Abstract
Background:
Little or no pharmacological or toxicological data are available for novel psychoactive substances when they first emerge, making their identification and interpretation in biological matrices challenging.
Materials & methods:
A new synthetic cathinone, α-pyrrolidinopentiothiophenone (α-PVT), was incubated with hepatocytes and samples were analyzed using liquid chromatography coupled to a Q ExactiveTM Orbitrap mass spectrometer. Authentic urine specimens from suspected α-PVT cases were also analyzed. Scans were data mined with Compound Discoverer™ for identification and structural elucidation of metabolites.
Results/conclusion:
Seven α-PVT metabolites were identified in hepatocyte incubations, and in the authentic urine samples, also with an additional monohydroxylated product and a glucuronide of low intensity. α-PVT dihydroxypyrrolidinyl, α-PVT 2-ketopyrrolidinyl, α-PVT hydroxythiophenyl and α-PVT thiophenol had the most intense in vivo signals.
Keywords: : α-PVT, hepatocytes, high-resolution MS, metabolism, novel psychoactive substances, synthetic cathinone
Novel psychoactive substances (NPS) are constantly emerging onto the illicit drug market, making it difficult to identify parent and/or metabolites of these substances as little pharmacological or toxicological data are available. The European Union's Early Warning System (EWS) identified 81 NPS in 2013 and 101 in 2014, bringing the total compounds monitored to over 450 [1]. The term NPS includes a wide range of substances, including synthetic cannabinoids, phenethylamines, cathinones, piperazines, ketamine, tryptamines and other plant-based psychoactive substances (i.e., Kratom, Salvia divinorum Epling & Játiva) [2]. Synthetic cathinone abuse may produce severe side effects such as tachycardia, hyperthermia, delusions and violent behavior [2–7]. In 2011 in USA, mephedrone (4-methylmethcathinone), methylone (3,4-methylenedioxymethcathinone) and 3,4-methylenedioxypyrovalerone (MDPV) were classified as schedule I controlled substances [8]. Ten additional cathinones were added, including 4-methyl-N-ethylcathinone, α-pyrrolidinobutiophenone (α-PBP) and α-pyrrolidinovalerophenone (α-PVP).
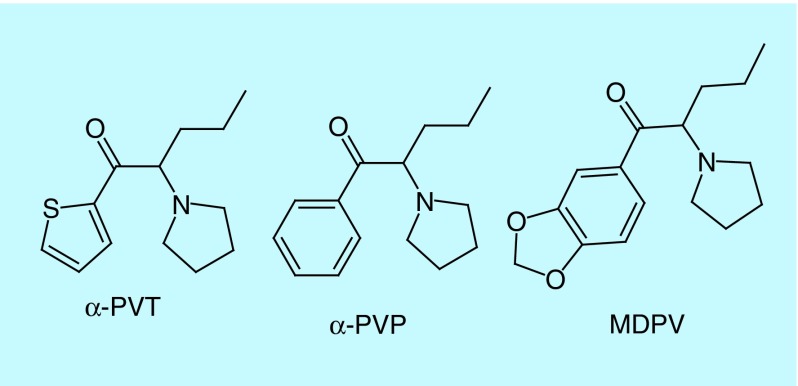
In mid-2012, reports of a new synthetic cathinone called α-pyrrolidinopentiothiophenone (α-PVT) appeared on several online drug forums. Later, α-PVT or 2-(pyrrolidin-1-yl)-1-(thiophen-2-yl)pentan-1-one was identified in illegal products purchased in Japan in 2013 [9] and in Germany in 2014 [10]. α-PVT is a substituted cathinone with structural similarities to schedule I controlled substances MDPV and α-PVP, except the benzene ring is a thiophene ring (Figure 1). The only available reports of α-PVT's effects are described on internet drug forums (e.g., bluelight.org, drugs-forum.com, reddit.com or chemsrus.com). The accounts often document the dose (10–100 mg), route (insufflation or oral), duration (up to 6 h) and side effects (positive: stimulation; negative: psychosis) experienced. However, self-reports are often unreliable as true compound identity is unknown.
Figure 1. . Structure of α-pyrrolidinopentiothiophenone and structurally related schedule I controlled substances.
As with many NPS, PD and PK profiles are nonexistent and controlled administrations or clinical trials are lacking, making identification and interpretation of toxicological findings difficult [11]. Between 2013 and April 2014, risk assessments were only performed on six NPS from the EWS compound list [12]. Pharmacologically, synthetic cathinones interact with monoamine transporters for dopamine (DAT), norepinephrine (NET) and serotonin [13–16]. Mephedrone and methylone increase extracellular dopamine and serotonin similar to 3,4-methylenedioxymethamphetamine [13]. MDPV is a potent DAT/NET transporter blocker, similar to cocaine [14,15]. α-PVP, α-PBP and α-pyrrolidinopropiophenone (α-PPP), three pyrrolidinophenones recently examined by Marusich et al. were determined to be DAT/NET transporter blockers with in vivo potency increasing with aliphatic chain length (α-PVP > α-PBP > α-PPP) [15]. Due to structural similarity to MDPV and α-PVP, it could be hypothesized that α-PVT would exhibit similar in vitro and in vivo pharmacological effects. If this is the case, α-PVT could cause severe side effects and pose risks for abuse and addiction. Characterizing NPS metabolic pathways is imperative for PK profiling and understanding PD effects. The metabolic pathways of other α-pyrrolidinophenones, including MDPV, α-PVP, α-PBP, α-PPP, 4-methyl-α-pyrrolidonophene, 4-methoxy-α-pyrrolidinophenone and 4-methyl-α-pyrrolidinohexiophenone were previously investigated in human liver microsomes (HLM) and rat urine [17–27]. In a recent review of pyrrolidinophenones’ pharmacology, Zaitsu et al. summarized the main metabolites to include ketone reduction to the corresponding alcohol and oxidation to the 2’-oxo metabolites [28]. In 2014, Takayama et al. characterized α-PVT's in vitro metabolism with HLM [29] and identified only three metabolites generated by hydroxylations on the pyrrolidine moiety, pyrrolidinoalkyl moiety and the thiophene ring. In silico, in vitro hepatocytes and in vivo metabolic studies for α-PVT are not available.
As a step toward NPS public education and awareness, complete metabolic profiles are necessary to identify unique metabolites as markers of intake and to link adverse effects to the causative agent. In order to generate the most comprehensive metabolic profile of a substance, in vitro human hepatocyte incubations, followed by analysis with liquid chromatography coupled to high-resolution MS (LC–HRMS) and software-assisted data mining were proven to be a promising approach [30–32]. Human hepatocytes offer a more complete metabolic profile than HLM since hepatocytes contain all hepatic enzymes involved in drug metabolism (both phase I and II transformations) as well as endogenous cofactors, provide the natural orientation of membrane enzymes and produce metabolites in concentrations similar to in vivo [33]. Analysis by HRMS is becoming more common in metabolic profiling assays and offers the ability to identify expected and unexpected metabolites with exact-mass capabilities. The incorporation of data-mining software decreases data analysis time and offers structural elucidation for a variety of HRMS data file types [34]. In silico prediction can assist in metabolite identification by offering accurate mass, fragmentation patterns and distribution constants (logD). Our objectives were to compare in silico metabolism predictions to HLM and human hepatocyte incubations analyzed by HRMS with software-assisted data mining for the identification and structural elucidation of α-PVT metabolites. Authentic urine specimens from α-PVT users also were analyzed for α-PVT and metabolites to confirm the metabolites identified in the in vitro samples.
Materials & methods
Chemicals & reagents
α-PVT HCl in powder form (5 mg) was obtained from Cayman Chemicals (Ann Arbor, MI, USA) and diclofenac (10 g) from Toronto Research Chemicals (Toronto, Canada). LC–MS grade water, acetonitrile (LC–MS grade) formic acid (LC–MS grade), LC grade methanol, glacial acetic acid, hydrochloric acid and ammonium hydroxide were acquired from Fisher Scientific (Fair Lawn, NJ, USA). Methylene chloride (dichloromethane) was purchased from J.T. Baker (Phillipsburg, NJ, USA). Dibasic and monobasic sodium phosphate and 2-propanol (LC–MS grade) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Water was purified in-house with an ELGA Purelab® Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA, USA). Pooled microsomes (n = 50), pooled cryopreserved human hepatocytes (n = 10), InVitroGRO HT and InVitroGRO Krebs–Henseleit buffer were obtained from BioReclamationIVT (Westbury, NY, USA). Solutions A and B (NADPH-regeneration system and Glu-6-phosphate dehydrogenase, respectively) were purchased from BD Biosciences (San Jose, CA, USA). Water bath incubations were performed on a Precision reciprocal shaking bath (Winchester, VA, USA) and hepatocytes were incubated in a FormaTM Steri-CycleTM CO2 incubator from Thermo Scientific.
