Abstract
CD8 T cells are key components of the immune response to viruses, but their roles in the pathogenesis of adenovirus respiratory infection have not been characterized. We used mouse adenovirus type 1 (MAV-1) to define CD8 T cell contributions to the pathogenesis of adenovirus respiratory infection. CD8 T cell deficiency in β2m−/− mice had no effect on peak viral replication in lungs, but clearance of virus was delayed in β2m−/− mice. Virus-induced weight loss and increases in bronchoalveolar lavage fluid total protein, IFN-γ, TNF-α, IL-10, CCL2, and CCL5 concentrations were less in β2m−/− mice than in controls. CD8 T cell depletion had similar effects on virus clearance, weight loss, and inflammation. Deficiency of IFN-γ or perforin had no effect on viral replication or inflammation, but perforin-deficient mice were partially protected from weight loss. CD8 T cells promote MAV-1-induced pulmonary inflammation via a mechanism that is independent of direct antiviral effects.
Keywords: Adenovirus, Respiratory infection, CD8 T cells, Interferon gamma, Perforin
Introduction
CD8 T cells contribute to the control of many viral infections (Adcock and Lane, 2003; Ehtisham et al., 1993; Kagi et al., 1994; Mullbacher et al., 1999; Walsh et al., 1994). After recognizing infected cells presenting virus-specific peptides by MHC class I, CD8 T cells kill them through the release of perforin (Pfn)- and granzyme (Gzm)-containing granules or by induction of apoptosis via interaction between Fas and Fas ligands (FasL) (Chavez-Galan et al., 2009; Harty et al., 2000; Hoves et al., 2010). CD8 T cells also produce a variety of antiviral and pro-inflammatory cytokines such as interferon (IFN)-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α. The collective action of these effectors aids in the control of viral replication during acute infection. However, the actions of CD8 T cells may also contribute to bystander tissue damage (Cannon et al., 1988). CD4 and CD8 T cell responses specific to human adenoviruses (HAdVs) have been described, and patients with acquired or inherited defects in cellular immune function are prone to more severe HAdV disease (Kojaoghlanian et al., 2003; Walls et al., 2003). However, the strict species-specificity of the adenoviruses has precluded extensive studies of the contributions of CD8 T cells to HAdV pathogenesis.
We use mouse adenovirus type 1 (MAV-1) to study the pathogenesis of an adenovirus in its natural host. We have established mouse models of adenovirus respiratory infection (McCarthy et al., 2014; Procario et al., 2012; Weinberg et al., 2005) and myocarditis (McCarthy et al., 2015a). Findings from our laboratory and our collaborators have provided insight into cellular immunity and adenovirus pathogenesis. CD4 and CD8 T cells are recruited to the lungs, heart, and brain of mice infected intranasally (i.n.) with MAV-1 (McCarthy et al., 2015a; Procario et al., 2012; Weinberg et al., 2007), and both CD4 and CD8 T cells recruited to lungs of MAV-1-infected mice include a significant population of CD62Llow effector memory cells (Procario et al., 2012). MAV-1-specific epitopes presented by MHC class I to CD8 T cells have not yet been defined, but we have demonstrated increased IFN-γ and GzmB production by CD8 T cells isolated from lungs of infected mice compared to mock-infected mice during acute infection (McCarthy et al., 2015b; McCarthy et al., 2014), suggesting that CD8 T cells respond specifically to MAV-1. α/β T cells are required for control of viral replication in the spleen and brain and for long-term survival following intraperitoneal (i.p.) inoculation (Moore et al., 2003).
Our recent work suggested the possibility that CD8 T cell dysfunction is associated with delayed virus clearance from the lungs of mice that received allogeneic bone marrow transplantation (BMT) (McCarthy et al., 2015c). However, the specific mechanisms by which CD8 T cells contribute to control of MAV-1 replication and to MAV-1-induced pulmonary inflammation are not yet completely defined. In this study, we demonstrate that clearance of MAV-1 DNA from the lungs was delayed in the absence of functioning CD8 T cells, but CD8 T cells were not essential for efficient control of MAV-1 replication in the lungs during acute infection. In contrast, MAV-1-induced airway inflammation and weight loss were markedly reduced in CD8 T cell-deficient mice. Effects of CD8 T cells on airway inflammation did not depend on Pfn or IFN-γ, but Pfn deficiency partially protected against virus-induced weight loss. Depletion of CD4 T cells had no effect on virus clearance or virus-induced inflammation. Even intact CD8 T cell function was insufficient to completely clear MAV-1 from the lungs. Collectively, our data suggest that CD8 T cells exert an immunomodulatory function in the lungs that is independent of their contributions to control of MAV-1 replication in the lungs.
Methods and Materials
Mice
C57BL/6J (B6), β2-microglobulin-deficient (β2m−/−, B6.129P2-B2mtm1Unc/J), CD8α-deficient (CD8α−/−; B6.129S2-CD8αtm1Mak/J), Pfn-deficient (Pfn−/−; C57BL/6-Prf1tm1Sdz/J), and IFN-γ-deficient (IFN-γ−/−, B6.129S7-Ifngtm1Ts/J) mice, all on a C57BL/6J background, were obtained from the Jackson Laboratory. In the experiments in Fig. 2, IFN-γ−/− mice bred at the University of Michigan (originally from the Jackson Laboratory, generously provided by Dr. Benjamin Segal) were used. C57BL/6J mice obtained from the Jackson Laboratory and bred at the University of Michigan were used for experiments involving antibody depletion of CD4 T-cells. All mice were maintained under specific pathogen-free conditions. All experiments were approved by the University of Michigan Institutional Animal Care and Use Committee.
Virus and Infections
MAV-1 was grown and passaged in NIH 3T6 fibroblasts, and titers of viral stocks were determined by plaque assay on 3T6 cells as previous described (Cauthen et al., 2007). Adult (6 to 8 weeks old) mice were anesthetized with ketamine and xylazine and then infected i.n with 105 plaque-forming units (pfu) of MAV-1 in 40 μl of sterile phosphate-buffered saline (PBS). Control mice were mock-infected i.n. with conditioned medium at an equivalent dilution in sterile PBS. In some experiments, mice were weighed on the day of infection and then intermittently throughout the course of the experiment. Mice were euthanized by pentobarbital overdose at the indicated time points. Organs were harvested, snap-frozen in dry ice and stored at −80°C.
CD4 and CD8 T Cell Depletion
A rat monoclonal antibody recognizing mouse CD8 T cells (clone YTS 169.4, BioXCell, Inc.) or CD4 T cells (clone GK1.5, BioXCell, Inc.) was administered i.p. at 200 μg/dose. Control mice received equivalent amounts of nonspecific rat IgG2b (clone LTF-2, BioXCell, Inc.). Antibody was administered on days -1, 3, 6, 10, and 13 relative to infection (day 0).
Isolation of DNA and RNA
RNA was isolated from homogenates of all organs as previously described (Nguyen et al., 2008). Portions of lung, heart, spleen, and brain were homogenized using sterile glass beads in a Mini-Beadbeater (Biospec Products) for 30 s in 1 ml of TRIzol (Invitrogen). RNA (all organs) and DNA (hearts, brains, and spleens) were then isolated from homogenates according to the manufacturer’s protocol. DNA was extracted from a separate portion of lung using the DNeasy Tissue kit (Qiagen Inc.).
Analysis of Host Gene Expression
Host gene expression was quantified using reverse transcriptase quantitative real-time PCR (RT-qPCR). RNA was reverse transcribed using MMLV reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. For measurements of CD4 (forward primer 5′-AGGTGATGGGACCTACCTCTC-3′; reverse primer 5′-GGGGCCACCACTTGAACTAC-3′) and CD8 (forward primer 5′-CCGTTGACCCGCTTTCTGT-3′; reverse primer 5′-CGGCGTCCATTTTCTTTGGAA-3′), and Pfn (forward primer 5′-AGCACAAGTTCGTGCCAGG-3′; reverse primer 5′-CTCCGTGATGGAAGACCACT-3′), 5 μl of cDNA were added to reactions containing Power SYBR Green PCR Mix (Applied Biosystems) and forward and reverse primers (each at 200 nM final concentration) in a 25 μl reaction volume. Separate reactions were prepared with primers for mouse GAPDH (forward primer 5′-TGCACCACCAACTGCTTAG-3′; reverse primer 5′-GGATGCAGGGATGATGTTC-3′). In all cases, RT-qPCR analysis consisted of 40 cycles of 15 s at 90°C and 60 s at 60°C. Quantification of target gene mRNA was normalized to GAPDH and expressed in arbitrary units as 2−ΔCt, where Ct is the threshold cycle and ΔCt = Ct(target) − Ct(GAPDH).
