Abstract
We used meropenem to successfully treat a patient with bacteremia due to ceftazidime-avibactam-resistant, meropenem- susceptible Klebsiella pneumoniae that carried mutant blaKPC-3. Meropenem was bactericidal against ceftazidime-avibactam- resistant K pneumoniae isolates in vitro. Nevertheless, the role of carbapenems in treating such infections remains uncertain, because meropenem resistance is selected readily during passage experiments.
Keywords: carbapenem-resistant Enterobacteriaceae, ceftazidime-avibactam resistance, Klebsiella pneumoniae carbapenemase, KPC mutations, sequence type 258 Klebsiella pneumoniae
CASE REPORT
A 67-year-old man with esophageal cancer underwent esophagectomy, which was complicated by kidney injury necessitating continuous renal replacement therapy (CRRT), respiratory failure, and ventilator-associated pneumonia due to carbapenem-resistant Klebsiella pneumoniae ([CR-Kp] isolate 4-A). Ventilator-associated pneumonia was treated with ceftazidime-avibactam (1.25 grams intravenous every 8 hours [q8hrs]) and inhaled gentamicin (80 milligrams q12hrs) for 15 days. Ten days after treatment, he developed leukocytosis. Computerized tomography scan revealed an intra-abdominal abscess. Ceftazidime-avibactam was restarted empirically. Drainage culture grew meropenem-susceptible K pneumoniae, reported as extended-spectrum β-lactamase (ESBL)-producing (isolate 4-B) [1]. Ceftazidime-avibactam was continued for 15 days, the abscess was surgically drained, and the patient improved. Several weeks later, the patient developed meropenem-susceptible, ESBL-producing K pneumoniae bacteremia (isolate 4-C). He was treated successfully with meropenem 1 gram intravenous q12hrs (adjusted for creatinine clearance ~40 mL/min) for 18 days and subsequently discharged.
CHARACTERIZATION OF ISOLATES
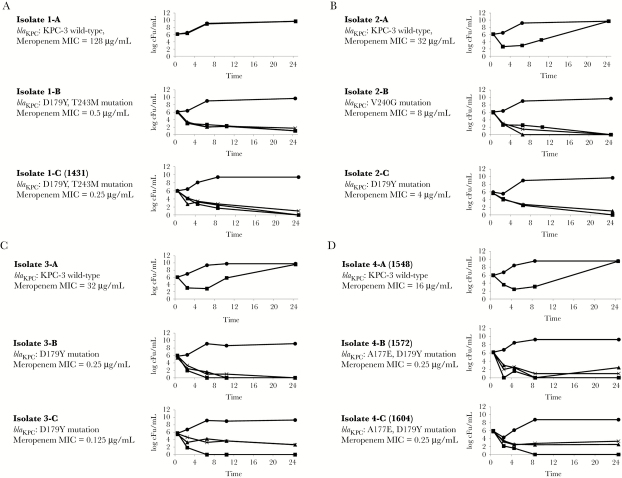
Isolate 4-A was resistant to all β-lactams except ceftazidime-avibactam (Table 1). Isolates 4-B and 4-C were ceftazidime-avibactam-resistant, but meropenem-susceptible (Figure 1); ceftriaxone, cefepime, and aztreonam MICs were reduced ≥16-fold from baseline. Isolates underwent whole-genome sequencing, as described previously [2]. Isolate 4-A was multilocus sequence type (ST)-258, K pneumoniae carbapenemase-3 (KPC-3)-producing. Isolates 4-B and 4-C were ST258, with glutamic acid for alanine and tyrosine for aspartic acid substitutions at KPC-3 Ambler positions 177 (A177E) and 179 (D179Y), respectively. Isolates clustered within a novel phylogenetic sublineage of clade II ST258, which also includes isolates from 3 other patients at our center in whom ceftazidime-avibactam resistance emerged [2].
Table 1.
