Abstract
Understanding how individual behaviour influences the spatial and temporal distribution of other species is necessary to resolve the complex structure of species assemblages. Mixed-species bird flocks provide an ideal opportunity to investigate this issue, because members of the flocks are involved in a variety of behavioural interactions between species. Willow tits (Poecile montanus) often produce loud calls when visiting a new foraging patch to recruit other members of mixed-species flocks. The costs and benefits of flocking would differ with individual foraging behaviours (i.e. immediate consumption or caching); thus, willow tits may adjust the production of loud calls according to their foraging intention. In this study, we investigated the link between foraging decisions and calling behaviour in willow tits and tested its influence on the temporal cohesion with members of mixed-species flocks. Observations at experimental foraging patches showed that willow tits produced more calls when they consumed food items compared with when they cached them. Playback experiments revealed that these calls attracted flock members and helped to maintain their presence at foraging patches. Thus, willow tits adjusted calling behaviour according to their foraging intention, thereby coordinating the associations with members of mixed-species flocks. Our findings demonstrate the influence of individual decision-making on temporal cohesion with other species and highlight the importance of interspecific communication in mixed-species flocking dynamics.
Keywords: flock dynamics, foraging decisions, mixed-species flocks, willow tits, vocal signals
1. Introduction
One of the greatest challenges in ecology and evolution is to understand how individual behaviour influences the spatial and temporal structuring of species assemblages [1–3]. Mixed-species bird flocks provide an excellent system to investigate this issue, because members of the flocks are involved in a variety of behavioural interactions between species, which may influence the flocking dynamics [3–6]. For example, individuals might follow heterospecific individuals to exploit their food items [7–11] or approach them to cooperatively drive off a predator [12–15]. Such organization of multi-species associations would reflect the individual decisions of flock members to maximize their own fitness benefits. However, knowledge is limited about how the decision of individuals is made in multi-species environments and how it influences social cohesion with other species.
Interspecific vocal communication might provide a way of linking the decisions of individuals with the temporal dynamics of mixed-species flocks [3,16]. For example, when separated from mixed-species flocks, greater racket-tailed drongos (Dicrurus paradiseus) attract flock members by mimicking the song and contact calls of other species [17]. By actively facilitating the social cohesion with other species, drongos can increase their foraging efficiency by catching insects flying out from the disturbance of other species and also by kleptoparasitism [18,19]. Similarly, in mixed-species flocks in Japan, willow tits (Poecile montanus) produce ‘tää’ calls (figure 1) when visiting a new foraging patch alone, which attracts both conspecific and heterospecific members of mixed-species flocks to the food source [20,21]. By recruiting other members of a mixed-species flock, willow tits may reduce the risk of predation [22,23]. However, tits do not typically produce calls when visiting the foraging patch as part of a flock, probably because redundant call production increases the risk of attracting predators [24]. Mixed-species flocks of tits are highly variable in their membership over short periods of time and may not be stable at a single foraging patch [25]. Therefore, the dynamic adjustment of calling behaviour may allow tits to control for the changes in temporal social cohesion with other individuals as well as to manage the immediate risk of predation.
Figure 1.
Sound spectrogram of willow tit ‘tää’ calls, consisting of multiple ‘tää’ notes.
Willow tits may adjust their calling behaviour based on subsequent foraging decisions, as well as the social context, because the costs and benefits of flocking would differ depending on the foraging decision. Willow tits have two different foraging behaviours: (i) eating food items immediately or (ii) caching food items for future use [26]. When handling and consuming food, willow tits might benefit from attracting other flock members, with associations increasing anti-predator benefits (e.g. sharing vigilance) [23]. In contrast, while caching food items, close associations with other flock members might increase the risk of cached food items being pilfered [27–29]. Tits might therefore reduce the risk of pilferage by caching items in highly dispersed locations [30]. As a result, the anti-predator benefits of attracting other individuals to food by calling would be greater for tits when consuming food items than when caching them. Such differences in the advantages of group foraging, in turn, might influence the temporal dynamics of mixed-species flocks.