Analysis of Viral Gene Expression
Viral gene expression was quantified using RT-qPCR. cDNA prepared as described above was amplified using primers targeting the MAV-1 tripartite leader (TPL) sequence (forward primer 5′-CGAGTCGCCTCCTGTGATACT-3′; reverse primer 5′-CAAGTCGATCTGTCGGAGCTT-3′). The 63 bp product amplified by these primers spans the 885 bp intron between exon 2 and exon 3 of the MAV-1 TPL sequence. Quantification of the target gene mRNA was normalized to GAPDH as described above.
Analysis of Viral Loads
MAV-1 viral loads were measured in organs using quantitative real-time polymerase chain reaction (qPCR) as previously described (McCarthy et al., 2015a; Procario et al., 2012). Results were standardized to the nanogram (ng) amount of input DNA. Each sample was assayed in triplicate.
Detection of Infectious Virus in Lung
Lungs were homogenized in sterile PBS (10% weight/volume) using sterile glass beads in a Mini-Beadbeater and then clarified by centrifugation after three freeze/thaw cycles. Plaque assay was then performed as previous described (Cauthen et al., 2007).
Lymphocyte Stimulation
Spleens were harvested from mice and single-cell suspensions of splenocytes were generated by passage through a 70 μm cell strainer followed by lysis of red blood cells with ACK lysis buffer. CD8 T cells were isolated from splenocytes by negative selection using antibody-coated magnetic beads (Mouse CD8α+ T cell kit; Miltenyi Biotec). CD8 T cells were seeded at a concentration of 3 × 105 cells/well in 96-well plates coated with anti-CD3 antibody (clone 145-2C11, BioLegend, 5 μg/ml) and incubated for 24 h. Cytokine protein concentrations in supernatants were determined by ELISA (Duoset Kits, R&D Systems) according to the manufacturer’s protocol.
Measurement of Cytokine Concentrations in Bronchoalveolar Lavage Fluid
Bronchoalveolar lavage fluid (BALF) was obtained by lavaging lungs three times with the same aliquot of 1 mL sterile PBS containing protease inhibitor (complete, Mini, EDTA-free tablets; Roche Applied Science). Cytokine concentrations were determined by ELISA (Duoset Kits, R&D Systems) according to the manufacturer’s directions. ELISA was performed by the University of Michigan Cancer Center Immunology Core.
Measurement of Total Protein in Bronchoalveolar Lavage Fluid
Total protein concentrations in BALF were determined using the Quick Start™ Bradford Protein Assay (BioRad) according to the manufacturer’s instructions.
Flow Cytometry
Lymphocyte depletion was confirmed by staining splenocytes with antibodies to TCR β chain (clone H57-597, BD Pharmingen), CD8 (clone 53-6.7, BD Pharmingen), and CD4 (clone RM4-5, BD Pharmingen). Cells were preincubated with anti-FcγR mAb 2.4G2 to block nonspecific binding before staining. Stained samples were analyzed by flow cytometry with a CyAn ADP flow cytometer (Beckman Coulter, Inc.) and FlowJo (Tree Start, Inc.) or Summit (Beckman Coulter, Inc.) software.
Histology
Lungs were harvested from a subset of mice and fixed in 10% formalin. Prior to fixation, lungs were inflated with PBS via the trachea to maintain lung architecture. After fixation, organs were embedded in paraffin and 5 μm sections were obtained for histopathology. Sections were stained with hematoxylin and eosin to evaluate cellular infiltration. To quantify cellular inflammation in the lungs, slides were examined in a blinded fashion to determine a pathology index as previous described (Procario et al., 2012). Separate sections used for immunohistochemistry were stained with anti-CD3 antibody (Thermo Scientific) to identify CD3-positive cells. Sectioning and staining were performed by the University of Michigan Unit for Laboratory Animal Medicine Pathology Cores for Animal Research. Digital images were obtained with an EC3 digital imaging system (Leica Microsystems) using Leica Acquisition Software (Leica Microsystems). Any adjustments to brightness and contrast in digital images were applied equally to all experimental and control images.
Statistics
Statistical analysis was performed using Prism 7 (GraphPad Software, Inc.). Viral loads were log-transformed for statistical analysis. Differences between two groups were analyzed using the Mann-Whitney rank sum test. Differences among more than two groups at a single time point were analyzed using the Kruskal-Wallis test with Dunn’s multiple comparison tests. Differences between groups at multiple time points were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests. P values of <0.05 were considered statistically significant.
Results
MAV-1-Induced CD8 T Cell Responses
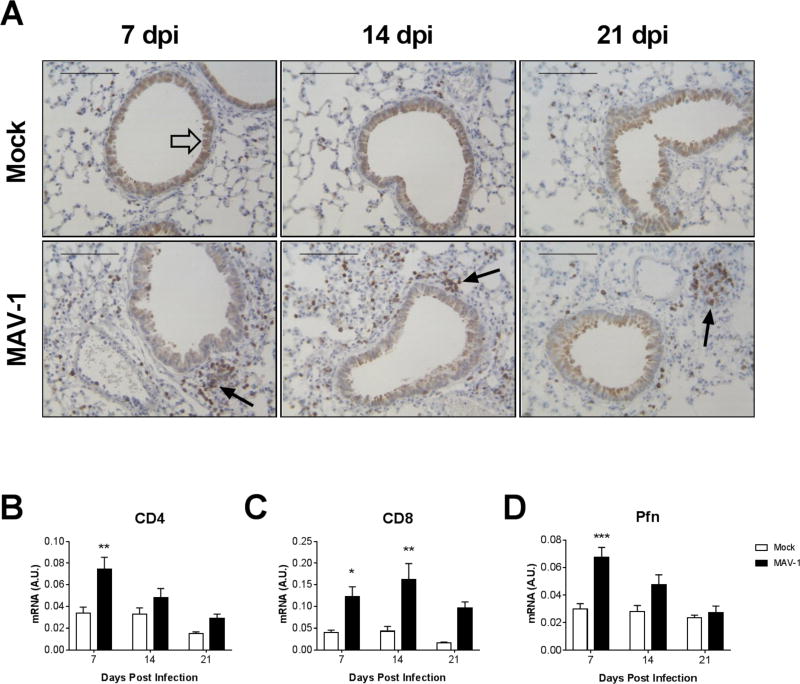
To characterize the kinetics and distribution of the CD8 T cell response to acute MAV-1 respiratory infection, we infected B6 mice i.n. with MAV-1 and used immunohistochemistry to identify CD3+ T cells in lungs of mock-infected and infected mice (Fig. 1A). A small number of scattered CD3+ cells were present in mock-infected lungs at all time points. There were substantially more CD3+ cells in the lungs of infected mice than in mock-infected mice at 7 days post infection (dpi). CD3+ cells tended to be concentrated around airways and blood vessels in lungs of infected mice. CD3+ cells were somewhat less abundant in the lungs of infected mice at 14 and 21 dpi than at 7 dpi, although there were still more than in lungs of mock-infected mice at each time point.
Figure 1.
T cell responses to acute MAV-1 respiratory infection. B6 mice were mock-infected or infected intranasally with MAV-1. (A) Lungs were harvested at the indicated time point. Sections from paraffin-fixed lungs CD3-stained sections were prepared from paraffin-embedded sections. CD3-positive cells are stained dark brown (examples indicated by black arrows). Respiratory epithelial cells lining larger airways exhibit lighter brown nonspecific staining (example indicated by open arrow). Scale bars, 100 μm. (B–D) RT-qPCR was used to quantify CD4, CD8, and Pfn mRNA levels in lungs. Data from n=5–16 mice per group combined from four or five independent experiments per time point are shown in arbitrary units standardized to GAPDH and presented as means ± S.E.M. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. *P<0.05, **P<0.01, and ***P<0.001.
