 1 May 2017) [In German].
- 100.Leuven RS, van der Velde G, Baijens I, Snijders J, van der Zwart C, Lenders HR, bij de Vaate A. 2009. The river Rhine: a global highway for dispersal of aquatic invasive species. Biol. Invasions 11, 1989–2008. (doi:10.1007/s10530-009-9491-7) [Google Scholar]
- 101.Panov VE, et al. 2009. Assessing the risks of aquatic species invasions via European inland waterways: from concepts to environmental indicators. Integr. Environ. Assess. Manag. 5, 110–126. (doi:10.1897/IEAM_2008-034.1) [DOI] [PubMed] [Google Scholar]
- 102.Deacon AE, Ramnarine IW, Magurran AE. 2011. How reproductive ecology contributes to the spread of a globally invasive fish. PLoS ONE 6, e24416 (doi:10.1371/journal.pone.0024416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prenter J, MacNeil C, Dick JT, Dunn AM. 2004. Roles of parasites in animal invasions. Trends Ecol. Evol. 19, 385–390. (doi:10.1016/j.tree.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 104.Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM. 2009. Parasite spillback: a neglected concept in invasion ecology? Ecology 90, 2047–2056. (doi:10.1890/08-1085.1) [DOI] [PubMed] [Google Scholar]
- 105.Nmor JC, Egwunyenga AO, Ake JE. 2003. Observations on the intestinal helminth parasites of cichlids in the upper reaches of River Orogodo, a freshwater body in Delta State, southern Nigeria. Trop. Freshwat. Biol. 12, 131–136. [Google Scholar]
- 106.King RP, Etim L. 2004. Reproduction, growth, mortality and yield of Tilapia mariae Boulenger 1899 (Cichlidae) in a Nigerian rainforest wetland stream. J. Appl. Ichthyol. 20, 502–510. (doi:10.1111/J.1439-0426.2004.00545.X) [Google Scholar]
- 107.Olurin KB, Somorin CA. 2006. Intestinal helminths of the fishes of Owa stream, South-West Nigeria. Res. J. Fish. Hydrobiol. 1, 6–9. [Google Scholar]
- 108.Honsig-Erlenburg W. 2001. Zum Fischbestand des Warmbaches in Villach. Carinthia II 191, 135–140 [In German]. [Google Scholar]
- 109.NOBANIS (European Network on Invasive Alien Species). 2017. See http://www.nobanis.org (accessed on 1 May 2017).
- 110.EASIN (European Alien Species Information Network). Version 5.7 of the EASIN Species Catalogue. See http://easin.jrc.ec.europa.eu/ (accessed on 1 May 2017).
- 111.DAISIE (Delivering Alien Invasive Species Inventories for Europe) European invasive alien species gateway database 2017. See http://www.europe-aliens.org (accessed on 1 May 2017).
- 112.FishBase. 2017. In Fishbase 2000: concepts, design and data sources (eds Froese R, Pauly D), p. 344 Los Baños, Laguna, Philippines: ICLARM; See www.fishbase.org (accessed on 1 May 2017). [Google Scholar]
- 113.Tierschutzgesetz. German Animal Welfare Act in the version of the promulgation of 18 May 2006 (Federal Law Gazette [BGBI.] Part I pp. 1206, 1313), most recently amended by Article 4 (87) of the Act of 18 July 2016 (Federal Law Gazett [BGBI.] Part I p. 1666). Available at: http://www.gesetze-im-internet.de/tierschg/BJNR012770972.html (accessed on 1 May 2017).
- 114.Lukas JAY, Jourdan J, Kalinkat G, Emde S, Miesen F, Jüngling H, Cocchiararo B, Bierbach D. 2017. Data from: On the occurrence of three non-native cichlid species including the first record of a feral population of Pelmatolapia (‘Tilapia’) mariae (Boulenger, 1899) in Europe. Dryad Digital Repository. (doi:10.5061/dryad.sd7vh) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lukas JAY, Jourdan J, Kalinkat G, Emde S, Miesen F, Jüngling H, Cocchiararo B, Bierbach D. 2017. Data from: On the occurrence of three non-native cichlid species including the first record of a feral population of Pelmatolapia (‘Tilapia’) mariae (Boulenger, 1899) in Europe. Dryad Digital Repository. (doi:10.5061/dryad.sd7vh) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Our data are deposited at Dryad (http://dx.doi.org/10.5061/dryad.sd7vh; [114]). mtDNA sequences are available at the NCBI GenBank under the following accession codes: KY565238–KY565240 and KY582461–KY582463. All specimens were integrated into the ichthyologic collection at the ZFMK in Bonn, Germany under the project numbers Lukas_Gillbach2016_01 to Lukas_Gillbach2016_15.