In this study, we investigated whether the calling behaviour of willow tits was related to their subsequent foraging decisions and if there was a concomitant effect on temporal cohesion with other members within mixed-species flocks. We predicted that willow tits would produce ‘tää’ calls only when they intended to consume a food item and not when they intended to cache the food. By creating artificial foraging patches, we examined how willow tits changed their foraging behaviour on repeated visits to the food source and how they adjusted the production of ‘tää’ calls according to their foraging intention. We also analysed the influence of social context on the production of ‘tää’ calls, since a previous study found that willow tits adjusted their calling behaviour according to the presence or absence of flock members [21]. Although ‘tää’ calls have been shown to function in attracting both conspecific and heterospecific flock members to a foraging patch [21], it remains unclear whether these calls function in maintaining temporal cohesion with other flock members. We conducted playback experiments to test whether these calls serve to attract other flock members and keep them at a foraging patch for longer than that with a silent control. We found that willow tits adjusted their calling behaviour according to their foraging intention and social context, thereby influencing the temporal cohesion with other species involved in mixed-species flocks.
2. Material and methods
2.1. Study site and subjects
The study was conducted in a mixed deciduous–coniferous forest in Karuizawa, Nagano, Japan (36°19–22′ N, 138°32–37′ E). In this forest, mixed-species flocks contained several passerine and non-passerine species. Out of these species, willow tits, Japanese tits (Parus minor), varied tits (Poecile varius) and nuthatches (Sitta europaea) used artificial feeders with sunflower seeds, and frequently consumed sunflower seeds [21]. There was a noticeable interspecific dominance hierarchy at the foraging sites, with nuthatches being the most dominant species, followed by varied tits, Japanese tits and willow tits [21]. In the present study, willow tits tended to arrive at novel food patches first (50%) (n = 54; see below), whereas other species (Japanese tits (33%), varied tits (13%) and nuthatches (4%)) tended to arrive later.
2.2. Observations at the artificial foraging patch
To investigate the relationship between the calling and foraging behaviours of willow tits, a forest block (approximately 500 × 500 m) was selected, in which most of the birds (willow tits, Japanese tits, varied tits and nuthatches) were captured using feeder traps and were colour-banded for individual identification during the course of a 3-year field research project. For willow tits, all of the individuals observed in the forest (n = 54) were individually identifiable with colour leg bands. Fifty-four forest openings were first chosen within the forest block, which were separated by at least 30 m from each other, and a forest opening was randomly assigned for each trial. Then, a wooden feeder (25 × 25 × 5 cm) was placed on the ground at each site and filled with 300 g sunflower seeds for each trial. The observation location was situated at a distance of 10–12 m from the feeder, which was ideal for collecting data on bird behaviour without causing disturbance [21].
The focal-observation method [31] was used to record the behaviour of individual willow tits. When a willow tit was within 15 m of the feeder, behavioural observations were started. Tits made a calling decision on reaching the tree branches around the feeder. Subsequently, they took a seed from the feeder, and then ate it or cached it (i.e. subsequent foraging behaviour). Tits typically continued to visit the feeder during the first 10 min after their initial arrival; therefore, behavioural observations lasted for approximately 10 min (see also [25]). The following variables were recorded: (i) whether the focal willow tit produced ‘tää’ calls before it took a seed from the feeder, (ii) whether it consumed the seed or cached it, and (iii) whether other flock members were present within a 5 m radius around the focal bird when it visited the feeder. It was often not possible to follow the foraging behaviours of willow tits when they flew a considerable distance away (more than 30 m from the feeder) after taking a seed. These instances (n = 26) were considered as caching, since a previous study showed that when flying such a distance, willow tits always cache food items inside tree trunks or branches [26]. Behavioural observations were recorded vocally using an LS370 parabolic microphone (Fuji Planning Co., Tokyo, Japan) connected to an MZ-RH1 Hi-MD Walkman (sampling wave files at 44.1 kHz, 16 bits; Sony Co., Tokyo, Japan).
Up to five trials (separated by at least 1 h) were conducted daily, resulting in 54 trials from 26 January to 9 February 2008. Data from 13 sites, in which willow tits never visited the feeder within the first 10 min of the arrival of the other species, were excluded from the analysis. In these trials, willow tits were not observed from the observation position; thus, they appeared to engage in foraging at a far distance. Therefore, the final dataset included data from 41 trials only. No experimental sites were used more than once to ensure that the birds visited novel foraging patches in all trials. All trials were conducted under calm and dry weather conditions from 08.00 to 15.00 h (Japan Standard Time). A total of 381 feeder visitations (up to 10 visitations in each trial) were recorded for 13 different willow tits that had unique combinations of colour-bands.