We previously demonstrated increases in the population of CD62Llow effector memory CD4 and CD8 T cells in the lungs of MAV-1 infected mice (Procario et al., 2012), and CD8 T cells isolated from lungs produce effectors such as IFN-γ and GzmB in response to MAV-1 infection (McCarthy et al., 2015b; McCarthy et al., 2014). Consistent with those reports, CD4 mRNA levels were significantly greater in lungs of infected mice compared to mock-infected mice at 7 dpi and then decreased to levels similar to those in mock-infected mice by 14 dpi (Fig. 1B). CD8 mRNA levels were greater in lungs of infected mice compared to mock-infected mice at 7, 14, and 21 dpi (Fig. 1C), although the difference was not statistically significant at 21 dpi. Pfn mRNA levels were significantly greater in lungs of infected mice compared to mock-infected mice at 7 dpi (Fig. 1D).
To further characterize CD8 T cell responses to MAV-1 respiratory infection, we isolated CD8 T cells from spleens of infected and mock-infected mice and restimulated them ex vivo with anti-CD3 antibody. IFN-γ production by CD8 T cells isolated from MAV-1-infected mice at both 7 and 14 dpi was significantly greater than production by CD8 T cells isolated from mock-infected mice (Supplemental Fig. 1). Collectively, these data suggest that CD8 T cells respond to MAV-1 infection during acute respiratory infection.
Effects of CD8 T Cells on MAV-1 Replication in Lungs
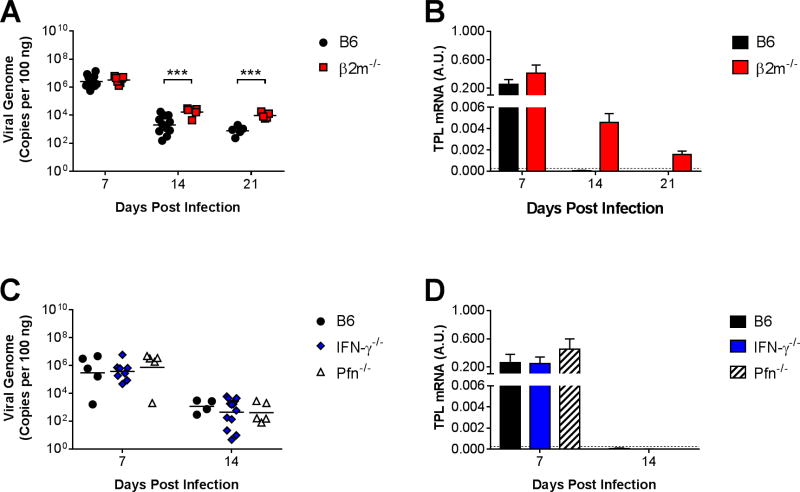
To determine whether CD8 T cells are essential for the control of viral replication in the lungs during acute infection, we infected β2m−/− mice, which are deficient in MHC class I expression and therefore largely deficient in CD8 T cell function (Koller et al., 1990). We used qPCR to quantify DNA viral loads in the lungs of infected B6 and β2m−/− mice (Fig. 2A). The highest lung viral loads in B6 mice were detected at 7 dpi, a time corresponding to peak viral replication in the lungs following i.n. inoculation (Procario et al., 2012). There was no significant difference between lung viral loads in B6 and β2m−/− mice at 7 dpi. Lung viral loads decreased from 7 to 14 dpi in B6 mice. Lung viral loads decreased to a lesser degree in β2m−/− mice, and they were significantly greater in β2m−/− mice compared to B6 mice at 14 dpi. Lung viral loads changed very little between 14 and 21 dpi in B6 and β2m−/− mice, and they remained significantly greater in β2m−/− mice compared to B6 mice at that time point.
Figure 2.
Effects of CD8 T cells on MAV-1 replication in the lung. B6 and β2m−/− mice were infected intranasally with MAV-1. (A) qPCR was used to quantify MAV-1 genome copies in the lungs at the indicated time points. DNA viral loads in the lungs of B6 and β2m−/− mice (n=5–12 per group combined from two independent experiments per time point) are expressed as copies of MAV-1 genome per 100 ng of input DNA. (B) RT-qPCR was used to quantify MAV-1 TPL mRNA levels in the lungs. Data are shown in arbitrary units standardized to GAPDH. In separate experiments, (C) lung viral loads and (D) TPL mRNA levels were quantified in infected B6, Pfn−/−, and IFN-γ−/− mice (n=4–13 per group from one independent experiment per time point). Individual circles represent values for individual mice, and horizontal bars represent means for each group. In B and D, horizontal dashed lines represent the limit of detection based on background levels detected in mock-infected B6 mice. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. ***P<0.001.
As an additional measure of viral replication, we used RT-qPCR to quantify viral gene expression in the lungs of B6 and β2m−/− mice. We used primers that amplified a region of the MAV-1 tripartite leader (TPL), similar to the HAdV three-exon untranslated TPL sequence that is present in late viral gene mRNAs (Logan and Shenk, 1984). We detected similar levels of TPL mRNA in the lungs of infected B6 and β2m−/− mice at 7 dpi (Fig. 2B). TPL mRNA levels in the lungs decreased substantially from 7 to 14 dpi in all infected mice. At 14 and 21 dpi, TPL mRNA was not detected above background levels in the lungs of B6 mice, while levels were detected but very low in β2m−/− mice. However, no infectious virus was detected by plaque assay in lungs of B6 or β2m−/− mice at 14 or 21 dpi (data not shown), suggesting that very little or no infectious virus was produced in the lungs at late time points.
To corroborate these data, we infected CD8α−/− mice, which are also deficient in functional CD8 T cells (Fung-Leung et al., 1991). CD8α−/− mice are also deficient in some non-migrating resident dendritic cells (DCs) that express CD8α (Shortman and Heath, 2010). Lung viral loads were slightly greater in CD8α−/− mice than in B6 mice at 7 dpi (Supplemental Fig. 2A). We detected a delay in clearance of viral DNA from lungs of CD8α−/− mice, with significantly greater lung viral loads at 14 dpi, although there was no difference between lung viral loads in B6 and CD8α−/− mice at 21 dpi. Likewise, TPL mRNA levels were not detected above background levels in lungs of CD8α−/− mice at 21 dpi (Supplemental Fig. 2B). Deficiency of specific CD8 T cell mediators in Pfn−/− or IFN-γ−/− mice was not associated with increased lung viral loads (Fig. 2C) or viral gene expression (Fig. 2D) compared to B6 mice, indicating that neither mediator was essential for clearance of viral DNA from the lungs or for the control of viral replication. Taken together, these measurements of viral loads and viral gene expression suggested that clearance of virus from the lungs was modestly impaired in the absence of CD8 T cells. Even in the presence of intact CD8 T cell function in B6 mice, MAV-1 DNA persisted in the lungs.