In silico metabolite prediction
The structure of α-PVT was imported into MetaSite software (v. 5.0.3; Molecular Discovery, Pinner, UK) and predictions were carried out using the cytochrome P450 (CYP450) liver model, reactivity correction and 39 CYP common biotransformations, including hydroxylation, dealkylation, carbonylation and ketone reduction. The CYP450 liver model provides a consensus of the sites of metabolism predictions from the three major liver isoforms CYP3A4, CYP2D6 and CYP2C9 and flavin containing monooxygenase 3 (FMO3). The computational procedure considers both thermodynamic and kinetic factors by simulating 3D docking of the substrate in the cavity of the CYP proteins. The software provides exact structures for the predicted metabolites, lists the corresponding biotransformation and monoisotopic mass and calculates logD values at pH 4, 7 and 9 as well as a probability score. Compounds with a probability score of 100% were imported into the software to generate a list of second-generation metabolites. Only metabolites >50 Da were considered in the final summary.
Additionally, the in silico prediction software generated a list of accurate mass m/z (inclusion list) for first-, second-, and third-generation predicted metabolites. This list was incorporated into the data-dependent MS2 (ddMS2) acquisition method operating in positive ESI, but identification of metabolites was not limited to this list. When a mass spectral peak on the inclusion list was detected, an MS/MS spectrum was automatically acquired. This list included 39 CYP and 40 non-CYP-mediated reactions, including oxidation, hydroxylation, carbonylation and glucuronidation. No structure, corresponding biotransformation, logD or prediction score was provided for the predicted m/z values.
Metabolic stability assessment with human liver microsomes
To determine α-PVT metabolism kinetics, 1 µmol/l drug was incubated with 50-donor-pooled HLM. The reaction mixture consisted of 780 µl water, 100 µl 0.5 M potassium phosphate buffer pH 7.4, 10 µl solution B and 10 µl α-PVT (100 µmol/l). After vortexing, 50 µl HLM (20 mg/ml suspension) was added to the mixture and preincubated for 3 min at 37°C. The reaction was initiated by the addition of 50 µl solution A. Samples (100 µl) were collected after 0, 3, 8, 13, 20, 30, 45 and 60 min and the reaction was stopped with an equal volume of ice-cold acetonitrile. The samples were centrifuged at 15,000 g for 5 min at 4°C and supernatant stored at -80°C until analysis. After thawing, HLM samples were diluted 100-fold with mobile phase A (0.1% formic acid in water) before injection (10 µl) onto the LC–HRMS. The experiment was prepared in duplicate and injected twice, allowing us to examine reproducibility.
Metabolite profiling in human hepatocytes
For metabolic profiling, 10 µmol/l α-PVT was incubated at 37ºC with pooled cryopreserved human hepatocytes. Hepatocytes were washed with InVitroGRO HT medium to remove dead cells by centrifugation at 50 g for 5 min at room temperature. Supernatant was aspirated and InVitroGRO Krebs–Henseleit buffer was added to the hepatocytes again. After centrifugation and supernatant removal, the cell pellet was resuspended in 2 ml buffer. Cell viability was assessed with Trypan blue exclusion method assuring >80% viability. The reaction mixture consisted of 250 µl α-PVT in buffer (20 µmol/l) and 250 µl cell suspension (2 × 106 cells/ml), yielding a final 10 µmol/l drug concentration. Samples (500 µl) were collected after 0, 30 and 120 min according to the HLM half-life derived from the previous HLM substrate depletion experiment. An equal volume of ice-cold acetonitrile was added to the samples immediately to stop the reaction. Diclofenac was incubated in the same manner as α-PVT as a positive control and evaluated for 4’-hydroxydiclofenac and diclofenac acyl glucuronide metabolites to ensure metabolic viability under our experimental conditions. A negative control (parent drug in buffer without hepatocytes) was incubated to rule out nonenzymatic transformation of α-PVT. Samples were stored at -80°C until analysis. After thawing, hepatocyte samples were centrifuged at 15,000 g at 4°C for 10 min to remove cell debris and supernatants diluted 1:5 with mobile phase A before injection (10 µl) onto the LC–HRMS.
LC–HRMS instrumentation
LC–HRMS was performed on a Thermo Scientific Ultimate™ 3000 RSLCnano system coupled to a Thermo Scientific Q Exactive™ mass spectrometer (Thermo Scientific, Fremont, CA, USA). The Ultimate™ 3000 RSLCnano system consisted of a degasser, a tertiary loading pump, a binary eluting pump, a column oven and an RS Autosampler. The Q Exactive™ was equipped with heated electrospray ionization source (HESI-II) and operated in positive ionization mode. The spray voltage was 3 kV, capillary temperature 350°C, heater temperature 425°C, S-lens RF level 50, sheath gas flow rate 50, auxiliary gas flow rate 13 and sweep gas 3 (manufacturer's units). Nitrogen was used for spray stabilization, for collision-induced dissociation experiments in the higher-energy collisional dissociation cell, and as the damping gas in the C-trap. The instrument was calibrated in the positive and negative mode every 25 h.
LC–HRMS for HLM incubations
HLM samples were separated on an Accucore™ C18 100 × 2.1 mm, 2.6 µm column (Thermo Scientific, Fremont, CA, USA) and identically packed defender guard cartridges (10 × 2.1 mm, 2.6 µm) in a thermostatted column oven at 35°C. Gradient elution was performed with mobile phase A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile) at a 0.4 ml/min flow rate. The initial composition (2% B) was maintained for 2 min, then increased from 2 to 10% B over 8 min, increased from 10 to 30% B over 4 min, increased from 30 to 95% B over 2 min, held at 95% for 1 min, returned to initial conditions over 1 min with a 2 min equilibration, yielding a total run time of 20 min. LC conditions and ionization parameters for HLM incubations were the same as our in-house validated stimulant method in urine [35] as the method has proven adequate to detect α-PVT.
The mass spectrometer acquired data using a high-resolution full-scan, data-dependent MS method. The MS acquired full scan MS spectra at a resolution of 35,000 (full width at half-maximum at m/z 200) from m/z 100 to 600. The resolution was not maximal in order to achieve a sufficient number of scan events for each chromatographic peak. Data were acquired in full-scan MS/ddMS2 mode: in each cycle, a full MS survey scan was acquired, followed by up to five MS2 events. When an ion was detected with an intensity above 8.3 × 104 (5% underfill ratio) in the survey scan, an MS2 event was triggered at the peak apex (3–8 s) and the ion was fragmented (isolation window m/z 3). Up to five ions with the most intense signal were fragmented. A 10 s delay was required for the same ion to trigger a new MS2 event to provide the opportunity for ions with the same RT and a lower intensity to be fragmented (dynamic exclusion). ddMS2 spectra were acquired at a resolution of 17,500 with a stepped normalized collision energy of 50 ± 30% to produce a high number of fragments.
LC–HRMS for hepatocyte incubations
In order to achieve better separation of metabolites, hepatocyte samples were separated on a Synergi™ Hydro-RP (150 × 2 mm, 4 µm) column (Phenomenex, Torrance, CA, USA) and a C18 end-capped guard in a thermostatted column oven at 30°C. Gradient elution was performed with mobile phase A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile) at a 0.4 ml/min flow rate. The initial composition (2% B) was maintained for 2 min, then increased from 2 to 95% B over 18 min, held at 95% B for 5 min, returned to initial conditions over 1 min with a 4 min equilibration, yielding a total run time of 30 min. For hepatocyte incubations, only one linear gradient was employed, with a longer column and a longer run time than HLM LC conditions to best separate the analytes while preserving good chromatography efficiency, as the aim of the experiment was to detect α-PVT and its metabolites. As we expected to detect a number of compounds with the same accurate mass and similar physical and chemical properties (close retention times), a longer gradient was necessary to provide chromatographic separation. In addition, MetaSite predicted metabolites that may elute after the parent compound, so the longer gradient allowed us to capture those compounds as well.
Hepatocyte samples were analyzed on the Q Exactive™ using two acquisition methods employing full scan and ddMS2 with and without precursors’ inclusion list. Full scan and ddMS2 (without an inclusion list) were acquired as previously described for HLM samples. For full scan and ddMS2 with an inclusion list, ddMS2 scans were triggered if the precursor ions from the inclusion list were detected above 8.3 × 104 intensity threshold. ddMS2 mode without an inclusion list was thus more suitable for unexpected metabolites with high signal intensity, while ddMS2 mode with an inclusion list was more suitable for expected metabolites with a low to high signal intensity.
Calculations
In vitro microsomal half-life (T1/2) was calculated in Excel by plotting the natural logarithm of % parent compound signal (area) remaining versus time. In vitro microsomal intrinsic clearance (CLint, micr), intrinsic clearance (CLint), predicted human hepatic clearance (CLhep) and extraction ratio (ER) were calculated in Excel based on the models described in Baranczewski et al. [36] and McNaney et al. [37], without consideration of plasma protein binding.