2.3. Playback experiments
Playback experiments were conducted to test whether the ‘tää’ calls of willow tits function to maintain temporal cohesion with other flock members. Playback stimuli were constructed from our recording libraries of 10 different willow tits. A single call composed of four ‘tää’ notes was chosen from each source individual. This call was repeated at a rate of one call per 6 s to create a 60 s calling bout. Thus, playback stimuli for call treatment were created so as to be typical of natural calling bout of tää’ calls produced in a food context [20,21]. The 60 s calling bout was preceded by a 30 s silent period and then these sounds were repeated 10 times, resulting in a 15 min (900 s) sound file. Background noise was used as a control stimulus, because it allowed us to assess the stability of mixed-species flocks without any playback of the call. Background noise was chosen from the same files for call playbacks, and was edited in the same way as the playback call files, i.e. a 90 s sound (60 s background noise preceded by a 30 s silent period) was repeated 10 times to fill a 15 min sound file. Call files were prepared for playing at a standardized volume (72 dB at 1 m from a loud speaker, measured using an SM-325 sound level meter; AS ONE Corporation, Osaka, Japan). Background noise files were prepared for broadcasting at the same amplitude as the background noise level in call playbacks (50 dB at 1 m).
Trials were conducted from 15 to 19 December 2015. First, we searched for a mixed-species flock in the forest. When a flock was found and a willow tit was seen, a loudspeaker was placed at the base of a tree. It was ensured that the loudspeaker was placed within approximately 15 m of the willow tit. Then, the observer moved to the observation location at 15 m from the loudspeaker, which was estimated using a tape measure, and the playback of either a call or a background noise was started. During the silent periods of each trial, the number of individuals within 15 m distance of the loudspeaker was counted, because, at this distance, all birds were visible from the observation position. This scanning method is generally acceptable for measuring the behavioural response of groups [31]. Trials were conducted at 20 sites, which were separated by at least 400 m, because previous observations of colour-banded individuals showed that this distance is enough to ensure that independent data are collected from different individuals [21]. The order of calls and background noise was alternated and counterbalanced across sites, so that call A at site 1, background noise A at site 2, background noise B at site 3, call B at site 4, and so on (capital letters correspond to the identity of the original recording files). Unique exemplars were used for each trial to avoid pseudoreplication [32].
2.4. Statistical analysis
All statistical analyses were carried out in R for Mac v. 3.1.2 [33]. Generalized linear mixed models (glmer function in the package lme4 [34]) with a binomial error structure and logit-link function were used to analyse foraging and calling behaviours. In the first model, the foraging behaviour (consume or cache) was modelled in response to the number of feeder visitations and presence or absence of other flock members. In the second model, calling behaviour (yes or no) was modelled in response to the number of feeder visitations, presence or absence of flock members, and subsequent foraging behaviour (consume or cache). In both models, the presence or absence of any flock members was included regardless of their species identity, because comparisons of Akaike's information criterion (AIC) values supported the view that this categorization better explains the adjustment of foraging and calling behaviour in willow tits; the models with the presence or absence of any flock members had smaller AIC values (first model: 282.8; second model: 241.9) compared with those including the presence or absence of each species of bird separately (first model: 287.9; second model: 243.6). For both models, the fixed terms were tested for possible multicollinearity by examining the variance inflation factor (VIF; function vif in the package DAAG [35]). The highest VIFs in the first and second models were 1.03 and 1.26, respectively, which were less than the cut-off value of 10 [36]. In addition, the maximum correlation coefficients between fixed terms were generally low for both models (first model: −0.16; second model: 0.24). Therefore, there was no multicollinearity effect on the fixed terms. All fixed terms were standardized using z-transformation to facilitate direct comparisons of the effect sizes before the analyses [37]. Since we collected data from 13 individual willow tits in 41 trials, all of the models included the individual identity of focal bird and the individual trials as random terms.