Effects of CD4 T Cells on Virus Clearance from the Lungs
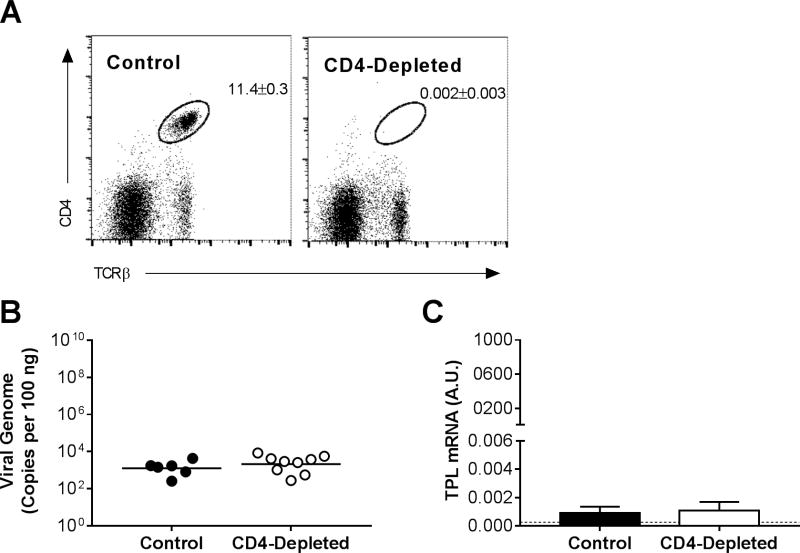
We detected increases in CD4 mRNA levels in the lungs of infected mice (Fig. 1), and the effector memory (CD62Llow) CD4 T cell population increases in lungs of MAV-1-infected mice (Procario et al., 2012). To determine whether CD4 T cells contributed to clearance of MAV-1 from the lungs, we measured lung viral loads and viral gene expression in B6 mice following antibody-mediated depletion of CD4 T cells. At 14 dpi, CD4+ cells comprised 11.4 ± 0.3% of splenocytes in control mice compared to 0.002 ± 0.003% of splenocytes in CD4-depleted mice (Fig. 3A; >99% reduction). In contrast to the effects of CD8 deficiency on lung viral loads and viral gene expression (Fig. 2A,B), depletion of CD4-positive cells had no effect on lung viral loads (Fig. 3B) or viral gene expression at 14 dpi compared to controls (Fig. 3C). Thus, CD4 T cells did not make essential contributions to clearance of viral DNA from or control of viral replication in the lungs during acute respiratory infection.
Figure 3.
Effects of CD4 T cells on MAV-1 replication in the lung. CD4 T cells were depleted from B6 mice using anti-CD4 antibody. Control mice received a nonspecific IgG control antibody. Mice were infected intranasally with MAV-1, and lungs were harvested at 14 dpi. (A) Flow cytometry was used to assess CD4 T cell depletion in a subset of mice. Splenocytes were analyzed for individual mice, and dot plots for one representative mouse of each group are shown. Percentages of CD4 T cells (CD4+, TCRβ+; n=2–4 mice per group; means ± S.E.M.) are indicated in the upper right quadrant for each group. (B) qPCR was used to quantify MAV-1 genome copies in the lungs. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. Individual circles represent values for individual mice from two independent experiments, and horizontal bars represent means for each group. (C) RT-qPCR was used to quantify MAV-1 TPL mRNA levels in the lungs. Data are shown in arbitrary units standardized to GAPDH (means ± S.E.M.). The horizontal dashed line represents the limit of detection based on background levels detected in mock-infected B6 mice. No statistically significant differences among groups in B or C were detected.
Effects of CD8 T Cell Deficiency on MAV-1-Induced Disease
The results of the experiments described above suggested that clearance of virus from the lungs during acute respiratory infection was somewhat impaired in the absence of CD8 T cells. To further evaluate the biological relevance of this effect, we assessed contributions of CD8 T cells to MAV-1-induced disease. We observed no mortality in infected B6 or β2m−/− mice up to 21 dpi (data not shown). We weighed B6 and β2m−/− mice during the course of infection to determine whether CD8 T cells contributed to this manifestation of disease. Compared to mock-infected B6 controls, infected B6 mice experienced significant weight loss by 7 to 8 dpi (Fig. 4). Infected B6 mice began to regain weight after 8 dpi but did not reach weights comparable to mock-infected controls by 20 dpi. In contrast, β2m−/− mice experienced no significant weight loss over the course of the experiment. Thus, although our data indicated that clearance of virus from the lungs was impaired in the absence of CD8 T cells, that effect was not associated with greater signs of disease. Instead, CD8 T cell deficiency protected from weight loss caused by MAV-1 infection.
Figure 4.
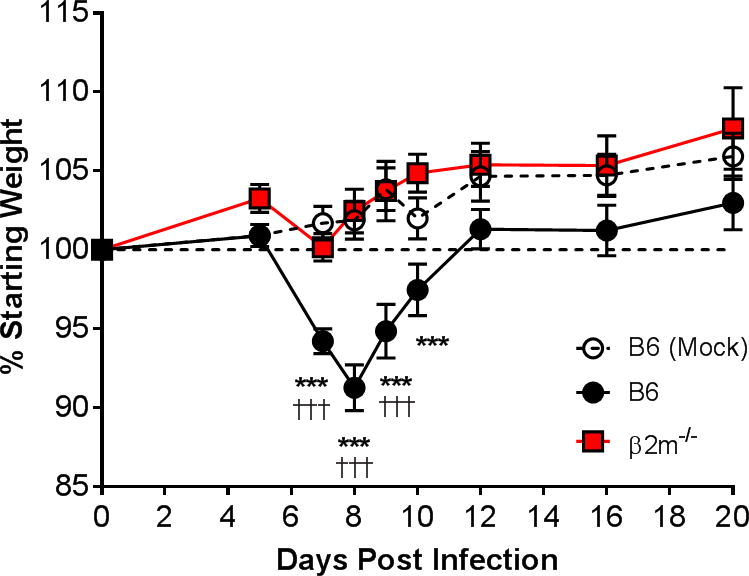
Effects of CD8 T cells on MAV-1-induced weight loss. A) Body weights were measured for mock infected and infected B6 mice and infected β2m−/− mice (n=5–28 mice per group combined from six independent experiments) at the indicated time points. Body weights are expressed as the percentage of starting weight. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. ***P<0.001 compared to mock-infected mice. †††P<0.001 compared to infected β2m−/− mice.
Effects of CD8 T Cells on Virus-Induced Airway Inflammation
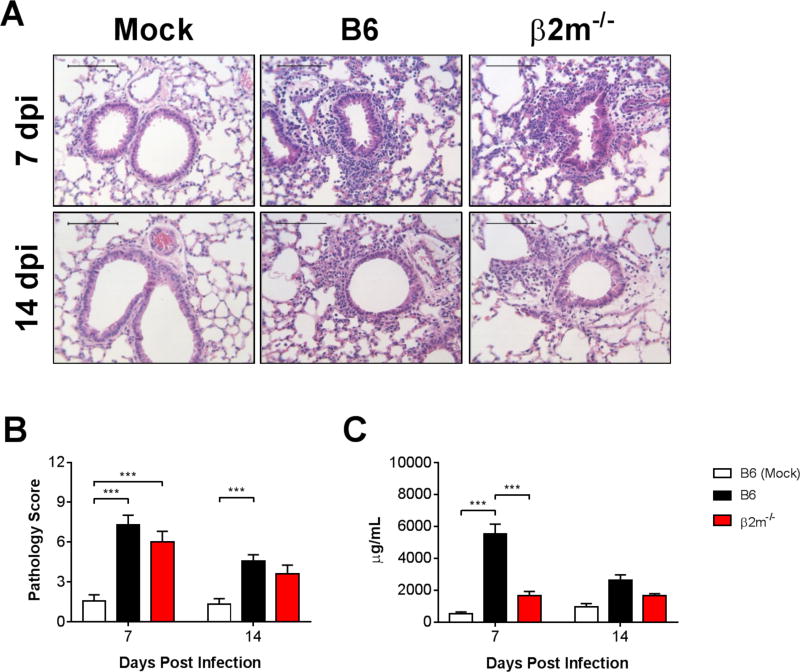
Because CD8 T cell-deficient mice did not lose weight despite impaired virus clearance, we hypothesized that they were protected from weight loss due to a decrease in virus-induced pulmonary inflammation. Histological evidence of pulmonary inflammation was qualitatively similar in infected B6 and β2m−/− mice at 7 and 14 dpi (Fig. 5A). There were no statistically significant differences between infected B6 and β2m−/− mice in lung pathology scores at 7 and 14 dpi (Fig. 5B). We also measured concentrations of total protein in BALF as an indicator of acute lung injury. The concentration of total protein in BALF was significantly greater in infected B6 mice than in mock-infected mice at 7 dpi (Fig. 5C). BALF protein concentrations were significantly lower in infected β2m−/− mice compared to infected B6 mice at 7 dpi. There were no significant differences between BALF protein concentrations in mock-infected mice and infected B6 or β2m−/− mice at 14 dpi.