Metabolite identification
Raw data files from the hepatocyte incubations (0, 0.5, 2 h in full scan-ddMS2 mode with and without an inclusion list) were imported into Compound Discoverer™ software (v. 1.0; Thermo Scientific, Fremont, CA, USA) to identify α-PVT metabolites. The software detects chromatographic peaks and the mass of the corresponding compound is compared with a list of generated theoretical metabolites (processing settings are summarized in Table 1). A fragment ion search (FISh) score was then assigned to determine the percentage match between theoretical and experimental fragmentation spectra (FISh coverage score = [Σper all scans number of matched fragments]/[Σper all scans number of centroids in the fragmentation scan that are above the signal/noise threshold] × 100). The apex triggering and the dynamic exclusion selected for the ddMS2 analysis limit the production of MS2 scans of low intensity that can affect the FISh score.
Table 1. . Compound discoverer settings: chromatographic peaks are detected and the mass of the corresponding compound is compared with a list of generated theoretical metabolites; a FISh score is then assigned to determine the percentage of match between theoretical and experimental MS2 spectra.
| Compound discoverer settings | |
|---|---|
|
Compound generator | |
| Parent compound |
α-PVT (C13H19NOS) |
| Phase I transformations |
Dehydration, desaturation, hydration, nitro reduction, oxidation, oxidative deamination to alcohol, oxidative deamination to ketone, oxidative debromination, oxidative dechlorination, oxidative defluorination, reduction, reductive debromination, reductive dechlorination, reductive defluorination, thiourea to urea |
| Phase II transformations |
Acetylation, arginine conjugation, glucoside conjugation, glucuronide conjugation, glutamine conjugation, glycine conjugation, GSH conjugation (on bromine), GSH conjugation (on chlorine), GSH conjugation (on fluorine), GSH conjugation 1, GSH conjugation 2, methylation, ornitine conjugation, palmitoyl conjugation, stearyl conjugation, sulfation, taurine conjugation |
| Maximal number of Phase II reactions |
1 |
| Maximal number of reactions |
3 |
| Ionization |
[M + H]+, [M + K]+, [M + Na]+ |
|
Expected finder | |
| Mass tolerance |
10 ppm |
| Intensity tolerance (isotope search) |
30% |
| Minimum peak intensity |
200,000 |
|
FISh scoring | |
| Signal/noise ratio threshold |
3 |
| Mass tolerance |
2.5 mDa |
| Use libraries | True |
α-PVT: α-pyrrolidinopentiothiophenone.
Potential metabolites were considered based on exact mass, MS2 fragmentation, isotopic pattern and retention time with respect to the parent compound (as determined by logD from in silico predictions). Relative abundance was considered for ranking and determining the most prominent metabolites. Potential metabolites were further compared with literature reports of structurally similar compounds and in silico predictions. Compounds with signal intensity <0.2% parent were judged insignificant and excluded from the analysis.
Authentic specimens
Hepatocyte incubation provides a good representation of hepatic metabolism but cannot mimic all in vivo postmetabolism processes (e.g., reabsorption, extrahepatic metabolism, enterohepatic circulation). Authentic blood or urine specimen analysis, when available, is highly valuable to complement in vitro experiments. Three authentic human urine specimens suspected to contain synthetic cathinones were analyzed by our in-house validated stimulant solid-phase extraction (SPE) method in urine [35], and the presence of α-PVT was confirmed and quantified. Additionally, a small volume of the 2 h hepatocyte sample also was extracted in order to determine if the generated metabolites could be successfully recovered. Briefly, the hepatocyte sample was thawed, vortexed, centrifuged and a portion diluted 1:5 with mobile phase A. Diluted hepatocyte sample (50 µl) was mixed with 1 ml 0.1 M phosphate buffer (pH 6), vortexed and centrifuged. For authentic specimens, 100 µl urine was fortified with 25 µl deuterated internal standard solution, mixed with 1 ml 0.1 M phosphate buffer (pH 6), vortexed and centrifuged. Supernatants were loaded onto SOLA CX SPE cartridges (10 mg/1 ml, Thermo Scientific, Fremont, CA, USA) preconditioned with methanol and phosphate buffer. Columns were washed with 1 M acetic acid and methanol before drying at 10 psi for 5 min. Analytes were eluted with two 1 ml aliquots 2% ammonium hydroxide in 95:5 dichloromethane:isopropanol (v/v). Eluents were acidified with 100 µl 1% HCl in methanol, dried under nitrogen and reconstituted in 200 µl mobile phase A; extracted hepatocytes were reconstituted in 250 µl to allow for direct comparison with 1:5 diluted unextracted hepatocyte samples). The extracted samples were analyzed using the same LC–HRMS method as described for the hepatocyte incubations. In addition, samples were analyzed with all-ion-fragmentation (AIF) acquisition (no quadrupole preselection) to identify metabolites whose intensity was not high enough to trigger an MS2 event in ddMS2 mode. AIF mode operated at a resolution of 35,000 and with a stepped normalized collision energy of 50 ± 30%. Raw data were processed using Compound Discoverer software with the same conditions as described above. Metabolites were considered identified if exact mass (±5 ppm) and retention time (±0.1 min) matched those found in hepatocytes.
Results
Metabolic stability assessment with human liver microsomes
α-PVT exhibited a 29.9 ± 2.2 min half-life. In vitro microsomal CLint micr was 23.3 ± 1.8 µl/min/mg and CLint was 21.9 ml/min/kg. CLhep was estimated at 10.5 ml/min/kg with a 0.52 ER.
Metabolite profiling in human hepatocytes
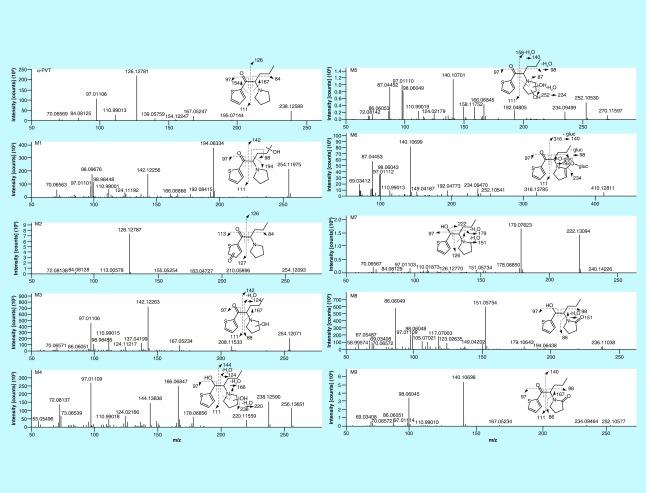
4’-hydroxydiclofenac and diclofenac acyl glucuronide were detected in the diclofenac hepatocyte incubations, demonstrating that the hepatocyte system was viable under our experimental conditions. No metabolites were identified in the negative control, demonstrating that nonenzymatic transformation of the parent drug did not occur. α-PVT MS peak area was 6.96 × 109, 5.63 × 109 and 4.91 × 109 at 0, 0.5 and 2 h, respectively. Seven phase I α-PVT metabolites were identified after 0.5 and 2 h incubations with mass measurement errors <1.3 (0.5 h) and <0.9 (2 h) ppm in full scan mode. Metabolites were named from M1 to M9 in ascending RT order, including the two additional metabolites identified in authentic urine samples (see below). MS2 fragmentation spectra are reported in Figure 2. Metabolites were mainly formed by hydroxylation at the thiophenyl (M2) or the pyrrolidine (M3, M4, M5, M8 and M9) rings or by ketone reduction (M4, M7 and M8). Elemental composition, accurate mass molecular ion, accurate mass diagnostic product ions, MS peak areas and RT of α-PVT and its metabolites are reported in Table 2. M5 (α-PVT dihydroxypyrrolidinyl), M9 (α-PVT 2-ketopyrrolidinyl) and M4 (α-PVT hydroxypyrrolidinylthiophenol) were the metabolites with the most intense signal respectively after 0.5 h incubation. M5, M9 and M2 (α-PVT hydroxythiophenyl) were the metabolites with the most intense signal respectively after 2 h incubation. M8 (α-PVT 2-ketopyrrolidinylthiophenol) signal was the signal of the in-source water loss only, as it represented more than 99% of the total signal.
Figure 2. . α-pyrrolidinopentiothiophenone and metabolites assigned fragmentation pattern.