Non-parametric statistics were used to analyse the data of the playback experiment. Mood's median tests were used to investigate whether birds were more likely to approach the loudspeaker with the playback of ‘tää’ calls than that with the playback of background noise, and whether they continued foraging for longer around the loudspeaker when exposed to calls than to background noise. All tests were two-tailed, and the statistical level was set at α = 0.05.
3. Results
3.1. Factors affecting foraging decisions
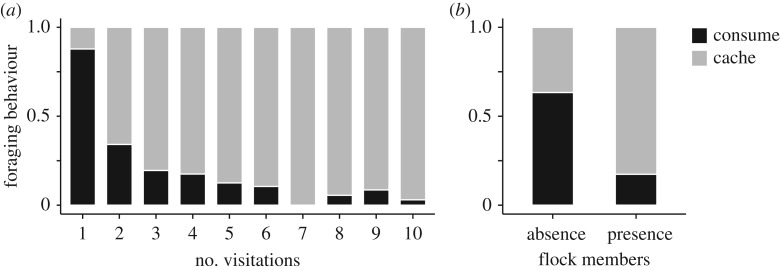
Willow tits changed their foraging behaviours when repeatedly visiting a feeder (table 1). For early visits, they consumed a seed close to the feeder. In later visits, they became more likely to carry seeds and cache them away from the feeder (figure 2a). Social context also affected the foraging behaviours of willow tits; they tended to consume seeds when they visited the feeder alone, whereas they typically cached the seeds when other flock members were already present around the feeder (figure 2b and table 1).
Table 1.
Factors affecting the foraging behaviour of willow tits analysed using the generalized linear mixed model. The foraging behaviour (consume or cache) was modelled in response to the number of repeated visits to the feeder and presence or absence of flock members. s.e.: standard error. Sample size: n = 381 feeder visitations by 13 individuals in 41 trials. All fixed terms were standardized before the analysis.
| model | estimate | s.e. | d.f. | χ2 | p |
|---|---|---|---|---|---|
| intercept | −1.85 | 0.35 | |||
| number of feeder visitations | −1.6 | 0.23 | 1 | 77.97 | <0.001 |
| flock members (presence/absence) | −0.44 | 0.15 | 1 | 9.12 | <0.01 |
Figure 2.
Factors affecting the foraging behaviours (consumption or caching) by willow tits. (a) Willow tits shifted their foraging modes from consumption to caching as they repeated feeder visitations. (b) Willow tits generally consumed seeds when they visited the feeder alone, but they tended to cache a seed in the presence of flock members. Sample size: n = 381 feeder visitations by 13 individuals in 41 trials.
3.2. Factors affecting calling behaviour
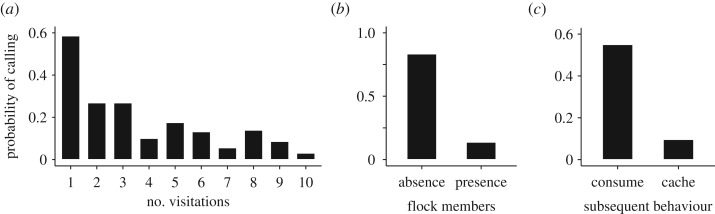
Willow tits changed their calling behaviour during the repeated visitations to the feeder (table 2). They frequently produced ‘tää’ calls on their first access to the feeder, but after the second visit to the feeder, they reduced their probability of calling (figure 3a). Social context affected the ‘tää’ calling in willow tits; they typically produced calls when visiting the feeder alone, whereas they rarely vocalized when other flock members were present (figure 3b and table 2). Foraging intention also had a significant effect on the calling behaviour of willow tits; they produced ‘tää’ calls particularly before they consumed a seed close to the feeder, whereas they typically did not call before caching a seed (figure 3c and table 2).
Table 2.