Figure 5.
Effects of CD8 T cells on MAV-1-induced lung inflammation. B6 and β2m−/− mice were infected intranasally with MAV-1. B6 mice were mock-infected as controls. Lungs were harvested at the indicated time points and hematoxylin-and-eosin-stained sections were prepared from paraffin-embedded specimens. (A) Representative images are shown from mock-infected and infected mice. Scale bars, 100 μm. (B) Pathology index scores were generated to quantify cellular inflammation in the lungs of mock-infected and infected mice. Data (n=8 to 13 mice per group combined from two independent experiments at each time point) are presented as means ± S.E.M. (C) Total protein concentration was measured in BALF. Data (n=4–12 mice per group, except n=2 mock-infected mice at 7 dpi) are presented as means ± S.E.M. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. ***P<0.001.
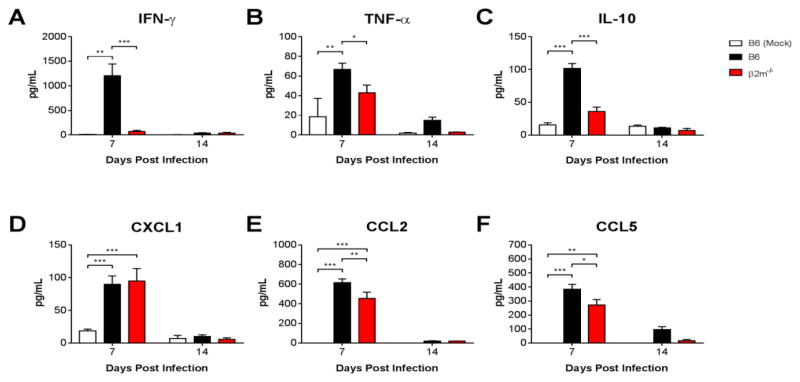
To further assess contributions of CD8 T cells to virus-induced inflammation during acute MAV-1 infection, we measured BALF concentrations of IFN-γ, the immunomodulatory cytokine IL-10, and proinflammatory cytokines and chemokines that are consistently induced by MAV-1 respiratory infection, including TNF-α, CXCL1, CCL2 and CCL5 (McCarthy et al., 2013; McCarthy et al., 2014; Procario et al., 2012). Concentrations of all cytokines and chemokines were significantly greater in infected B6 mice compared to mock-infected mice at 7 dpi and then decreased to levels close to those detected in mock-infected mice at 14 dpi (Fig. 6A–F). BALF IFN-γ concentrations were significantly lower in infected β2m−/− mice than in infected B6 mice, remaining close to levels detected in mock-infected mice at 7 and 14 dpi. BALF concentrations of TNF-α, IL-10, CCL2, and CCL5 were also lower in infected β2m−/− mice than in infected B6 mice at 7 dpi, although the magnitude of the effect was less than for IFN-γ. There was no difference between BALF CXCL1 concentrations in B6 and β2m−/− mice. Together with measurements of BALF total protein (Fig. 5C), these data indicate that CD8 T cells exert a proinflammatory effect in the airways of mice infected with MAV-1.
Figure 6.
Effects of CD8 T cells on MAV-1-induced cytokine production. B6 and β2m−/− mice were infected intranasally with MAV-1. B6 mice were mock-infected as controls. ELISA was used to quantify cytokine and chemokine protein concentrations in BALF obtained from infected and mock-infected mice at the indicated times. Data (n=3–12 mice per group combined from two independent experiments at each time point, except n=2 mock-infected mice at 7 dpi) are presented as means ± S.E.M. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. ***P<0.001 **P<0.01, and *P<0.05.
Effects of CD8 T Cell Depletion on Virus Clearance and Airway Inflammation
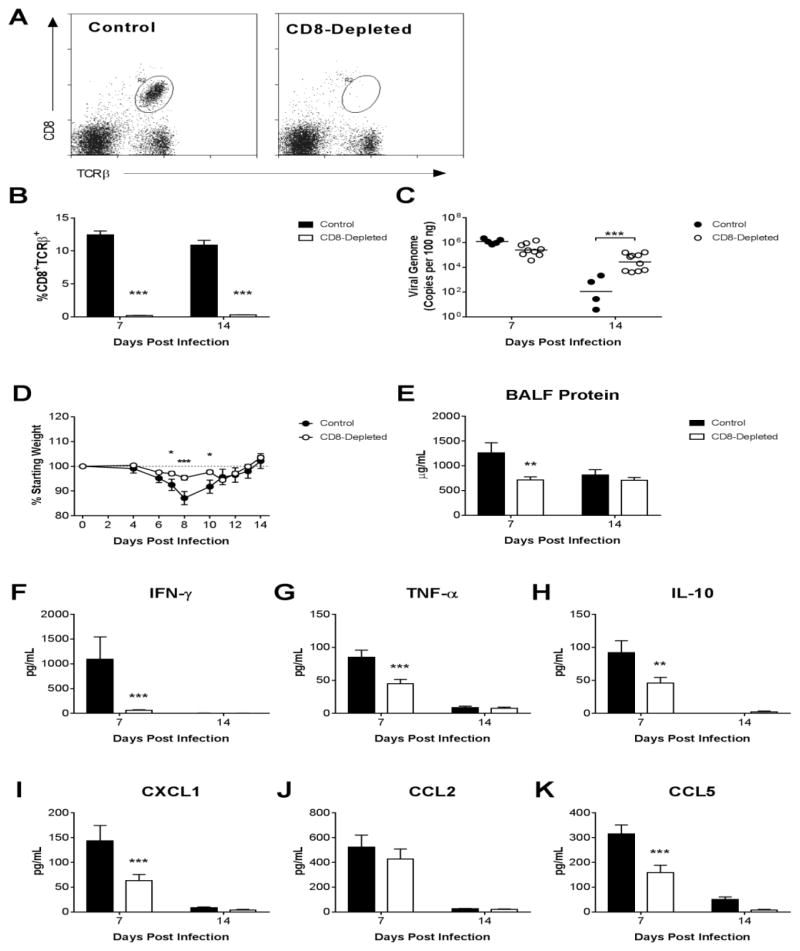
β2m−/− mice have defective natural killer (NK) cells (Liao et al., 1991) and are deficient in natural killer T (NKT) cells in addition to deficiency of CD8 T cells (Bendelac et al., 1994; Bix et al., 1993). We used antibody depletion of CD8 T cells from B6 mice to further confirm that effects observed in β2m−/− mice were due to their lack of CD8 T cells. Successful depletion of CD8 T cells was maintained through 14 dpi (>95% depletion at each time point; Fig. 7A,B). Lung viral loads in CD8-depleted mice were equivalent to those in control mice at 7 dpi but significantly greater than those in control mice at 14 dpi (Fig. 7C). CD8-depleted mice were protected from virus-induced weight loss (Fig. 7D), although protection was not as complete as it was in β2m−/− mice (Fig. 3). BALF protein concentrations were significantly lower in CD8-depleted mice than in infected controls at 7 dpi (Fig. 7E). BALF IFN-γ concentrations were significantly lower in infected CD8-depleted mice than in infected controls at 7 dpi (Fig. 7F). BALF concentrations of TNF-α, IL-10, CXCL1, and CCL5 were also significantly lower in infected CD8-depleted mice than in infected controls at 7 dpi, while there was no difference between groups in CCL2 concentrations (Fig. 7G–K). Collectively, the effects of CD8 T cell depletion closely resembled the effects observed in β2m−/− mice.
Figure 7.