Table 2. . Elemental composition, accurate mass molecular ion, nominal mass diagnostic product ions, MS peak areas and retention time of α-pyrrolidinopentiothiophenone and its metabolites in human hepatocyte incubations and authentic urine specimens.
| Peak ID | Biotransformation | Elemental formula | Experimental molecular ion (m/z) | Mass error (ppm) | Diagnostic product ions (m/z) | Peak area, hepatocyte, 0.5 h | Peak area, hepatocyte, 2 h | Peak area, urine #1 | Peak area, urine #2 | Peak area, urine #3 | RT (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-PVT |
Parent |
C13H19NOS |
238.1260 |
+0.21 |
97, 111, 126, 167 |
5.63 × 109 |
4.91 × 109 |
6.80 × 107 |
9.25 × 109 |
2.74 × 109 |
7.88 |
| M1 |
Hydroxylation |
C13H19NO2S |
254.1206 |
+1.38 |
97, 98, 111, 142, 194 |
ND |
ND |
ND |
3.04 × 107 |
1.27 × 107 |
6.2 |
| M2 |
Hydroxylation |
C13H19NO2S |
254.1207 |
+0.78 |
84, 113, 126, 127 |
2.21 × 107 |
8.14 × 107 |
3.15 × 104† |
2.69 × 108 |
2.25 × 108 |
6.88 |
| M3 |
Hydroxylation |
C13H19NO2S |
254.1207 |
+0.78 |
97, 98, 111, 142, 167 |
1.37 × 107 |
2.69 × 107 |
ND |
3.64 × 107 |
9.89 × 106† |
7.38 |
| M4 |
Hydroxylation + reduction |
C13H21NO2S |
256.1364 |
+0.63 |
97, 124, 144, 166, 238 |
2.41 × 107 |
2.56 × 107 |
ND |
6.52 × 107 |
8.14 × 106 |
7.55 |
| M5 |
Dihydroxylation |
C13H19NO3S |
270.1157 |
+0.32 |
97, 98, 140, 166, 252 |
1.05 × 108 |
2.73 × 108 |
2.19 × 106† |
8.72 × 108 |
5.45 × 108 |
7.67 |
| M6 |
Hydroxylation + dehydrogenation + glucuronidation |
C19H25NO8S |
428.1369 |
+1.15 |
97, 98, 140, 192, 234 |
ND |
ND |
ND |
1.87 × 107 |
8.49 × 106 |
7.75 |
| M7 |
Reduction |
C13H21NOS |
240.1415 |
+0.63 |
97, 126, 151, 179, 222 |
1.47 × 107 |
5.79 × 107 |
8.27 × 104† |
1.92 × 108 |
4.56 × 107 |
8.33 |
| M8 |
Hydroxylation + dehydrogenation + reduction |
C13H19NO2S |
236.1103‡ |
+0.04 |
86, 97, 98, 151 |
8.23 × 106 |
3.63 × 107 |
ND |
1.76 × 106 |
6.06 × 105 |
11.07 |
| M9 | Hydroxylation + dehydrogenation | C13H17NO32S | 252.1052 | +0.14 | 86, 98, 140, 167 | 7.29 × 107 | 1.47 × 108 | 1.03 × 104† | 3.33 × 107 | 2.18 × 107 | 13.12 |
Peak area for α-PVT parent at 0 h was 6.96 × 109. Metabolites are ranked by ascending RT.
†No MS2 confirmation.
‡In-source water loss (– m/z 18.0105) (>99% of the signal).
ND: Not detected; RT: Retention time.
Metabolic confirmation & profiling in authentic urine samples
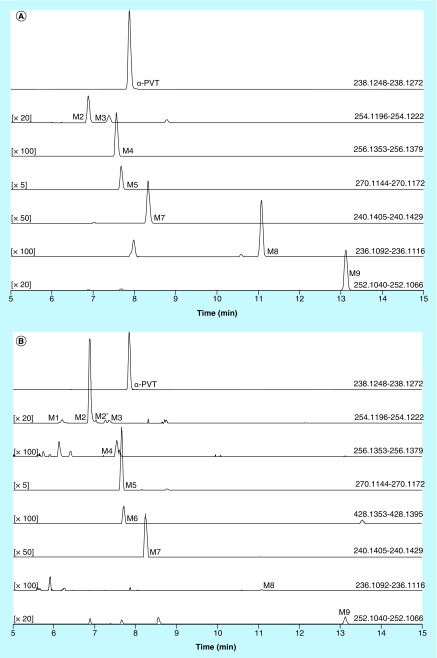
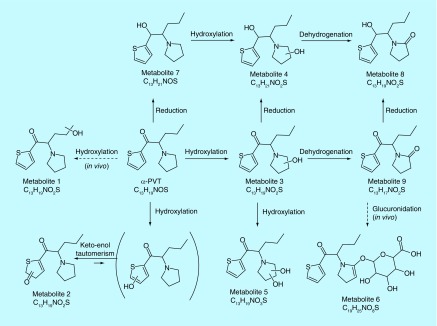
Three authentic urine specimens previously tested positive for α-PVT by a validated Q-TOF screening method were selected to evaluate the proposed metabolic profile. The urine samples were analyzed by our in-house validated stimulant SPE method in urine, and the presence of α-PVT and other stimulants were confirmed and quantified [35]. Urine samples were α-PVT-positive at 5, >500 and 251 µg/l in specimens 1, 2 and 3, respectively. Sample 1 also was positive for 6-APB (8.3 µg/l), normephedrone (79 µg/l), 4-methylethcathinone (> 500 µg/l), α-PVP (> 500 µg/l) and MDPV (6.8 µg/l). Samples 2 and 3 also were positive for α-PVP (> 500 µg/l). α-PVT and all seven metabolites identified in hepatocytes incubations were found in samples 2 and 3 (Figure 3). MS peak areas are reported in Table 2. α-PVT MS peak area was 6.80 × 107, 9.25 × 109 and 2.74 × 109 in samples 1, 2 and 3, respectively. M5, M2 and M7 were the metabolites with the most intense signal in the three samples. Two additional metabolites were identified in samples 2 and 3: M1 (α-PVT hydroxypropyl) and M6 (α-PVT 2-ketopyrrolidinyl-glucuronide) (Table 2 & Figures 2 & 3). A suggested metabolic pathway is described in Figure 4.
Figure 3. . Extracted ion chromatograms obtained from hepatocyte incubation with α-pyrrolidinopentiothiophenone (2 h) (A) and from an authentic α-PVT-positive urine sample (specimen #3) (B).
α-PVT metabolites are numbered one through nine in ascending order of retention time in hepatocyte incubations and urine samples (M1–M9). The chromatographic peaks with no assignment were not identified as α-PVT metabolites or presented below the signal intensity threshold set for the metabolite identification. M1 and M6 were not detected in hepatocyte incubations.
α-PVT: α-pyrrolidinopentiothiophenone.
Figure 4. . α-pyrrolidinopentiothiophenone suggested metabolic pathway.
Plain arrow, observed in human hepatocyte incubations; dashed arrow, observed in authentic urine samples.
In silico predictions
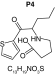
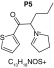
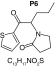
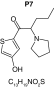
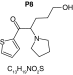
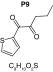
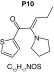
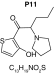
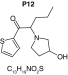
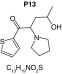
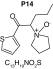
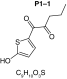
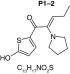
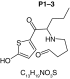
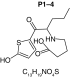
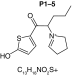
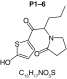
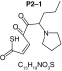
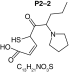
MetaSite software predicted the formation of 14 metabolites with a probability score above 20% using the CYP450 liver model (P1–P14 in ascending probability score order). Predicted structure, biotransformation, accurate mass, logD at pH = 4 (closest pH to gradient conditions) and probability score for each molecule are reported in Table 3. Thiophenyl (P1, P7 and P11) and alkyl (P8 and P13) hydroxylation and N-dealkylation (P3 and P4) were the most common reactions. P1 (thiophenyl hydroxylation) and P2 (thiophenyl hydroxylation and rearrangement) presented with a probability score of 100%. P3 (N-dealkylation and oxidation to carbonyl), P4 (N-dealkylation and oxidation to carboxylic acid) and P5 (iminium formation) presented with a score of 57%. Six and two second-generation metabolites were generated from P1 and P2, respectively, as they both showed a 100% score.