Meropenem Time-Kill Results Against Klebsiella pneumoniae That Developed Ceftazidime-Avibactam Resistancea
| Isolate | ST | KPC Variant | MIC (µg/mL)b | Log-Kill at 24 Hours in Presence of Meropenemc | ||||
|---|---|---|---|---|---|---|---|---|
| Ceftazidime-Avibactam | Ceftazidime | Meropenem | 4× MIC | 8× MIC | 16 µg/mL | |||
| 1-A | 258 | KPC-3 | 2 (S) | 512 | 128 | d | d | +3.53 |
| 1-B | 258 | D179Y, T243M | 256 | >512 | 0.5 (S) | −4.97 | −4.27 | −4.97 |
| 1-C | 258 | D179Y, T243M | 256 | >512 | 0.25 (S) | −5.94 | −4.94 | −5.96 |
| 2-A | 258 | KPC-3 | 4 (S) | 256 | 32 | d | d | +3.57 |
| 2-B | 258 | V240G | 32 | >512 | 8 | −6.14 | −6.14 | −6.14 |
| 2-C | 258 | D179Y | >256 | >512 | 4 | −4.64 | −5.64 | −4.64 |
| 3-A | 258 | KPC-3 | 2 (S) | 256 | 32 | d | d | +3.52 |
| 3-B | 258 | D179Y | 128 | 512 | 0.25 (S) | −5.98 | −5.98 | −5.98 |
| 3-C | 258 | D179Y | 64 | 512 | 0.125 (S) | −3.18 | −3.18 | −5.83 |
| 4-A | 258 | KPC-3 | 1 (S) | 256 | 16 | d | d | +3.52 |
| 4-B | 258 | A177E, D179Y | 256 | 256 | 0.25 (S) | −3.70 | −5.16 | −6.16 |
| 4-C | 258 | A177E, D179Y | 128 | 256 | 0.25 (S) | −3.26 | −2.54 | −5.96 |
Abbreviations: cfu, colony-forming units; CLSI, Clinical Laboratory Standards Institute; KPC, Klebsiella pneumoniae carbapenemase; MIC, minimum inhibitory concentration; S, susceptible based on CLSI interpretive criteria; ST, sequence type. Bolded rows represent the baseline isolate from each of the 4 patients.
aTime-kill assays were performed in duplicate for each isolate, using a 1 × 106 cfu/mL inoculum in cation-adjusted Mueller-Hinton broth. Bactericidal responses were defined as a ≥3-log decrease in cfu/mL from the starting inoculum at 24 hours.
bMICs were determined by broth microdilution according to reference methods (1). CLSI interpretative criteria were applied to define susceptibility as follows: ceftazidime-avibactam, ≤8 µg/mL; ceftazidime, ≤4 µg/mL; meropenem, ≤1 µg/mL.
cDifference in concentration (log10) compared with baseline (time 0). Data are shown for a representative replicate of each isolate.
dNot tested.
Figure 1.
Time-kill responses of ceftazidime-avibactam-resistant Klebsiella pneumonia to meropenem. NOTE: Time-kill results in the presence of meropenem. Circle = control (no drug), triangle = 4× minimum inhibitory concentration (MIC), cross = 8× MIC, square = 16 μg/mL.
Time-kill assays were performed on longitudinal isolates from our 4 patients, using meropenem at 16 µg/mL (achievable serum concentration) [3], 4× minimum inhibitory concentration (MIC), and 8× MIC (Table 1; Figure 2). Meropenem (16 µg/mL) did not inhibit CR-Kp with wild-type blaKPC-3, but it was bactericidal at 24 hours against blaKPC-3 mutants (4× MIC, 8× MIC, and 16 µg/mL log-kills: −3.18 to −5.98, −2.54 to −6.14, and −4.64 to −6.16 log10, respectively).
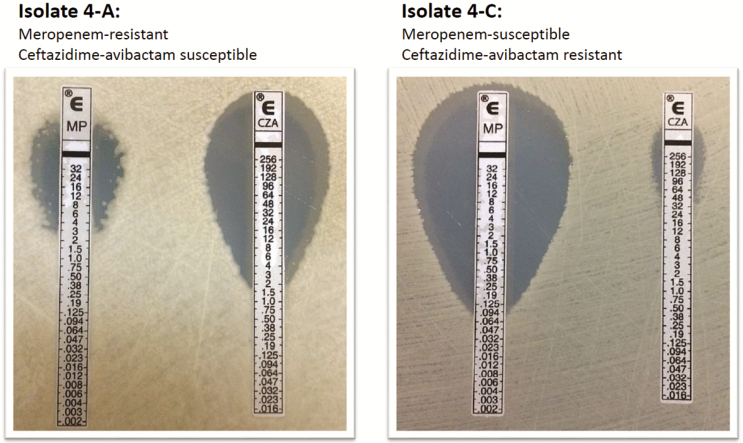
Figure 2.
Restoration of meropenem susceptibility among ceftazidime-avibactam-resistant Klebsiella pneumonia. Etest results for meropenem and ceftazidime-avibactam against baseline isolate 4-A (left) and follow-up isolate 4-C (right).