Effects of CD8 depletion on virus clearance and airway inflammation. Following intranasal infection with MAV-1, CD8 T cells were depleted from B6 mice using anti-CD8 antibody. Control mice received a nonspecific IgG control antibody. (A) Flow cytometry was used to identify splenocytes that were CD8 T cells (CD8+, TCRβ+). Representative dot plots at 7 dpi are shown. (B) Flow cytometry data are summarized to indicate the percentages of splenocytes that were CD8 T cells at the indicated time points. (C) qPCR was used to quantify MAV-1 genome copies in the lungs. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. (D) Body weights are expressed as the percentage of starting weight. (E) Total protein concentration was measured in BALF. (F–K) ELISA was used to quantify cytokine and chemokine protein concentrations in BALF. In B individual circles represent values for individual mice one experiment, and horizontal bars represent means for each group. Data in E-K are presented as means ± S.E.M. (n=4–10 mice per group at each time point from one experiment; in D, n=8–19 per group up to 7 dpi, then n=4–10 per group up to 14 dpi). Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. *P<0.05, **P<0.01, and ***P<0.001.
Effects of CD8 T Cell Effectors on Virus-Induced Airway Inflammation
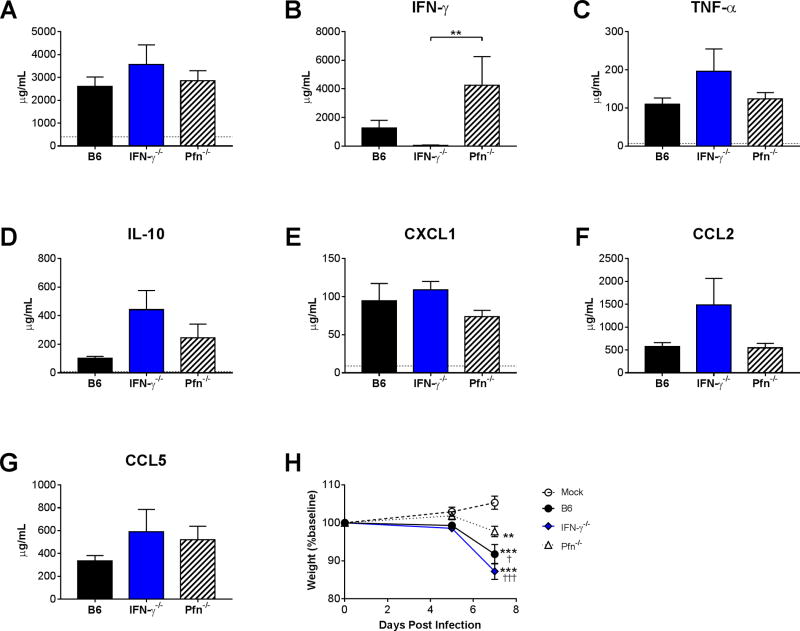
Although the CD8 T cell effectors Pfn and IFN-γ were not essential for control of viral replication in the lungs (Fig. 2C,D), we reasoned that either or both could mediate CD8 T cell-induced immunopathology during MAV-1 respiratory infection. To address this possibility, we assayed measures of pulmonary inflammation and weight loss in a subset of B6, Pfn−/−, and IFN-γ−/− mice at 7 dpi, when we detected the greatest effects of CD8 T cell deficiency. Histological evidence of lung inflammation and lung pathology scores did not differ between infected B6, Pfn−/−, and IFN-γ−/− mice at 7 dpi (data not shown), and there were no statistically significant differences between BALF total protein concentrations in infected B6, Pfn−/−, and IFN-γ−/− mice (Fig. 8A). As expected, IFN-γ was not detected in IFN-γ−/− mice (Fig. 8B). In no cases were cytokine or chemokine concentrations lower in IFN-γ−/− or Pfn−/− mice compared to B6 mice (Fig. 8B–G). However, virus-induced weight loss was significantly less in Pfn−/− mice compared to B6 or IFN-γ−/− mice at 7 dpi (Fig. 8H). While Pfn and IFN-γ were not essential for CD8 T cell promotion of proinflammatory cytokine production in the airways, effects mediated by Pfn contributed to virus-induced weight loss.
Figure 8.
Effects of Pfn and IFN-γ on MAV-1-induced airway inflammation. B6, Pfn−/−, and IFN-γ−/− mice (n=3–5 per group combined from two independent experiments) were infected intranasally with MAV-1. B6 mice were mock-infected as controls. (A) Total protein concentration was measured in BALF. (B–G) ELISA was used to quantify BALF cytokine and chemokine protein concentrations. Horizontal dotted lines in A–G (in some cases not visible if close to the X axis) indicate background levels detected in mock-infected B6 mice. Data in B–H are presented as means ± S.E.M, and statistical comparisons were made using Kruskal-Wallis test followed by Dunn’s multiple comparisons test. ***P<0.001. (H) Body weights are expressed as the percentage of starting weight and presented as means ± S.E.M. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. *P<0.05 and ***P<0.001 compared to mock-infected mice. †P<0.05 and †††P<0.001 compared to infected Pfn−/− mice.
Discussion
CD8 T cells are important components of host defense against a variety of pathogens. The cytolytic activity of CD8 T cells facilitates immune-mediated clearance of many types of viruses, but CD8 T cell activity can also contribute to pathology (Cannon et al., 1988). In this study, we defined the role of CD8 T cells in acute MAV-1 respiratory infection. Our results indicate that CD8 T cells contributed to clearance of viral DNA from the lungs of mice infected with MAV-1, but they were not essential for control of viral replication in the lungs. IFN-γ and Pfn, effector molecules produced by CD8 T cells, were each dispensable for control of viral replication. However, CD8 T cells did make substantial contributions to local inflammatory responses in the airways of infected mice. Additionally, CD8 T cells were required for virus-induced weight loss, a systemic manifestation of disease. We therefore conclude that the overall effect of CD8 T cells during acute MAV-1 respiratory infection is to promote local production of inflammatory cytokines in the airways rather than provide a protective antiviral effect.
CD8 T cell deficiency had no effect on the control of peak viral replication in the lungs, as assessed by measurements of viral loads and viral gene expression at 7 dpi. Clearance of viral DNA from the lungs was delayed in β2m−/− and CD8α−/− mice, consistent with the role for CD8 T cells suggested by increased MAV-1 lung viral loads and T cell dysfunction in mice following allogeneic bone marrow transplantation (McCarthy et al., 2015c). However, here we did not detect a substantial effect of CD8 T cell deficiency on MAV-1 replication in the lungs when using viral gene expression as a measure of viral replication. The discrepancy between an effect on measurements of viral DNA and viral gene expression could reflect a role for CD8 T cells in clearing dead cells that contain viral DNA but no longer support viral replication. Alternatively, the presence of viral DNA without detectable viral gene expression could represent a reservoir of persistent MAV-1. If this were the case, our data would support the possibility that MAV-1 persists in the lung in a non-replicative state, and that CD8 T cell function modulates the size of the reservoir of persistent virus as it is established in the lungs early during acute infection.
Data from other models indicate that virus-specific CD8 T cell phenotype and function can differ depending on the specific organ or compartment studied (Palendira et al., 2008; Piet et al., 2011; Remmerswaal et al., 2012). Moore et al. demonstrated a delay in clearance of replicating MAV-1 from the brains of β2m−/− mice at 8 dpi following i.p. inoculation (Moore et al., 2003), suggesting that there may be roles for CD8 T cells during acute MAV-1 infection that are specific to route of inoculation or to a particular organ. As Moore et al. described in the brain, we found that Pfn was not essential for control of MAV-1 replication in the lungs or for clearance of viral DNA from the lungs. Likewise, IFN-γ deficiency did not impair clearance of viral DNA or control of replication in this study. MAV-1 lung viral loads are slightly greater in IFN-γ-deficient mice on a BALB/c background (Procario et al., 2012), indicating that the effects of IFN-γ may depend on background mouse strain. It is possible that Pfn and IFN-γ exert overlapping functions in the context of MAV-1 infection, as has been described for IFN-γ and Pfn with murine gammaherpesvirus 68 infection (Tsai et al., 2011). CD8 T cells can control viral infection via other effector mechanisms, such as interactions between Fas and FasL (Chavez-Galan et al., 2009; Harty et al., 2000; Hoves et al., 2010), although work with influenza indicates some redundancy in the contributions of Pfn and Fas to control of viral replication (Topham et al., 1997). Future work using our model will allow us to determine whether Fas/FasL interactions or other CD8 T cell effector mechanisms, such as TNF-α production or TNF-related apoptosis-inducing ligand (TRAIL) (Brincks et al., 2008), contribute to the effects of CD8 T cells on clearance of viral DNA that we describe in this study.