Table 3. . In silico predictions by MetaSite software for α-pyrrolidinopentiothiophenone metabolism.
| First-generation metabolites | |||||
|---|---|---|---|---|---|
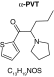 |
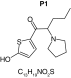 |
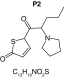 |
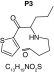 |
 |
|
| Biotransformation | Parent | Hydroxylation | Hydroxylation + tautomerism | N-dealkylation | N-dealkylation + hydroxylation |
| Mass (Da) | 237.1187 | 253.1136 | 253.1136 | 253.1136 | 269.1086 |
| Theoretical logD (pH = 4) | -0.09 | -0.05 | 1.21 | -0.3 | -0.25 |
| Probability score (%) |
NA |
100 |
100 |
57 |
57 |
 |
 |
 |
 |
 |
|
| Biotransformation | Iminium formation | Carbonylation | Hydroxylation | Hydroxylation | N-dealkylation |
| Mass (Da) | 236.1109 | 251.0979 | 253.1136 | 253.1136 | 182.0401 |
| Theoretical logD (pH = 4) | 1.76 | 2.49 | -0.54 | -1.15 | 1.74 |
| Probability score (%) |
57 |
57 |
53 |
40 |
36 |
 |
 |
 |
 |
 |
|
| Biotransformation | Dehydrogenation | Hydroxylation | Hydroxylation | Hydroxylation | N-oxidation |
| Mass (Da) | 235.1031 | 253.1136 | 253.1136 | 253.1136 | 253.1136 |
| Theoretical logD (pH = 4) | 0.47 | -0.64 | -0.67 | -1.01 | 2.7 |
| Probability score (%) |
36 |
25 |
23 |
22 |
20 |
|
Second-generation metabolites | |||||
 |
 |
 |
 |
 |
|
| Biotransformation | N-dealkylation | Dehydrogenation | N-dealkylation | N-dealkylation | Iminium formation |
| Mass (Da) | 198.0351 | 251.098 | 269.1086 | 285.1035 | 252.1058 |
| Theoretical logD (pH = 4) | 1.8 | 0.17 | -0.92 | -0.45 | 1.83 |
| Probability score (%) |
83.3 |
83.3 |
66.7 |
66.7 |
66.7 |
 |
 |
 |
|||
| Biotransformation | Carbonylation | S-dealkylation | Thioester cleavage | ||
| Mass (Da) | 267.0929 | 269.1086 | 271.1242 | ||
| Theoretical logD (pH = 4) | 2.57 | -0.88 | -0.47 | ||
| Probability score (%) | 66.7 | 100 | 100 | ||
Metabolites are ranked according to their probability score (>20%). P1 and P2 second-generation metabolites were included (when probability score >50%). Theoretical logD was indicated at pH = 4 as it was the pH closest to gradient conditions.
NA: Not applicable.
MetaSite also generated a list of 237 m/z values of predicted metabolites in HESI+ (theoretical accurate mass). This list was incorporated into the acquisition mode identifying expected metabolites with a low to high signal intensity: ddMS2 mode with an acquisition list.
Discussion
Metabolic stability assessment with human liver microsomes
α-PVT can be considered as an intermediate-clearance drug. McNaney et al. considers compounds with CLint 15–40 ml/min/kg as intermediate-clearance drugs [37], and Lavé et al. classify compounds with an ER between 0.3 and 0.7 as intermediate-clearance drugs [38]. Based on these results, we hypothesize that α-PVT metabolites may be found in urine for a few days after intake. However, CLint and ER are predicted values as the calculations make several assumptions. Hepatic clearance is expected to be sensitive to changes in plasma protein binding, drug metabolism/elimination and hepatic blood flow [39].
Metabolite profiling in human hepatocytes
Takayama et al. previously reported formation of M2 and M3 along with another hydroxypryrrolidinopropyl metabolite using HLM, but the last metabolite was not detected in the present hepatocyte incubations [29]. However, it is difficult to make direct comparisons between HLM and hepatocytes results, as metabolites and their relative abundance formed via HLM incubations may not be representative of metabolic profiles produced in vitro or in vivo. Despite structural similarities to α-PVP (phenyl analog of α-PVT), a few biotransformations were not detected in vitro, including N-dealkylation and pyrrolidine ring opening and oxidation to corresponding carboxylic acid as detected in vitro (HLM) and in vivo (urine) in previous α-PVP studies [40–42]. The differences between α-PVP and α-PVT can be attributed to a number of factors, including the different metabolic systems employed (HLM vs hepatocytes), binding affinity and different structural properties (benzene vs thiophene ring). In hepatocyte incubations experiments, ddMS2 mode with an inclusion list identified the same metabolites as ddMS2 mode without an inclusion list plus several more, indicating that MetaSite software predicted all the metabolites produced in vitro with a high signal intensity and ddMS2 mode with an inclusion list allowed identification of lower intensity metabolites.
α-PVT fragmentation pattern
α-PVT fragmentation pattern is reported in Figure 2 (m/z 238.1260, RT: 7.88 min). Major ions were produced by fragmentation on both sides of the α-carbon: fragments m/z 97.0106 and 110.9899 were related to the thiophenyl (thiophenylmethylium ion) and thiophenone (thiophenylmethylidyneoxonium ion) moieties respectively and fragment m/z 126.1277 was related to the pyrrolidinoalkyl group (butylidenepyrrolidinium ion). Fragments m/z 70.0651 and 84.0808 were produced by the pyrrolidinyl (dihydropyrrolium ion) and the methylpyrrolidinyl (methylenepyrrolidinium ion) moieties respectively and fragment m/z 167.0525 was produced by the pentothiophenone group (oxothiophenylpentanylium ion).
Hydroxylated metabolites
M2 and M3 (m/z 254.1209, RT: 6.88 and 7.38 min, respectively) were hydroxyl metabolites of α-PVT as suggested by the increase of 15.9949 Da from the parent m/z (Figure 3). Butylidenepyrrolidinium ion (m/z 126.1277) was by far the most intense fragment in M2 MS2 spectrum, while the intact thiophenyl fragment was replaced by a hydroxythiophenyl group (m/z 97.0106 + 15.9949 = m/z 113.0055), indicating the formation of α-PVT hydroxythiophenyl. The structure is not stable, however, and resulted in a keto-enol tautomerism in favor of the keto form as suggested by the absence of water loss during fragmentation. The 73% FISh score indicates a good match between the suggested structure of M2 and the theoretical MS2 spectrum but the hydroxyl position on the thiophene ring could not be determined by MS. The M3 MS2 spectrum included the intact thiophenyl and the hydroxypyrrolidinyl (m/z 88.0757) fragments, while the pyrrilidinoalkyl group was replaced by a hydroxypyrrolidinoalkyl fragment (m/z 126.1277 + 15.9949 = m/z 142.1226), indicating the formation of α-PVT hydroxypyrrolidinyl. The position of the hydroxyl group on the pyrrolidine ring cannot be determined even though the absence of loss of water molecule during fragmentation may indicate the formation of α-PVT 2-hydroxypyrrolidinyl (or α-PVT 5-hydroxypyrrolidinyl), the C–O bond being reinforced by the co-sharing of electrons between the oxygen and nitrogen atoms. Both M2 and M3 were already reported in previous in vitro studies using HLM incubations [29].
M5 (m/z 270.1158, RT: 7.67 min) was formed by dihydroxylation of α-PVT as suggested by the addition of 31.9898 Da from the parent drug and the two water molecules loss during fragmentation (Figure 2). In silico studies predicted a metabolite with the exact same mass by pyrrolidine ring opening and N-butanoic acid formation (Table 3, P4) but the fragmentation pattern was not indicative of the expected acetic acid loss [40]. Therefore, we suggest that both hydroxylation reactions occurred on the pyrrolidine ring as indicated by the presence of the intact thiophenyl fragment (m/z 97.0106), appearing in parent MS2 spectrum, the replacement of the pyrrolidinoalkyl fragment by a dihydroxy-substituted group (m/z 126.1277 + 31.9898 = m/z 158.1176) and the formation of butaniminium (m/z 72.0808) and aminothiophenylpentenylium (m/z 166.0685) ions. These two last fragments are noteworthy as the formation of the primary amine (or iminium) structure is favored by hydroxylation at the pyrrolidine ring. These two fragments were not present in parent MS2 spectrum. The 60% FISh score indicates a good match between the proposed structure of M5 and theoretical MS2 spectra.
Metabolites with β-ketone reduction
The water loss in M7 (m/z 240.1417, RT: 8.33 min) MS2 spectrum and addition of 2.0157 Da from parent compound suggested β-ketone reduction (Figure 2). This metabolite was highly expected as ketone reduction was reported as the main α-PVP metabolite in urine samples [40,41] and other β-keto cathinones [43]. Twenty to 30% of signal was produced by in-source water loss (m/z 222.1311). The structure of the most intense M7 fragment at m/z 179.0769 could not be elucidated through the software; nevertheless, the FISh score for the metabolite indicated a good match between the suggested M7 and theoretical MS2 spectra (43%). This fragment was not the result of contamination as the full-scan spectrum did not show any co-eluting additional masses in the range selected for M7 fragmentation. Moreover, the same fragment was observed in the MS2 spectrum of m/z 222.1311 (in-source water loss of M7). In a window of 5 ppm, an experimental m/z 179.0769 should have the following elemental composition: C10H13NS. We hypothesized that the fragment was formed by the loss of the hydroxyl group and the alkyl chain. M7 eluted after parent although it is theoretically more polar than α-PVT in these analysis conditions (logD at pH = 4: -1.00 according to MetaSite). However, the ketone reduction remains the most likely reaction, considering the literature and the fragmentation pattern. It is noteworthy that the same retention time order was observed with M3 and M4.