Factors affecting the production of ‘tää’ calls by willow tits analysed using the generalized linear mixed model. Calling behaviour (yes or no) was modelled in response to the number of repeated visits to the feeder, subsequent foraging behaviour (consume or cache), and presence or absence of flock members. s.e.: standard error. Sample size: n = 381 feeder visitations by 13 individuals in 41 trials. All fixed terms were standardized before the analysis.
| model | estimate | s.e. | d.f. | χ2 | p |
|---|---|---|---|---|---|
| intercept | −2.67 | 0.44 | |||
| number of feeder visitations | 0.97 | 0.21 | 1 | 7.52 | <0.01 |
| flock members (presence/absence) | −0.64 | 0.25 | 1 | 15.33 | <0.001 |
| subsequent foraging behaviour (consume/cache) | −0.86 | 0.26 | 1 | 25.21 | <0.001 |
Figure 3.
Factors affecting the production of ‘tää’ calls by willow tits. (a) The probability of focal tits producing calls declined as they repeated feeder visitations. (b) The probability of calling declining due to the presence of flock members. (c) The probability of calling being affected by subsequent foraging behaviour. Sample size: n = 381 feeder visitations by 13 individuals in 41 trials.
3.3. Playback experiments
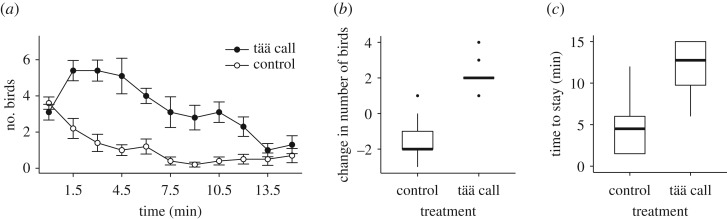
Playback experiments showed that ‘tää’ calls attracted both conspecific and heterospecific flock members to the loudspeaker, and maintained the cohesion of flock members at a foraging patch (figure 4a). During the first 1.5 min of the playback, more birds approached during the playback of the ‘tää’ calls than when the background noise was played (figure 4b; Mood's median test: p < 0.001). Willow tits (6/10 trials), Japanese tits (6/10 trials) and coal tits (4/10 trials) were the most common species that approached the ‘tää’ calls during the first 1.5 min of playback. The time that birds continued foraging within a 15 m distance of the loudspeaker was significantly longer during the playback of ‘tää’ calls than the background noise (figure 4c; Mood's median test: p = 0.023), indicating that calls facilitated temporal cohesion with flock members at the foraging patch.
Figure 4.
Responses of birds to the playback of ‘tää’ calls of willow tits and background noise. (a) Number of birds (mean ± s.e.) within a 15 m distance of the loudspeaker. (b) Changes in the number of birds during the first 1.5 min of the playback. (c) Time to stay within a 15 m distance of the loudspeaker after the beginning of the playback. Sample size: n = 10 for both treatments.
4. Discussion
Willow tits produced ‘tää’ calls when they focused on consuming a food item (figure 3c), and these calls were also shown to facilitate social cohesion with both conspecific and heterospecific flock members at a foraging patch (figure 4). In contrast, willow tits rarely produced calls when caching a food item (figure 3c). Therefore, the temporal stability of mixed-species flocks at a patch was maintained particularly when willow tits consumed a seed and produced ‘tää’ calls. Attracting other flock members may provide anti-predator advantages to the callers. For example, close associations with other flock members allow individuals to share vigilance costs while foraging [23,38]. In addition, individuals within mixed-species flocks may recognize heterospecific alarm calls, which enhance predator detection and choosing an appropriate anti-predator behaviour [12,13]. Furthermore, even when approached by a predator, an increase in the number of surrounding individuals reduces an individual's probability of being attacked by the predator [22]. These anti-predator benefits of flocking would therefore be larger for willow tits consuming food items close to the feeder than those caching them, because tits caching food typically fly a considerable distance from the feeder [26].
Willow tits change their foraging and calling behaviours according to the temporal social cohesion with other flock members (figures 2b and 3b). When alone, willow tits typically produce calls and consume seeds close to the feeder. In contrast, when other flock members are present, willow tits silently take and cache the seeds. Although attracting other flock members may allow individuals to reduce the risk of predation [22,23], the production of loud calls, such as ‘tää’ calls, may also attract predators [24]. Therefore, the adjustment of call production under different social environments may serve to manage immediate predation risk. When caching food items, willow tits travel a considerable distance from the feeder in order to reduce the risk of pilferage by other individuals [26,30]. Therefore, willow tits caching seeds alone are expected to be more isolated from the flocks than those caching seeds with other flock members around. This may explain why willow tits rarely cache food items when other flock members are absent around the feeder (figure 2b).