While CD8 T cells were not essential for control of MAV-1 replication in the lungs during acute infection, our data indicate that that CD8 T cells drive local inflammatory responses in the airways during the peak of viral replication in the lungs. CD8 T cells contribute to immunopathology associated with other respiratory viruses, such as influenza, RSV, and pneumonia virus of mice (Ostler et al., 2002; Walsh et al., 2014; Wells et al., 1981). MAV-1-induced airway inflammatory cytokine responses were associated with lung injury, as indicated by increased total protein concentrations in BALF. The effects of CD8 T cells on pulmonary inflammation were limited to the airways, with no substantial differences in overall histological evidence of virus-induced pulmonary inflammation. This is consistent with CD8 T cell responses that are specific to the site of replication during acute MAV-1 respiratory infection, which includes respiratory epithelial cells (Weinberg et al., 2005). The CD8 T cell effector molecules Pfn and IFN-γ were not required for control of MAV-1 replication in the lungs, and our results indicate that those effectors are also dispensable for the proinflammatory effects of CD8 T cells in the lungs of mice acutely infected with MAV-1. Although IFN-γ promotes cardiac inflammation in neonatal mice infected with MAV-1 (McCarthy et al., 2015a), virus-induced pulmonary inflammation in adult mice was not affected by IFN-γ deficiency in this study. Likewise, we observed no effect of Pfn deficiency on MAV-1-induced pulmonary inflammation. Other CD8 T cell effectors such as TNF-α may be more important contributors to airway inflammation induced by MAV-1 infection, as others have shown in models of infection with RSV and influenza (DeBerge et al., 2013; Hussell et al., 2001; Peper and Van Campen, 1995; Xu et al., 2004). Pfn−/− mice were partially protected from weight loss at 7 dpi. This apparent disconnect between pulmonary inflammation and weight loss is likely due to Pfn-dependent effects of CD8 T cells on MAV-1-induced inflammation outside of the lung. For instance, Pfn deficiency is associated with reduced inflammation in the brains of mice infected i.p. with MAV-1 without an effect on viral replication (Moore et al., 2003).
HAdV shedding can be detected in respiratory and fecal samples for extended periods (Fox et al., 1969; Fox et al., 1977), and HAdV persistence in the lungs has been linked to the pathogenesis of chronic obstructive pulmonary disease and asthma (Hogg, 2001). Studies in human lymphocyte and monocyte cell lines suggest that those cell types may support persistent infection (Chu et al., 1992; Zhang et al., 2010). In vivo, HAdV persists in mucosal lymphoid tissue, predominantly in T lymphocytes (Garnett et al., 2002; Garnett et al., 2009). HAdV DNA can also be found in nonlymphoid cells, such as respiratory epithelial cells (Eissa et al., 1994; Elliott et al., 1995). Like HAdV, MAV-1 persists in an infected host following the resolution of acute infection. For instance, MAV-1 DNA is detected in hearts up to 12 weeks post infection in hearts following i.n. infection of neonatal B6 mice (McCarthy et al., 2015a), and MAV-1 is shed in the urine of Swiss Webster mice infected i.p. as adults for up to 55 weeks post infection (Smith et al., 1998b). Our data indicate that MAV-1 persists in the lungs of infected mice even in the presence of intact CD8 T cell function. It remains to be seen whether CD8 T cells are essential for long-term control of persistent MAV-1.
In summary, our data indicate that CD8 T cells were not essential for efficient control of MAV-1 replication at the peak of infection in the lungs, but CD8 T cell deficiency was associated with delayed clearance of virus from the lungs. CD8 T cells were important contributors to airway inflammatory responses during acute MAV-1 respiratory infection. It remains possible that other immune cells, such as NK and NKT cells, made small contributions to the effects on virus clearance and inflammation. However, our combined data from CD8 T cell-deficient and CD8 T cell-depleted mice indicate that CD8 T cells are the predominant immune cells that are essential for those effects. Although not directly addressed in this study, it is possible that MAV-1-specific CD8 T cells in an immune host may exert a protective effect against repeat infection, as we have shown for CD4 T cells (McCarthy et al., 2013). This would be consistent with effects of virus-specific CD8 T cell function in humans, as suggested by the successful use of adoptive transfer of virus-specific cytotoxic T cells as therapy for immunocompromised patients with HAdV infection (Chakupurakal et al., 2013; Feuchtinger et al., 2004; Geyeregger et al., 2014; Heemskerk et al., 2003; Leen et al., 2009; Leen et al., 2004; Smith et al., 1996; Smith et al., 1998a). It will be important to further define correlates of protection and disease in order to optimize this type of therapy. An increased understanding of CD8 T cell function during adenovirus infection will aid in efforts to enhance protective antiviral effects while minimizing deleterious inflammatory effects of virus-specific cells.
Supplementary Material
Highlights.
CD8 T cells were not essential for control of peak MAV-1 replication in the lungs.
Clearance of virus from the lungs was delayed in CD8 T cell-deficient mice.
CD8 T cells were required for virus-induced weight loss and airway inflammation.
IFN-γ or perforin deficiency did not affect viral replication or airway inflammation.
Acknowledgments
Expert technical assistance from Joel Whitfield in the University of Michigan Cancer Center Immunology Core is greatly appreciated. We thank Mary McCarthy, Kathy Spindler, and Bethany Moore for helpful review of the manuscript. This research was supported by R01 AI083334, R21 AI103452, a University of Michigan Amendt-Heller Award for Newborn Research, and a University of Michigan Charles Woodson Accelerator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock IM, Lane SJ. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol. 2003;178:347–355. doi: 10.1677/joe.0.1780347. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Bix M, Coles M, Raulet D. Positive selection of V beta 8+ CD4-8- thymocytes by class I molecules expressed by hematopoietic cells. J Exp Med. 1993;178:901–908. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauthen AN, Welton AR, Spindler KR. Construction of mouse adenovirus type 1 mutants. Methods in molecular medicine. 2007;130:41–59. doi: 10.1385/1-59745-166-5:41. [DOI] [PubMed] [Google Scholar]
- Chakupurakal G, Onion D, Bonney S, Cobbold M, Mautner V, Moss P. HLA-peptide multimer selection of adenovirus-specific T cells for adoptive T-cell therapy. J Immunother. 2013;36:423–431. doi: 10.1097/CJI.0b013e3182a8029e. [DOI] [PubMed] [Google Scholar]
- Chavez-Galan L, Arenas-Del Angel MC, Zenteno E, Chavez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009;6:15–25. doi: 10.1038/cmi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Sperber K, Mayer L, Hsu MT. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology. 1992;188:793–800. doi: 10.1016/0042-6822(92)90534-v. [DOI] [PubMed] [Google Scholar]
- DeBerge MP, Ely KH, Cheng GS, Enelow RI. ADAM17-mediated processing of TNF-alpha expressed by antiviral effector CD8+ T cells is required for severe T-cell-mediated lung injury. PLoS One. 2013;8:e79340. doi: 10.1371/journal.pone.0079340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtisham S, Sunil-Chandra NP, Nash AA. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa NT, Chu CS, Danel C, Crystal RG. Evaluation of the respiratory epithelium of normals and individuals with cystic fibrosis for the presence of adenovirus E1a sequences relevant to the use of E1a- adenovirus vectors for gene therapy for the respiratory manifestations of cystic fibrosis. Hum Gene Ther. 1994;5:1105–1114. doi: 10.1089/hum.1994.5.9-1105. [DOI] [PubMed] [Google Scholar]
- Elliott WM, Hayashi S, Hogg JC. Immunodetection of adenoviral E1A proteins in human lung tissue. Am J Respir Cell Mol Biol. 1995;12:642–648. doi: 10.1165/ajrcmb.12.6.7766428. [DOI] [PubMed] [Google Scholar]