M4 (m/z 256.1366, RT: 7.55 min) was a secondary metabolite produced by both β-ketone reduction, which had led to M7, and pyrrolidine hydroxylation, which also generated M3, as attested by the two water losses during fragmentation and the increase of 18.0106 Da from α-PVT (Figure 2). An intact thiophenyl fragment (m/z 97.0106), an aminothiophenylpentenylium ion (m/z 166.0685) also observed in the M5 MS2 spectrum and a thiophenyletheniminium ion (m/z 124.0215) observed in both M3 and M5 fragmentation spectra were produced during M4 fragmentation, demonstrating that hydroxylation occurred on the pyrrolidine ring. The hydroxyl position on the pyrrolidine ring could not be determined.
Metabolites with γ-lactam formation
M9 (m/z 252.1053, RT: 13.12 min) was formed by hydroxylation and dehydrogenation of α-PVT (+13.9793 Da). The MS2 spectrum included the intact thiophenyl fragment along with substituted pyrrolidinoalkyl (m/z 126.1277 + 13.9793 = m/z 140.1070) and pyrrolidinyl (m/z 84.0808 + 13.9793 = m/z 98.0600) fragments suggesting an oxidation of the pyrrolidine ring. HRMS allowed the distinction between fragments m/z 98.0600 and 98.0964, the last one being present in parent MS2 spectrum (vinylpyrrolidinium ion) (Figure 2). Hydroxylation followed by dehydrogenation to the corresponding ketone is more likely than dehydrogenation of a C–C bond. Moreover, γ-lactam formation is a major metabolic pathway of α-PVP [40,41] and other pyrrolidine compounds [44]. M9 eluted 5.25 min after the parent, supporting the hypothesis of formation of α-PVT pyrrolidinin-2-one (or α-PVT pyrrolidinin-5-one), a γ-lactam, the carbonyl group being a hindrance to hydrogen bonding with the nitrogen atom and decreasing M9 polarity and alkalinity. M8 (m/z 254.1209, RT: 11.07 min) was a tertiary metabolite produced by β-ketone reduction, pyrrolidine hydroxylation and then dehydrogenation to the corresponding lactam. Interestingly, the M8 signal was drastically reduced due to in-source fragmentation, 99.5% of the signal being produced by the water loss at m/z 236.1104. The MS2 spectrum of ion m/z 236.1104 contained an intact thiophenyl fragment (m/z 97.0106), an oxopyrrolidinium (m/z 86.0600) and a methyleneoxopyrrolidinium (m/z 98.0600) ion, which were also present in the M9 MS2 spectrum, pointing toward a γ-lactam formation. It also contained a thiophenylpentenylium ion (m/z 151.0576), previously observed in the M7 MS2 spectrum and indicating a β-ketone reduction (Figure 2). This metabolite was expected as the reaction is a major α-PVP biotransformation [40].
Metabolic confirmation & profiling in authentic urine samples
The three samples were positive for α-PVT and one to five other cathinones (6-APB, normephedrone, 4-methylethcathinone, α-PVP and MDPV). All seven α-PVT metabolites previously elucidated in hepatocyte incubations contained a thiophene ring and would not be produced from the other detected cathinones. α-PVT and its seven metabolites were identified in samples 2 and 3 (Figure 3); however, M3 in sample 3 and M7 in both samples were not confirmed as the low signal did not trigger an MS2 event in ddMS2 mode and the diagnostic MS2 ions were not detected in AIF mode. M2, M5, M7, M9 and parent were identified in sample 1 but, similarly, M2, M7 and M9 were not confirmed due to low signal intensity. This is consistent with the lower α-PVT concentration in urine specimen 1. Interestingly, M2 and M4 chromatographic peaks presented with shoulders in authentic samples, suggesting the presence of potential positional isomers. The M2 shoulder peak (M2’, RT: 7.03 min) is supposed to be a M2 isomer as it produced the same fragments. Both M2 and M2’ were included in the MS peak area calculations (Table 2). The M4 shoulder peak (RT: 7.60 min) was not intense enough to trigger an MS2 scan so its identity was not confirmed and was not included in peak area calculations.
In order to protect the instrument, we utilized SPE for the urine samples validated for cathinone parent compounds [35]. The signal intensities of each metabolite were consistent with hepatocyte incubation results (Table 2). However, relative intensities do not necessarily reflect metabolite concentrations, as ionization, extraction recovery and matrix effect could vary depending on the compound. As certified reference standards are not yet available for these metabolites, recovery and matrix effect cannot be evaluated. The extraction procedure utilized in this study proved useful for the recovery of a wide variety of phenethylamines, pyrrolidinophenones and their respective metabolites (>80% recovery), as seen in our publications [35,45]. A gross estimation of SPE recoveries in hepatocyte samples was made by comparing areas from extracted and unextracted incubations (representing same sample volume). α-PVT, M3, M4 and M7 recoveries ranged from 75 to 95%, while M2, M5, M8 and M9 recoveries ranged from 0.5 to 3.5% (α-PVT extraction recovery in urine is 93.1 – 94.7% [35]). It is important to keep in mind that estimations were made on a single experiment and may also be biased by matrix effect in both SPE and dilution experiments. A strong positive matrix effect in dilution samples and/or a strong negative matrix effect in SPE samples for M2, M5, M8 and M9 could alone explain the low recovery values. Hypothesizing that the extraction recoveries are similar in urine and hepatocyte incubations and regardless of the difference in signal production and matrix effect, M2, M5 and M9 would be the main metabolites of α-PVT in vivo. M5, M2, parent and M9 would be respectively the most concentrated α-PVT-related compounds in urine specimens 2 and 3, which present a high α-PVT concentration. M2, M9, M5 and parent would be respectively the most abundant α-PVT-related compounds in urine specimen 1, which presents a low α-PVT concentration. There is currently no mention of α-PVT concentrations in biological matrices in the scientific literature; however, samples 2 and 3 urine concentrations may be indicative of recent drug use or larger doses while sample 1 may not, based on the PK patterns of other cathinones [46].
Two additional metabolites, M1 and M6, were identified in samples 2 and 3 using ddMS2 acquisition mode with an inclusion list (Figure 3). M1 (m/z 254.1209, RT: 6.20 min) was formed by hydroxylation, as suggested by the increase of 15.9949 Da from parent (Figure 2). The presence of the intact thiophenyl and pyrrolidine fragments and the substituted pyrrolidinoalkyl fragment (m/z 126.1277 + 15.9949 = m/z 142.1226) indicated a hydroxylation on the alkyl chain. M1 may be the α-PVT hydroxypyrrolidinopropyl metabolite that was previously identified by Takayama et al. using HLM incubations [29]. The chromatographic peak presented a small shoulder on both sides, suggesting the presence of 2 positional isomers, but the low abundances did not trigger MS2 events. The shoulder peaks were not included in MS peak area calculations. M6 (m/z 428.1374, RT: 7.75 min) was the glucuronide of M9 as evidenced by the increase by 176.0321 Da from α-PVT and its MS2 spectrum, which contained the main M9 fragments along with M9 itself and substituted pyrrolidinoalkyl chain (m/z 126.1277 + 13.9793 + 176.0321 = m/z 316.1391) (Figure 2). Both newly identified metabolites showed only low abundance signals (Table 2).
No other phase II metabolites were detected in vitro or in vivo. The parent drug is a polar compound and the analyte with the most intense signal detected in urine specimens. The only glucuronide detected in the urine sample was the conjugated form of M9, which is the least polar α-PVT metabolite identified. We did not detect any glucuronidated metabolites in hepatocyte incubations, meaning that glucuronidation does not occur or did not have time to occur in the present 2 h experiments. Identification of diclofenac acyl glucuronide in the positive control samples confirms that the hepatocytes were viable to produce phase II metabolites. The lack of phase II metabolites also was observed with 4-MeO-α-PVP [30].