In the course of repeated visits to the feeder, willow tits changed their foraging modes and calling behaviour (figures 2a and 3a). Initially, birds produced calls and consumed seeds when they arrived at a feeder. In later visits they would reduce their calling and take seeds in order to cache. Because the experimental foraging patches were created at novel sites, advertising the location of the feeder by calling might be important for food discoverers to direct other flock members to the patches [21,39]. This result is also consistent with our previous observations that willow tits produce calls more often when they find food items at a permanent feeder alone compared with when other individuals are present [21]. In addition to the calling, feeding around the feeder might aid tits to advertise the exact location of the feeder to other birds. The change in foraging behaviours may also be explained by individuals' physiological condition. In winter, birds lose heat from their body surface, and require more energy to support high metabolic expenditure [28,40]. Therefore, consuming seeds first might allow tits to cover the energy cost of subsequent flights for caching.
The playback of willow tit ‘tää’ calls attracted both conspecific and heterospecific flock members (figure 4a,b). Since the discovery of profitable foraging patches is difficult and often stochastic [9–11], approaching ‘tää’ calls may allow flock members to find food sources more effectively [16,23]. This is consistent with previous studies showing that parids are often followed by other species that exploit the same food items [4]. In general, parids have large vocal repertoires [41,42] and produce different call types in different contexts, such as when driving away a predator [43–45] or when in flight [46]. These calls may be recognized by other species and evoke behavioural responses [12–14], but the community-level outcomes of interspecific communication remain poorly understood [3]. Uncovering the link between sophistication in communication and its influence on other species may advance our understanding about mechanisms underlying the temporal stability and movement patterns of mixed-species flocks.
This study demonstrates that the foraging decisions of individuals are linked with their calling behaviour, which influences the temporal cohesion with other species involved in mixed-species flocks. Our findings support the view that individuals within mixed-species flocks have the potential to actively coordinate the associations with other species [17,21] and highlight the importance of interspecific vocal signalling in the flock dynamics. Future detailed studies on interspecific social interactions may not only improve our present understanding of the costs and benefits of mixed-species flocking but also provide insight into the evolution of social foraging strategies in mixed-species environments.
Supplementary Material
Acknowledgements
We are grateful to Craig RA Barnett, Damien Farine, Friederike Hillemann and Matthew Silk for valuable comments on the manuscript.
Ethics
All experiments were performed in accordance with the relevant guidelines and regulations. Field observations and colour-ringing were approved by the Ministry of the Environment and the Forestry Agency of Japan and playback experiments by the Animal Care and Use Committees at the Graduate University for Advanced Studies. All the procedures adhered to the Guidelines for the Use of Animals in Research of the Animal Behaviour Society/Association for the Study of Animal Behaviour.
Data accessibility
Supporting data are available as electronic supplementary material.
Authors' contributions
T.N.S. conceived and designed the study, collected the data, and analysed the data. T.N.S. and N.K. discussed the results and wrote the manuscript. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
This study was supported by a grant-in-aid for scientific research (KAKENHI; no. 25-3391) provided by the Japan Society for the Promotion of Science.