- Feuchtinger T, Lang P, Hamprecht K, Schumm M, Greil J, Jahn G, Niethammer D, Einsele H. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Experimental hematology. 2004;32:282–289. doi: 10.1016/j.exphem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Fox JP, Brandt CD, Wassermann FE, Hall CE, Spigland I, Kogon A, Elveback LR. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969;89:25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- Fox JP, Hall CE, Cooney MK. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977;105:362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76:10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett CT, Talekar G, Mahr JA, Huang W, Zhang Y, Ornelles DA, Gooding LR. Latent species C adenoviruses in human tonsil tissues. J Virol. 2009;83:2417–2428. doi: 10.1128/JVI.02392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyeregger R, Freimuller C, Stemberger J, Artwohl M, Witt V, Lion T, Fischer G, Lawitschka A, Ritter J, Hummel M, Holter W, Fritsch G, Matthes-Martin S. First-in-man clinical results with good manufacturing practice (GMP)-compliant polypeptide-expanded adenovirus-specific T cells after haploidentical hematopoietic stem cell transplantation. J Immunother. 2014;37:245–249. doi: 10.1097/CJI.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annual review of immunology. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- Heemskerk B, Veltrop-Duits LA, van Vreeswijk T, ten Dam MM, Heidt S, Toes RE, van Tol MJ, Schilham MW. Extensive cross-reactivity of CD4+ adenovirus-specific T cells: implications for immunotherapy and gene therapy. J Virol. 2003;77:6562–6566. doi: 10.1128/JVI.77.11.6562-6566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC. Role of latent viral infections in chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med. 2001;164:S71–S75. doi: 10.1164/ajrccm.164.supplement_2.2106063. [DOI] [PubMed] [Google Scholar]
- Hoves S, Trapani JA, Voskoboinik I. The battlefield of perforin/granzyme cell death pathways. J Leukoc Biol. 2010;87:237–243. doi: 10.1189/jlb.0909608. [DOI] [PubMed] [Google Scholar]
- Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Sili U, Savoldo B, Jewell AM, Piedra PA, Brenner MK, Rooney CM. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103:1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Logan J, Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984;81:3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Levine RE, Procario MC, McDonnell PJ, Zhu L, Mancuso P, Crofford LJ, Aronoff DM, Weinberg JB. Prostaglandin E2 induction during mouse adenovirus type 1 respiratory infection regulates inflammatory mediator generation but does not affect viral pathogenesis. PLoS One. 2013;8:e77628. doi: 10.1371/journal.pone.0077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Procario MC, Twisselmann N, Wilkinson JE, Archambeau AJ, Michele DE, Day SM, Weinberg JB. Proinflammatory effects of interferon gamma in mouse adenovirus 1 myocarditis. J Virol. 2015a;89:468–479. doi: 10.1128/JVI.02077-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Procario MC, Wilke CA, Moore BB, Weinberg JB. Prostaglandin E2 production and T cell function in mouse adenovirus type 1 Infection following allogeneic bone marrow rransplantation. PLoS One. 2015b;10:e0139235. doi: 10.1371/journal.pone.0139235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Procario MC, Wilke CA, Moore BB, Weinberg JB. Prostaglandin E2 production and T cell function in mouse adenovirus type 1 infection following allogeneic bone marrow transplantation. PLoS ONE. 2015c;10:e0139235. doi: 10.1371/journal.pone.0139235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Zhu L, Procario MC, Weinberg JB. IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection. Virology. 2014;456–457:259–267. doi: 10.1016/j.virol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ML, Brown CC, Spindler KR. T cells cause acute immunopathology and are required for long-term survival in mouse adenovirus type 1-induced encephalomyelitis. J Virol. 2003;77:10060–10070. doi: 10.1128/JVI.77.18.10060-10070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullbacher A, Hla RT, Museteanu C, Simon MM. Perforin is essential for control of ectromelia virus but not related poxviruses in mice. J Virol. 1999;73:1665–1667. doi: 10.1128/jvi.73.2.1665-1667.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Y, McGuffie BA, Anderson VE, Weinberg JB. Gammaherpesvirus modulation of mouse adenovirus type 1 pathogenesis. Virology. 2008;380:182–190. doi: 10.1016/j.virol.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostler T, Davidson W, Ehl S. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur J Immunol. 2002;32:2117–2123. doi: 10.1002/1521-4141(200208)32:8<2117::AID-IMMU2117>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Palendira U, Chinn R, Raza W, Piper K, Pratt G, Machado L, Bell A, Khan N, Hislop AD, Steyn R, Rickinson AB, Buckley CD, Moss P. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood. 2008;112:3293–3302. doi: 10.1182/blood-2008-02-138040. [DOI] [PubMed] [Google Scholar]
- Peper RL, Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog. 1995;19:175–183. doi: 10.1006/mpat.1995.0056. [DOI] [PubMed] [Google Scholar]
- Piet B, de Bree GJ, Smids-Dierdorp BS, van der Loos CM, Remmerswaal EB, von der Thusen JH, van Haarst JM, Eerenberg JP, ten Brinke A, van der Bij W, Timens W, van Lier RA, Jonkers RE. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011;121:2254–2263. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procario MC, Levine RE, McCarthy MK, Kim E, Zhu L, Chang CH, Hershenson MB, Weinberg JB. Susceptibility to acute mouse adenovirus type 1 respiratory infection and establishment of protective immunity in neonatal mice. J Virol. 2012;86:4194–4203. doi: 10.1128/JVI.06967-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmerswaal EB, Havenith SH, Idu MM, van Leeuwen EM, van Donselaar KA, Ten Brinke A, van der Bom-Baylon N, Bemelman FJ, van Lier RA, Ten Berge IJ. Human virus-specific effector-type T cells accumulate in blood but not in lymph nodes. Blood. 2012;119:1702–1712. doi: 10.1182/blood-2011-09-381574. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Smith CA, Woodruff LS, Kitchingman GR, Rooney CM. Adenovirus-pulsed dendritic cells stimulate human virus-specific T-cell responses in vitro. J Virol. 1996;70:6733–6740. doi: 10.1128/jvi.70.10.6733-6740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Woodruff LS, Rooney C, Kitchingman GR. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum Gene Ther. 1998a;9:1419–1427. doi: 10.1089/hum.1998.9.10-1419. [DOI] [PubMed] [Google Scholar]
- Smith K, Brown CC, Spindler KR. The role of mouse adenovirus type 1 early region 1A in acute and persistent infections in mice. J Virol. 1998b;72:5699–5706. doi: 10.1128/jvi.72.7.5699-5706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- Tsai CY, Hu Z, Zhang W, Usherwood EJ. Strain-dependent requirement for IFN-gamma for respiratory control and immunotherapy in murine gammaherpesvirus infection. Viral Immunol. 2011;24:273–280. doi: 10.1089/vim.2011.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls T, Shankar AG, Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect Dis. 2003;3:79–86. doi: 10.1016/s1473-3099(03)00515-2. [DOI] [PubMed] [Google Scholar]
- Walsh CM, Matloubian M, Liu CC, Ueda R, Kurahara CG, Christensen JL, Huang MT, Young JD, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci U S A. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Teijaro JR, Brock LG, Fremgen DM, Collins PL, Rosen H, Oldstone MB. Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J Virol. 2014;88:6281–6293. doi: 10.1128/JVI.00464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Jensen DR, Gralinski LE, Lake AR, Stempfle GS, Spindler KR. Contributions of E1A to mouse adenovirus type 1 pathogenesis following intranasal inoculation. Virology. 2007;357:54–67. doi: 10.1016/j.virol.2006.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Stempfle GS, Wilkinson JE, Younger JG, Spindler KR. Acute respiratory infection with mouse adenovirus type 1. Virology. 2005;340:245–254. doi: 10.1016/j.virol.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MA, Albrecht P, Ennis FA. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981;126:1036–1041. [PubMed] [Google Scholar]
- Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J Immunol. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang W, Ornelles DA, Gooding LR. Modeling adenovirus latency in human lymphocyte cell lines. J Virol. 2010;84:8799–8810. doi: 10.1128/JVI.00562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.