In silico predictions
MetaSite predicted a list of 14 first-generation and 8 second-generation metabolites, based on a CYP450 liver model (Table 3). A prediction score thus was assigned to each compound depending on the simulations of phase I metabolism. However, in silico prediction software is incapable of predicting second- and third-generation metabolites or non-CYP- or FMO3-mediated biotransformations, such as aldehyde oxidase, sulfotransferase etc. In addition, the liver model takes into account the sites of metabolism based on only four metabolic enzymes (CYP3A4, CYP2D6, CYP2C9 and FMO3), potentially introducing a bias in predictions. The predictions should be considered carefully as they might influence the judgment of the operator during metabolite identification. It is noteworthy that P1 and P1–1 to P1–6 are not stable in the conditions of the analysis as they represent the enol form of a keto-enol tautomerism (P2 is the keto P1 form). Similarly, P5 is not stable in aqueous conditions and may lose its pyrrolidine group to produce P9. Both P1 and P5 presented with a high prediction score (100 and 57%, respectively) but were not detected in the present experiments, possibly due to instability. Four metabolites identified in hepatocyte incubations and authentic urine samples matched at least one metabolite predicted by MetaSite software: M1, M2, M3 and M9. M2 matched P2, which presented with a 100% score, and appeared to be, indeed, a major metabolite in in vitro and in vivo experiments. Lactam formation is a common reaction for pyrrolidine compounds [43,44] leading to M9 in the present experiments. M9 matched P6, which was predicted with a 57% score. The position of the hydroxyl group in M1 could not be elucidated in the conditions of the analysis and the compound could match either P8 or P13, which were respectively predicted with a 40 and 22% score. Similarly the hydroxylation site of M3 could not be elucidated but the metabolite matched P12, which was predicted with a 23% score and was the only predicted α-PVT hydroxypyrrolidine. N-dealkylation reactions were not detected in vitro or in vivo. Five metabolites were detected in hepatic or/and urine samples but were not predicted by MetaSite software (score <20%): M4, M5, M6, M7 and M8. M5, the metabolite with the most intense signal in hepatic incubations and urine samples, did not match any predicted metabolite as it was a second-generation metabolite from P12, which presented with a 23% score. M6 did not match any predicted metabolite as it was the result of a phase II reaction (glucuronidation). More interestingly, M4, M7 and M8 did not match any predicted metabolite although ketone reduction is a common biotransformation for β-keto cathinones [40,41,43].
HRMS inclusion list
Beside the list of the 22 most expected metabolites based on the CYP450 liver model, MetaSite generated a more exhaustive list of 237 m/z values of predicted metabolites in HESI+ for ddMS2 analysis. This list included not only the 39 biotransformations generated by the CYP450 liver model, but also 40 additional non-CYP mediated reactions as well as second- and third-generation metabolites. However, the structure, the corresponding biotransformations and the prediction score assigned to these m/z were not provided. No additional metabolites were identified in ddMS2 acquisition mode without an inclusion list compared with ddMS2 mode with an inclusion list, indicating that the inclusion list contained the m/z value of all the metabolites that were detected in hepatic and urine samples. Moreover, the ddMS2 mode with an inclusion list allowed the elucidation of a higher number of metabolites, indicating that the list of m/z generated by MetaSite software was crucial in the identification of metabolites with lower signal intensity.
Conclusion
For the first time, a comprehensive metabolic profile of α-PVT was generated using in vitro HLM and human hepatocyte incubations, and confirmation in authentic urine specimens with HRMS detection. α-PVT was predicted as an intermediate-clearance drug with a 30 min half-life in HLM. Nine metabolites were elucidated in hepatocyte incubations and urine samples. Observed metabolic patterns were comparable to other pyrrolidinophenones, including hydroxylation, ketone reduction, lactam formation and combinations thereof. In addition to parent drug, analytical methods should monitor M2 (α-PVT hydroxythiophenyl), M5 (α-PVT dihydroxypyrrolidinyl), M7 (α-PVT thiophenol) and M9 (α-PVT 2-ketopyrrolidinyl) to serve as markers for α-PVT intake in forensic and clinical samples. Results were compared with in silico predictions by MetaSite software. MetaSite CYP450 liver model appeared to be unsuitable for α-PVT metabolites prediction, as only four of nine predicted metabolites achieved a score higher than 20%. However, the software also generated a more exhaustive list of theoretical m/z values of predicted metabolites in HESI+ that proved crucial for the identification of metabolites with low intensity signal in HRMS experiments using ddMS2 acquisition mode with an inclusion list.
Future perspective
In the last several years, more than 450 NPS were identified by the EWS. As the drug market evolves, reference standards for new NPS and metabolites are not always readily and quickly available, making it difficult for forensic and clinical laboratories to maintain validated analytical assays for identifying and quantifying these markers in biological specimens. The advent of HRMS allowed laboratories to screen for hundreds of additional compounds against NPS libraries. However, knowledge of metabolites can increase detection windows for many drugs. Since controlled administration studies are not yet permissible, in vitro studies are the fastest, most robust assays for determining metabolic profiles. Identification of prominent metabolites can assist reference standard manufacturers in deciding which compounds to produce and publishing of product ion spectra can help laboratories confirm NPS intake. Over the next several years, in silico predictions and in vitro assays will become routine in mapping NPS metabolic pathways. Understanding drug clearance and production of potential toxic metabolites will assist practitioners in understanding the PD and PK profiles of emerging compounds. Knowledge of several drugs in a particular class also will provide insight into how other analogs may behave.
Executive summary.
Little pharmacological or toxicological data are available for constantly emerging novel psychoactive substances (NPS).
Characterizing NPS metabolic pathways is necessary for improving identification of NPS intake.
Human hepatocyte drug incubations, followed by analysis with liquid chromatography coupled to high-resolution MS and software-assisted data mining is a promising approach to generating comprehensive NPS metabolic profiles.
Results
We generated the metabolic profile for α-pyrrolidinopentiothiophenone (α-PVT), a novel synthetic cathinone and identified seven metabolites in vitro and two additional metabolites in vivo.
The primary biotransformations were hydroxylation, ketone reduction, lactam formation and combinations thereof.
The most intense α-PVT metabolites and suitable markers for documenting α-PVT intake in addition to the parent drug were hydroxythiophenyl, dihydroxypyrrolidinyl, thiophenol and 2-ketopyrrolidinyl α-PVT.
Acknowledgements
The authors would like to thank Tim Moeller of Bioreclamation IVT for his assistance with the incubations, Ismael Zamora and his team at Molecular Discovery for the MetaSite software and assistance, and Caroline Ding and her team at Thermo for the Compound Discoverer software and training.
Footnotes
Financial & competing interests disclosure
This research was funded by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.European Monitoring Center for Drugs and Drug Addiction. New psychoactive substances in Europe - An update from the EU Early Warning System (March 2015) Publications Office of the European Union; Luxembourg: 2015. European Monitoring Centre for Drugs and Drug Addiction Publications Office of the European Union. [Google Scholar]
- 2.European Monitoring Center for Drugs and Drug Addiction. Publications Office of the European Union; Luxembourg: World Drug Report 2013. United Nations Office on Drugs and Crime Sales No. E.13.XI.6 18 (2013)www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf [Google Scholar]
- 3.Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3(7–8):439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- 4.Gregg RA, Rawls SM. Behavioral pharmacology of designer cathinones: a review of the preclinical literature. Life Sci. 2014;97(1):27–30. doi: 10.1016/j.lfs.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capriola M. Synthetic cathinone abuse. Clin. Pharmacol. 2013;5:109–115. doi: 10.2147/CPAA.S42832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunderson EW, Kirkpatrick MG, Willing LM, Holstege CP. Substituted cathinone products: a new trend in “bath salts” and other designer stimulant drug use. J. Addict. Med. 2013;7(3):153–162. doi: 10.1097/ADM.0b013e31829084b7. [DOI] [PubMed] [Google Scholar]
- 7.Lehner KR, Baumann MH. Psychoactive ‘bath salts’: compounds, mechanisms, and toxicities. Neuropsychopharmacology. 2013;38(1):243–244. doi: 10.1038/npp.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrigan T. Schedules of controlled substances: temporary placement of 10 synthetic cathinones into schedule I. Fed. Regist. 2014;79(45):12938–12943. [PubMed] [Google Scholar]
- 9.Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y. Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol. 2013;31(2):223–240. [Google Scholar]; • First identification of α-pyrrolidinopentiothiophenone (α-PVT) in herbal material sold in Japan in 2013.