DISCUSSION
Ceftazidime-avibactam, a novel β-lactam/β-lactamase inhibitor, is US Food and Drug Administration-approved for treating complicated intra-abdominal infections (cIAI) and urinary tract infections (cUTI). The agent is active against carbapenem-resistant Enterobacteriaceae (CRE) expressing KPCs, but not metallo-β-lactamases. Ceftazidime-avibactam is likely to be widely used against off-label CRE infections. We used ceftazidime-avibactam to treat 37 CRE-infected patients (29 without cIAI or cUTI) [4]. Thirty-day clinical success and mortality rates were 59% (22 of 37) and 76% (28 of 37), respectively. In a multicenter series, 36 CRE-infected patients (20 without cIAI or cUTI) received compassionate-use ceftazidime-avibactam [5]. Clinical cure and in-hospital survival rates were 69% (25 of 36) and 61% (22 of 36), respectively. Results did not differ in either study if ceftazidime-avibactam was combined with another antibiotic. Outcomes were comparable to those reported previously for CRE-infected patients treated with ≥2 in vitro active agents [6]. Acute kidney injury was described in only 3 patients between studies, suggesting lower toxicity than with colistin or aminoglycosides.
Ceftazidime-avibactam resistance has emerged in ~10% of CR-Kp-infected patients treated at our center [2, 4]. Thus far, these are the only cases reported to develop during treatment. Resistance has been diagnosed after 10–19 days of drug exposure, exclusively in KPC-3-producing ST258 isolates. It is conferred by plasmid-borne blaKPC-3 mutations, which reduce MICs of carbapenems (often restoring susceptibility) and other β-lactams [2, 7]. The D179Y variant, alone or in combination with other mutations, predominates in patients and after in vitro passage [8, 9], and manifests the strongest phenotypes [2, 7]. Most mutations have arisen within the KPC Ω-loop (positions 165–179), thereby enhancing ceftazidime affinity and possibly restricting avibactam binding [9]. The A177E mutation is newly reported. As in our case, K pneumoniae with mutant blaKPC-3 may be identified as ESBL-producers (rather than KPC-producers) if carbapenemase screening is triggered by elevated carbapenem MICs. Failure to detect blaKPC in a timely fashion may facilitate nosocomial dissemination.
Meropenem at clinically achievable concentrations was bactericidal against ceftazidime-avibactam-resistant K pneumoniae in vitro, and it eradicated isolate 4-C from our patient’s bloodstream. However, meropenem resistance has emerged in ceftazidime-avibactam-resistant isolates from our patients during in vitro passage at subinhibitory meropenem concentrations [10]. In most passage experiments, resistance to ceftazidime-avibactam and other β-lactams was retained. Therefore, until more data are available, the role of carbapenems in treating patients is unclear. Combination regimens merit investigation. Of note, ceftazidime-avibactam dosing is not established for CRRT; our patient received half the conventional dose. Better pharmacokinetic data are needed in CRRT and other specialized populations and for various types of invasive infections.
CONCLUSIONS
In conclusion, clinicians should be alert for the inevitable emergence of more widespread ceftazidime-avibactam resistance. To best use and preserve ceftazidime-avibactam, resistant isolates should be studied for resistance mechanisms and genome and plasmid content.
Acknowledgments
Financial support. This work was funded, in part, by grants from the National Institutes of Health (K08AI114883 [to R. K. S.], R21AI117338 [to L. C.], R01AI090155 [to B. N. K.], R21AI128338 [to M. H. N.], and R21AI111037 [to C. J. C.]).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 26th ed M100S. Wayne, PA: 2017. [Google Scholar]
- 2. Shields RK, Chen L, Cheng S et al. . Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2017; 61:pii: e02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaruratanasirikul S, Sriwiriyajan S, Punyo J. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob Agents Chemother 2005; 49:1337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shields RK, Potoski BA, Haidar G et al. . Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63: 1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Temkin E, Torre-Cisneros J, Beovic B et al. . Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 2017; 61:pii: e01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzouvelekis LS, Markogiannakis A, Piperaki E et al. . Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014; 20:862–72. [DOI] [PubMed] [Google Scholar]
- 7. Haidar G, Clancy CJ, Shields RK et al. . Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob Agents Chemother 2017; 61:pii: e02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Livermore DM, Warner M, Jamrozy D et al. . In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 2015; 59:5324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winkler ML, Papp-Wallace KM, Bonomo RA. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother 2015; 70:2279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shields RK, Nguyen MH, Press EG et al. . In vitro selection of meropenem resistance among ceftazidime-avibactam resistant, meropenem susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother 2017; 61:pii: e.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]