References
- 1.Fryxell J, Lundber P. 1998. Individual behavior and community dynamics. New York, NY: Chapman and Hall. [Google Scholar]
- 2.Morin PJ. 2009. Community ecology. Malden, MA: Blackwell. [Google Scholar]
- 3.Goodale E, Beauchamp G, Magrath RD, Nieh JC. 2010. Interspecific information transfer influences animal community structure. Trends Ecol. Evol. 25, 354–361. (doi:10.1016/j.tree.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 4.Morse DH. 1970. Ecological aspects of some mixed-species foraging flocks of birds. Ecol. Monogr. 40, 119–168. (doi:10.2307/1942443) [Google Scholar]
- 5.Silk MJ, Croft DP, Tregenza T, Bearhop S, Lens L. 2014. The importance of fission-fusion social group dynamics in birds. Ibis 156, 701–715. (doi:10.1111/ibi.12191) [Google Scholar]
- 6.Farine DR, Aplin LM, Garroway C, Mann RP, Sheldon BC. 2014. Collective decision making and social interaction rules in mixed-species flocks of songbirds. Anim. Behav. 95, 173–182. (doi:10.1016/j.anbehav.2014.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasvári L. 1979. Observational learning in great, blue and marsh tits. Anim. Behav. 27, 767–771. (doi:10.1016/0003-3472(79)90012-5) [Google Scholar]
- 8.Oommen MA, Shanker K. 2010. Shrewd alliances: mixed foraging associations between treeshrews, greater racket-tailed drongos and sparrowhawks on Great Nicobar Island, India. Biol. Lett. 6, 304–307. (doi:10.1098/rsbl.2009.0945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aplin LM, Farine DR, Morand-Ferron J, Sheldon BC. 2012. Social networks predict patch discovery in a wild population of songbirds. Proc. R. Soc. B 279, 4199–4205. (doi:10.1098/rspb.2012.1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farine DR, Aplin LM, Sheldon BC, Hoppitt W. 2015. Interspecific social networks promote information transmission in wild songbirds. Proc. R. Soc. B 282, 20142804 (doi:10.1098/rspb.2014.2804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeberg TM, Eppert SK, Sieving KE, Lucas JR. 2017. Diversity in mixed species groups improves success in a novel feeder test in a wild songbird community. Sci. Rep. 7, 43014 (doi:10.1038/srep43014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Templeton CN, Greene E. 2007. Nuthatches eavesdrop on variations in heterospecific chickadee mobbing alarm calls. Proc. Natl Acad. Sci. USA 104, 5479–5482. (doi:10.1073/pnas.0605183104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetrick SA, Sieving KE. 2011. Antipredator calls of tufted titmice and interspecific transfer of encoded threat information. Behav. Ecol. 23, 83–92. (doi:10.1093/beheco/arr160) [Google Scholar]
- 14.Suzuki TN. 2016. Referential calls coordinate multi-species mobbing in a forest bird community. J. Ethol. 34, 79–84. (doi:10.1007/s10164-015-0449-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheatcroft D, Gallego-Abenza M, Qvarnström A. 2016. Species replacement reduces community participation in avian antipredator groups. Behav. Ecol. 27, 1499–1506. (doi:10.1093/beheco/arw074) [Google Scholar]
- 16.Gil MA, Emberts Z, Jones H, St. Mary CM. 2017. Social information on fear and food drives animal grouping and fitness. Am. Nat. 189, 227–241. (doi:10.1086/690055) [DOI] [PubMed] [Google Scholar]
- 17.Goodale E, Kotagama SW. 2006. Vocal mimicry by a passerine bird attracts other species involved in mixed-species flocks. Anim. Behav. 72, 471–477. (doi:10.1016/j.anbehav.2006.02.004) [Google Scholar]
- 18.Hino T. 1998. Mutualistic and commensal organization of avian mixed-species foraging flocks in a forest of western Madagascar. J. Avian Biol. 29, 17–24. (doi:10.2307/3677336) [Google Scholar]
- 19.King DI, Rappole JH. 2001. Kleptoparasitism of laughing thrushes Garrulax by greater racket-tailed drongos Dicrurus paradiseus in Myanmar. Forktail 17, 121–122 [Google Scholar]
- 20.Suzuki TN. 2012. Calling at a food source: context-dependent variation in note composition of combinatorial calls in willow tits. Ornithol. Sci. 11, 103–107. (doi:10.2326/osj.11.103) [Google Scholar]
- 21.Suzuki TN. 2012. Long-distance calling by the willow tit, Poecile montanus, facilitates formation of mixed-species foraging flocks. Ethology 118, 10–16. (doi:10.1111/j.1439-0310.2011.01982.x) [Google Scholar]
- 22.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Sridhar H, Beauchamp G, Shanker K. 2009. Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Anim. Behav. 78, 337–347. (doi:10.1016/j.anbehav.2009.05.008) [Google Scholar]