- 10.Roesner P. Designer drugs online news: alpha-PVT. Email Newsletter. May 8 (2014) www.designer-drugs.de [Google Scholar]
- 11.Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci. Int. 2014;243C:55–60. doi: 10.1016/j.forsciint.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 12.European Monitoring Centre for Drugs and Drug Addiction Publications Office of the European Union. European Drug Report 2014: Trends and Developments. Luxembourg; 2014. [Google Scholar]
- 13.Baumann MH, Ayestas MA, Jr, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumann MH, Partilla JS, Lehner KR, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha K, Partilla JS, Lehner KR, et al. ‘Second-generation’ mephedrone analogs, 4-MEC and 4-MePPP, differentially affect monoamine transporter function. Neuropsychopharmacology. 2015;40(6):1321–1331. doi: 10.1038/npp.2014.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Springer D, Fritschi G, Maurer HH. Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4’-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;796(2):253–266. doi: 10.1016/j.jchromb.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Springer D, Fritschi G, Maurer HH. Metabolism and toxicological detection of the new designer drug 3’,4’-methylenedioxy-alpha-pyrrolidinopropiophenone studied in urine using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;793(2):377–388. doi: 10.1016/s1570-0232(03)00350-7. [DOI] [PubMed] [Google Scholar]
- 19.Springer D, Paul LD, Staack RF, Kraemer T, Maurer HH. Identification of cytochrome p450 enzymes involved in the metabolism of 4’-methyl-alpha-pyrrolidinopropiophenone, a novel scheduled designer drug, in human liver microsomes. Drug Metab. Dispos. 2003;31(8):979–982. doi: 10.1124/dmd.31.8.979. [DOI] [PubMed] [Google Scholar]
- 20.Springer D, Peters FT, Fritschi G, Maurer HH. New designer drug 4’-methyl-alpha-pyrrolidinohexanophenone: studies on its metabolism and toxicological detection in urine using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;789(1):79–91. doi: 10.1016/s1570-0232(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 21.Springer D, Peters FT, Fritschi G, Maurer HH. Studies on the metabolism and toxicological detection of the new designer drug 4’-methyl-alpha-pyrrolidinopropiophenone in urine using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;773(1):25–33. doi: 10.1016/s1570-0232(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 22.Springer D, Peters FT, Fritschi G, Maurer HH. New designer drug 4’-methyl-alpha-pyrrolidinohexanophenone: studies on its metabolism and toxicological detection in urine using gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003;5(789):79–91. doi: 10.1016/s1570-0232(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 23.Springer D, Staack RF, Paul LD, Kraemer T, Maurer HH. Identification of cytochrome P450 enzymes involved in the metabolism of 4’-methoxy-alpha-pyrrolidinopropiophenone (MOPPP), a designer drug, in human liver microsomes. Xenobiotica. 2003;33(10):989–998. doi: 10.1080/00498250310001602775. [DOI] [PubMed] [Google Scholar]
- 24.Springer D, Staack RF, Paul LD, Kraemer T, Maurer HH. Identification of cytochrome P450 enzymes involved in the metabolism of 3’,4’-methylenedioxy-alpha-pyrrolidinopropiophenone (MDPPP), a designer drug, in human liver microsomes. Xenobiotica. 2005;35(3):227–237. doi: 10.1080/00498250400028239. [DOI] [PubMed] [Google Scholar]
- 25.Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC–MS and LC–high-resolution MS and its detectability in urine by GC–MS. J. Mass Spectrom. 2010;45(12):1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- 26.Sauer C, Peters FT, Haas C, Meyer MR, Fritschi G, Maurer HH. New designer drug α-pyrrolidinovalerophenone (PVP): studies on its metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J. Mass Spectrom. 2009;44(6):952–964. doi: 10.1002/jms.1571. [DOI] [PubMed] [Google Scholar]
- 27.Peters FT, Meyer MR, Theobald DS, Maurer HH. Identification of cytochrome P450 enzymes involved in the metabolism of the new designer drug 4’-methyl-alpha-pyrrolidinobutyrophenone. Drug Metab. Dispos. 2008;36(1):163–168. doi: 10.1124/dmd.107.017293. [DOI] [PubMed] [Google Scholar]
- 28.Zaitsu K, Katagi M, Tsuchihashi H, Ishii A. Recently abused synthetic cathinones, α-pyrrolidinophenone derivatives: a review of their pharmacology, acute toxicity, and metabolism. Forensic Toxicol. 2014;32(1):1–8. [Google Scholar]; • Comprehensive review of α-pyrrolidinophenone compounds’ pharmacology and metabolism.
- 29.Takayama T, Suzuki M, Todoroki K, et al. UPLC/ESI-MS/MS-based determination of metabolism of several new illicit drugs, ADB-FUBINACA, AB-FUBINACA, AB-PINACA, QUPIC, 5F-QUPIC and alpha-PVT, by human liver microsome. Biomed. Chromatogr. 2014;28(6):831–838. doi: 10.1002/bmc.3155. [DOI] [PubMed] [Google Scholar]; • First elucidation of three α-PVT hydroxylated metabolites using HLM.
- 30.Ellefsen K, Wohlfarth A, Swortwood M, Diao X, Concheiro M, Huestis M. 4-methoxy-α-PVP: in silico prediction, metabolic stability, and metabolite identification by human hepatocyte incubation and high-resolution mass spectrometry. Forensic Toxicol. 2015 doi: 10.1007/s11419-015-0287-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First study to report metabolites of synthetic cathinones after incubation with human hepatocytes.
- 31.Wohlfarth A, Scheidweiler KB, Pang S, et al. Metabolic characterization of AH-7921, a synthetic opioid designer drug: in vitro metabolic stability assessment and metabolite identification, evaluation of in silico prediction, and in vivo confirmation. Drug Test Anal. 2015 doi: 10.1002/dta.1856. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diao X, Wohlfarth A, Pang S, Scheidweiler KB, Huestis MA. High-resolution mass spectrometry for characterizing the metabolism of synthetic cannabinoid THJ-018 and Its 5-fluoro analog THJ-2201 after incubation in human hepatocytes. Clin. Chem. 2015 doi: 10.1373/clinchem.2015.243535. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Li AP. Human hepatocytes: isolation, cryopreservation and applications in drug development. Chem. Biol. Interact. 2007;168(1):16–29. doi: 10.1016/j.cbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Strano-Rossi S, Anzillotti L, Dragoni S, et al. Metabolism of JWH-015, JWH-098, JWH-251, and JWH-307 in silico and in vitro: a pilot study for the detection of unknown synthetic cannabinoids metabolites. Anal. Bioanal. Chem. 2014;406(15):3621–3636. doi: 10.1007/s00216-014-7793-9. [DOI] [PubMed] [Google Scholar]
- 35.Concheiro M, Anizan S, Ellefsen K, Huestis MA. Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography-high resolution mass spectrometry. Anal. Bioanal. Chem. 2013;405(29):9437–9448. doi: 10.1007/s00216-013-7386-z. [DOI] [PubMed] [Google Scholar]; • A comprehensive quantification method for 28 synthetic cathinones in urine, including α-PVT.
- 36.Baranczewski P, Stanczak A, Sundberg K, et al. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol. Rep. 2006;58(4):453–472. [PubMed] [Google Scholar]
- 37.Mcnaney CA, Drexler DM, Hnatyshyn SY, et al. An automated liquid chromatography-mass spectrometry process to determine metabolic stability half-life and intrinsic clearance of drug candidates by substrate depletion. Assay Drug Dev. Technol. 2008;6(1):121–129. doi: 10.1089/adt.2007.103. [DOI] [PubMed] [Google Scholar]
- 38.Lave T, Dupin S, Schmitt C, et al. The use of human hepatocytes to select compounds based on their expected hepatic extraction ratios in humans. Pharm. Res. 1997;14(2):152–155. doi: 10.1023/a:1012036324237. [DOI] [PubMed] [Google Scholar]
- 39.Atkinson AJ, Jr, Kushner W. Clinical pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 1979;19:105–127. doi: 10.1146/annurev.pa.19.040179.000541. [DOI] [PubMed] [Google Scholar]
- 40.Tyrkko E, Pelander A, Ketola RA, Ojanpera I. In silico and in vitro metabolism studies support identification of designer drugs in human urine by liquid chromatography/quadrupole-time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2013;405(21):6697–6709. doi: 10.1007/s00216-013-7137-1. [DOI] [PubMed] [Google Scholar]; • The authors elucidated seven metabolites of α-PVP, phenyl analog of α-PVT, using in silico predictions, HLM incubations and authentic urine specimens analysis.
- 41.Shima N, Katagi M, Kamata H, et al. Metabolism of the newly encountered designer drug α-pyrrolidinovalerophenone in humans: identification and quantitation of urinary metabolites. Forensic Toxicol. 2014;32(1):59–67. [Google Scholar]
- 42.Negreira N, Erratico C, Kosjek T, et al. In vitro phase I and phase II metabolism of alpha-pyrrolidinovalerophenone (alpha-PVP), methylenedioxypyrovalerone (MDPV) and methedrone by human liver microsomes and human liver cytosol. Anal. Bioanal. Chem. 2015;407(19):5803–5816. doi: 10.1007/s00216-015-8763-6. [DOI] [PubMed] [Google Scholar]
- 43.Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr. Drug Metab. 2010;11(5):468–482. doi: 10.2174/138920010791526042. [DOI] [PubMed] [Google Scholar]
- 44.Vickers S, Polsky SL. The biotransformation of nitrogen containing xenobiotics to lactams. Curr. Drug Metab. 2000;1(4):357–389. doi: 10.2174/1389200003338929. [DOI] [PubMed] [Google Scholar]
- 45.Concheiro M, Castaneto M, Kronstrand R, Huestis MA. Simultaneous determination of 40 novel psychoactive stimulants in urine by liquid chromatography-high resolution mass spectrometry and library matching. J. Chromatogr. A. 2015;1397:32–42. doi: 10.1016/j.chroma.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J. Anal. Toxicol. 2013;37(3):135–146. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]