- 24.Krams I. 2001. Communication in crested tits and the risk of predation. Anim. Behav. 61, 1065–1068. (doi:10.1006/anbe.2001.1702) [Google Scholar]
- 25.Farine DR, et al. 2015. The role of social and ecological processes in structuring animal populations: a case study from automated tracking of wild birds. R. Soc. open sci. 2, 150057 (doi:10.1098/rsos.150057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura H, Wako Y. 1988. Food storing behaviour of willow tit Parus montanus. J. Yamashina Inst. Ornithol. 20, 21–36. (doi:10.3312/jyio1952.20.21) [Google Scholar]
- 27.Carrascal LM, Moreno E. 1993. Food caching versus immediate consumption in the nuthatch: the effect of social context. Ardea 81, 135–141. [Google Scholar]
- 28.Brodin A. 2007. Theoretical models of adaptive energy management in small wintering birds. Phil. Trans. R. Soc. B 362, 1857–1871. (doi:10.1098/rstb.2006.1812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pravosudov VV. 2008. Mountain chickadees discriminate between potential cache pilferers and non-pilferers. Proc. R. Soc. B 275, 55–61. (doi:10.1098/rspb.2007.1281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Male LH, Smulders TV. 2007. Hyperdispersed cache distributions reduce pilferage: a field study. Anim. Behav. 73, 717–726. (doi:10.1016/j.anbehav.2006.06.017) [Google Scholar]
- 31.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–266. (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 32.Kroodsma DE, Byers BE, Goodale E, Johnson S. 2001. Pseudoreplication in playback experiments, revisited a decade later. Anim. Behav. 61, 1029–1033. (doi:10.1006/anbe.2000.1676) [Google Scholar]
- 33.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 34.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4 R package version 1.1-7.
- 35.Maindonald JH, Braun J. 2014. DAAG: data analysis and graphics data and functions R package version 1.20.
- 36.Petraitis PS, Dunham AE, Niewlarowski PH. 1996. Inferring multiple causality: the limitations of path analysis. Funct. Ecol. 10, 421–431. (doi:10.2307/2389934) [Google Scholar]
- 37.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. (doi:10.1111/j.2041-210X.2010.00012.x) [Google Scholar]
- 38.Elgar MA. 1986. House sparrows establish foraging flocks by giving chirrup calls if the resources are divisible. Anim. Behav. 34, 169–174. (doi:10.1016/0003-3472(86)90020-5) [Google Scholar]
- 39.Mahurin EJ, Freeberg TM. 2009. Chick-a-dee call variation in Carolina chickadees and recruiting flockmates to food. Behav. Ecol. 20, 111–116. (doi:10.1093/beheco/arn121) [Google Scholar]
- 40.Farine DR, Lang SDJ. 2013. The early bird gets the worm: foraging strategies of wild songbirds lead to the early discovery of food sources. Biol. Lett. 9, 20130578 (doi:10.1098/rsbl.2013.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas JR, Freeberg TM. 2007. ‘Information’ and the chick-a-dee call: communicating with a complex vocal system. In Ecology and behavior of chickadees and titmice: an integrated approach (ed. Otter KA.), pp. 199–213. Oxford, UK: Oxford University Press. [Google Scholar]
- 42.Krams I, Krama T, Freeberg TM, Kullberg C, Lucas JR. 2012. Linking social complexity and vocal complexity: a parid perspective. Phil. Trans. R. Soc. B 367, 1879–1891. (doi:10.1098/rstb.2011.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Templeton CN, Greene E, Davis K. 2005. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937. (doi:10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- 44.Sieving KE, Hetrick SA, Avery ML. 2010. The versatility of graded acoustic measures in classification of predation threats by the tufted titmouse Baeolophus bicolor: exploring a mixed framework for threat communication. Oikos 119, 264–276. (doi:10.1111/j.1600-0706.2009.17682.x) [Google Scholar]
- 45.Suzuki TN. 2014. Communication about predator type by a bird using discrete, graded and combinatorial variation in alarm calls. Anim. Behav. 87, 59–65. (doi:10.1016/j.anbehav.2013.10.009) [Google Scholar]
- 46.Freeberg TM, Mahurin EJ. 2013. Variation in note composition of chick-a-dee calls is associated with signaler flight in Carolina chickadees, Poecile carolinensis. Ethology 119, 1086–1095. (doi:10.1111/eth.12169) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data are available as electronic supplementary material.